Summary
Muscle atrophy contributes to the poor prognosis of many pathophysiological conditions, but pharmacological therapies are still limited. Muscle activity leads to major swings in mitochondrial [Ca2+] which control aerobic metabolism, cell death and survival pathways. We have investigated in vivo the effects of mitochondrial Ca2+ homeostasis in skeletal muscle function and trophism, by overexpressing or silencing the Mitochondrial Calcium Uniporter (MCU). The results demonstrate that both in developing and in adult muscles MCU-dependent mitochondrial Ca2+ uptake has a marked trophic effect that does not depend on aerobic control, but impinges on two major hypertrophic pathways of skeletal muscle, PGC-1α4 and IGF1-AKT/PKB. In addition, MCU overexpression protects from denervation-induced atrophy. These data reveal a novel Ca2+-dependent organelle-to-nucleus signaling route, which links mitochondrial function to the control of muscle mass and may represent a possible pharmacological target in conditions of muscle loss.
Introduction
Loss of muscle mass and performance, together with important metabolic changes, occurs during pathophysiological conditions such as aging (sarcopenia), disuse and denervation, starvation and cancer (cachexia). Therapeutic interventions aimed at preserving muscle mass are of key importance, but they are still limited. Skeletal muscle size is determined by the equilibrium between protein synthesis and degradation which in turn is controlled by different signaling. In particular, the IGF1-AKT/PKB pathway controls muscle size by impinging both on protein translation via mTOR and GSKβ and on protein degradation via the ubiquitin-proteasome and the autophagy-lysosome pathways (Mammucari et al., 2008). In addition, a novel isoform of the mitochondria-related PGC-1α family of transcription coactivators, namely PGC-1α4, has been recently shown to trigger muscle hypertrophy (Ruas et al., 2012).
Mitochondria play a central role in skeletal muscle function by providing ATP largely consumed by SERCA activity and actomyosin contraction. The tight coupling of mitochondrial ATP production to the requirements of a contracting muscle is ensured by effects of the ubiquitous second messenger Ca2+ on aerobic metabolism. In a wide variety of cell types, including primary cultures of skeletal myotubes (Brini et al., 1997) and muscle fibers in situ (Rudolf et al., 2004), cytosolic Ca2+ transients generated by physiological stimuli, elicit large increases in the [Ca2+] of the mitochondrial matrix ([Ca2+]mt), which in turn stimulate the Ca2+-sensitive dehydrogenases of the Krebs cycle. At the same time [Ca2+]mt rises have been shown to inhibit autophagy (Cardenas et al., 2010) and sensitize cells to apoptosis and necrotic challenges (for review see (Rizzuto et al., 2012)).
The recent identification of the Mitochondrial Calcium Uniporter (MCU) (Baughman et al., 2011; De Stefani et al., 2011), the highly selective channel responsible for Ca2+ entry into mitochondria allow to investigate in detail its role in different aspects of skeletal muscle biology. Genetic ablation of MCU in the germline, however, displayed a mild phenotype (Pan et al., 2013). A clear indication of the importance of MCU-dependent mitochondrial Ca2+ accumulation in skeletal muscle function was the recent identification of a mutation of MICU1, a direct modulator of MCU, in patients affected by proximal muscle weakness, learning difficulties and extrapyramidal motor disorder (Logan et al., 2014).
In this contribution, we addressed the role of MCU in skeletal muscle by overexpressing or silencing MCU after birth, in order to rule out compensatory effects during prenatal development. The results showed that MCU expression triggers hypertrophy, both during post-natal growth and in adulthood, by controlling protein synthesis through PGC-1α4 and IGF1-AKT/PKB pathways. Finally, MCU exerts a protective effect against atrophy, suggesting that modulation of mitochondrial Ca2+ uptake may represent a novel area of therapeutic intervention to combat muscle mass loss.
