Summary
In E. coli, outer-membrane stress causes a transcriptional response through a signaling cascade initiated by DegS cleavage of a transmembrane anti-sigma factor. Each subunit of DegS, an HtrA-family protease, contains a protease domain and a PDZ domain. The trimeric protease domain is autoinhibited by the unliganded PDZ domains. Allosteric activation requires binding of unassembled outer-membrane proteins (OMPs) to the PDZ domains and protein-substrate binding. Here, we identify a set of DegS residues that cluster together at subunit-subunit interfaces in the trimer, link the active sites and substrate-binding sites, and are crucial for stabilizing the active enzyme conformation in response to OMP signaling. These residues are conserved across the HtrA-protease family, including orthologs linked to human disease, supporting a common mechanism of allosteric activation. Indeed, mutation of residues at homologous positions in the DegP quality-control protease also eliminates allosteric activation.
INTRODUCTION
Allostery, in which binding of an effector molecule at one site alters activity at a distinct site, controls the biological activities of many enzymes, including proteases. In the periplasm of Escherichia coli, for example, heat and other environmental stresses activate the DegS protease, which is anchored to the inner membrane, and initiate an intramembrane proteolytic cascade that results in transcription of stress-response genes in the cytoplasm (Ades, 2008). DegS is a trimeric HtrA-family protease, in which each subunit contains a trypsin-like protease domain and a regulatory PDZ domain (Fig. 1A). The trimer is stabilized by packing between the protease domains. The principal substrate of DegS is the periplasmic domain of RseA, a transmembrane protein with a cytoplasmic domain that binds and inhibits the σE transcription factor (Alba and Gross, 2004). Two stress-induced molecular signals are required for DegS cleavage of RseA in vivo. First, unassembled outer-membrane proteins (OMPs) accumulate in the periplasm during stress, and Tyr-Xxx-Phe (YxF) peptides at the C termini of these OMPs bind to the PDZ domains of DegS and stimulate proteolytic activity (Walsh et al., 2003). Second, lipopolysaccharides bind the RseB regulatory protein, releasing it from RseA, which allows cleavage of RseA at a single site by OMP-activated DegS (Lima et al., 2013). This initial cleavage event results in subsequent cleavage of RseA by other proteases, freeing σE to activate transcription of stress-response genes (Ades et al., 1999; Kanehara et al., 2002; Akiyama et al., 2004; Flynn et al., 2004; Chaba et al., 2007; Li et al., 2009).
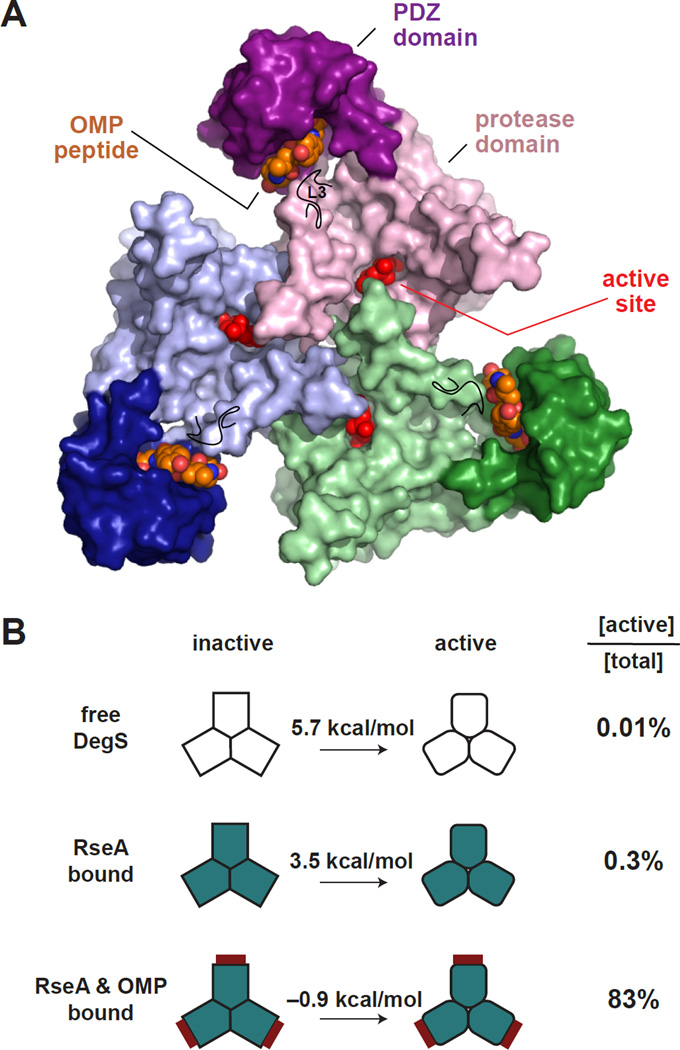
Figure 1. The DegS Trimer Equilibrates Between Inactive and Active Structures.
(A) Structure of a DegS trimer in the active conformation with OMP peptides bound to the PDZ domains (pdb code 3GDS). Each DegS subunit (surface representation colored green, purple, or blue) consists of a protease domain (lighter colors), which pack together to form the trimer, and a peripheral PDZ domain (darker colors). Bound OMP peptides are shown in CPK representation with orange carbons. An isopropyl phosphate group covalently bound to the active-site serine is shown in CPK representation and colored red. The position of the L3 loop is marked by a black line.
(B) Free energies for conversion of inactive to active DegS in the free enzyme, the substrate-saturated enzyme, and the substrate and OMP-peptide saturated enzyme (Sohn and Sauer, 2009). These values would be decreased by ~4 kcal/mol for H198P DegS (Sohn et al., 2009; 2010). The column on the right lists the percentage of total DegS that assumes the active conformation for the unbound and ligand bound states.
In the absence of stress, DegS cleaves RseA at a low rate in vivo (Ades, 2008). Similarly, in the absence of OMP peptides in vitro, DegS cleaves the periplasmic domain of RseA >100-fold more slowly than in their presence (Walsh et al. 2003; Sohn et al., 2007; Sohn and Sauer, 2009). Crystal structures of DegS or DegSΔPDZ trimers show two distinct conformations, one inactive because of a malformed oxyanion hole and one with a functional His-Asp-Ser catalytic triad and oxyanion hole (Wilken et al., 2004; Zeth, 2004; Hasselblatt et al., 2007; Sohn et al., 2007; 2009; 2010). The PDZ domain has two major roles, keeping OMP-free DegS in an autoinhibited state (DegSΔPDZ has robust protease activity) and binding OMP peptide to allow activation. In structures of active DegS, the PDZ-bound OMP peptide is ~25 Å from the closest active site in the trimer. Allosteric activation over this distance is partially explained by remodeling of the protease-domain L3 loop, which is spatially adjacent to bound OMP peptide (Fig. 1A). This remodeling breaks autoinhibitory interactions between the L3 loop and the PDZ domain or other regions of DegS and allows formation of alternative contacts that appear to stabilize active DegS. Experiments using DegS enzymes with mixtures of full-length and ΔPDZ subunits show that all subunits in the trimer experience a strongly coupled energetic landscape, with peptide binding to a single PDZ domain stimulating proteolytic cleavage both in the attached protease domain and in neighboring domains (Mauldin and Sauer, 2013).
