Abstract
Low functioning MAOA genotypes have been reliably linked to increased reactive aggression, yet the psychological mechanisms of this effect remain largely unknown. The low functioning MAOA genotype’s established link to diminished inhibition and greater reactivity to conditions of negative affect suggest that negative urgency, the tendency to act impulsively in the context of negative affect, may fill this mediating role. Such MAOA carriers may have higher negative urgency, which may in turn predict greater aggressive responses to provocation. To test these hypotheses, 277 female and male participants were genotyped for an MAOA SNP yet to be linked to aggression (rs1465108), and then reported their negative urgency and past aggressive behavior. We replicated the effect of the low functioning MAOA genotype on heightened aggression, which was mediated by greater negative urgency. These results suggest that disrupted serotonergic systems predispose individuals towards aggressive behavior by increasing impulsive reactivity to negative affect.
Keywords: MAOA, aggression, negative urgency, UPPS model, genetics, impulsivity
1. Introduction
Aggression, the act of harming others against their will, is a ubiquitous and resilient phenomenon (Anderson & Bushman, 2002). Although numerous situational factors can increase aggression, substantial evidence suggests that people have a disposition to behave aggressively, with approximately half of this tendency being genetically inherited (e.g., Moffitt, 2005). To reduce aggression, it is crucial to determine the factors that give rise to such dispositional aggression tendencies.
Of the various genetic predictors of aggression, low functioning allelic variants of the monoamine oxidase A (MAOA) gene have emerged as uniquely potent correlates with violence (Gallardo-Pujol, Andrés-Pueyo, & Maydeu-Olivares, 2013; Kuepper, Grant, Wielpuetz, & Hennig, 2013; McDermott, Tingley, Cowden, Frazzetto, & Johnson, 2009; Raine, 2008). However, the psychological mechanisms that explain this genetic link to aggression remain largely unexamined. To fill this gap, we sought to identify personality traits that arise from the MAOA gene. Specifically, we sought to implicate negative urgency, the tendency to act rashly when experiencing negative affect, as a mechanism through which the MAOA minor allele predicts aggression (e.g., McDermott et al., 2009).
1.1 MAOA and Aggression
The MAOA gene encodes monoamine oxidase A, an enzyme that breaks down monoamine neurotransmitters, chiefly serotonin, into their constituent molecular compounds (Buckholtz & Meyer-Lindenberg, 2008; Raine, 2008). Providing the initial evidence of a link between this gene and aggression, MAOA knockout mice showed greater instances of aggression against conspecifics (Cases et al., 1995). Humans with a low functioning mutation of this gene show greater levels of aggression (Brunner, Nelen, Breakefield, Ropers, & van Oost, 1993; Meyer-Lindenberg et al., 2006; Raine, 2008), whereas those with a higher functional allelic variant show greater prosociality (Mertins, Schote, Hoffeld, Griessmair, & Meyer, 2011). This antisocial tendency of low functioning MAOA genotypic individuals is exacerbated among those with adverse early life experiences of maltreatment (Caspi et al., 2002; Kim-Cohen et al., 2006).
Crucially, this aggressive tendency among low functioning MAOA genotypic people is most pronounced and perhaps specific to situations characterized by interpersonal exclusion or provocation (Gallardo-Pujol et al., 2013; Kuepper et al., 2013; McDermott et al., 2009). Thus, the MAOA gene, also known from earlier research as “warrior gene”, may not be associated with aggression per se but with reactive, retaliatory aggression to provocative situations. This specificity fits well with the gene-environment interactionist approach to behavioral genetics (e.g., McDermott et al., 2009) as well as contemporary meta-theories of aggression such as the General Aggression Model (Anderson & Bushman, 2002; DeWall, Anderson, & Bushman, 2011) and I3 Theory (Finkel, in press; Slotter & Finkel, 2011). However, it remains largely unknown what psychological dispositions contribute to this link between low functioning MAOA genotype and retaliatory aggression. A psychological phenotype marked by heightened reactivity to provocative situations may partially account for the relationship between low functioning MAOA and greater aggression.
