Summary
Bariatric surgical procedures such as vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) are the most potent treatments available to produce sustained reductions in body weight and improvements in glucose regulation. While traditionally these effects are attributed to mechanical aspects of these procedures such as restriction and malabsorption, a growing body of evidence from mouse models of these procedures points to physiological changes that mediate the potent effects of these surgeries. In particular, there are similar changes in gut hormone secretion, bile acid levels and composition after both of these procedures. Moreover, loss-of-function of the nuclear bile acid receptor (FXR) greatly diminishes the effects of VSG. Both VSG and RYGB are linked to profound changes in the gut microbiome that also mediate at least some of these surgical effects. We hypothesize that surgical rearrangement of the gastrointestinal tract results in enteroplasticity caused by the high rate of nutrient presentation and altered pH in the small intestine that contribute to these physiological effects. Identifying the molecular underpinnings of these procedures provides new opportunities to understand the relationship of the gastrointestinal tract to obesity and diabetes as well as new therapeutic strategies to harness the effectiveness of surgery with less invasive approaches.
Introduction
Advancements in modern medical treatment are often thought to be the result of meticulously thought out hypotheses that are carefully tested. New therapies are then supposed to be developed based on these new understandings. Indeed, a number of Nobel prizes for medicine fall into this category. The finding that Helicobacter pylori is a primary cause of peptic ulcers has forever altered the way these ulcers are treated with many fewer patients having to face the business end of a scalpel as treatment for their ulcers. In this case, an innovative hypothesis led directly to better therapies that saved money and lives.
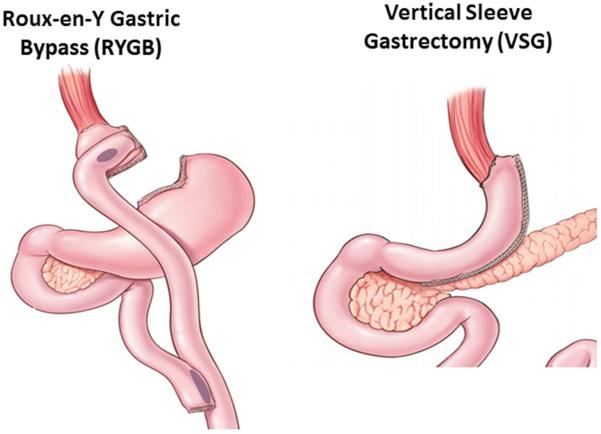
Unfortunately, even in the 21st century, much of what we use for therapy is not nearly so connected to an understanding of a disease process or even how the therapy impacts the body. This does not mean that these therapies are not genuinely effective but rather that we know much less than we think about why they are effective. Take bariatric surgery as an example. One of the most common types of bariatric surgery is a Roux-en-Y gastric bypass (RYGB). This surgery involves making a small pouch just under the esophagus and then bypassing the remaining stomach and part of the small intestine by connecting the jejunum directly to the small pouch (see Figure 1). Ironically, this procedure was initially used to treat peptic ulcers and was made mostly obsolete by therapies that targeted Helicobacter pylori. However, surgeons performing these procedures did notice that many patients had sustained weight loss after these procedures(Mason, 2005).
Figure 1. The two most common bariatric surgeries in the United States.
The first is a Roux-en-Y Gastric Bypass (RYGB) in which a small pouch is created just beneath the esophagus that is not in contact with the rest of the stomach. The jejunum is anastomosed to this small pouch so that ingested food “bypasses” the remnant stomach and upper small intestine and flows directly into the jejunum. The second is a Vertical Sleeve Gastrectomy (VSG) where roughly 80% of the stomach along the greater curvature is removed turning the pouch of the stomach into a “sleeve”.
These were important observations and they have led to the use of RYGB and other related procedures as direct therapies for obesity and related metabolic conditions such as type 2 diabetes mellitus (T2DM). Not surprisingly, the explanations from surgeons on how a RYGB exerted these powerful effects focused on mechanical hypotheses related to the execution of the surgery itself. The idea was that making the small pouch was “restrictive”, i.e. that the small pouch physically limited the number of calories that could be consumed at least over short intervals. The second hypothesis was that by bypassing some of the absorptive capacity of the intestine, such procedures were “malabsorptive”, i.e. that calories could be furtively taken out of the body in the feces and thereby create negative energy balance.
Unfortunately these mechanical hypotheses do not provide an adequate explanation for what occurs after bariatric surgery. The arguments against these mechanical hypotheses are numerous and have been made elsewhere (Miras and le Roux, 2013; Stefater et al., 2012; Thaler and Cummings, 2009) so we will not detail all of them here. However, the most fundamental argument is that after bariatric surgery, patients are less hungry even after they have lost substantial amounts of weight (le Roux and Bueter, 2014). This is exactly the opposite of what you would expect if we either restricted an individual’s ability to ingest or absorb calories. Under such circumstances animals become hungrier as a consequence of neuroendocrine changes that accompany negative energy balance (Ahima et al., 2000). Rather, what occurs after bariatric surgery is best explained as a lowering of the level of body weight/body fat that the body defends. This becomes apparent in experiments in which rats had lost significant amounts of weight after a bariatric procedure termed Vertical Sleeve Gastrectomy (VSG, see Figure 1) and then were forced to lose more weight via further food restriction. Once the VSG rats had adlib access to food again, the rats overate and regained the weight lost due to food restriction (Stefater et al., 2010). VSG rats actively defended a body weight, albeit a lower one, in a manner that was identical to rats that had received a sham version of the procedure.
This misunderstanding is not without consequences. By not identifying the real mediators of these surgical effects, we are unable to improve upon them to make them even more effective and/or less invasive. For example, some surgeons adjust the length of the bypassed limb of a RYGB according to a patient’s BMI. They hypothesize that surgeries for heavier patients need to be “more malabsorptive” in order to achieve greater weight loss. This misunderstanding also leads to patients being exposed to revision surgeries that seek to impact the mechanical aspects of the surgery. In patients who have not achieved some arbitrary definition of “adequate weight loss”, surgeons sometimes evaluate the patient for potential dilations of the small pouch and propose revision surgery if they find them. In this case, the hypothesis that the surgery must “restrict” stomach size to be effective leads to clinical decisions that may not benefit the patient.