Proteolytic activation of DegS largely conforms to a two-state model of MWC allostery, in which the preferential binding of RseA substrates and OMP peptides to active DegS provides ~2.2 and ~4.4 kcal/mol of stabilization, respectively, relative to the inactive enzyme (Fig. 1B; Monod et al., 1965; Sohn and Sauer, 2009). Thus, only ~0.3% of substrate-saturated DegS assumes the active conformation, whereas ~83% of substrate-saturated and OMP-saturated DegS is active. DegSΔPDZ still behaves as an allosteric enzyme with respect to RseA cleavage (Sohn and Sauer, 2009; Sohn et al., 2007; 2010), indicating that the protease-domain trimer still adopts inactive and active conformations and thus contains the basic machinery required for allostery. Interestingly, brief soaking of active DegS crystals to remove OMP peptide results in a transient state in which the protease domain remains in an “active” conformation but some autoinhibitory contacts between the L3 loop and PDZ domain reform (Wilken et al., 2004). This hybrid state is metastable and assumes the inactive conformation after prolonged soaking. These results suggest that this hybrid conformation is unlikely to be substantially populated at equilibrium compared to inactive or active conformations, explaining why the two-state MWC model accounts well for proteolytic activation of DegS. The energetic basis for the relative stabilities of the active and inactive states is unclear, however, and dynamic factors and/or ensembles of states may play roles in the allosteric transition (Sohn et al., 2010; Motlagh et al., 2014).
Previous studies identified a handful of mutations that preferentially stabilize the inactive conformation compared to the active conformation of DegS or vice versa, and inspection of crystal structures suggested additional residues that may be involved in DegS allostery (Wilken et al., 2004; Hasselblatt et al., 2007; Sohn et al., 2007; 2010; Sohn and Sauer, 2009). However, there has been no comprehensive test of the importance of these residues in mediating the allosteric switch between DegS conformations. Here, we identify a critical set of amino acids needed for allosteric activation of DegS, providing a foundation for understanding regulation by substrate and OMP-peptide binding. These critical residues cluster at each subunit-subunit interface in the trimer and adjoin the active sites. Bound substrates would link these clusters and active sites around the trimer, providing a direct mechanism for allosteric activation by substrate. By contrast, bound OMP peptide appears to activate DegS indirectly by breaking autoinhibitory contacts. The cluster residues most important for DegS allostery are highly conserved within the HtrA-protease family, strongly suggesting that all of these enzymes function in similar fashions. Indeed, based on homology with DegS, we identify and characterize mutations that prevent allosteric activation of E. coli DegP, a trimeric HtrA quality-control protease that assembles into proteolytically active polyhedral cages in the presence of protein substrates (Jiang et al. 2008; Krojer et al., 2008; Kim et al., 2011). These results have broad implications, as mutant forms of human HtrA homologs have been linked to cancer, vascular disease, arthritis, macular degeneration, and neurodegenerative disease (Grau et al., 2005; Coleman et al., 2008; Milner et al., 2008; Vande Walle et al., 2008; Chien et al., 2009; Hara et al., 2009; Bowden et al., 2010).
RESULTS
Allosteric activation of DegS requires linkage between OMP-peptide binding to the PDZ domains, substrate binding to the protease domain, and the conformations of the active sites in each protease domain of the trimer (Sohn et al. 2007; Sohn and Sauer, 2009; Mauldin and Sauer, 2013). Based on interactions observed in DegS crystal structures and/or previous proposals, 16 side chains were selected for mutagenic analysis of allosteric activation (Table 1). Most of these amino acids lie between the binding sites for OMP peptide in the PDZ domains and the proteolytic active sites. In the sections below, we discuss evidence for or against these residues playing important roles in allosteric switching.
Table 1.
Second-order rate constants for RseA cleavage and KD’s for OMP-peptide binding are listed for DegS and variants in otherwise wild-type (WT) backgrounds or in the H198P DegS (HP) background.
| activity WT background (M−1 s−1) | activity HP background (M−1 s−1) | KD for OMP peptide (µM) | ||||
|---|---|---|---|---|---|---|
| variant enzyme |
basal | with YYF | basal | with YYF | WT background |
HP background |
| WT DegS | <4.5 | 2300 ± 60 | 540 ± 42 | 13900 ± 2100 | 4.0 ± 0.4 | 2.5 ± 0.3 |
| P161A | <4.5 | <4.5 | <4.5 | <4.5 | 1.5 ± 0.1 | 2.5 ± 0.4 |
| Y162A | <4.5 | <4.5 | <4.5 | <4.5 | 1.9 ± 0.4 | n.d. |
| L164A | <4.5 | <4.5 | <4.5 | <4.5 | 2.8 ± 0.6 | 2.5 ± 0.5 |
| T167V | <4.5 | <4.5 | <4.5 | <4.5 | 1.8 ± 0.1 | 1.9 ± 0.7 |
| T169A | <4.5 | <4.5 | <4.5 | <4.5 | 1.8 ± 0.1 | 2.0 ± 0.2 |
| R178A | <4.5 | <4.5 | 160 ± 28 | 3400 ± 470 | 3.6 ± 0.2 | 4.0 ± 0.1 |
| I179A | <4.5 | 230 ±130 | 260 ± 150 | 9000 ± 2400 | 2.3 ± 0.4 | 1.6 ± 0.1 |
| Q187A | <4.5 | 740 ± 74 | 800 ± 330 | 21000 ± 5100 | 2.3 ± 0.1 | 1.5 ± 0.1 |
| Q191A | <4.5 | <4.5 | <4.5 | <4.5 | 3.4 ± 1.3 | 1.4 ± 0.1 |
| N197A | <4.5 | 49 ± 24 | <4.5 | 300 ± 68 | 1.4 ± 0.1 | 0.7 ± 0.1 |
| F220A | <4.5 | 46 ± 1 | 50 ± 11 | 1200 ± 11 | 2.1 ± 0.1 | 1.6 ± 0.1 |
| D221A | <4.5 | 740 ± 140 | 1400 ± 390 | 12000 ± 3700 | 3.2 ± 0.2 | 1.7 ± 0.1 |
| P229A | <4.5 | 2900 ± 270 | 640 ± 420 | 28000 ± 2500 | 1.6 ± 0.2 | 2.0 ± 0.1 |
| E230A | <4.5 | 230 ± 50 | 300 ± 150 | 14000 ± 2000 | 5.7 ± 1.3 | 1.8 ± 0.4 |
| I232A | <4.5 | <4.5 | <4.5 | <4.5 | 2.2 ± 0.3 | 2.7 ± 0.3 |
| F234A | <4.5 | <4.5 | <4.5 | 70 ± 15 | 5.9 ± 0.6 | 2.9 ± 0.1 |
(n.d.) not determined
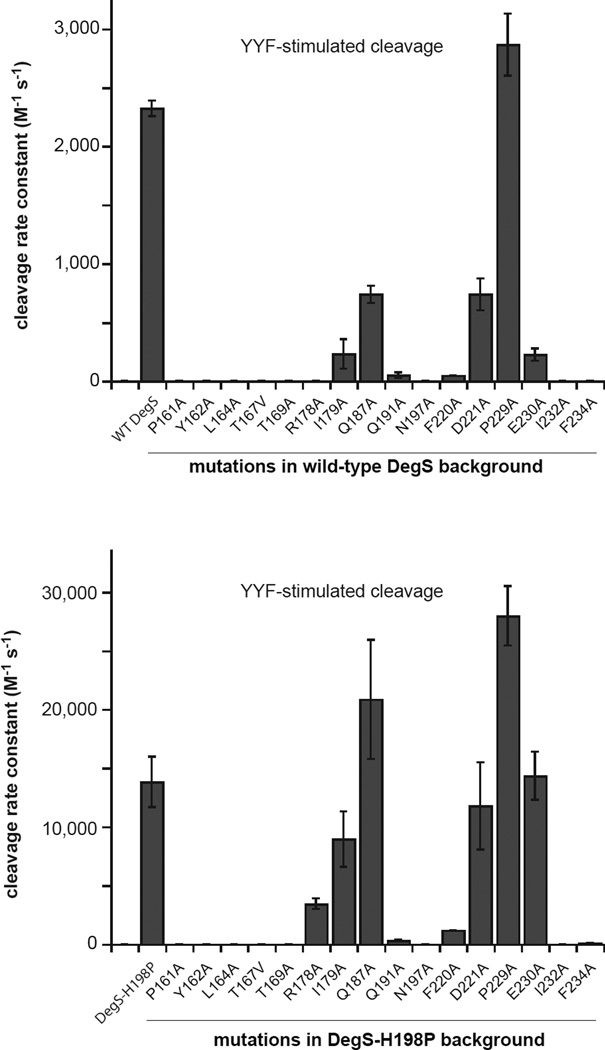
To monitor proteolytic activity in vitro, we purified wild-type or mutant variants of DegS without the membrane anchor and determined second-order rate constants (kcat/KM) for cleavage of sub-KM concentrations of a 35S-labeled protein fragment corresponding to the periplasmic domain of RseA in the presence of a high concentration of Tyr-Tyr-Phe (YYF) peptide (Sohn et al., 2007). The effects of different mutations were measured in otherwise wild-type backgrounds and also in combination with the H198P mutation, which stabilizes active DegS (Sohn et al., 2009; 2010) and increased the proteolytic activity of otherwise wild-type DegS ~6-fold under our assay conditions (Fig. 2). The H198P mutation increased the relative proteolytic activities of some but not all mutations that diminish DegS activity (compare top and bottom panels in Fig. 2), allowing the relative importance of different mutations for proteolysis and allosteric switching to be determined.