1.2 Mechanisms Underlying the MAOA-Aggression Link
Reactive aggression often results from a combination of the discrete elements of provocation, heightened emotional reactivity to a provocative event, and impaired inhibition (e.g., Chester et al., 2014; see Denson, DeWall, & Finkel, 2012). Crucially, these factors interact with one another to further exacerbate one another. Individuals with the low functioning MAOA genotype possess a neural makeup that would establish just such a perfect storm of heightened emotional reactivity and impaired inhibition. Low functioning MAOA genotypes show reduced levels of monoamine oxidase A which results in greater, dysregulated levels of circulating central serotonin (for a more detailed account see Buckholtz & Meyer-Lindenberg, 2008). These heightened serotonin levels predispose neural regions that produce and regulate affective responses to social stimuli to behave in a dysregulated and labile manner (Buckholtz & Meyer-Lindenberg, 2008).
A seminal neuroimaging study demonstrated that the low expression MAOA allelic variant was associated with hyper-reactivity of the amygdala and hypo-reactivity of the dorsal lateral prefrontal cortex (DLPFC) during an emotionally arousing task (Meyer-Lindenberg et al., 2006). This association between low functioning MAOA genotype and hyper reactivity of the amygdala to negatively valenced affective stimuli was recently replicated, using an ecologically valid provocation paradigm, and shown to predict greater subsequent effort required to control anger (Denson, Dobson-Stone, Ronay, von Hippel, & Schira, 2014). This effect of MAOA genotype on anger control also held for the dorsal anterior cingulate cortex (dACC), a neural region implicated in responding to events characterized by negative affect (Denson et al., 2014). Further, the association between low functioning MAOA genotype and aggression was mediated by greater reactivity of the dACC during an instance of social rejection (Eisenberger, Way, Taylor, Welch, & Lieberman, 2007). Combining these findings with the behavioral literature on the MAOA-aggression link suggests that the disruption of the serotonergic system that is associated with the low functioning allelic variants of the MAOA gene predisposes individuals to experience greater negative affect in response to interpersonal threat.
According to balance theory, the LPFC maintains a self-regulatory balance by inhibiting activity in the amygdala and other regions such as the dACC (Heatherton & Wagner, 2011). But when this balance is tipped in favor of the amygdala, possibly by genetic influences from the MAOA gene, self-regulation fails and increases aggression. This unbalanced combination of greater amygdala and blunted LPFC activity during negative affect is prevalent in highly aggressive populations (Coccaro, McCloskey, Fitzgerald, & Phan, 2007). Specifically, this maladaptive neural mechanism may underpin a unique facet of impulsivity called negative urgency, which is characterized by both deficits in inhibition and negative behavioral outcomes such as aggression (Cyders & Smith, 2008).
1.3 Negative Urgency as a Mechanism
Negative urgency refers to the tendency to react impulsively to experiences of negative affect (Cyders & Smith, 2008; Whiteside & Lynam, 2001). Negative urgency is one of four facets of impulsivity that also include the lack of perseverance, the lack of premeditation, and sensation-seeking (Whitesyde & Lynam, 2001). We focus on negative urgency for two key reasons. First, this facet of impulsivity is predictive of aggressive responses to provocation and threat (not aggression per se) above and beyond other features of impulsivity (Anestis, Anestis, Selby, & Joiner, 2009; Cyders & Smith, 2008; Derefinko, DeWall, Metze, Walsh, & Lynam, 2011; Settles et al., 2012). Second, negative urgency has been previously linked to a low functioning serotonergic genotype using the 5HTTLPR gene (Carver, Johnson, Joorman, Kim, & Nam, 2011; Carver, LeMoult, Johnson, & Joormann, 2014). This evidence, combined with the effect of MAOA on tipping the balance of the self-regulatory neural network that likely elicits negative urgency suggests that negative urgency (Eisenberger et al., 2007; Meyer-Lindenberg et al., 2006) might relate to having a low functioning MAOA genotype. Further, this expression of a low functioning MAOA genotype as greater negative urgency may help explain why MAOA most often relates to aggression under conditions of negative affect (e.g., McDermott et al., 2009).
