Summary
18F-labelled–fluorodeoxyglucose positron emission tomography (FDG-PET) findings are challenging to interpret for residual disease versus complete response in paediatric patients with non-Hodgkin lymphoma (NHL). A biopsy is often warranted to confirm the presence or absence of viable tumour if there is clinical or radiographic evidence of residual disease. In this study, we compared conventional imaging and FDG-PET/computerized tomography (CT) findings with biopsy results in 18 children with NHL. Our goal was to provide additional data to establish more reliable criteria for response evaluation. Residual disease was suspected after conventional imaging alone in 8 patients, after FDG-PET/CT alone in 3 and after both modalities in 7 patients. Biopsy confirmed the presence of viable tumour in 2 patients. Two additional patients experienced progressive disease or relapse. The sensitivity and negative predictive value of FDG-PET/CT using the London criteria to indicate residual tumour detectable by biopsy were 100%, but specificity was low (60%), as was the positive predictive value (25%). Thus, in this study, a negative FDG-PET/CT finding was a good indicator of complete remission. However, because false-positive FDG-PET/CT findings are common, biopsy and close monitoring are required for accurate determination of residual disease in individual patients.
Keywords: non-Hodgkin Lymphoma, Positron-emission tomography, Neoplasm, residual, biopsy
Introduction
During the past 15-20 years, the use of 18F-labelled–fluorodeoxyglucose positron emission tomography (FDG-PET) in the management of patients with lymphomas has continued to increase. Because FDG-PET measures regional glucose uptake, it is extremely sensitive in detecting metabolically active tissue. Thus, in combination with a computerized tomography (CT) scan for lesion localization, it has gained popularity for initial staging, response evaluation, and follow-up. In 2007, FDG-PET/CT data was incorporated in the revised criteria for response evaluation of malignant lymphomas that was published by the International Harmonization Project (i.e., revised Cheson criteria) (Cheson, et al 2007). In patients with Hodgkin lymphoma, interim FDG-PET/CT is highly sensitive and specific for predicting survival, and multiple trials to study FDG-PET/CT response–adapted therapy are ongoing (Hutchings 2014, Moskowitz, et al 2010a). However, results of FDG-PET/CT are not widely used to guide therapy in patients with non-Hodgkin lymphoma (NHL) (Moskowitz, et al 2010b), primarily because of heterogeneity and lack of evidence of a reasonable positive predictive value (Moskowitz, et al 2010a, Terasawa, et al 2009). Therefore, additional studies are required to provide data to formulate guidelines for incorporating FDG-PET/CT findings in response assessment in paediatric NHL (Kluge, et al 2013, Shankar, et al 2008).
Patients with NHL undergo imaging during therapy and at the completion of therapy to assess response. In certain subtypes of NHL in adults, such as diffuse large B-cell lymphoma (DLBCL), limited data suggest that patients whose imaging results indicate a rapid, early response may have outcomes superior to those of patients whose imaging results indicate a slower response (Mikhaeel, et al 2005, Safar, et al 2012). Depending on the subtype of NHL, an interim evaluation to assess complete response (i.e., a CR evaluation) is performed after 3-5 cycles of chemotherapy. Finding residual disease at this time often guides further therapy decisions and necessitates upstaging to more aggressive therapy. If clinical examination or imaging findings are suggestive of residual disease, then standard practice involves a biopsy of the residual mass for pathological confirmation of viable tumour prior to a decision to escalate therapy.
Here, we focus on the utility of FDG-PET/CT in detecting residual disease at the time of response evaluation, with the understanding that invasive biopsy procedures may not be necessary if FDG-PET/CT is proven to be highly sensitive and specific for detecting residual tumour. Therefore, we retrospectively evaluated patients with NHL in whom clinical or radiographic findings were suggestive of a residual tumour and a biopsy was performed, comparing the FDG-PET/CT and conventional imaging findings with the biopsy results.
Patients and Methods
Patients
Diagnostic imaging and pathology databases were reviewed retrospectively for children with mature B-cell NHL (B-NHL) and anaplastic large cell lymphoma (ALCL) treated at our institution from August 2004 to May 2012. These two subtypes of NHL were chosen as they are both treated with cyclic chemotherapy regimens and CR evaluation is done at similar time-points (10-15 weeks). Patients were included in this study if two criteria were fulfilled: 1) They underwent biopsy because of clinical or radiographic findings suggestive of residual disease (relapse in 1 case), and 2) FDG-PET/CT images obtained within the 2 weeks prior to the biopsy were available. This study was approved by the institutional review board of St. Jude Children's Research Hospital.
Therapy
Therapy was based on contemporary approaches specific to the subtype of NHL. For example, patients with mature B-cell lymphoma received French-American-British Mature B-cell lymphoma 96 (FAB LMB)–based regimens (Cairo, et al 2007, Patte, et al 2007), and patients with ALCL received NHL- Berlin-Frankfurt-Munster (BFM) 90 (Seidemann, et al 2001) or APO (vincristine, adriamycin, prednisone) (Weinstein, et al 1984)-based regimens. The time of each response evaluation was driven by the guidelines of each patient's respective therapeutic study. Multidisciplinary meetings that included oncologists and radiologists were held to review all patient data and images. Surgeons participated in these meetings if residual disease was suspected.