Beyond Restriction and Malabsorption: Hormones
The obvious alternative to these mechanical explanations is to posit that specific bariatric procedures result in an alteration in the communication between gut and key metabolic organs including the brain that are important for the regulation of both body weight and various aspects of metabolism including glucose levels. It is not a given that the body weight and metabolic effects of these procedures are driven by the same mechanisms. However, throughout this review, we will make the assumption that there is at least considerable overlap between these two outcomes and so discuss them concurrently. We acknowledge that this assumption may not be borne out ultimately by the data.
The two gut hormones that have received the most attention are ghrelin and glucagon-like peptide (GLP)-1 since both regulate key aspects of energy homeostasis. Secreted in response to changes in acute nutritional flux, these factors affect numerous metabolic processes to influence meal-size, nutrient absorption, and glucose-handling. VSG and RYGB profoundly affect the pattern of release of many gastrointestinal hormones. The magnitude of these changes is impressive, and provides a compelling basis for the perceived role of these hormones in the metabolic outcome(s) of procedures like RYGB and VSG. Interpreting the significance of such changes, however, requires careful consideration and knowing more than whether levels of these hormones are altered by various procedures.
Ghrelin
Ghrelin was among the first candidates to be identified as a potentially important endocrine target in VSG and RYGB procedures. Given exogenously, ghrelin regulates activity in areas of the central nervous system (CNS)implicated in reward and the homeostatic regulation of long-term energy stores such as the hypothalamus (Kojima et al., 1999) and nucleus accumbens (Cone et al., 2014). Pharmacologically, ghrelin increases food intake in humans (Wren et al., 2001) and rodents (Tschöp et al., 2000), but also modulates peripheral glucose metabolism through both central and peripheral actions (Heppner et al., 2014) in ways that inhibit glucose stimulated insulin release (Reimer et al., 2003; Tong et al., 2010), and promote insulin resistance in muscle (Vestergaard et al., 2008). Removing ghrelin, therefore, provides a plausible basis for reduced food cravings as well as improved glycaemia in some bariatric procedures. This is particularly true in the case of VSG where the major source of ghrelin is removed with the removal of much of the stomach along the greater curvature. We studied circulating levels of ghrelin in rat models of VSG and RYGB, and found plasma ghrelin levels were substantially reduced after VSG, but not after RYGB (Chambers et al., 2012). We then compared the effects of VSG on food intake, body weight, dietary fat preference, and glucose tolerance in ghrelin-deficient- and wild-type mice and found that VSG was equally effective in both strains (Chambers et al., 2012). While loss-of-function studies such as these leave open the possibility of functional and developmental compensations that could potentially obscure, or distort, ghrelin’s role in these outcomes, it is nonetheless clear that reduced ghrelin signaling is not necessary for the weight loss and improved glucose regulation that result from VSG.
GLP-1
Secreted from intestinal L-cells, GLP-1 increases insulin and decreases glucagon production, delays gastric emptying and intestinal transit, and reduces meal-size through a G-coupled protein receptor specific to GLP-1. Administration of exogenous GLP-1 or GLP-1 analogues results in weight loss and improvements in glucose regulation in T2DM patients (Vilsbøll et al., 2012). Post-prandial levels of GLP-1 are dramatically increased after both VSG both patients and rodent models and RYGB (Chambers et al., 2014; Jiménez et al., 2013, 2014; Umeda et al., 2011) in suggesting that these changes are important to the metabolic benefit of these procedures. Consistent with this hypothesis, post-surgical increases in prandial GLP-1 are associated with greater insulin release (Umeda et al., 2011)and greater weight loss (le Roux et al., 2007) after RYGB surgery in humans. In some human studies, short-term infusion of a pharmacological antagonist of the GLP-1 receptor can reduce the increased insulin secretion observed after RYGB (Salehi et al., 2011).
However, functional studies, designed to assess the influence of GLP-1 signaling per se on these outcomes have produced mixed results. Pharmacologic blockade of the GLP-1 receptor after RYGB or VSG greatly inhibits prandial insulin release(Jiménez et al., 2013, 2014; Salehi et al., 2014; Shah et al., 2014). The corresponding impairment in glycaemia, however, is modest by comparison, indicating that the contribution of endogenous GLP-1 to overall β-cell function after these surgeries may be relatively minor. The importance of endogenous GLP-1 signaling to the anorectic effect of bariatric surgery is also unclear. For example, rats that underwent RYGB or a sham-operation showed similar responses in terms of food intake and weight change when chronically infused with a GLP-1 receptor antagonist in the brain. In other words, surgical increases in GLP-1 signaling in the CNS are not uniquely responsible for the body weight-lowering effect of this surgery (Ye et al., 2014) but it remains possible that GLP-1 signaling on the vagus may be enhanced after these bariatric procedures. However, mice with genetic loss-of-function of the GLP-1 receptor respond normally to VSG (Wilson-Pérez et al., 2013)and RYGB (Mokadem et al., 2014) both in terms of weight loss and improvements in glucose regulation. Such an outcome indicates that increases in GLP-1 receptor signaling are not necessary for the major metabolic outcomes of either VSG or RYGB. One possibility is that activation of L-cells may not drive the weight or metabolic benefits but may be an emergency response to the high gastric emptying levels where increased GLP-1 (and PYY) may be an ineffective attempt to reduce gastric emptying. Alternatively, undigested chyme in the the ileum may signal the need to increase absorptive capacity of the small intestine and increased GLP-2 that is co-secreted with GLP-1 may be an attempt to drive such increased absorptive capacity. In this possibility, increased GLP-1 would be an epiphenomena to the attempt to alter gut morphology to alleviate increased nutrient presentation in the ileum.
These data cannot exclude the possibility that both increases in GLP-1, decreases in ghrelin, and a myriad of other factors are part of a broader set of hormonal changes that in concert work to mediate the potent effects of these procedures. Other factors that have been hypothesized to be altered after one or more of these procedures include prandial secretion of cholecystokinin (Jacobsen et al., 2012; Peterli et al., 2012), glucose inhibitory peptide (Lee et al., 2013; Romero et al., 2012), glucagon (Romero et al., 2012), GLP-2 (Jacobsen et al., 2012; Romero et al., 2012), peptide YY (Dimitriadis et al., 2013; Peterli et al., 2009), and perhaps others (Dimitriadis et al., 2013; Santoro et al., 2008). Determining the relative contribution of these different factors to surgical benefits on glucose tolerance and weight loss remains an important research goal. What is clear, however, is that changes in the secretion of GLP-1 or ghrelin do not explain nearly as much of the phenomena as we and others had hypothesized.