1.4 Current Study
The current study sought to replicate and examine a psychological phenotype that may help explain why the low functioning genotype of the MAOA gene often relates to greater aggression. Further, the study sought to test the novel hypothesis that the positive association between low functioning MAOA genotype and greater aggression would be mediated by heightened negative urgency. Finally, we aimed to genotype individuals on a single nucleotide polymorphism (SNP; rs1465108; Figure 1) of the MAOA gene that has yet to be linked to aggression. The functionality of this SNP has yet to be fully established, a fruitful avenue for future MAOA research.
Figure 1.
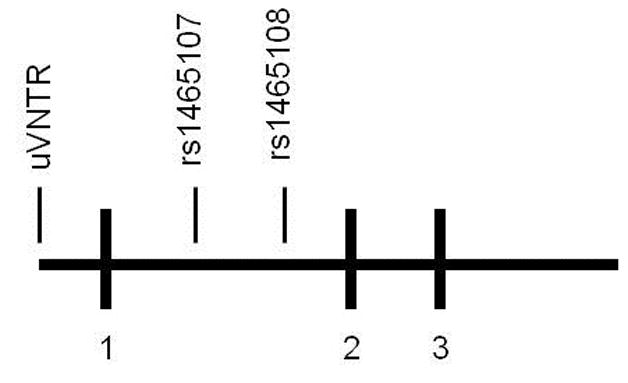
Spatial schematic of the MAOA gene’s VNTR promoter region and adjacent SNPs, including the SNP genotyped in this study (i.e., rs1465108). Single-digit numbers represent the location of exons.
To achieve these aims, undergraduate students were genotyped on the rs1465108 SNP and reported their levels of negative urgency, relevant personality traits, and aggressive behavior. To disentangle the effects of negative urgency from those of closely-related personality constructs, participants also reported their general levels of negative affect (i.e., neuroticism), other facets of impulsivity and personality, and dispositional self-control, which were accounted for in all analyses. These hypotheses were tested among both females and males as previous research has observed associations between MAOA genotype and aggression across both groups (e.g., Kuepper et al., 2013).
2. Materials and Methods
2.1 Participants
Participants were originally 376 female and male undergraduates recruited from introductory psychology courses and received both course credit and monetary incentives for participation. “High risk” participants were over-recruited to ensure sufficient variability in conduct problems (e.g., aggression). Participants were determined to be “high risk” if they fell within the upper quartile of a 12-item composite measure of conduct problems administered in a screening session prior to recruitment. Due to the relatively small numbers of racial minorities in this sample and the variance in MAOA allelic frequency among these groups, racial minorities were excluded from the sample to avoid population stratification. Participants were 277 Caucasian undergraduates (50.9% female; Age: M = 18.88, SD = 0.47) of whom approximately 25% were categorized as “high risk”.
2.2 Measures
2.2.1. Aggression composite score
Items from two different measures were aggregated to form a composite measure of aggression. Items included those from the screening measure that assessed aggression (e.g., Before the age of 18, did you ever pick on smaller peers or threaten or tease those who were too scared to fight you?; Before the age of 18, did you ever take part in a fight where a group of your friends were against another group?), and three additional aggression items from the Crime and Analogous Behavior Scale (CAB; Lynam, Whiteside, & Jones, 1999), including: Ever been in a physical fight?; Ever hurt someone intentionally to the extent that they needed bandages or a doctor?; and Ever attacked someone with intent of seriously hurting or killing them? All five items from the aggression composite were scored ‘yes’ or ‘no’ (1 and 0, respectively). Values were then summed across the five items to create an aggression index that could range from 0 to 5.