Imaging
All patients underwent baseline contrast-enhanced CT of the neck, chest, abdomen and pelvis and had FDG-PET/CT scans that showed metabolically active disease at diagnosis. These studies and, when indicated, ultrasound (US) or magnetic resonance imaging (MRI) were obtained at the time of response evaluation. For this study, baseline and follow-up FDG-PET/CT and contrast-enhanced CT, US or MRI underwent joint, retrospective review by 2 experienced paediatric imaging physicians who were blinded to the biopsy results. From the CT, US and MRI examinations, bidirectional measurements of residual masses were obtained in the axial plane. For the FDG-PET/CT image acquisition, 5.5 MBq/kg FDG (maximum, 444 MBq) was injected intravenously. Blood glucose was verified to be normal before FDG injection. Patients were kept in a quiet, dark room after injection and were instructed to relax in a recumbent position with their arms at their sides. Approximately 1 hour later, a GE Discovery LS PET/CT system (GE Medical Systems, Waukesha, WI) was used to acquire transmission CT images and PET emission images for attenuation correction and lesion localization. CT acquisition parameters were as follows: tube rotation, 0.8 second; slice thickness, 0.5 cm; table speed, 1.5 cm/rotation; pitch, 1.5:1; 120 kV; 90 mA, with dose modulation. Whole-body PET images were obtained from the top of skull to the feet for 5 minutes per bed position in two-dimensional mode. All scans but one included extremities. Scans obtained after July 2011 were acquired on a GE Discovery 690 PET/CT system in three-dimensional mode with CT parameters similar to those used with the GE Discovery LS PET/CT system.
The maximum standardized uptake values (SUVmax) of residual masses were measured from FDG-PET/CT images, and the London criteria (5-point scale) were used to subjectively assess post-baseline FDG-PET/CT (Le Roux, et al 2011, Meignan, et al 2009). The London criteria are defined as follows: 1= no uptake above background; 2= uptake equal to or lower than mediastinum; 3= uptake between mediastinum and liver uptake; 4=uptake moderately increased compared to the liver and 5= uptake markedly increased compared to the liver.
Biopsy
Biopsies were performed by paediatric surgeons or interventional radiologists. The site and approach were chosen based on safety of the procedure and access to sufficient material for analysis. In patients with suspicious findings by FDG-PET/CT, biopsy was done of the most representative PET-avid site (nodal or extranodal), which, in many cases, was the only site of activity. All biopsies were reviewed by a paediatric haematopathologist using standard diagnostic methods, including immunohistochemical staining to characterize the tumour at initial presentation.
Statistical Analysis
A London score of 4 or 5 was considered to be a positive FDG-PET/CT result; a score of 3 was indeterminate, and a score of 1 or 2 was considered to be negative. An FDG-PET/CT study was considered true-positive if biopsy confirmed the presence of malignant cells. The sensitivity, specificity, and positive and negative predictive values for FDG-PET/CT were calculated by using standard methods (Altman and Bland 1994a, Altman and Bland 1994b). To evaluate the utility of FDG-PET/CT in identifying patients who experience progressive disease or relapse, a separate analysis was done wherein FDG-PET/CT was considered true-positive if the patient experienced an event (progressive disease or relapse), irrespective of biopsy findings. All other analyses were descriptive.
Results
Patient Characteristics
Seventy-three patients with B-NHL and ALCL were treated at our institution between August 2004 and May 2012 (26 with Burkitt lymphoma, 19 with DLBCL, 9 with primary mediastinal large cell lymphoma and 19 with ALCL). Of these, 18 (25%) underwent concurrent conventional and FDG-PET/CT imaging and pathological examination of samples from biopsy of a residual mass (Table I). Median age of the patients at diagnosis was 15.7 years (range 2.7–20.6 years). For 16 patients, biopsies were done at the time of response evaluation during or at the end of therapy according to the guidelines of the therapeutic protocols. One patient (Patient 4) underwent biopsy during the post-therapy follow-up period, and another patient (Patient 10) underwent biopsy at the time of early response evaluation for suspicion of a new site of disease. Fifteen patients had mature B-cell lymphoma (Burkitt lymphoma, DLBCL or primary mediastinal B-cell lymphoma) and 3 ALCL. The median time from diagnosis to disease evaluation for all patients was 2.3 months. According to the therapeutic study and treatment arm, for patients with B-NHL, the median time from diagnosis to evaluation was 2.4 months (range, 1.7 to 4.7 months). Evaluations for the 3 patients with ALCL were done at 3.4 months, 14.6 months and 2.1 months respectively. At the time of evaluation, findings suggestive of residual disease were detected by conventional imaging (with or without FDG-PET/CT findings) in 15 patients and by FDG-PET/CT alone in 3 patients.