Beyond Restriction and Malabsorption: Bile Acids and Gut Microbiota
Bile acids are made in the liver and secreted into the duodenum particularly in response to fat ingestion where they act as necessary surfactants so that lipids can be absorbed and either stored or moved to the tissues that will utilize them as fuel. In addition to this role in lipid absorption, a wide range of evidence points to bile acids as hormones. Two receptors have been identified that respond to bile acids. The first is a G-protein coupled receptor found on the cell surface termed TGR5 and the second is a ligand-activated transcription factor farnesoid-X-receptor (FXR) (Lefebvre et al., 2009). In a RYGB, bile acids secreted into the duodenum do not mix with food until the two limbs of the RYGB become the common channel in the distal jejunum. Such surgical manipulation has been shown to alter both the composition and levels of bile acids in different compartments including in general circulation in a weight-independent manner (Kohli et al., 2013; Patti et al., 2009). Like for many other hormonal changes, VSG and RYGB look similar on this front with VSG also resulting in increased circulating bile acids in both rodents (Myronovych et al., 2014) and humans (Kohli et al., 2013).
Such results open up the possibility that an important underpinning of the effects of bariatric surgery is its ability to alter bile acid signaling. We directly tested this hypothesis by comparing the effects of VSG in wild-type (WT) and FXR knockout (FXRKO) mice. While FXRKO mice initially reduced their food intake and body weight after VSG, after 4 weeks they had begun overeating and by 11 weeks regained all of the lost weight and body fat compared to sham operated FXRKO mice(Ryan et al., 2014). The importance of FXR signaling was not limited to the effect on body weight. FXRKO mice also failed to show the potent effects of VSG to reduce fasting blood glucose and improve glucose tolerance. These experiments point to an important role of FXR as a molecular target for the potent effects of VSG.
FXR plays an important role in a wide range of G.I. functions. One target of FXR signaling is the gut bacterial community (Sayin et al., 2013). Inside our gut is approximately 3 trillion bacteria and several recent findings point to these bacteria having an impact on host metabolism including susceptibility to obesity and T2DM (Sommer and Bäckhed, 2013). Both VSG and RYGB represent large perturbations in the environment of the GI tract and so not surprisingly they exert potent changes on which bacteria are most prevalent in the gut (Aron-Wisnewsky and Clement, 2014). Recent evidence implicates these changes as a driver for the effects of RYGB (Liou et al., 2013). When germ-free mice were given bacteria containing fecal transplants from RYGB-treated mice, those mice lost weight while germ-free mice given fecal transplants from sham-treated mice gained weight. It is difficult to say from these experiments the size of the gut-bacteria driven effect of RYGB but it is clear that independent of other impacts of the surgery, changes in gut bacteria after RYGB are sufficient to alter the body weight of the host organism.
A key question that results from these findings is the relationship between the effects of bariatric surgery on levels/composition of bile acids, FXR signaling and the gut bacteria. In FXRKO mice, some of the effects of VSG to alter the gut bacteria community were blunted including entirely obviating the effect of VSG on some strains of bacteria (Ryan et al., 2014). However, this does not mean that FXR is strictly “upstream” of the effect of surgery on the gut bacteria. Bile acids and the resulting changes in pH are important regulators of the environment that promote some bacteria to thrive and others to whither independent of their effect on receptors such as FXR. One target gene for FXR is a gut hormone termed FGF19 (in human and its mouse ortholog FGF15). FGF19 has potent effects to reduce bile acid secretion both at the level of the liver and the gallbladder (Kir et al., 2011; Potthoff et al., 2011). Consequently, FXR can exert indirect effects on gut bacteria by manipulating the levels of bile acids. The gut bacteria are not passive recipients of bile acids either. Gut bacteria can impact the levels of bile acids by a variety of bile-acid degrading pathways and the composition of the bile acids by altering their conjugation (Sayin et al., 2013). In turn, alterations in levels and types of bile acids can alter the amount of FXR signaling in the intestine and beyond (Sayin et al., 2013). The important point here is that we simply do not know the sequence of events that alters the bile acids, FXR signaling and gut bacteria that all appear to contribute to the effects of surgery on obesity and diabetes.
The role of enteroplasticity in the mechanisms underlying bariatric surgery
It is interesting to consider that our most successful strategy towards treating obesity involves manipulation of one of the body’s most complex biological systems. Traditionally, the primary function of the intestine was focused on ensuring maximal macro- and micro-nutrient and water absorption into the body. Without this capacity, malnourishment ensues and becomes one of the most confounding health problems in intestinal disease. In fact, the diarrhea and dehydration that accompanies GI infections causes millions of deaths per year (Kosek et al., 2003).
To accomplish this task, the intestinal mucosa is highly plastic system where in humans epithelial cells turnover every 3-5 days (Groos et al., 2001). Consequently, the intestinal mucosa has an enormous capacity to respond to internal and external stimuli (Shaw et al., 2012). This process called intestinal adaptation, or enteroplasticity (Drozdowski et al., 2009), has been extensively studied in response to massive small bowel resection where the mucosa displays profound proliferation in patients with more positive outcomes (Shaw et al., 2012). Such enteroplasticity occurs in diabetes, aging, with fasting and with malnutrition (Fedorak et al., 1987; Ferraris and Carey, 2000). While enteroplasticity can have positive outcomes for patients after small bowel resection (Sturm et al., 1997), it can have negative outcomes for patients with diabetes (Burant et al., 1994). Further, high fat diets have been suggested to play a role in changes in gastrointestinal physiology that then contribute to the metabolic complications associated with obesity (Cani et al., 2007).