Figure 2. Mutational Effects on DegS Cleavage of RseA.
Effects of DegS mutations in an otherwise wild-type background (upper panel) or in an H198P background (lower panel) on OMP-peptide stimulated proteolytic activity, expressed as the second-order rate constant (kcat/KM) for RseA cleavage. Values are averages of two or more independent trials ± SEM. Cleavage reactions contained different sub-KM concentrations of 35S-labelled RseA, DegS or variants (1 µM trimer), and YYF OMP peptide (230 µM). Initial cleavage rates normalized by total enzyme were plotted as a function of the RseA concentration, and the second-order rate constant was determined from the slope of a linear fit.
Residues that Play Major Roles in Allosteric Switching
The side chains of Pro161, Tyr162, Leu164, Thr167, Thr169, Gln191, Asn197, Ile232, and Phe234 in DegS play very important roles in allosteric switching by the following criteria. The P161A, Y162A, L164A, T167V, T169A, Q191A, N197A, I232A, and F234A mutants had less than 2% of YYF-stimulated wild-type cleavage activity, and combining these mutations with the H198P mutation did not rescue cleavage activity appreciably (Fig. 2; Table 1). The Arg178 and Phe220 side chains of DegS play somewhat important roles, as the R178A and F220A mutants had almost no cleavage activity, but the R178A/H198P and F220A/H198P mutants had activities between 9–24% of the parental enzyme. All of the mutant enzymes were soluble and well-behaved during purification, suggesting that the mutations had no substantial effects on folding or trimerization, and previously determined crystal structures of ΔPDZ variants of Y162A, Y162A/H198P, T167V/H198P, and Q191A showed that these mutants form stable trimers with the protease domain in the proteolytically inactive conformation (Sohn et al., 2010).
By monitoring changes in the anisotropy of a fluorescein-labeled OMP peptide as a function of increasing concentrations of mutant DegS variants (Sohn et al., 2007), we determined binding affinities for the P161A, Y162A, L164A, T167V, T169A, R178A, Q191A, N197A, F220A, I232A, and F234A proteins in wild-type and H198P enzyme backgrounds. Each mutant bound OMP peptide with a KD similar to or slightly stronger than wild-type DegS or DegSH198P (Table 1). Thus, the observed proteolytic defects of these mutants are not a consequence of an inability to bind OMP peptide. Rather, OMP-peptide binding to these mutants is not sufficient to shift most enzymes from the inactive to the active protease conformation.
Residues that Play Minor Roles or No Role in Allosteric Switching
Based on the crystal structures of active and inactive DegS, the side chains of Ile179, Pro183, Gln187, Asp221, and Glu227 were proposed to play important roles in activation (Wilken et al., 2004; Hasselblatt et al., 2007). Both the P183A and E227A DegS mutants were previously found to have OMP-stimulated activities similar to or slightly higher than wild-type DegS (Sohn et al., 2007), indicating that these side chains play no significant role in allosteric switching. Here, we found that I179A, Q187A, and D221A DegS displayed reduced YYF-stimulated cleavage activity compared to wild-type DegS (Fig. 2), but I179A/H198P, Q187A/H198P, and D221A/H198P DegS had YYF-stimulated cleavage activities that were 65% or more of the H198P parent (Fig. 2; Table 1). Thus, the Ile179, Gln187, and Asp221 side chains play minor roles in stabilizing active compared to inactive DegS but are not critical determinants of allosteric activation.
Pro229 and Glu230 assume different conformations in active and inactive DegS structures (Sohn et al., 2010). We found that the P229A mutant was more active than wild-type DegS and H198P/P229A was more active than H198P (Fig. 2). Thus, the pyrrolidine ring of Pro229 is not important for allosteric activation. The E230A mutant had ~10% of wild-type activity, whereas H198P/E230A had activity within error of H198P (Fig. 2). Hence, the Glu230 side chain plays a minor role in DegS activation.
Structural Roles of Key Allosteric Residues
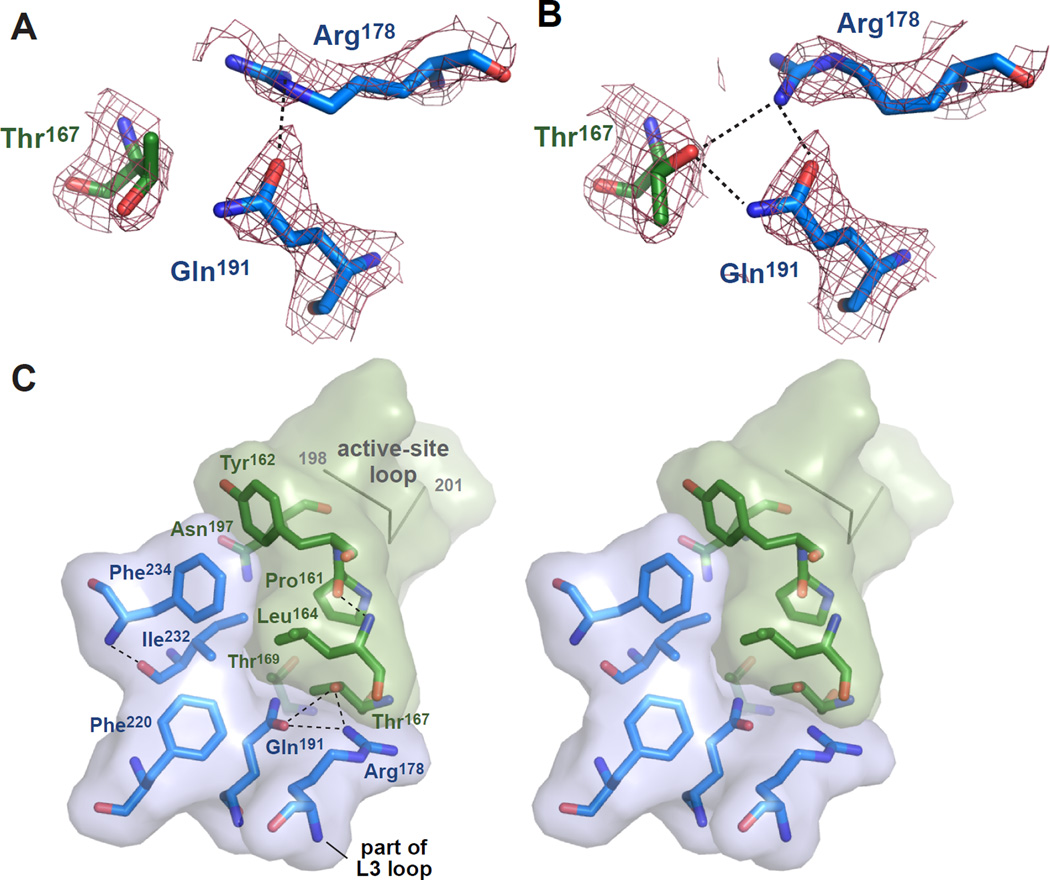
Prior to examination of the structural roles of DegS side chains that play important roles in allosteric switching, we sought to resolve apparent discrepancies among active and inactive crystal structures determined by different groups. For example, the side chains of Thr167, Arg178, and Gln191 form a hydrogen-bond network in the all of the active DegS structures determined in our lab (Sohn et al., 2007; 2009; 2010), but a badly eclipsed rotamer of Thr167 prevents hydrogen bonding in the 1SOZ structure determined by another group (Fig. 3A; Wilken et al., 2004; Hasselblatt et al., 2007). When we re-refined this structure, improving R factors and geometry (Table 2), the side chains of Thr167, Arg178, and Gln191 formed a hydrogen-bonding network (Fig. 3B) similar to those in our previous DegS structures. We also re-refined several inactive or hybrid structures of DegS to improve geometry. As determined by MolProbity analysis (Chen et al., 