2.2.2 UPPS-P Impulsivity Scale
The UPPS-P (Lynam, Smith, Whiteside, & Cyders, 2006; Whiteside & Lynam, 2001) includes 59 items, scored on a 4-point Likert-style scale, assessing five distinct personality pathways to impulsive behavior: negative urgency (the tendency to behave rashly when distressed), lack of premeditation (failure to think about consequences of behavior before acting), lack of perseverance (failure to persist in tasks or obligations), sensation seeking (preference for stimulation and excitement), and positive urgency (tendency to act rashly when feeling positive emotion). Internal consistency is good to excellent for all of the subscales in previous research (Cyders & Smith, 2010; Whiteside, Lynam, & Miller, 2005) and in the present study, α = .82–.93. Because of the high intercorrelation between negative and positive urgency, r(275) = .75, p < .001, the lack of any research on positive urgency and MAOA genotype, and the fact that negative, rather than positive, urgency has been shown to relate to greater aggressive reactivity to provocation (e.g., Derefinko et al., 2011) positive urgency scores were not included in any subsequent analyses.
2.2.3 Revised NEO Personality Inventory
The NEO-PI-R (Costa & McCrae, 1992) is a self-report questionnaire assessing general personality dimensions based on the Five Factor Model of personality. The NEO-PI-R consists of 240 items, which are rated on a 5-point scale, anchored by 1 (strongly disagree) and 5 (strongly agree). The inventory provides scores for each of the five personality domains (Agreeableness, Conscientiousness, Extraversion, Neuroticism, and Openness to Experience), with 48 questions per domain, as well as six facet scores per domain. An extensive research base supports the reliability and validity of the NEO-PI-R (Costa & McCrae, 1992; 2010). The Agreeableness, Conscientiousness, Extraversion, Neuroticism, and Openness to Experience domain scores demonstrated excellent internal consistency in the present sample, α = .87–.92.
2.2.4 Self-Control Scale
The Self-Control Scale is a 36-item self-report questionnaire developed by Tangney, Baumeister, & Boone (2004) to assess individual differences in multiple aspects of self-control. Items are rated on a 5-point scale, from ‘Not At All Like Me’ to ‘Very Much Like Me’. The total score demonstrated good internal consistency in the present sample (α = .90). The construct of trait self-control is different than conscientiousness as this trait reflects the dispositional ability and tendency to effortfully inhibit prepotent impulses and action tendencies (Hoffman, Friese, & Strack, 2009).
2.3 Procedure
This study represents data from the first year of a 3-year longitudinal data collection in which data were collected annually. With the exception of the trait self-control data which was acquired in the second year, all data were obtained from the first year precluding any longitudinal analyses. All study procedures were reviewed and approved by the University of Kentucky’s IRB and a federal Certificate of Confidentiality was acquired. After providing informed consent, participants were asked to voluntarily provide a saliva sample for genotyping. Then, participants completed a battery of computerized questionnaires which included the aggression items, UPPS-P impulsivity scale, and NEO-PI-R personality scale. Participants returned for the second year in which they completed another battery of computerized questionnaires which included the Self-Control Scale.
Saliva samples were collected from the participants who signed additional consent forms for genotyping at the time of the experiment. The subjects were de-identified for genetic analysis. DNA was extracted from saliva samples in the genetic laboratory at the University of Kentucky’s College of Medicine. The de-identified DNA samples were sent to Yale University’s Center for Genetics for genotyping.
2.3.1 Genotyping
DNA was purified from Oragene saliva collection kits according to the manufacturer’s directions (DNA Genotek). DNA was quantified by UV absorbance at 260 nm, diluted to 10 ng/μl and MAOA rs1465108 and MAOB SNPs were genotyped by Sequenom MassARRAY iPLEX technology (W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University; http://ycga.yale.edu/). This MAOA SNP, located at the position 43294463 MFA 0.338, has previously been linked to antisocial personality disorder in adult females (Ducci et al., 2008), inattention-hyporeactivity levels among children (Karmakar et al., 2014), autism spectrum disorder (Verma et al., 2014), and the efficacy of antidepressants (Peters, Slager, McGrath, Knowles, & Hamilton, 2004). This SNP has yet to be linked to aggression, though its inclusion in the MAOA gene suggests it may exhibit just such an association. Because the MAOA gene is X-linked, heterozygous genotypes (i.e., GA) were only possible among females.