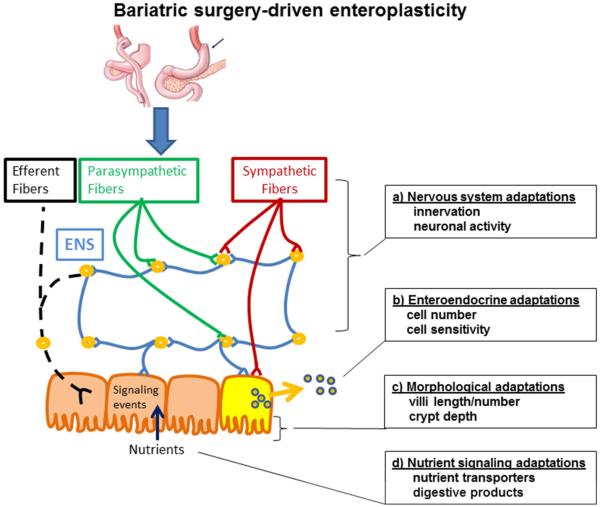
Most macronutrients are absorbed in the proximal small intestine (duodenum and jejunum) while most micronutrients are absorbed distally in the ileum. This functional change from the proximal to distal gut is dictated in part by the types of epithelial columnar cells that form the intestinal brush border. Development and turnover of these cells progress from crypt to villus units that contain absorptive (enterocytes), secretory (goblet, Paneth, tuft, and enteroendocrine), progenitor, and stem cells (Spence et al., 2011). Tight junction proteins located between enterocytes and mucous secreted from goblet cells also provide an additional physical barrier between the luminal contents and the internal milieu. Recently, a deeper understanding of the mucous layer has revealed a much higher ordered organization and thus a more important role in immunuology than previously thought. Within the stomach and colon there is a looser outer layer and an inner more stratified layer while the small intestine has a more discontinuous and less defined nature (Johansson et al., 2011). The divergent layers are formed by a large class of proteins of various sizes and structures call mucins. In the outer loose layer is where the commensal bacteria is found. As discussed above, these bacterial species that play a key role in immune function and also influence other biological systems as well. Lastly, the intestine is highly innervated by the CNS but also has its own enteric nervous system. Thus, there are countless possible morphological, cellular and systemic adaptations that can take place when the intestinal integrity is challenged and with that there are multi-system consequences (see Figure 2).
Figure 2. Bariatric surgery-driven enteroplasticity.
Bariatric surgery involves surgical manipulation of the body’s most complex biological systems. We hypothesize that intestinal adaptation, or enteroplasticity, underlies the benefits of bariatric surgery. Some of the possible adapations that could be involved include a) the nervous system via changes in innervation or neuronal activity, b) enteroendocrine adaptations via increases in enteroendocrine cell number or sensitivity to stimuli, c) morphological changes that increase nutrient absorptive capacity via increases in villi length and/or number and/or increased crypt depth, and d) adaptations in nutrient signaling processes either by increases nutrient transport or by the production of digestive products that stimulate intracellular signaling processes.
Enteroplasticity: Morphology
The rapid cell-turnover in the intestine is regulated by signaling pathways that dictate rates of proliferation and atrophy. Changes in the balance of proliferation to atrophy leads to changes in overall mucosal mass (Shaw et al., 2012). The process of enterocyte proliferation can involve one or more of the following: increases in villus height, crypt depth, mucosal surface area, and intestinal weight (Drozdowski et al., 2009). In the context of removal of surface area with surgery, proliferation is beneficial because it creates more absorptive cells for macro and micronutrients. This occurs independent of increasing nutrient transporters, per se. For example, a 70% resection of the proximal bowel in rats leads to an increase in ileal glucose uptake that was associated with an increase in villus height and intestinal length rather than increased gene expression of glucose transporters (Iqbal et al., 2008). The important point here is that when one part of the GI tract is compromised, another part adapts to take up that function.
There are many reasons to speculate that bariatric surgery drives important changes in morphological enteroplasticity. First, there is the research demonstrating the wide-ranging impact of small bowel resection on intestinal adaptation. Second, some early studies found that diet-induced obesity was associated with increased intestinal length (Dameto et al., 1991), and multiple rodent models of obesity display increased intestinal cell proliferation (Ishizuka et al.; Kageyama et al., 2003) and permeability (Brun et al., 2007; Cani et al., 2007). However, in most of these experiments there was no attempt to control for food intake. Consequently, it is not entirely clear whether such enteroplasticity is an effect of the diet per sé or the result of handling additional calories. Nevertheless, if obesity results in alterations of GI morphology, it is intriguing to consider that some bariatric surgical procedures might directly or indirectly reverse these effects on the GI tract.
Multiple types of bariatric surgeries demonstrate some degree of enteroplasticity in rodent models. For example, in Zucker rats, duodenal jejunal bypass, a surgery where the stomach is left intact and the upper gut is bypassed from nutrient exposure, causes atrophy in the bypassed limb but hyperplasia in the portion of jejunum now exposed to nutrients(Li et al., 2013). In Zucker Diabetic rats, placement of a duodenal-endoluminal sleeve, a flexible tube that is inserted within the intestine and prevents nutrient to tissue interactions in the duodenum, increases villus length through the upper intestine compared to pair-fed rats (Habegger et al., 2013). Another surgery where a piece of the ileum is interpositioned within the jejunum leads to a “jejunization” of the transposed piece (Kohli et al., 2010). Lastly, in rats, RYGB significantly increases bowel width, villus height, crypt depth, and cell proliferation (le Roux et al., 2010; Taqi et al., 2010) in the alimentary and common intestinal limbs while the biliopancreatic limb only demonstrates an increase in bowel width (Taqi et al., 2010). Taken together, these data point to important restructuring of the GI anatomy after bariatric surgical procedures that includes distinct cell-structural changes.
Enteroplasticity: Nervous system changes
The small intestine is richly innervated by the autonomic nervous system (ANS), but also contains an enteric nervous system (ENS). This independently functioning nervous system is composed of neural circuits that control intestinal motor functions, blood flow, mucosal transport and secretions, and modulates immune and endocrine functions. The ENS spans the length of the GI tract within the myenteric and submucosal plexus which is also innervated by the ANS (Bitar et al., 2014). Given the extreme surgical restructuring of the GI tract involved in some bariatric surgeries, it would seem reasonable to hypothesize that enteroplastic adaptations after the surgery could involve the nervous system.