2010), for example, the 1TE0 structure of inactive DegS (Zeth, 2004) had extremely poor geometry (23% poor rotamers; 7% Ramachandran outliers; 13% Cβ deviations; 3% bad backbone bonds; 3% bad backbone angles), suggesting a highly strained conformation or a poorly refined structure. By contrast, the 1SOT structure of full-length inactive DegS (Wilken et al., 2004) had somewhat better geometry (6% poor rotamers; 3% Ramachandran outliers; 0.4% Cβ deviations; 0.1% bad backbone bonds; 0.4% bad backbone angles), suggesting less strain or better refinement. The hybrid 1VCW structure of DegS after removal of bound OMP peptide by brief soaking (Wilken et al., 2004) also had geometric issues (8% poor rotamers; 4% Ramachandran outliers; 1% Cβ deviations; 0.2% bad backbone bonds; 0.8% bad backbone angles). Our re-refined 1TE0, 1SOT, and 1VCW structures had improved R factors and no poor rotamers, Ramachandran outliers, Cβ deviations, or bad backbone bonds or angles (Table 2). Thus, inactive or hybrid DegS does not assume an obviously strained conformation.
Figure 3. Structural Determinants of DegS Activation.
(A) Electron-density map (2FO–FC; contoured at 1.3 σ) for Thr167 in chain B (dark green carbons) and Arg178 and Gln191 in chain C (marine carbons) in the 1SOZ DegS trimer. Thr167 is modeled in an eclipsed conformation (χ 116°) found in only 0.1% of high-resolution structures. A hydrogen bond is shown as a dashed black line.
(B) Electron-density map (same residues and parameters as panel A) but for the 4RQZ re-refined structure of 1SOZ. Thr167 is modeled as a rotamer (χ 290°) found in 18.7% of high-resolution structures and forms hydrogen bonds (dashed black lines) with Arg178 and Gln191.
(C) Stereo view of residues identified as being very important or somewhat important for DegS allostery cluster between the active-site loop containing the catalytic Ser201 and oxyanion hole (top right) and the L3 loop (bottom center). Residues with marine-colored carbons are from one subunit of the 4RQZ trimer and those with dark-green carbons are from another subunit; transparent surfaces are shown for both subunits to emphasize the position of the interface. The residues shown represent one of the three activation clusters in the DegS trimer. Hydrogen bonds are represented as dashed black lines.
Table 2.
Statistics for refinement and geometry of original structures (1SOZ, 1SOT, 1TE0, and 1VCW) and the same structures re-refined here (4RQZ; 4RR1, 4RQY, and 4RR0).
| PDB ID | ref. | ligand | resolution (Å) |
Rwork / Rfree | clash percentile |
poor rotamers % |
Rama. outliers % |
Rama. favored % |
bad backbone bonds |
bad backbone angles |
Cβ deviations >0.25 Å |
MolProbity score (rank percentile) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1SOZ | (1) | OMP peptide | 2.4 | 0.231 / 0.272 | 40 | 5.3 | 3.5 | 87 | 3 | 7 | 30 | 3.05 (21) |
| 4RQZ (a) | (2) | OMP peptide | 2.4 | 0.196 / 0.226 | 100 | 0 | 0 | 98 | 0 | 0 | 0 | 0.96 (100) |
| 1SOT | (1) | apo | 2.3 | 0.198 / 0.248 | 29 | 8.9 | 3.3 | 86 | 1 | 1 | 9 | 3.34 (13) |
| 4RR1 (b) | (2) | apo | 2.3 | 0.184 / 0.217 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0.75 (100) |
| 1TE0 | (3) | apo | 2.2 | 0.245 / 0.295 | 8 | 23 | 7 | 85 | 73 | 140 | 186 | 3.81 (1) |
| 4RQY (c) | (2) | apo | 2.2 | 0.179 / 0.223 | 100 | 0 | 0 | 99 | 0 | 0 | 0 | 0.75 (100) |
| 1VCW | (1) | OMP peptide removed | 3.05 | 0.233 / 0.301 | 42 | 8.2 | 4.4 | 82 | 9 | 11 | 73 | 3.55 (36) |
| 4RR0 (d) | (2) | OMP peptide removed | 3.05 | 0.226 / 0.256 | 100 | 0 | 0 | 98 | 0 | 0 | 0 | 0.89 (100) |
re-refinement of 1SOZ
re-refinement of 1SOT
re-refinement of 1TE0
re-refinement of 1VCW
This work
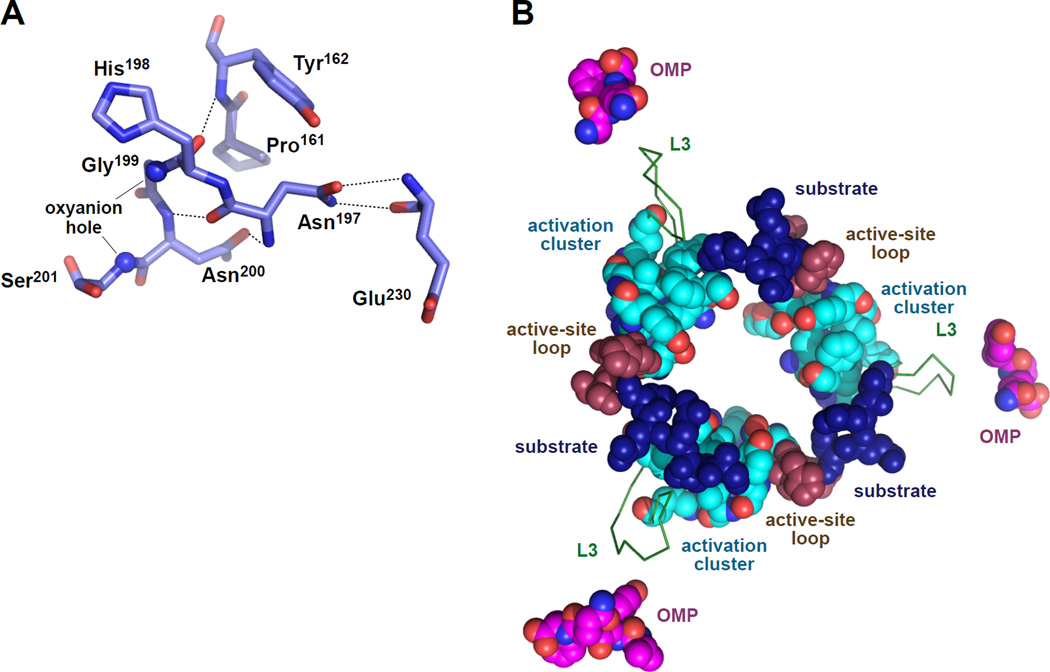
The residues we identified as playing the most important roles in allosteric activation cluster together at the subunit-subunit interfaces of the active conformation of the DegS trimer (Fig. 3C). The cluster is stabilized by hydrophobic packing between the side chains of Tyr162, Leu164, Phe220, Ile232, and Phe234, and by hydrogen bonds involving side-chain atoms of Thr167, Arg178, and Gln191 or main-chain atoms of Pro161 Leu164, Phe220, and Ile232. OMP-peptide binding causes repositioning of the L3 loop, which includes Arg178, on the outer edge of the cluster. Main-chain and side-chain atoms from Pro161, Tyr162, and Asn197 on the inner edge of the cluster contact and stabilize the functional conformation of the active-site loop (residues 198–201) that forms the oxyanion hole and contains the catalytic Ser201 (Fig. 4A). Importantly, these "activation clusters" are present in all crystal structures of DegS in the active conformation, including DegS with bound OMP peptides and DegSΔPDZ (Wilken et al., 2004; Zeth, 2004; Hasselblatt et al., 2007; Sohn et al., 2007; 2009; 2010). Moreover, activation clusters with very similar structural features are present in the functional conformations of a DegS ortholog from Mycobacterium tuberculosis (2Z9I; Mohamedmohaideen et al., 2008) and in E. coli DegP (3OU0; 3OTP; Kroger et al., 2008; Kim et al., 2011).