3. Results
3.1 Descriptives
Genotyping results on the rs1465108 SNP of the MAOA gene indicated that of the 277 participants, 58.5% were of the GG genotypes, 22.0% were of the GA genotype (all female), and 19.5% were of the AA genotype. The rs1465108 SNP was within Hardy-Weinberg equilibrium, χ2 = 1.86, p > .05. To assess the specificity of the MAOA gene, we also genotyped participants on the rs295791 SNP of the MAOB gene, which indicated that all participants were of the CC genotype. Because there was no variability in the MAOB genotype, no effects on aggression were tested. Across all MAOA genotypes, aggression levels (which could range from 0 to 5) showed substantial variability, M = 0.87, SD = 1.13, observed range = 0 – 5. To reflect genotypes that were associated with low functioning MAOA, a dummy code was created in which GG genotypes were coded as 0 and GA and AA genotypes were coded as 1. Thus, higher values represented the presence of the MAOA minor allele (i.e., the A allele).
3.2 Mediation Model
A bias-corrected, bootstrapped mediation model (Preacher & Hayes, 2008) was fit to the data using 1,000 bootstrap samples in which the dummy code for the MAOA minor allele was the independent variable, negative urgency was the mediator, and the aggression index was the dependent variable. Gender, personality traits relevant to aggression (i.e., agreeableness, conscientiousness, extraversion, openness to experience, neuroticism, self-control), and the other three facets of impulsivity (i.e., lack of perseverance, lack of premeditation, sensation seeking) were included as covariates of no interest. Of the 277 participants, five participants were missing NEO-PI-R data and two participants were missing trait self-control data. Thus the mediation model was run on the remaining 270 participants.
The mediation model explained a significant portion of variance in aggression, F(12,257) = 8.48, p < .001, adjusted R2 = .25. Replicating previous research, the MAOA minor allele was marginally associated with greater aggression, B = .25, t(258) = 1.92, p = .056. Supporting our mediation hypotheses, low functioning MAOA genotype exhibited an indirect effect on aggression through greater levels of negative urgency (95% confidence interval: .007, .135; Figure 2); this indirect effect account for an estimated 24% of the total effect of the genotype on aggression. Specifically, MAOA minor allele was associated with greater negative urgency, B = .11, t(258) = 2.14, p = .033, which was in turn associated with greater aggression, B = .48, t(258) = 3.05, p = .003. Controlling for this indirect effect substantially reduced the effect of MAOA minor allele on aggression, B = .19, t(258) = 1.53, p = .127, providing additional evidence for mediation. Among the control variables, being female was significantly associated with lesser aggression, B = −.54, t(258) = −3.68, p < .001, as was agreeableness, B = −.48, t(258) = −2.79, p = .006. All other control variables failed to reach significance, Bs < 0.32, ps > .07. Using the mediation model described above, no other facet of impulsivity significantly mediated the effect of low functioning MAOA genotype on aggressive behavior.
Figure 2.
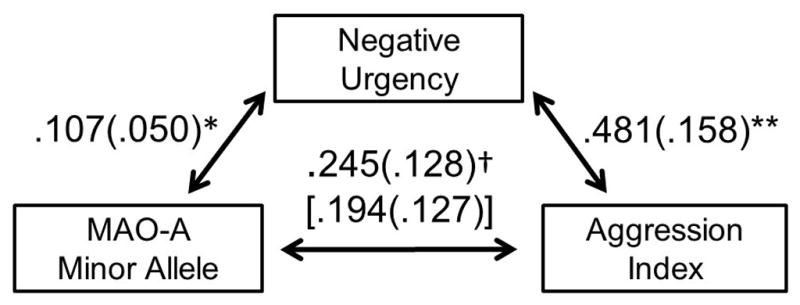
Bootstrapped mediation model whereby greater negative urgency mediated the positive association between MAOA minor allele and aggression. Non-parenthesized values represent partial, unstandardized regression coefficients. Parenthesized values represent standard errors of the regression coefficients. Bracketed values represent the direct effect after controlling for the indirect path. †p < .06, *p < .05, **p < .005.