Table I. Patient Characteristics and Results of Imaging and Biopsy (N =18).
| Patient | Age (years) |
Diagnosis | Therapy | Diagnosis to evaluation (months) |
SUV max |
London criteria |
FDG-PET result |
Conventional imaging |
Biopsy Result | Biopsy site (modality) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.4 | Burkitt lymphoma | LMB 96 | 3.8 | 17.5 | 5 | positive | positive (CT) 1.9 × 1.1 | positive | periaortic nodes (excisional) | Progressive disease |
| 2 | 18.1 | MLBCL | LMB 96 | 4.7 | 4.8 | 5 | positive | positive (MRI) 1.1 × 0.8 nodule | positive | mediastinum (resection) | Relapse |
| 3 | 15.3 | ALCL | COG NHL0131 | 3.4 | 10.9 | 5 | positive | positive (CT) 3.8 × 3.2 | negative- necrosis | mediastinum (excisional) | Relapse |
| 4 | 2.7 | ALCL | NHL-BFM 90 | 14.6 | 2.9 | 4 | positive | negative (CT) 1.7 × 0.6 | negative-lymphoid tissue | cervical node (core) | Relapse |
| 5 | 15.7 | MLBCL | LMB 96 | 1.9 | 5.4 | 4 ; 5 | positive | positive (CT) 5.0 × 3.5 | negative-xanthomatous pseudotumour | mediastinum (multiple) | CR |
| 6 | 19.9 | Burkitt lymphoma | LMB 96 | 3.1 | 10.4 | 5 | positive | positive (CT) 3.4 × 4.2 | negative-fibrosis/necrosis | pelvic mass (excisional) | CR |
| 7 | 12.1 | MLBCL | LMB 96 | 1.8 | 3.8 | 4 | positive | positive (CT) 6.2 × 5.9 | negative- necrosis | mediastinum (core) | CR |
| 8 | 20.6 | MLBCL | LMB 96 | 2.3 | 4.9 | 5 | positive | negative (CT) 5.5 × 2.7 (thymus) | negative-fibrosis/necrosis | mediastinum (excisional) | CR |
| 9 | 15.8 | DLBCL | LMB 96 | 2.4 | 1.9 | 3 | indeterminate | positive (CT) 3.8 × 2.4 | negative-fibrosis | inguinal node (excisional) | CR |
| 10 | 16 | Burkitt lymphoma | LMB 96 | 0.5 | - | 1 | negative | positive (CT) thickening of stomach wall | negative-inflammation | stomach (excisional) | CR |
| 11 | 5.4 | Burkitt lymphoma | LMB 96 | 1.7 | 0.7 | 1 | negative | positive (CT) 1.6 × 1.2 | negative-fibrosis | kidney (core) | CR |
| 12 | 12.9 | DLBCL | LMB 96 | 2.0 | 1.9 | 1 | negative | positive (CT) 7.1 × 5.3 | negative-necrosis | retroperitoneal mass (excisional) | CR |
| 13 | 3.1 | Burkitt lymphoma | LMB 96 | 2.4 | 1.2 | 1 | negative | positive (CT) 2.0 × 1.1 | negative-fibrosis | ovary (excisional) | CR |
| 14 | 18.7 | DLBCL | LMB 96 | 1.7 | 1.7 | 1 | negative | positive (CT) 3.6 × 1.8 | negative-fibroadipose tissue | perirectal mass (core) | CR |
| 15 | 15.7 | DLBCL | LMB 96 | 2.4 | 1.3 | 1 | negative | positive (CT) 1.9 × 1.0 | negative-lymphoid tissue | paracaval node (excisional) | CR |
| 16 | 8.4 | Burkitt lymphoma | LMB 96 | 2.6 | 0.9 | 2 | negative | positive (US) 2.3 × 1.1 | negative-lymphoid tissue | testicle (core) | CR |
| 17 | 17.2 | DLBCL | LMB 96 | 2.3 | 1.6 | 1 | negative | positive (CT) 1.8 × 1.2 | negative-fibrosis | femoral node (excisional) | CR |
| 18 | 16.9 | ALCL | APO regimen | 2.1 | 1.1 | 1 | negative | negative (CT) 1.1 × 0.9 | negative-lymphoid tissue | inguinal node (core) | CR |
Abbreviations: ALCL, anaplastic large-cell lymphoma; APO doxorubicin, prednisone, vincristine, 6-mercaptopurine, methotrexate; BFM, Berlin-Frankfurt-Munster; cm, centimetres; COG, Children's Oncology Group; CR, complete remission; CT, computerized tomography; DLBCL, diffuse large B-cell lymphoma; FDG-PET, 18F-labelled–fluorodeoxyglucose positron emission tomography; LMB 96, French-American-British Mature B-cell lymphoma 96 study regimen; MLBCL, mediastinal large B-cell lymphoma; MRI, magnetic resonance imaging; NHL, Non-Hodgkin lymphoma; SUV, standardized uptake value; US, ultrasound
Time from diagnosis to evaluation
Imaging
The conventional imaging modalities used for the response evaluation included contrast-enhanced CT in 16 patients, US in 1 and MRI in 1. These findings were suggestive of residual disease in 15 patients, but the other 3 patients did not have abnormal findings (e.g., normal-sized lymph nodes and thymus) (Figure 1). FDG-PET/CT findings were positive in 8 patients (London criteria 4 or 5) and indeterminate in 1 (London criteria 3); SUVmax ranged from 2.9-17.5. Conventional imaging and FDG-PET/CT findings were concordant in 8 of 18 (44%) patients.