Figure 4. Allosteric Ligands and Catalytic and Regulatory Elements in Active DegS.
(A) Main-chain and side-chain atoms from activation-cluster residues Pro161, Tyr162, and Asn197 stabilize the functional conformation of the active-site loop, including the catalytic Ser201 and the oxyanion-hole nitrogens (small spheres). Hydrogen bonds are shown as dashed lines.
(B) Positions of OMP peptides, L3 loops, activation clusters, active-site loops, and modeled substrate in an active DegS trimer (4RQZ). The position of substrate was modeled by aligning 4RQZ with a DegS ortholog from Mycobacterium tuberculosis with a bound substrate-cleavage product (2Z9I; Mohamedmohaideen et al., 2008). Although the PDZ-bound OMP peptides are adjacent to the L3 loops, there are no critical residues between the OMP peptide and the activation clusters, suggesting that OMP-peptide binding destabilizes autoinhibitory interactions rather than directly stabilizing the active conformation. Substrate binding ties together all of the active-site loops and activation clusters in the trimer, providing a mechanism for allosteric substrate stabilization of the active conformation.
In the inactive conformation of full-length DegS, represented by the re-refined 1TE0 structure, many residues of the activation cluster occupy substantially different positions than in the active conformation. For example, the side chains of Arg178, Phe220, and Tyr162 move more than 10 Å, disrupting numerous packing and polar interactions. Indeed, all of the hydrogen bonds present in the functional activation cluster are missing in the inactive conformation. As a consequence of these structural rearrangements, the 198–201 active-site loop adopts an altered conformation which destroys the oxyanion hole. The R178A mutation has two effects. It destabilizes the activation cluster in active DegS by removing part of a hydrogen-bond network but also removes an autoinhibitory salt-bridge with Asp320 in the unliganded PDZ domain in inactive DegS (Sohn et al., 2007), which is likely to ameliorate the severity of this mutation. Interestingly, after removal of OMP peptide from active DegS by brief crystal soaking (Wilken et al., 2004), the activation clusters remained intact, except for Arg178, which again formed an autoinhibitory saltbridge with Asp320 in the PDZ domain, and Phe220, which became disordered. Like R178A, F220A was one of the least severe of the activation-cluster mutations (Fig. 2). By the principle of microscopic reversibility, the Arg178 and Phe220 side chains would be expected to be late additions to the activation cluster following OMP-peptide stimulation.
Parts of the RseA substrate are likely to interact directly with the activation cluster. For example, the carbonyl oxygen of the scissile peptide bond would bind in the oxyanion hole, which is mimicked by fluorophosphate modification of the active-site Ser201 side chain in many structures (Sohn et al., 2007; 2009; 2010). The S5-S1 positions of RseA are also likely to interact with parts of the DegS activation cluster, based upon structures of orthologs with peptide fragments bound to the active site (Mohamedmohaideen et al., 2008; Kim et al., 2011). Indeed, some effects of the F220A mutation on RseA cleavage may arise from weaker substrate binding. As shown in Fig. 4B, part of a bound substrate is predicted to contact the active-site loop and adjacent activation cluster. Substrate could not bind to the distorted oxyanion hole of inactive DegS and additional substrate contacts may also be weakened in inactive DegS, explaining allosteric activation by preferential substrate binding to active DegS.
Fig. 4B shows that bound OMP peptides are not in close physical proximity to the activation clusters. Moreover, mutations in L3-loop residues that lie between bound OMP peptide and the activation cluster do not have substantial defects in proteolytic activity, supporting a model in which OMP binding serves only to relieve autoinhibitory interactions that stabilize the inactive state and does not play a role in directly stabilizing the active conformation of the protease domain (Sohn et al. 2007; 2010). This loss-of-inhibition model also explains why the conformations of the residues of the active site and activation cluster are almost identical in DegSΔPDZ and in OMP-peptide bound DegS.