4. Discussion
Aggression costs humankind lives, resources, and suffering. Genetic markers can predispose certain people to behave aggressively, but the psychological phenotypes underlying this association remain underexplored. This study fills this gap in the literature by identifying a psychological mechanism through which the low functioning MAOA allele exerts its influence on aggression.
Consistent with previous research on the MAOA-aggression link, the positive association between the low functioning, MAOA genotype and aggression (e.g., Eisenberger et al., 2007; Gallardo-Pujol et al., 2013; Kuepper et al., 2013; McDermott et al., 2009). We were also able to substantiate the link between low functioning MAOA allelic variants on a new SNP that has never before been linked to aggression. Demonstrating this effect across SNPs on the MAOA gene emphasizes the aggression-promoting effect of this location on the genotypic map.
Most notably, our findings built upon this established correlation by demonstrating statistical mediation of the effect of the MAOA minor allele on aggressive behavior via heightened negative urgency, while controlling for potential confounds. These findings suggest a unique psychological phenotype, impulsivity under conditions of negative affect, which helps explain how the low functioning MAOA genotype influences aggression. This finding meshes well with previous research on the MAOA gene showing that it predicts greater retaliatory aggression after exclusion or provocation (Gallardo-Pujol et al., 2013; Kuepper et al., 2013; McDermott et al., 2009), both of which can be readily construed as an induction of negative affect (Anderson & Bushman, 2002; Williams, 2009). This empirical emphasis on context specificity fits within the gene-by-environment interactionist approaches to behavioral genetics that has yielded great gains and promises to yield much more. Our findings add to a growing body of literature that demonstrates that the MAOA gene increases the likelihood of aggression only elicited under conditions of negative affect such as provocation or social rejection (McDermott et al., 2009).
Our findings also continue to implicate negative urgency as a personality trait and facet of impulsivity that is uniquely potent in the domain of violence (e.g., Derefinko et al., 2011). More so, our ability to implicate negative urgency as a mechanism of the MAOA-aggression link and not other facets of impulsivity (e.g., sensation seeking) supports the UPPS model of impulsivity, in which impulsivity is comprised of distinct facets which differentially predict such negative outcomes as aggression (Cyders & Smith, 2008; Whiteside & Lynam, 2001). This multifaceted view of impulsivity’s relationship with aggression informs potential interventions for aggressive behavior, suggesting that targeting reactivity in the context of negative affect and not impulsivity more generally, may be useful to reduce violence and other problematic behavioral tendencies.
4.1 Limitations and Future Directions
Our findings were limited in several ways that suggest future avenues for research. Due to differences in allelic frequencies between racial groups, our findings are limited to the individuals with Caucasian ancestry and future research should assess whether these effects hold across racial categories. Second, aggressive behavior was reported and not observed or measured objectively, thus reports may have been prone to biases in participants’ recollection. Future research may assess these effects using valid aggression measurements such as the Taylor Aggression Paradigm (Anderson & Bushman, 1997) or the voodoo doll task (DeWall et al., 2013). In addition, future neuroimaging measurements will serve as intermediate phenotypes of affect-related brain circuits between genetics and behavior (Parasuraman & Jiang, 2012). Third, we were unable to tease apart aggressive behavior that was due to provocation and that which was not because our measure did not specify this distinction. Because provocation is the most reliable predictor of aggression (Anderson & Bushman, 2002), it is safe to assume that much of the aggression reported by our participants was retaliatory in nature. Fourth, the direct effect of MAOA genotype on aggressive behavior was only marginally statistically significant. However, the direct path of mediation models are often statistically underpowered compared to the indirect effect (Kenny & Judd, 2013). Thus our marginally significant direct effect may be due to the nature of the mediation test and less a reflection of the actual nature of the association between MAOA genotype and aggression.