The ENS is not the only innervation of the GI tract. The vagus nerve provides both afferent and efferent communication from the brain to the gut and abnormal vagal activity has been implicated in obesity (de Lartigue et al., 2011, 2014). To attempt to understand the impact of surgery on the vagal nerve, one study performed RYGB in a mouse model that expresses a reporter protein that could be easily visualized using IHC specifically in vagal neurons (Gautron et al., 2013). The results demonstrated that innervation was lost at all surgical anastomoses within the stomach and intestine while innervation of the intact intestinal segments and liver were normal. Vagal fibers displayed morphological abnormalities predominantly in the myenteric plexus of the stomach, including swollen axons and terminals and abnormally shaped preganlionic endings. The physiological implications of this remodeling is unknown. Additional studies have sought to determine the role of the vagus in the success of bariatric surgery. Clinical studies have demonstrated that the threshold for vagal tension sensations were negatively correlated with meal size after RYGB (Björklund et al., 2010). In rats, a RYGB surgery that spares the vagus resulted in greater weight loss and reduced food intake compared to a surgery where the vagus was cut (Bueter et al., 2010). Counter to previous research that suggested that portal vein neuronal glucose-sensing is necessary for the improvements in glucose homeostasis with bariatric surgery (Troy et al., 2008), common hepatic branch vagotomy, which ablates innervation of the liver, portal vein, and proximal duodenum did not prevent weight loss after RYGB in rats (Shin et al., 2012). However, a more specific lesion of the celiac branch of the vagus, which specifically innervates the intestinal tract moderates early post-surgical weight loss after RYGB (Hao et al., 2014). Together with the data by Gautron et al. (Gautron et al., 2013), these data suggest that the innervation to the intestine may not only be intact after RYGB, but that neural innervation may be necessary for the outcome of the surgery . It is also interesting that the changes in GI innervation or neuronal activity could influence GI peptide secretion (Hansen et al., 2004) and/or may play a role in the negative side-effects of the surgery such as dumping syndrome.
Enteroplasticity: Enteroendocrine changes
Nutrient entry into the GI tract initiates a myriad of physiological responses including secretion of several GI peptides that have paracrine, endocrine, and neuroendocrine action and function to aid in the processing and systemic assimilation of nutrients. As discussed above, it is consistently demonstrated that both RYGB and VSG cause significant elevations in some of the same GI peptides (Peterli et al., 2012). However, as also reviewed above, changes in individual GI peptides (GLP-1, PYY, CCK) are not necessary for the beneficial outcomes of bariatric surgery. We predict that the changes in the level of these peptides are a product of surgery rather than a driver of the metabolic adaptations and thus are representative of enteroplasticity. In this section we will review the potential enteroplastic mechanisms that drive the increase in GI peptide secretion following RYGB and VSG.
Given the profound anatomical differences between RYGB and VSG, the mechanism(s) that underlie this effect in both procedures is not obvious. One hypothesis is that both procedures compromise the ability of the stomach to meter chyme into the small intestine. This high gastric emptying rate would result in an increase in the amount of nutrients reaching the distal small intestine where these hormones are thought to be secreted. However, recent data from our laboratory suggest that this explanation may be too simplistic. To examine intestinal “sensitivity” to nutrients, GLP-1 responses were measured in response to nutrients infused directly into the duodenum at the same rate and volume in sham versus VSG surgery animals (Chambers et al., 2014). Despite this control over gastric emptying rate, the VSG animals maintained significant increases in nutrient-induced GLP-1 secretion. This would support the notion of enteroplasticity rather than just altered nutrient presentation as a driver of the increased nutrient response.
Therefore, we believe this response to be a physiologic adaptation borne from the increased metabolic demands produced by chronically high gastric emptying rates. One consequence of this enteroplasticity appears to be elevated nutrient-induced GI hormone secretion such as GLP-1. If so, we would predict that compensations such as changes in the secretion of prandial hormones will occur in discreet regions of the gut that are most affected by surgery, i.e., regions of the gut that face the greatest increase in metabolic demand, as opposed to homogenous changes throughout the gut. Indeed, when Nguyen et al. infused nutrients directly into the bypassed segment of RYGB patients, a region in which the metabolic demands of the tissue actually decrease, prandial GLP-1 responses appeared normal relative to those of control subjects (Nguyen et al., 2014). The same patients, however, showed robust increases in prandial GLP-1 when nutrients were presented to the common limb via the stomach.
It is the common limb that bears the brunt of the increased metabolic demand caused by faster gastric emptying and the exclusion of the proximal foregut. Consistent with this observation, the number of enteroendocrine cells that express GLP-1, CCK, serotonin and PYY is greatly increased in the common limb, but not in the segment of the proximal intestine that is bypassed in a rat model of RYGB surgery (Mumphry et al. 2013). Moreover, the common limb is also the region in which the greatest morphological changes occur in terms of increased villus height and greater overall surface area (le Roux et al., 2010). A similar effect on increasing enteroendocrine cell numbers is seen after ileal interposition (Hansen et al., 2014). Thus, at least with VSG, but we believe with other surgeries as well, an alternative explanation to the distal gut hypothesis is that chronically high gastric emptying rates drive adaptive enteroplasticity. A consequence of this enteroplasticity is elevated nutrient-induced GI hormone secretion.
Other hormones, growth factors, and cytokines that are associated with enteroplasticity have also been shown to increase after RYGB. The preproglucagon gene produces GLP-1 but also co-secretes other peptides including glucagon-like-peptide-2 (GLP-2). GLP-2 has been shown to have a physiological role in intestinal growth (Hartmann et al., 2002), and long-acting GLP-2 agonists (eg. Teduglutide) are effective treatments in patients with diseases that cause intestinal insufficiency (Jeppesen et al., 2001; Shaw et al., 2012). Additionally, IGF-1, fibroblast growth factors and epidermal growth factor have all been shown to increase in rats following RYGB (Taqi et al., 2010), and all have been shown to have physiological or pharmacological roles in intestinal growth and proliferation (Brubaker et al., 1997; Houchen et al., 1999). Together these data suggest that enteroendocrine plasticity results in an increase in several gut-secreted peptides that have positive metabolic and intestinal morphology outcomes.
Enteroplasticity: Nutrient sensing
That nutrient flow through the GI tract is essential for maintaining intestinal integrity is highlighted by the fact that when nutrients are no longer presented to the intestinal lumen, for example due to starvation or total parental nutrition (IV nutrients), the intestinal mucosa drastically atrophies due both to increased apoptosis and decreased proliferation (Tappenden, 2006; Yang et al., 2003). This atrophy compromises barrier function (Yang et al., 2003) resulting in high rates of infection and sepsis in patients on total parental nutrition. In most cells of the body, including the intestine, nutrients act not only as fuel but also as signaling molecules(Ryan and Seeley, 2013). In this manner, nutrients could directly influence intestinal adaptation. Indirect actions are also possible. Enhanced nutrient-induced stimulation of gut peptide secretion can result in alterations in associated neuroendocrine and paracrine signaling pathways. The result is that changes in nutrient-sensing could play a key role in the many biological processes regulated by the intestinal tract.