Figure 1. Correlation of imaging and biopsy findings.
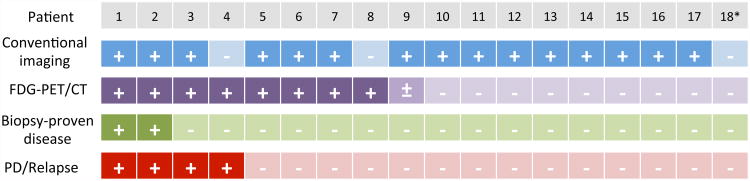
Findings of conventional imaging, FDG-PET/CT and biopsy for individual patients are indicated in the figure. Four of 9 patients with FDG-PET/CT findings suggestive of residual disease developed progressive disease or relapse, 2 of whom had negative biopsies at the time of response evaluation.
+, positive result; -, negative result; ±, indeterminate result
*FDG-PET/CT for Patient 18 was reported to be positive at the time of response evaluation, but negative at the time of retrospective review for this study.
FDG-PET/CT, 18F-labelled–fluorodeoxyglucose positron emission tomography with computerized tomography; PD, progressive disease.
Biopsy
A core biopsy was performed under radiographic guidance in 6 patients. Excisional biopsies were performed in 10 patients and complete resection was performed in 1. One patient (Patient 5) underwent multiple sequential procedures (core biopsy, incisional biopsy and complete resection) and has been reported previously (Otto, et al 2012). Biopsy confirmed residual disease in 2 patients and showed fibrotic and/or necrotic changes in 9, inflammatory changes in 1, normal lymphoid tissue in 4 and fibro-adipose tissue in 1. Patient 5 was found to have a xanthomatous pseudotumour.
Correlation of Conventional Imaging, FDG-PET/CT, and Biopsy Findings
Figure 1 summarizes imaging and pathology findings and clinical outcome for all 18 patients. Residual disease was suspected after conventional imaging (CT, US or MRI) in 15 patients. Of these, FDG-PET/CT findings were positive in 6 patients, indeterminate in 1 and negative in 8. Of the 3 patients in whom conventional imaging was not suggestive of residual disease, 2 had positive findings on FDG-PET/CT. The third patient (Patient 18) underwent biopsy because the FDG-PET/CT was reported to be positive at the time of disease evaluation (although found to be negative by retrospective review for this study).
Of the 8 patients who had positive findings on FDG-PET/CT, biopsy results confirmed the presence of residual tumour presence in 2 patients and did not reveal tumour in 6. Two of the latter 6 patients (Patients 3 and 4) developed early relapses. Relapse was detected in Patient 3 by re-biopsy of the mediastinal mass following an additional cycle of chemotherapy (1 month). Patient 4 was monitored closely with monthly FDG-PET/CT studies. The third follow-up study showed development of new sites of disease and increased avidity in the site of the first biopsy (cervical node). She underwent biopsy of an inguinal node, which revealed relapse. The other 4 patients with positive findings on FDG-PET/CT and negative biopsies remain disease-free at a median follow up of 4.7 years (range 2.7-6.8 years). Of these 4 patients, the subsequent post-biopsy (i.e. end of therapy) FDG-PET/CT findings were negative in 2 patients, and the remaining 2 patients (Patients 7 and 8) continued to have abnormal findings on multiple follow-up FDG-PET/CT studies, which ultimately normalized after 4 and 9 months after completion of therapy, respectively. Of the 2 patients with biopsy-proven residual disease, 1 died from progressive disease. Therapy was intensified for the third patient (Patient 2); he achieved complete remission, but his disease relapsed 6 months later.
Biopsy results did not show residual tumour in the 9 patients with normal FDG-PET/CT or in the 1 patient in whom FDG-PET/CT findings were indeterminate: All 10 of these patients are alive, and their disease in complete remission. Thus, the sensitivity and negative predictive value of subjectively assessing FDG-PET/CT images by using the London criteria with a score of 4 or 5 as indicative of a residual tumour detectable by biopsy were 100%, but specificity and positive predictive value of this approach were low, at 60% and 25%, respectively (Table II). A true-negative FDG-PET/CT study is illustrated in Figure 2 and a false-positive study is shown in Figure 3. Similarly, the sensitivity of FDG-PET/CT for predicting progressive disease or early relapse (irrespective of biopsy findings) was 100%, with specificity of 69%. However, the specificity of conventional imaging in detecting residual disease detectable by biopsy was quite poor at 20%, while sensitivity was 100% (N=2 patients). The size of the residual mass measured on conventional imaging did not correlate with FDG-PET/CT or biopsy results. Of note, 2 patients with residual nodules < 2 cm in maximum diameter had positive biopsies. The 2 patients with biopsy-proven residual disease had tumour SUVmax measurements of 17.5 and 4.8 while 3 patients with negative biopsies had tumour SUVmax measurements > 5. Thus, SUVmax was not able to distinguish benign from malignant histology in residual masses.