5. Conclusions
Aggression plagues mankind and is a hallmark of many psychopathologies. Genetic influences on aggressive behavior may cause some to lose hope of ever reducing violence, yet understanding how genes such as the MAOA gene express themselves as personality phenotypes allow the opportunity to gain traction on these effects. We showed that the association between the low functioning variant of the MAOA gene and aggression likely occurs through increases in impulsivity that is specific to conditions of negative affect. By understanding the nuanced conditions through which genes code for aggressive personalities, we may be able to impede the tide of aggressive acts.
Highlights.
Low functioning MAOA genotype was linked to greater past aggression
Low functioning MAOA genotype was linked to greater negative urgency
The effect of MAOA genotype on aggression was mediated by greater negative urgency
Acknowledgments
This research was supported by funding from the National Institutes of Health (grant P50-DA05312) to University of Kentucky’s Center for Drug Abuse Research Translation and from the University of Kentucky’s Department of Behavioral Science. The authors also gratefully acknowledge research support from the National Institutes on Drug Abuse (DA007304 and T32DA035200) and National Center for Advancing Translational Sciences (UL1TR000117) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We also thank Richard Milich for his assistance in developing the study and in data collection, and Ke Xu for comments in an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CA, Bushman BJ. External validity of “true” experiments: The case of laboratory aggression. Review of General Psychology. 1997;1(1):19–41. [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annual Review of Psychology. 2002;53(1):27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Anestis MD, Anestis JC, Selby EA, Joiner TE. Anger rumination across forms of aggression. Personality and Individual Differences. 2009;46(2):192–196. [Google Scholar]
- Brunner H, Nelen M, Breakefield X, Ropers H, van Oost B. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends in Neurosciences. 2008;31(3):120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268(5218):1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychological Science. 2011;22(5):589–595. doi: 10.1177/0956797611404085. [DOI] [PubMed] [Google Scholar]
- Carver CS, LeMoult J, Johnson SL, Joormann J. Gene effects and G × E interactions in the differential prediction of three aspects of impulsiveness. Social Psychological and Personality Science. 2014 1948550614527116. [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Richman SB, Bushman BJ, DeWall CN. The interactive effect of social pain and executive functioning on aggression: An fMRI experiment. Social Cognitive and Affective Neuroscience. 2014;9(5):699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62(2):168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Costa PT, McCrae RR. Bridging the gap with the five-factor model. Personality Disorders: Theory, Research, and Treatment. 2010;1(2):127–130. doi: 10.1037/a0020264. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dispositions to rash action: Positive and negative urgency. Psychological Bulletin. 2008;134(6):807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Longitudinal validation of the urgency traits over the first year of college. Journal of Personality Assessment. 2010;92(1):63–69. doi: 10.1080/00223890903381825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, DeWall CN, Finkel EJ. Self-control and aggression. Current Directions in Psychological Science. 2012;21(1):20–25. [Google Scholar]
- Denson TF, Dobson-Stone C, Ronay R, von Hippel W, Schira MM. A functional polymorphism of the MAOA gene is associated with neural responses to induced anger control. Journal of Cognitive Neuroscience. 2014;26(7):1418–1427. doi: 10.1162/jocn_a_00592. [DOI] [PubMed] [Google Scholar]
- Derefinko K, DeWall CN, Metze AV, Walsh EC, Lynam DR. Do different facets of impulsivity predict different types of aggression? Aggressive Behavior. 2011;37(3):223–233. doi: 10.1002/ab.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, Anderson CA, Bushman BJ. The general aggression model: Theoretical extensions to violence. Psychology of Violence. 2011;1(3):245–258. [Google Scholar]