Although it is not clearly understood, the physical changes with bariatric surgery could influence mechanical and physiological processing of nutrients and thus could alter the types of nutrient by-products and signaling that occurs with food ingestion. We do know that individual macronutrients and macronutrient products do influence morphological enteroplasticity. Withdrawal of protein restricts intestinal growth (Sanderson and Naik, 2000) while supplementation of total parental nutrition (intravenous nutrients) with oral glutamine (Kessel et al., 2008) or oral arginine (Koppelmann et al., 2012) can protect the intestine from endotoxin-induced injury. Moreover, compared to total parental nutrition, enteral infusion of higher concentrations of sucrose maintain body weight and mucosal mass (Weser et al., 1986). Although it is unclear if this is due to the increasing calories or to the carbohydrate exposure itself, it has been demonstrated that dietary carbohydrate rapidly stimulates its own uptake into the intestinal epithelium by increasing active transport processes (Cheeseman and Maenz, 1989; Diamond et al., 1984; Ferraris et al., 1992). All of these observations are consistent with data demonstrating atropy of the bypassed limb after RYGB (Li et al., 2013).
Carbohydrates could also support intestinal function in another way. Fermentation of prebiotics and carbohydrates within the colon produces short chain fatty acids which then support colonic enteroplasticity (Roy et al., 2006). Supplementation of total parental nutrition with short chain fatty acids (sodium acetate, propionate, butyrate) maintains intestinal mass in rats (Koruda et al., 1988); although not to the level of the chow-fed control animals (Murakoshi et al., 2011). With both amino acids and short chain fatty acids it is interesting that direct luminal nutrient exposure is not necessary to support intestinal morphology underscoring the integrative role of the intestine in physiological regulation.
Multiple studies do suggest that intestinal nutrient sensing is altered by bariatric surgery. Earlier research suggested that the bulk of this was sensed directly within the portal vein (Troy et al., 2008). However, additional research suggests that a duodenal-jejunal bypass surgery in rats leads to improved nutrient-sensing within the gut that contributed to enhanced CCK secretion (Rasmussen et al., 2012). One study has found that ex vivo 3H-glucose uptake from the lumen into enterocytes of the Roux limb was reduced compared to sham operated rats after RYGB (Stearns et al., 2009). However, more recent in vivo data found that RYGB causes the intestine to become a major site of glucose disposal, even when tissue mass was taken into consideration, and that intestinal glucose metabolic pathways are reprogrammed to support tissue growth (Saeidi et al., 2013). The discrepancies between the two studies could be methodological or could implicate systemic influences, neuronal or endocrine for example, on regulation of intestinal glucose disposal; influences that would be missing in the ex vivo studies performed by Stearns et. al. (Stearns et al., 2009). If the results of Saeidi et al. are true, a reasonable alternative explanation could be that the hypertrophy and increased glucose uptake could be in response to the fact that the remaining intestine has an increased workload when processing nutrients.
The critical point here is that surgical rearrangement of the GI tract such as occurs with bariatric surgery necessitates a number of gut adaptions. Such enteroplasticity could be the result of increased nutrient presentation that results from high gastric emptying rates that alter nutrient presentation, dramatic changes in pH, altered physical forces within the GI tract or some combination of these. Importantly, from this perspective the changes in both the gut microbiota and bile acids that clearly contribute to the physiological effects of the surgery can be seen as reflections of this surgically-induced enteroplasticity. Changes in the gut bacterial community are likely a result of the physical accommodations the gut is making that changes the environment and thereby shifts the bacterial population to ones that are best suited for that new environment. While it remains unclear just why bile acid composition and levels in the plasma are altered, it seems likely that this is also the result of intestinal adaptation that alters bile acid handling. Determining how enteroplasticity is linked to altered bile acid secretion, absorption or reuptake is an important research goal upon which our group and others are focusing.
Conclusions
The potent effects of bariatric surgery to cause dramatic and sustainable reductions in body weight and improvements in glucose regulation remain incompletely understood. Ultimately, bariatric surgery is not just an effective therapeutic tool but a platform that will yield new insights into both the etiology of metabolic diseases and like the insights from Helicobacter pylori reduce the need for surgical interventions. However, progress can only be made with a new framework for understanding these effects that moves past the rationale that drove the development of these successful procedures, i.e. mechanical restriction and malabsorption. This new framework must focus on linking the surgical procedures to the physiological systems and molecular pathways that are ultimately responsible for the benefits on weight and metabolism. We have forwarded the hypothesis that this link involves gut adaptation driven by the modified environment of the surgically altered GI tract. Identifying the common enteroplastic changes that occur after diverse bariatric procedures is likely to yield significant advances in how we got into the twin epidemics of obesity and T2DM and how we might get out of them as well.
Highlights.
Bariatric surgery is highly effective for treating obesity and diabetes.
These procedures alter gut function in important ways that contribute to their potent effects on weight and glucose metabolism.
Gut adaptation or “enteroplasticity” are likely contributors to the effects of these surgeries on energy balance and metabolism.
Acknowledgements
Both RJS and DAS have received research funding from Ethicon Surgical Care that supported work on this topic. RJS has received research funding and worked as a consultant for Novo Nordisk. RJS has also worked as a consultant for Boehringer Ingelheim, Novartis, Takeda Eisai and Sanofi. RJS was supported by NIH grant 7R01DK093848. DAS was supported by 5R01DK08248.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
DAS contributed to discussion of the ideas that make up this review, generated substantial text and provided edits for other sections.
APC contributed to discussion of the ideas that make up this review, generated substantial text and provided edits for other sections.
RJS contributed to discussion of the ideas that make up this review, generated substantial text and provided edits for other sections.