Mutations that Block Allosteric Switching in DegP
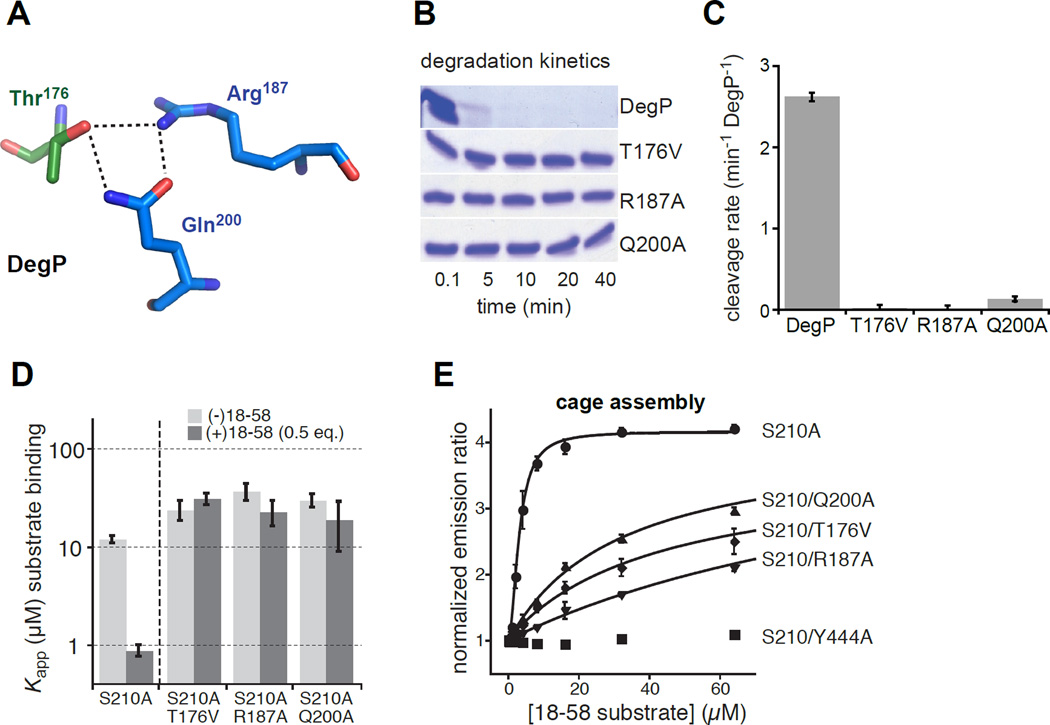
The DegP protease differs from DegS in several ways. First, the allosteric transition from inactive to proteolytically active DegP trimers is driven by binding of one degron in a protein substrate to the active site and by binding of a second C-terminal degron in the same substrate to a tethering site in the DegP PDZ1 domain (Kim et al., 2011). Second, proteolytic activation is accompanied by assembly of DegP trimers into polyhedral cages with 12, 18, 24, or 30 subunits (Jiang et al., 2008; Kroger et al., 2008; Kim et al., 2011), although cage formation is not required for proteolytic activation (Kim and Sauer, 2012). To test if DegP uses a pathway of allosteric activation similar to DegS, we focused on DegP residues Thr176, Arg187, and Gln200, which form a hydrogen-bond network in active DegP (Fig. 5A) but not in the inactive enzyme (Kroger et al., 2002; 2008; Kim et al., 2011). These residues correspond to Thr167, Arg178, and Gln191 in DegS, and the networks formed by each set of residues in the active structures are similar (cf. Figs. 3B and 5A). If these DegP interactions are important for allosteric switching, then mutation of these residues should prevent or greatly diminish proteolytic activity, weaken substrate binding, and reduce or eliminate the positive cooperativity of substrate binding. To test these predictions, we made individual T176V, R187A, and Q200A mutations in DegP. Each mutation severely compromised substrate degradation. For example, purified DegPT176V, DegPR187A, and DegPQ200A exhibited no detectable degradation of lysozyme denatured by carboxymethylation of cysteines after 40 min, whereas wild-type DegP degraded most of this substrate within 5 min (Fig. 5B). To assay degradation activity more quantitatively, we used a fluorescent 23-residue peptide (p23) derived from lysozyme at a concentration ~20-fold above the wild-type KM. DegPQ200A cleaved p23 at a rate much lower than wild-type DegP, whereas the rate of cleavage of p23 by DegPT176V and DegPR187A was negligible (Fig. 5C).
Figure 5. Effects of activation-cluster mutations on DegP activity and cage assembly.
(A) The side chain of Thr176 from one DegP subunit (dark-green carbons) forms hydrogen bonds with the side chains of Arg187 and Gln200 in an adjacent subunit (marine carbons) in the protease domain of an active DegP trimer (3OTP).
(B) Kinetics of degradation of carboxymethylated lysozyme (5 µM) by wild-type DegP and the T176V, R187A, or Q200A variants (1 µM) at room temperature was assayed by SDS-PAGE and staining with Coomassie Blue.
(C) Rates of cleavage of the p23 substrate (40 µM) by wild-type DegP or variants (1 µM) were determined at room temperature. Error bars are averages ±1 SD (N = 3).
(D) Apparent equilibrium dissociation constants (Kapp) for binding of different concentrations of proteolytically inactive DegPS210A or variants to a fluorescent substrate (flClys18–58; 50 nM) were determined in the absence or presence of unlabeled lys18–58 at one-half of the DegP concentration by changes in fluorescence anisotropy (Kim et al., 2011; Kim and Sauer, 2012). Positive cooperativity makes binding stronger in the presence of unlabeled lys18–58 for variants, like DegPS210A, that can switch between the inactive and active conformations. The T167V, R187A, and Q200A mutations weakened binding and eliminated positive cooperativity. Bars represent the error of fitting of individual binding curves to a hyperbolic binding equation.
(E) Polyhedral-cage assembly of DegP variants as a function of added lys18–58 substrate was monitored by increases in FRET between trimers labeled with donor and acceptor dyes (Kim et al., 2011). Emission ratios were normalized to data in the absence of substrate and fitted to a Hill equation (y = y0 + max • [lys18–58]h/(Kapph + [lys18–58]h)) for DegPS210A or to a hyperbolic equation (y = y0 + max • [lys18–58]/(Kapp + [lys18–58])) for variants containing the T176V, R187A, or Q200A mutations. No assembly of the DegPS210A/Y444A variant was observed, as expected because the Y444A mutation removes a critical interaction that stabilizes cages (Kim et al., 2011). Error bars are averages ±1 SD (N = 3).
To assay substrate binding, we monitored the change in fluorescence anisotropy of a fluorescent peptide derived from residues 18–58 of lysozyme (lys18–58) upon addition of DegP variants. In one set of experiments, equilibrium binding curves and apparent affinities were determined after incubation of a low concentration of fluorescent lys18–58 with different concentrations of DegPS210A (a proteolytically inactive variant missing the catalytic serine), DegPS210A/T176V, DegPS210A/R187A, or DegPS210A/Q200A. In a second set of experiments, binding mixtures were supplemented with non-fluorescent lys18–58 at one-half of the enzyme concentration, which should result in stronger binding for variants that display positively cooperative substrate binding (Kim et al., 2011). Addition of unlabeled substrate strengthened the apparent affinity of DegPS210A for fluorescent lys18–58 from ~12 µM to ~1 µM (Fig. 5D). By contrast, DegPS210A/T176V, DegPS210A/R187A, and DegPS210A/Q200A displayed comparable affinities (~30 µM) in the presence and absence of unlabeled substrate (Fig. 4D). Thus, the T176V, R187A, and Q200A mutations almost completely eliminate degradation, weaken substrate binding, and abolish positive cooperativity, as expected if these variants are largely trapped in the inactive state, even when substrate is bound.
Formation of DegP cages was monitored by fluorescence resonance energy transfer (FRET) between trimers labeled with donor dyes and trimers labeled with acceptor dyes as described (Kim et al., 2011). Mixtures of acceptor and donor labeled trimers were prepared in the S210A, S210A/T176V, S210A/R187A, and S210A/Q200A backgrounds and FRET was assayed as a function of increasing quantities of lys18–58 substrate (Fig. 5E). Notably, we observed substrate-dependent increases in FRET for the T176V, R187A, and Q200A variants, although compared to the S210A experiment substantially more lys18–58 was required to drive an equivalent level of cage assembly by the variants. As expected, no change in FRET was observed for a variant bearing the Y444A mutation, which removes contacts required for cage formation (Kim et al., 2011). These results support a model in which the T176V, R187A, and Q200A variants assemble into cages in which the component trimers remain in the “inactive” conformation, albeit requiring higher substrate concentrations because of weaker and non-cooperative substrate binding.