Table II. Sensitivity and specificity of FDG-PET/CT and conventional imaging in diagnosing residual tumour by biopsy and predicting progressive disease or relapse.
| Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
|---|---|---|---|---|
| *FDG-PET for positive biopsy | 100 | 60 | 25 | 100 |
| Conventional imaging for positive biopsy | 100 | 20 | 14 | 100 |
| *FDG-PET for progressive disease or relapse | 100 | 69 | 50 | 100 |
| Conventional imaging for progressive disease or relapse | 75 | 15 | 21 | 67 |
Abbreviations: FDG-PET/CT, 18F-labelled–fluorodeoxyglucose positron emission tomography with computerized tomography
Patient 9 had indeterminate findings on FDG-PET and was not included in this analysis.
Figure 2. True-negative FDG-PET/CT study.
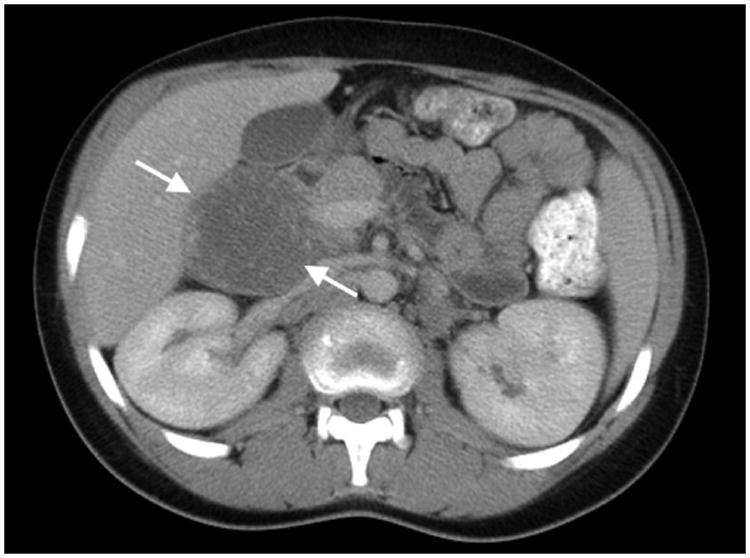
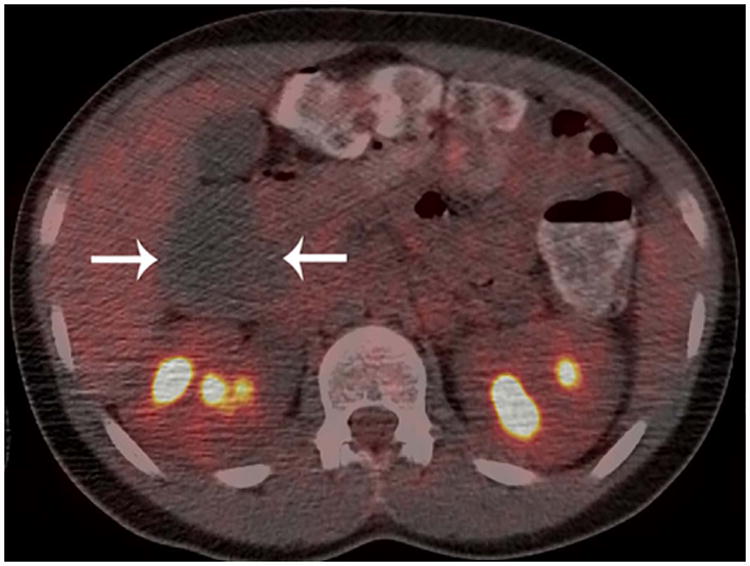
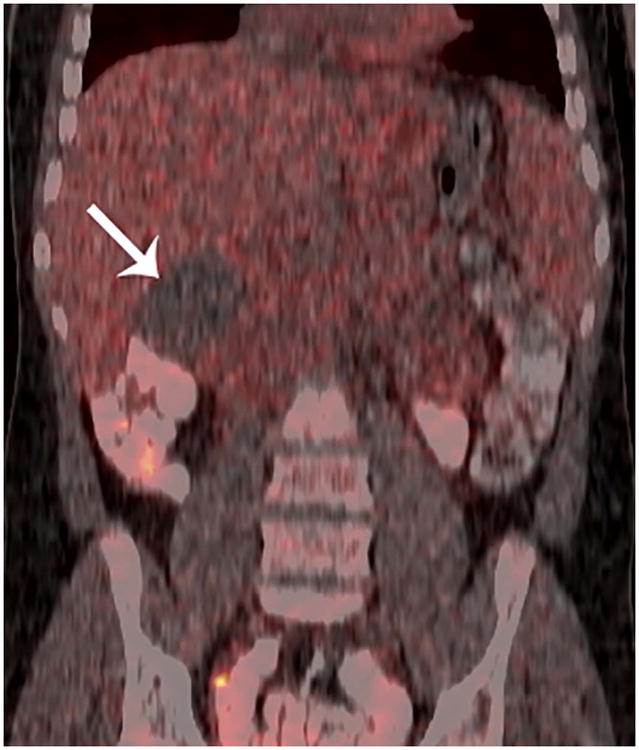
Patient 12 (13-year-old boy treated for diffuse large B-cell lymphoma). A) Conventional abdominal computerized tomography (CT) shows a residual retroperitoneal mass that measured 7.1 × 5.3 cm. (arrows). B) Axial and C) coronal 18F-labelled–fluorodeoxyglucose positron emission tomography with computerized tomography (FDG-PET/CT) images show the mass to be non-FDG avid (arrows). The mass was negative for viable tumour on histopathological inspection.