References
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front. Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Aron-Wisnewsky J, Clement K. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity. Curr. Atheroscler. Rep. 2014;16:454. doi: 10.1007/s11883-014-0454-9. [DOI] [PubMed] [Google Scholar]
- Bitar KN, Raghavan S, Zakhem E. Tissue engineering in the gut: developments in neuromusculature. Gastroenterology. 2014;146:1614–1624. doi: 10.1053/j.gastro.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund P, Laurenius A, Een E, Olbers T, Lönroth H, Fändriks L. Is the Roux limb a determinant for meal size after gastric bypass surgery? Obes. Surg. 2010;20:1408–1414. doi: 10.1007/s11695-010-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- Bueter M, Löwenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, Lutz T, le Roux CW. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes. Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burant CF, Flink S, DePaoli AM, Chen J, Lee WS, Hediger MA, Buse JB, Chang EB. Small intestine hexose transport in experimental diabetes. Increased transporter mRNA and protein expression in enterocytes. J. Clin. Invest. 1994;93:578–585. doi: 10.1172/JCI117010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, et al. The Effects of Vertical Sleeve Gastrectomy in Rodents are Ghrelin Independent. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, Lasance K, Woods SC, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am. J. Physiol. Endocrinol. Metab. 2014;306:E424–E432. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman CI, Maenz DD. Rapid regulation of D-glucose transport in basolateral membrane of rat jejunum. Am. J. Physiol. 1989;256:G878–G883. doi: 10.1152/ajpgi.1989.256.5.G878. [DOI] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J. Neurosci. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameto MC, Rayó JM, Esteban S, Planas B, Tur JA. Effect of cafeteria diet on the gastrointestinal transit and emptying in the rat. Comp. Biochem. Physiol. A. Comp. Physiol. 1991;99:651–655. doi: 10.1016/0300-9629(91)90145-3. [DOI] [PubMed] [Google Scholar]
- Diamond JM, Karasov WH, Cary C, Enders D, Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J. Physiol. 1984;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann. Surg. 2013;257:647–654. doi: 10.1097/SLA.0b013e31826e1846. [DOI] [PubMed] [Google Scholar]
- Drozdowski LA, Clandinin MT, Thomson ABR. Morphological, kinetic, membrane biochemical and genetic aspects of intestinal enteroplasticity. World J. Gastroenterol. 2009;15:774–787. doi: 10.3748/wjg.15.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak RN, Chang EB, Madara JL, Field M. Intestinal adaptation to diabetes. Altered Na-dependent nutrient absorption in streptozocin-treated chronically diabetic rats. J. Clin. Invest. 1987;79:1571–1578. doi: 10.1172/JCI112991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris RP, Carey HV. Intestinal transport during fasting and malnutrition. Annu. Rev. Nutr. 2000;20:195–219. doi: 10.1146/annurev.nutr.20.1.195. [DOI] [PubMed] [Google Scholar]
- Ferraris RP, Villenas SA, Hirayama BA, Diamond J. Effect of diet on glucose transporter site density along the intestinal crypt-villus axis. Am. J. Physiol. 1992;262:G1060–G1068. doi: 10.1152/ajpgi.1992.262.6.G1060. [DOI] [PubMed] [Google Scholar]
- Gautron L, Zechner JF, Aguirre V. Vagal innervation patterns following Roux-en-Y gastric bypass in the mouse. Int. J. Obes. (Lond) 2013;37:1603–1607. doi: 10.1038/ijo.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos S, Hünefeld G, Luciano L. Epithelial cell turnover--extracellular matrix relationship in the small intestine of human adults. Ital. J. Anat. Embryol. 2001;106:353–361. [PubMed] [Google Scholar]
- Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J, Yi C-X, Gao Y, Lehti M, Ottaway N, et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut. 2013 doi: 10.1136/gutjnl-2013-304583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CF, Vassiliadis E, Vrang N, Sangild PT, Cummings BP, Havel P, Jelsing J. The effect of ileal interposition surgery on enteroendocrine cell numbers in the UC Davis type 2 diabetes mellitus rat. Regul. Pept. 2014;189:31–39. doi: 10.1016/j.regpep.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Hansen L, Lampert S, Mineo H, Holst JJ. Neural regulation of glucagon-like peptide-1 secretion in pigs. Am. J. Physiol. Endocrinol. Metab. 2004;287:E939–E947. doi: 10.1152/ajpendo.00197.2004. [DOI] [PubMed] [Google Scholar]
- Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud H-R. Vagal Innervation of Intestine Contributes to Weight Loss After Roux-en-Y Gastric Bypass Surgery in Rats. Obes. Surg. 2014 doi: 10.1007/s11695-014-1338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B, Thulesen J, Hare KJ, Kissow H, Orskov C, Poulsen SS, Holst JJ. Immunoneutralization of endogenous glucagon-like peptide-2 reduces adaptive intestinal growth in diabetic rats. Regul. Pept. 2002;105:173–179. doi: 10.1016/s0167-0115(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Heppner KM, Piechowski CL, Müller A, Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi R, Biebermann H, et al. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2014;63:122–131. doi: 10.2337/db13-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am. J. Physiol. 1999;276:G249–G258. doi: 10.1152/ajpgi.1999.276.1.G249. [DOI] [PubMed] [Google Scholar]
- Iqbal CW, Qandeel HG, Zheng Y, Duenes JA, Sarr MG. Mechanisms of ileal adaptation for glucose absorption after proximal-based small bowel resection. J. Gastrointest. Surg. 2008;12:1854–1864. doi: 10.1007/s11605-008-0666-9. discussion 1864–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Senoo A, Hayashi K, Sasaki K, Kako M, Suzuki Y, Imazeki N, Shimizu H, Kobayashi Y, Haba R, et al. Ventromedial hypothalamic lesions enhance small intestinal cell proliferation in mice. Obes. Res. Clin. Pract. 6:e175–e262. doi: 10.1016/j.orcp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Worm D, Almdal T, Naver LS, Hvolris LE, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg. 2012;22:1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care. 2013;36:2062–2069. doi: 10.2337/dc12-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and Glucose Tolerance After Sleeve Gastrectomy in Morbidly Obese Subjects with Type 2 Diabetes. Diabetes. 2014;63:3372–3377. doi: 10.2337/db14-0357. [DOI] [PubMed] [Google Scholar]
- Johansson MEV, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, Subramani DB, Holmén-Larsson JM, Thomsson KA, Bergström JH, et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama H, Kageyama A, Endo Y, Osaka T, Nemoto K, Hirano T, Namba Y, Shioda S, Inoue S. Ventromedial hypothalamus lesions induce jejunal epithelial cell hyperplasia through an increase in gene expression of cyclooxygenase. Int. J. Obes. Relat. Metab. Disord. 2003;27:1006–1013. doi: 10.1038/sj.ijo.0802325. [DOI] [PubMed] [Google Scholar]