DISCUSSION
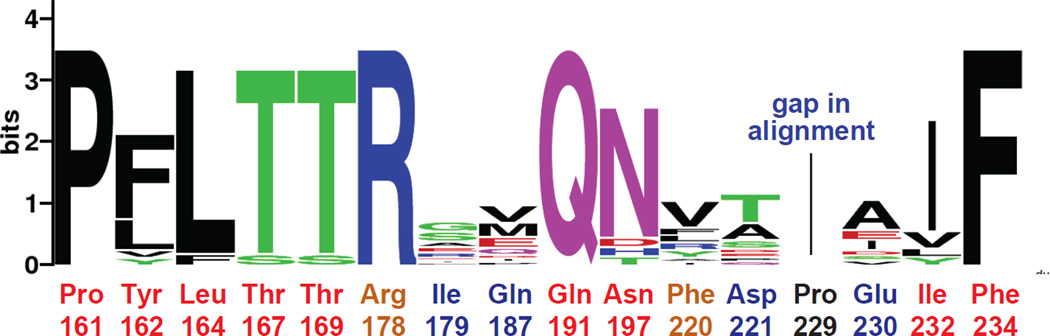
Our results identify a set of amino acids that play critical roles in allosteric activation of the E. coli DegS protease. Mutation of these residues almost completely eliminates proteolytic activity in the presence of an OMP peptide that robustly activates substrate cleavage by wild-type DegS. From an energetic perspective, if a single mutation in one activation cluster destabilizes active DegS by ~1–2 kcal/mol relative to inactive DegS, then the active trimer would be destabilized by 3–6 kcal/mol and little or no activation by substrate and OMP-peptide binding would be expected. The activation clusters of DegS and DegP are very similar. Indeed, we found that mutation of some of these “activation cluster” residues in DegP eliminated or dramatically decreased proteolytic activity, as well as reducing the affinity and eliminating the positive cooperativity of substrate binding. Strikingly, the amino acids that are critical determinants of allosteric activation of DegS are also highly conserved in homologous proteases from all kingdoms of life (Fig. 6). Thus, we propose that a conserved mechanism of allosteric activation is a fundamental feature of this entire family of proteases, including human HtrA enzymes linked to a wide spectrum of diseases.
Figure 6. Side Chains Important for DegS Allostery Are Conserved in the HtrA Family.
Residues shown here to be very important (red labels) or somewhat important (orange labels) for allosteric activation of DegS are largely conserved in HtrA proteases from all kingdoms of life. Residues that play minor roles (blue labels) or no role (black label) in DegS activity are far less conserved. HtrA sequences from E. coli (3), H. sapiens (4), Synechocystis (3), D. rerio, A. thaliana, C. reinhardtii, D. melanogaster, B. dendrobatidis, A. pseudotrichonymphae, and K. cryptofilum were aligned using ClustalW2 (Larkin et al., 2007) and submitted to WebLogo (Crooks et al., 2004) for visualization of conservation information. A higher number of bits indicates greater conservation for a given position.
Why is allosteric activation of HtrA proteases so important? The activities of all intracellular proteases must be carefully regulated to allow them to degrade the proper target substrates but to avoid degrading proteins required for cellular function. By linking proteolytic activation to assembly of specific enzyme-substrate or enzyme-substrate-activator complexes, these potentially dangerous enzymes can be maintained in an inactive state until the appropriate substrates and/or activators are present. Moreover, because allosteric activation is reversible, these proteases switch back to inactive conformations once protein substrates are degraded or activators are removed. Cage formation provides a second level of cellular protection for many HtrA proteases, as activation leads to assembly of complexes in which the proteolytic active sites are sequestered and only accessible to unfolded proteins that can enter the degradation chamber. Indeed, recent studies show that DegP mutations that prevent cage formation and facilitate allosteric activation result in rogue proteolysis that kills cells (Kim and Sauer, 2014).
For DegS, an unresolved question is how OMP-peptide binding to the PDZ domains transmits a signal that results in remodeling the protease domain into an active conformation. An early model suggested that contacts between residues of the OMP peptide and the L3 loop of DegS directly stabilized the active conformation (Wilken et al., 2004). This model, however, is inconsistent with numerous results, including the robust activity of DegSΔPDZ and crystal structures of OMP-bound active DegS that show no conserved contacts between the OMP peptide and the L3 loop (Sohn et al., 2007; 2010). Nevertheless, OMP-peptide binding is a key step in activation of full-length DegS and thus these binding events must either destabilize inactive DegS or stabilize active DegS by some mechanism. One possibility is that OMP-peptide binding causes conformational changes in the PDZ domain that then result in clashes with the L3 loop, initiating the conformational change in the protease domains (Sohn and Sauer, 2009). OMP-peptide binding to a single PDZ domain stimulates proteolytic activation of both the attached and neighboring protease domains in the DegS trimer (Mauldin and Sauer, 2013). The clusters of DegS residues that mediate allosteric switching are located at subunit-subunit interfaces in the protease domain, providing a simple mechanism by which binding of one OMP peptide could change the conformations of all subunits in the DegS trimer.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Wild-type and mutant variants of E. coli DegS (residues 27–355) with an N-terminal His6 tag replacing the wild-type membrane anchor and an 35S-labeled variant of the periplasmic domain of E. coli RseA (residues 121–216) with a C-terminal His6 tag were expressed in E. coli strain X90 from IPTG-inducible plasmids. Cultures were collected by centrifugation, resuspended in lysis buffer (50 mM sodium phosphate [pH 8], 300 mM NaCl, 10 mM imidazole), and lysed by sonication. Cleared lysates were applied to a pre-equilibrated Ni++-NTA (Qiagen) affinity column, washed with lysis buffer, and eluted in elution buffer (50 mM sodium phosphate [pH 8], 300 mM NaCl, 300 mM imidazole) as described (Walsh et al., 2003; Sohn et al., 2007; Cezairliyan and Sauer, 2009). Proteins were then dialyzed against two changes of storage buffer (50 mM sodium phosphate [pH 8], 200 mM NaCl, 1 mM EDTA, 10% glycerol) before being stored at −80 °C. YYF peptide was synthesized by GenScript and purified by reverse-phase high-pressure liquid chromatography on a C18 column. His6-tagged variants of DegP were expressed in E. coli strain X90(DE3) bearing a deletion of the chromosomal degP gene, cells were lysed by sonication, and proteins were purified by Ni++-NTA affinity chromatography, concentrated by Amicon filtration (Millipore), and purified by size-exclusion chromatography on a Superose-6 column (GE Healthcare) as described (Kim et al., 2011). Protein concentrations were determined by UV absorbance using extinction coefficients calculated from the amino-acid sequence.
Enzymatic and Biochemical Assays
DegS cleavage of RseA and OMP-peptide binding assays were performed at room temperature in buffer containing 150 mM NaHPO4 [pH 8.3], 380 mM NaCl, 10% glycerol, and 4 mM EDTA as described (Sohn et al., 2007; Cezairliyan and Sauer, 2009). Cleavage was performed by incubating DegS3 or variants (1 µM) with or without YYF tripeptide (230 µM) and three different sub-KM concentrations of 35S-RseA for different times. Reactions were terminated by quenching in 10% trichloroacetic acid, and acid-soluble radioactive cleavage products were quantified by scintillation counting to calculate cleavage rates. Initial cleavage rates normalized by total enzyme were plotted as a function of the RseA concentration, and the second-order rate constant was determined from the slope of a linear fit. Binding assays were performed by titrating increasing DegS or variants against a fixed concentration of the fluorescent OMP peptide fluorescein-DNRDGNVYYF and monitoring changes in fluorescence anisotropy (excitation 480 nm; emission 520 nm) after correction for protein scattering. Binding curves were fitted to a hyperbolic equation using Prism (GraphPad). Substrate cleavage by DegP, assayed by fluorescence or SDS-PAGE, binding of fluorescent substrate to proteolytically inactive DegP, assayed by fluorescence anisotropy, and substrate-mediated cage assembly, assayed by Förster resonance energy transfer, were performed at room temperature in 50 mM sodium phosphate (pH 8), 100 mM NaCl as described (Kim et al., 2011; Kim and Sauer, 2012).
Structural Refinement
Structure factors for 1SOT, 1SOT, 1TE0, and 1VCW were obtained from the Protein Data Bank. Re-refinement was performed using COOT (Emsley and Cowtan, 2004) for model building, MolProbity (Chen et al., 2010) for assessment of model geometry, and PHENIX (Adams et al., 2010) for refinement.