Figure 3. False-positive FDG-PET/CT study.
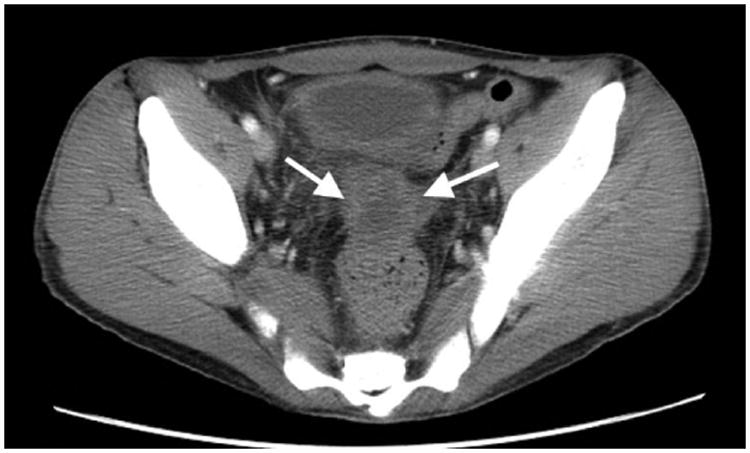
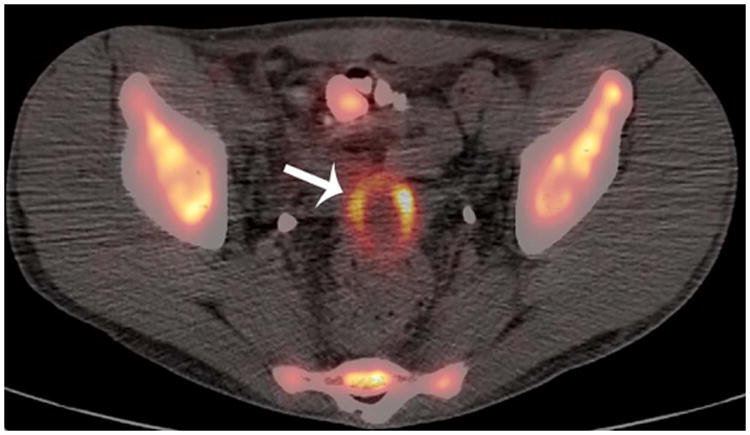
Patient 6 (20-year-old young man treated for Burkitt lymphoma. A) Diagnostic pelvic computerized tomography (CT) shows a residual pelvis mass (arrows). B) Axial positron emission tomography (PET)/CT shows the mass to have intense peripheral fluorodeoxyglucose avidity (arrow). The mass was resected and found to contain necroinflammatory tissue but no viable tumour on histopathological examination.
Discussion
The decision to proceed with a biopsy for pathological examination of a residual mass seen on imaging in patients with NHL often poses a clinical dilemma. This scenario is particularly true in the case of mediastinal or deep-seated abdominal masses because surgical procedures in these locations can be associated with complications. This study reports the largest series of paediatric patients with NHL who underwent concurrent FDG-PET/CT and biopsy for the work-up of a residual mass at the time of response evaluation. It demonstrates the excellent negative predictive value of FDG-PET/CT for residual disease. It also identifies limitations of imaging and of biopsy and suggests that integration of all modalities is required to accurately determine whether individual patients with NHL have residual disease.
Findings on conventional imaging and FDG-PET/CT scans correlated with each other in only 44% of patients. This is a biased estimate, as patients with concordant negative findings on both modalities did not undergo a biopsy and are not included in this study. Even when conventional imaging and FDG-PET/CT findings were concordant in our study cohort, both modalities were not specific for detecting residual tumour by biopsy (2 true-positive, 1 true-negative, 5 false-positive). In addition to multiple false positive findings, limitation of conventional imaging was noted for Patient 4. CT images did not demonstrate findings suggestive of residual disease and core biopsy did not show residual disease; however, multiple FDG-PET/CT studies were positive during close follow up, and the patient developed an overt relapse within 4 months, indicating the likely persistence of residual disease. In addition to Patient 4, the limitation of biopsy was noted for Patient 3, who underwent a core biopsy of a residual mediastinal mass. Pathological examination showed only necrotic tissue; however, CT and FDG-PET/CT findings remained abnormal, and the patient's disease relapsed within a month. Sampling errors during the biopsy procedure do not occur frequently, but it is prudent to monitor patients with abnormal imaging findings closely. Although FDG-PET/CT was not specific for detection of residual disease or relapse, it was highly sensitive. Moreover, a negative FDG-PET/CT at the time of response evaluation was reassuring as there were no false negative studies in our patients.