- DeWall CN, Finkel EJ, Lambert NM, Slotter EB, Bodenhausen GV, Pond RS, Fincham FD. The voodoo doll task: Introducing and validating a novel method for studying aggressive inclinations. Aggressive Behavior. 2013;39(6):419–439. doi: 10.1002/ab.21496. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular Psychiatry. 2007;13(3):334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: Clues from the brain’s response to social exclusion. Biological Psychiatry. 2007;61(9):1100–1108. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Finkel EJ. The I3 Model: Metatheory, theory, and evidence. In: Olson JM, Zanna MP, editors. Advances in experimental social psychology. Vol. 49. San Diego: Academic Press; (in press) [Google Scholar]
- Gallardo-Pujol D, Andrés-Pueyo A, Maydeu-Olivares A. MAOA genotype, social exclusion and aggression: an experimental test of a gene–environment interaction. Genes, Brain and Behavior. 2013;12(1):140–145. doi: 10.1111/j.1601-183X.2012.00868.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science. 2009;4(2):162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Karmakar A, Maitra S, Verma D, Chakraborti B, Goswami R, Ghosh P, Mukhopadhyay K. Potential contribution of monoamine oxidase A gene variants in ADHD and behavioral co-morbidities: Scenario in Eastern Indian probands. Neurochemical Research. 2014;39(5):843–852. doi: 10.1007/s11064-014-1276-4. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Judd CM. Power anomalies in testing mediation. Psychological Science. 2014;25(2):334–339. doi: 10.1177/0956797613502676. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kuepper Y, Grant P, Wielpuetz C, Hennig J. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behavioural Brain Research. 2013;247:73–78. doi: 10.1016/j.bbr.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. Technical Report. West Lafayette, IN: Purdue University; 2006. The UPPS-P: Assessing five personality pathways to impulsive behavior. [Google Scholar]
- Lynam DR, Whiteside S, Jones S. Self-reported psychopathy: A validation study. Journal of Personality Assessment. 1999;73(1):110–132. doi: 10.1207/S15327752JPA730108. [DOI] [PubMed] [Google Scholar]
- McDermott R, Tingley D, Cowden J, Frazzetto G, Johnson DDP. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proceedings of the National Academy of Sciences. 2009;106(7):2118–2123. doi: 10.1073/pnas.0808376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins V, Schote AB, Hoffeld W, Griessmair M, Meyer J. Genetic susceptibility for individual cooperation preferences: the role of monoamine oxidase A gene (MAOA) in the voluntary provision of public goods. PLoS ONE. 2011;6:e20959. doi: 10.1371/journal.pone.0020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. FOCUS: The Journal of Lifelong Learning in Psychiatry. 2006;4(3):360–368. [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: Gene–environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131(4):533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Jiang Y. Individual differences in cognition, affect, and performance: Behavioral, neuroimaging, and molecular genetic approaches. NeuroImage. 2012;59:70–82. doi: 10.1016/j.neuroimage.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Molecular Psychiatry. 2004;9(9):879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Raine A. From Genes to Brain to Antisocial Behavior. Current Directions in Psychological Science. 2008;17(5):323–328. [Google Scholar]
- Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, Smith GT. Negative urgency: A personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. Journal of Abnormal Psychology. 2012;121(1):160–172. doi: 10.1037/a0024948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotter EB, Finkel EJ. I3 theory: Instigating, impelling, and inhibiting factors in aggression. In: Shaver PR, Mikulincer M, editors. Human aggression and violence: Causes, manifestations, and consequences. Washington, DC: American Psychological Association; 2011. pp. 35–52. [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Verma D, Chakraborti B, Karmakar A, Bandyopadhyay T, Singh AS, Sinha S, Rajamma U. Sexual dimorphic effect in the genetic association of monoamine oxidase A (MAOA) markers with autism spectrum disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;50:11–20. doi: 10.1016/j.pnpbp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. European Journal of Personality. 2005;19(7):559–574. [Google Scholar]
- Williams KD. Ostracism: A temporal need-threat model. In: Zanna Mark P., editor. Advances in Experimental Social Psychology. Vol. 41. New York, NY: Academic Press; 2009. pp. 275–314. [Google Scholar]