Sequence Alignment
The sequences for the following HtrA proteins (with associated accession numbers) were downloaded from UniProt: E. coli DegS (P0AEE3), E. coli DegP (P0C0V0), E. coli DegQ (P39099), Synechocystis HtrA (HTRA_SYNY3), Synechocystis HhoA (P72780), Synechocystis HhoB (P73940), H. sapiens HtrA1 (Q92743), H. sapiens HtrA2 (O43464), H. sapiens HtrA3 (P83110), H. sapiens HtrA4 (P83105), D. rerio HtrA1a (Q6GMI0), A. thaliana DegP1 (O22609), D. melanogaster HtrA2 (Q9VFJ3), C. reinhardtii DegP-type protease (A8HQB3) B. dendrobatidis putative uncharacterized protein (F4P0R9), A. pseudotrichonymphae DegQ (B6YQJ5) and K. cryptofilum Serine protease Do (B1L493). Sequences were aligned in ClustalW2 (Larkin et al., 2007). Alignments at positions highlighted in this paper were collected and submitted to WebLogo (Crooks et al., 2004) for visualization.
Highlights.
Residues critical for DegS-protease activation cluster at the trimer interfaces
Activation-cluster residues adjoin the active sites but not OMP-peptide binding sites
Activation-cluster residues are conserved throughout the HtrA-protease family
Mutation of “cluster” residues in DegP prevents allosteric activation by substrates
ACKNOWLEDGEMENTS
Supported by NIH grant AI-16892 and a Charles A. King Trust Postdoctoral Fellowship to S.K. T.A.B. is an employee of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
The re-refined 1SOT, 1SOT, 1TE0, and 1VCW structures of DegS have been deposited in the Protein Data Bank with accession codes 4RQZ, 4RR1, 4RQY, and 4RR0, respectively.
References
- Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades SE, Connolly LE, Alba BM, Gross CA. The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor. Genes Dev. 1999;13:2449–2461. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades SE. Regulation by destruction: design of the σE envelope stress response. Curr. Opin. Microbiol. 2008;11:535–540. doi: 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Kanehara K, Ito K. RseP (YaeL), an Escherichia coli RIP protease, cleaves transmembrane sequences. EMBO J. 2004;23:4434–4442. doi: 10.1038/sj.emboj.7600449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- Bowden MA, et al. High-temperature requirement factor A3 (Htra3): a novel serine protease and its potential role in ovarian function and ovarian cancers. Mol. Cell. Endocrinol. 2010;327:13–18. doi: 10.1016/j.mce.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chaba R, Grigorova IL, Flynn JM, Baker TA, Gross CA. Design principles of the proteolytic cascade governing the sigmaE-mediated envelope stress response in Escherichia coli: keys to graded, buffered, and rapid signal transduction. Genes Dev. 2007;21:124–136. doi: 10.1101/gad.1496707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr. Cancer Drug Targets. 2009;9:451–468. doi: 10.2174/156800909788486704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HR, Chan CC, Ferris F, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Research. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JF, Levchenko I, Sauer RT, Baker TA. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Grau S, et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA. 2005;102:6021–6026. doi: 10.1073/pnas.0501823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N. Engl. J. Med. 2009;360:1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- Hasselblatt H, Kurzbauer R, Wilken C, Krojer T, Sawa J, Kurt J, Kirk R, Hasenbein S, Ehrmann M, Clausen T. Regulation of the sigmaE stress response by DegS: how the PDZ domain keeps the protease inactive in the resting state and allows integration of different OMP-derived stress signals upon folding stress. Genes Dev. 2007;21:2659–2670. doi: 10.1101/gad.445307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Zhang X, Chen Y, Wu Y, Zhou ZH, Chang Z, Sui SF. Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proc. Natl. Acad. Sci. USA. 2008;105:11939–11944. doi: 10.1073/pnas.0805464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehara K, Ito K, Akiyama Y. YaeL (EcfE) activates the sigma(E) pathway of stress response through a site-2 cleavage of anti-sigma(E), RseA. Genes Dev. 2002;16:2147–2155. doi: 10.1101/gad.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sauer RT. Cage assembly of DegP protease is not required for substrate-dependent regulation of proteolytic activity or high-temperature cell survival. Proc. Natl. Acad. Sci. USA. 2012;109:7263–7268. doi: 10.1073/pnas.1204791109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sauer RT. Distinct regulatory mechanisms balance DegP proteolysis to maintain cellular fitness during heat stress. Genes Dev. 2014;28:902–911. doi: 10.1101/gad.238394.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Grant RA, Sauer RT. Covalent linkage of distinct substrate degrons controls assembly and disassembly of DegP proteolytic cages. Cell. 2011;145:67–78. doi: 10.1016/j.cell.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature. 2002;416:455–459. doi: 10.1038/416455a. [DOI] [PubMed] [Google Scholar]
- Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, Clausen T. Structural basis for the regulated protease and chaperone function of DegP. Nature. 2008;453:885–890. doi: 10.1038/nature07004. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. ClustalW and ClustalX version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Li X, Wang B, Feng L, Kang H, Qi Y, Wang J, Shi Y. Cleavage of RseA by RseP requires a carboxyl-terminal hydrophobic amino acid following DegS cleavage. Proc. Natl. Acad. Sci. USA. 2009;106:14837–14842. doi: 10.1073/pnas.0903289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S, Guo MS, Chaba R, Gross CA, Sauer RT. Dual molecular signals mediate the bacterial response to outer-membrane stress. Science. 2013;340:837–841. doi: 10.1126/science.1235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin RV, Sauer RT. Allosteric regulation of DegS protease subunits though a shared energy landscape. Nat. Chem. Biol. 2013;9:90–96. doi: 10.1038/nchembio.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JM, Patel A, Rowan AD. Emerging roles of serine proteinases in tissue turnover in arthritis. Arthritis Rheum. 2008;58:3644–3656. doi: 10.1002/art.24046. [DOI] [PubMed] [Google Scholar]
- Mohamedmohaideen NN, Palaninathan SK, Morin PM, Williams BJ, Braunstein M, Tichy SE, Locker J, Russell DH, Jacobs WR, Sacchettini JC. Structure and function of the virulence-associated high-temperature requirement A of Mycobacterium tuberculosis. Biochemistry. 2008;47:6092–6102. doi: 10.1021/bi701929m. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Motlagh HN, Wrabl JO, Li J, Hilser VJ. The ensemble nature of allostery. Nature. 2014;508:331–319. doi: 10.1038/nature13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Sauer RT. OMP peptides modulate the activity of DegS protease by differential binding to active and inactive conformations. Mol. Cell. 2009;33:64–74. doi: 10.1016/j.molcel.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Grant RA, Sauer RT. Allosteric activation of DegS, a stress sensor PDZ protease. Cell. 2007;131:572–583. doi: 10.1016/j.cell.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Sohn J, Grant RA, Sauer RT. OMP peptides activate the DegS stress-sensor protease by a relief of inhibition mechanism. Structure. 2009;17:1411–1421. doi: 10.1016/j.str.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Grant RA, Sauer RT. Allostery is an intrinsic property of the protease domain of DegS: implications for enzyme function and evolution. J. Biol. Chem. 2010;403:420–429. doi: 10.1074/jbc.M110.135541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Walle L, Lamkanfi M, Vandenabeele P. The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 2008;15:453–460. doi: 10.1038/sj.cdd.4402291. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- Wilken C, Kitzing K, Kurzbauer R, Ehrmann M, Clausen T. Crystal structure of the DegS stress sensor: How a PDZ domain recognizes misfolded protein and activates a protease. Cell. 2004;117:483–494. doi: 10.1016/s0092-8674(04)00454-4. [DOI] [PubMed] [Google Scholar]
- Zeth K. Structural analysis of DegS, a stress sensor of the bacterial periplasm. FEBS Lett. 2004;569:351–358. doi: 10.1016/j.febslet.2004.06.012. [DOI] [PubMed] [Google Scholar]