Prior studies on the utility of FDG-PET/CT in paediatric lymphomas include only a small number of patients with NHL as they focus on Hodgkin lymphoma (Depas, et al 2005, Hines-Thomas, et al 2008, Miller, et al 2006, Riad, et al 2010a). Additionally, these reports combine results of FDG-PET/CT studies at various time points, including initial staging and long-term follow up (Nakatani, et al 2012). Limited data are available to aid decision-making in patients with concerning findings at the time of CR evaluation, and biopsy results are known for only a handful of these patients. In general, most studies demonstrate that a true-negative FDG-PET/CT finding is a reliable indicator of CR. Similar to our study, 2 others showed that patients with negative interim FDG-PET/CT findings remained in continued CR at the time of the respective reports (Amthauer, et al 2005, Mody, et al 2007). This finding is in agreement with the revised Cheson criteria for response evaluation in adult lymphomas wherein resolution of FDG avidity in a nodal mass is considered to be CR (Cheson, et al 2007). However, in a reported series of 8 paediatric patients, disease progressed in 3 patients despite the fact that all 8 patients had negative FDG-PET/CT findings during early response evaluation or at the end of therapy (Depas, et al 2005). It is not clear why the sensitivity of FDG-PET/CT was poor in this series. False-positive FDG-PET/CT studies are relatively common in clinical practice, with reported rates between 50% and 75% in paediatric NHL (Nakatani, et al 2012, Riad, et al 2010b). Reasons for false-positive FDG-PET/CT are many and commonly include infection/inflammation, thymic rebound and trauma (Shankar, et al 2008). However during therapy, fibrosis and/or necrosis were the most common cause of false-positive FDG-PET/CT in our study and other reports (Riad, et al 2010b). For FDG-PET/CT studies obtained during therapy, the imaging subcommittee of the International Harmonization Project for lymphoma recommends performing the study as close as possible prior to the subsequent cycle to minimize false positive findings related to recent therapy (Juweid, et al 2007). Therefore, at this time, changing therapy on the basis of a positive FDG-PET/CT finding alone is not recommended in children with NHL, and biopsy confirmation is required.
On the other hand, excluding the 3 patients in the report by Depas et al (2005), available data suggests that the false negative rate of FDG-PET/CT is extremely low. Therefore, avoiding biopsy may be reasonable for paediatric patients with a negative FDG-PET/CT. By this practice, 8 of 18 (44%) patients in our series would have been spared a surgical procedure for biopsy. Moreover, if additional data can support the high sensitivity of FDG-PET/CT alone, one could perhaps avoid the higher dose radiation exposure needed with conventional CT and still be assured of detecting any active sites of disease.
Our study has several limitations. Because of its retrospective nature, there was slight variability in image and data acquisition. Furthermore, because most children with NHL respond well to therapy, only a small number continue to have residual masses for further investigation. NHL is a heterogeneous disease and larger studies will be required to study individual biological subtypes for optimal clinical utilization of FDG-PET/CT, as different subtypes of NHL may have varied FDG uptake. In the ongoing Intergroup trial for paediatric B-NHL (NCT01516567), FDG-PET/CT data is being collected prospectively in a large group of patients. Analysis of this data may provide helpful information on the utility of FDG-PET/CT for response evaluation in this common subtype of paediatric NHL. Nevertheless, an integrated approach is important for the accurate determination of residual disease in paediatric NHL. In addition to conventional imaging and FDG-PET/CT, multi-disciplinary discussions that include the oncologist, radiologist, surgeon and pathologist are helpful in the management of disease in individual patients.
Acknowledgments
The authors thank Cherise Guess from the Department of Scientific Editing, St Jude Children's Research Hospital for assistance with editing the manuscript.