- Kessel A, Toubi E, Pavlotzky E, Mogilner J, Coran AG, Lurie M, Karry R, Sukhotnik I. Treatment with glutamine is associated with down-regulation of Toll-like receptor-4 and myeloid differentiation factor 88 expression and decrease in intestinal mucosal injury caused by lipopolysaccharide endotoxaemia in a rat. Clin. Exp. Immunol. 2008;151:341–347. doi: 10.1111/j.1365-2249.2007.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in Liver Metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011;76:139–144. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- Kohli R, Kirby M, Setchell KDR, Jha P, Klustaitis K, Woollett LA, Pfluger PT, Balistreri WF, Tso P, Jandacek RJ, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G652–G660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight Loss Induced by Roux-en-Y Gastric Bypass But Not Laparoscopic Adjustable Gastric Banding Increases Circulating Bile Acids. J. Clin. Endocrinol. Metab. 2013;98:E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I. Dietary L-arginine supplementation reduces Methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol. 2012;12:41. doi: 10.1186/1471-230X-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koruda MJ, Rolandelli RH, Settle RG, Zimmaro DM, Rombeau JL. Effect of parenteral nutrition supplemented with short-chain fatty acids on adaptation to massive small bowel resection. Gastroenterology. 1988;95:715–720. doi: 10.1016/s0016-5085(88)80019-2. [DOI] [PubMed] [Google Scholar]
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab. 2011;301:E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol. Metab. 2014;3:595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Brown T, Magnuson TH, Egan JM, Carlson O, Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J. Clin. Endocrinol. Metab. 2013;98:E1208–E1212. doi: 10.1210/jc.2013-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li B, Lu Y, Srikant CB, Gao Z-H, Liu J-L. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal-jejunal bypass surgery in Zucker fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G635–G645. doi: 10.1152/ajpgi.00275.2012. [DOI] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano J-M, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005687. 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason EE. History of obesity surgery. Surg. Obes. Relat. Dis. 2005;1:123–125. doi: 10.1016/j.soard.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013;10:575–584. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab. 2014;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumphrey MB, Patterson LM, Zheng H, Berthoud H-R. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 2013;25:e70–e79. doi: 10.1111/nmo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi S, Fukatsu K, Omata J, Moriya T, Noguchi M, Saitoh D, Koyama I. Effects of adding butyric acid to PN on gut-associated lymphoid tissue and mucosal immunoglobulin A levels. JPEN. 2011;35:465–472. doi: 10.1177/0148607110387610. [DOI] [PubMed] [Google Scholar]
- Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014;22:390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption, and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 2014;22:2003–2009. doi: 10.1002/oby.20791. [DOI] [PubMed] [Google Scholar]
- Patti M-E, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann. Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes. Surg. 2012;22:740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BA, Breen DM, Luo P, Cheung GWC, Yang CS, Sun B, Kokorovic A, Rong W, Lam TKT. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology. 2012;142:834–843.e3. doi: 10.1053/j.gastro.2011.12.053. [DOI] [PubMed] [Google Scholar]
- Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc. 2012;26:2231–2239. doi: 10.1007/s00464-012-2166-y. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Bueter M. The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp. Physiol. 2014;99:1128–1132. doi: 10.1113/expphysiol.2014.078378. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJB. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann. Surg. 2010;252:50–56. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr. Clin. Pract. 2006;21:351–366. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Seeley RJ. Physiology. Food as a hormone. Science. 2013;339:918–919. doi: 10.1126/science.1234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Gastaldelli A, D’Alessio DA. Blockade of Glucagon-like Peptide 1 Receptor Corrects Postprandial Hypoglycemia After Gastric Bypass. Gastroenterology. 2014;146:669–68.e2. doi: 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson IR, Naik S. Dietary regulation of intestinal gene expression. Annu. Rev. Nutr. 2000;20:311–338. doi: 10.1146/annurev.nutr.20.1.311. [DOI] [PubMed] [Google Scholar]
- Santoro S, Milleo FQ, Malzoni CE, Klajner S, Borges PCM, Santo MA, Campos FG, Artoni RF. Enterohormonal changes after digestive adaptation: five-year results of a surgical proposal to treat obesity and associated diseases. Obes. Surg. 2008;18:17–26. doi: 10.1007/s11695-007-9371-0. [DOI] [PubMed] [Google Scholar]
- Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Shah M, Law JH, Micheletto F, Sathananthan M, Dalla Man C, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. Contribution of Endogenous Glucagon-Like Peptide 1 to Glucose Metabolism After Roux-en-Y Gastric Bypass. Diabetes. 2014;63:483–493. doi: 10.2337/db13-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D, Gohil K, Basson MD. Intestinal mucosal atrophy and adaptation. World J. Gastroenterol. 2012;18:6357–6375. doi: 10.3748/wjg.v18.i44.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Berthoud H-R. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann. Surg. 2012;255:294–301. doi: 10.1097/SLA.0b013e31823e71b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev. Dyn. 2011;240:501–520. doi: 10.1002/dvdy.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns a. T., Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. AJP Gastrointest. Liver Physiol. 2009;297:G950–G957. doi: 10.1152/ajpgi.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater M. a, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval D. a, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. 2436.e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All Bariatric Surgeries Are Not Created Equal: Insights from Mechanistic Comparisons. Endocr. Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Layer P, Goebell H, Dignass AU. Short-bowel syndrome: an update on the therapeutic approach. Scand. J. Gastroenterol. 1997;32:289–296. doi: 10.3109/00365529709007674. [DOI] [PubMed] [Google Scholar]
- Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130:S93–S99. doi: 10.1053/j.gastro.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Taqi E, Wallace LE, de Heuvel E, Chelikani PK, Zheng H, Berthoud H-R, Holst JJ, Sigalet DL. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J. Pediatr. Surg. 2010;45:987–995. doi: 10.1016/j.jpedsurg.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes. Surg. 2011;21:896–901. doi: 10.1007/s11695-011-0412-3. [DOI] [PubMed] [Google Scholar]
- Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JOL, Schmitz O. Acute effects of ghrelin administration on glucose and lipid metabolism. J. Clin. Endocrinol. Metab. 2008;93:438–444. doi: 10.1210/jc.2007-2018. [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weser E, Babbitt J, Hoban M, Vandeventer A. Intestinal adaptation. Different growth responses to disaccharides compared with monosaccharides in rat small bowel. Gastroenterology. 1986;91:1521–1527. [PubMed] [Google Scholar]
- Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical Sleeve Gastrectomy is Effective in Two Genetic Mouse Models of Glucagon-like Peptide-1 Receptor Deficiency. Diabetes. 2013;62:2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G629–G637. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Munzberg H, Morrison CD, Drucker DJ, Berthoud H-R. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R352–R362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]