Funding: National Institutes of Health grant P30-CA021765 and the American Lebanese Syrian Associated Charities
Footnotes
Financial disclosures: None
Contributions: DB, MBM, BLS, JTS designed the research study
DB, MBM, JS, BLS, JTS acquired the data
DB, MBM, JKC, MLM, HI, AMD, RG, BLS, JTS analysed and interpreted the data
All authors wrote the manuscript and approved the submitted version
References
- Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ. 1994a;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ. 1994b;309:102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthauer H, Furth C, Denecke T, Hundsdoerfer P, Voelker T, Seeger K, Stover B, Henze G. FDG-PET in 10 children with non-Hodgkin's lymphoma: initial experience in staging and follow-up. Klin Padiatr. 2005;217:327–333. doi: 10.1055/s-2005-872517. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C FAB LMB96 International Study Committee. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Depas G, De Barsy C, Jerusalem G, Hoyoux C, Dresse MF, Fassotte MF, Paquet N, Foidart J, Rigo P, Hustinx R. 18F-FDG PET in children with lymphomas. Eur J Nucl Med Mol Imaging. 2005;32:31–38. doi: 10.1007/s00259-004-1604-z. [DOI] [PubMed] [Google Scholar]
- Hines-Thomas M, Kaste SC, Hudson MM, Howard SC, Liu WA, Wu J, Kun LE, Shulkin BL, Krasin MJ, Metzger ML. Comparison of gallium and PET scans at diagnosis and follow-up of pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer. 2008;51:198–203. doi: 10.1002/pbc.21574. [DOI] [PubMed] [Google Scholar]
- Hutchings M. FDG-PET response-adapted therapy: is 18F-fluorodeoxyglucose positron emission tomography a safe predictor for a change of therapy? Hematol Oncol Clin North Am. 2014;28:87–103. doi: 10.1016/j.hoc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD Imaging Subcommittee of International Harmonization Project in, L. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- Kluge R, Kurch L, Montravers F, Mauz-Korholz C. FDG PET/CT in children and adolescents with lymphoma. Pediatr Radiol. 2013;43:406–417. doi: 10.1007/s00247-012-2559-z. [DOI] [PubMed] [Google Scholar]
- Le Roux PY, Gastinne T, Le Gouill S, Nowak E, Bodet-Milin C, Querellou S, Mahe B, Dubruille V, Blin N, Salaun PY, Bodere-Kraeber F. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging. 2011;38:1064–1071. doi: 10.1007/s00259-011-1741-0. [DOI] [PubMed] [Google Scholar]
- Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–1523. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- Miller E, Metser U, Avrahami G, Dvir R, Valdman D, Sira LB, Sayar D, Burstein Y, Toren A, Yaniv I, Even-Sapir E. Role of 18F-FDG PET/CT in staging and follow-up of lymphoma in pediatric and young adult patients. J Comput Assist Tomogr. 2006;30:689–694. doi: 10.1097/00004728-200607000-00022. [DOI] [PubMed] [Google Scholar]
- Mody RJ, Bui C, Hutchinson RJ, Frey KA, Shulkin BL. Comparison of (18)F Flurodeoxyglucose PET with Ga-67 scintigraphy and conventional imaging modalities in pediatric lymphoma. Leuk Lymphoma. 2007;48:699–707. doi: 10.1080/10428190601179783. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Zelenetz A, Schoder H. An update on the role of interim restaging FDG-PET in patients with diffuse large B-cell lymphoma and Hodgkin lymphoma. J Natl Compr Canc Netw. 2010a;8:347–352. doi: 10.6004/jnccn.2010.0023. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, Straus D, Noy A, Palomba ML, O'Connor OA, Horwitz S, Weaver SA, Meikle JL, Filippa DA, Caravelli JF, Hamlin PA, Zelenetz AD. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010b;28:1896–1903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Nakamoto Y, Watanabe K, Saga T, Higashi T, Togashi K. Roles and limitations of FDG PET in pediatric non-Hodgkin lymphoma. Clin Nucl Med. 2012;37:656–662. doi: 10.1097/RLU.0b013e318238f72b. [DOI] [PubMed] [Google Scholar]
- Otto M, Shulkin BL, Kundu M, Sandlund JT, Snyder SE, Metzger ML. Histiocyte-rich xanthomatous pseudotumor mimicking relapse on positron emission tomography imaging in an adolescent with primary mediastinal diffuse large B-cell lymphoma. J Pediatr Hematol Oncol. 2012;34:232–235. doi: 10.1097/MPH.0b013e3182281c54. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, Cairo MS Committee, F.L.I.S. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad R, Omar W, Kotb M, Hafez M, Sidhom I, Zamzam M, Zaky I, Abdel-Dayem H. Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging. 2010a;37:319–329. doi: 10.1007/s00259-009-1276-9. [DOI] [PubMed] [Google Scholar]
- Riad R, Omar W, Sidhom I, Zamzam M, Zaky I, Hafez M, Abdel-Dayem HM. False-positive F-18 FDG uptake in PET/CT studies in pediatric patients with abdominal Burkitt's lymphoma. Nucl Med Commun. 2010b;31:232–238. doi: 10.1097/MNM.0b013e328334fc14. [DOI] [PubMed] [Google Scholar]
- Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, Vera P, Copie-Bergman C, Rahmouni A, Tilly H, Meignan M, Haioun C. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, Dorffel W, Zimmermann M, Mann G, Gadner H, Parwaresch R, Riehm H, Reiter A. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97:3699–3706. doi: 10.1182/blood.v97.12.3699. [DOI] [PubMed] [Google Scholar]
- Shankar A, Fiumara F, Pinkerton R. Role of FDG PET in the management of childhood lymphomas--case proven or is the jury still out? Eur J Cancer. 2008;44:663–673. doi: 10.1016/j.ejca.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, Nihashi T, Nagai H. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin's lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27:1906–1914. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- Weinstein HJ, Lack EE, Cassady JR. APO therapy for malignant lymphoma of large cell “histiocytic” type of childhood: analysis of treatment results for 29 patients. Blood. 1984;64:422–426. [PubMed] [Google Scholar]