Results
MCU overexpression or silencing in vivo affects mitochondrial Ca2+ uptake in muscle fibers
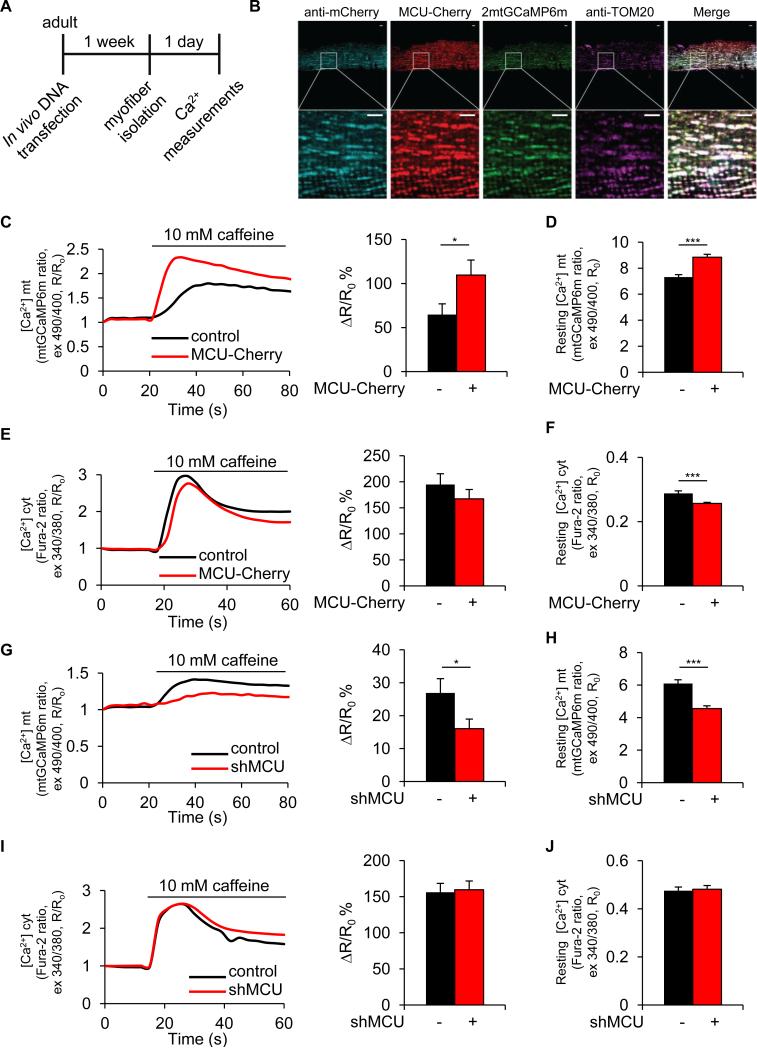
In cultured cells, modulation of MCU expression determines the amplitude of mitochondrial Ca2+ uptake upon physiological stimuli (De Stefani et al., 2011). In this work, we decided to specifically alter mitochondrial Ca2+ uptake in vivo by AAV9-based transduction or muscle transfection with MCU plasmids. To verify the efficacy of this approach, we transfected adult flexor digitorum brevis (FDB) mouse muscles in vivo with plasmids encoding a green fluorescent protein (GFP)-based Ca2+ probe targeted to mitochondria, mtGCaMP6m (Logan et al., 2014), in combination with a plasmid encoding mCherry (control) or mCherry-tagged MCU (MCU-Cherry). Eight days later, real-time imaging experiments were performed on isolated single myofibers (Figure 1A). Both MCU-Cherry and mtGCaMP6m colocalize with the mitochondrial protein TOM20 in muscle fibers (Figure 1B). After assessment of basal Ca2+ concentrations, a cytosolic, and hence mitochondrial [Ca2+] rise were evoked by discharging the SR pool with caffeine. MCU overexpression caused a marked increase in the caffeine peak and a modest elevation of the resting [Ca2+]mt (Figures 1C and 1D). Cytosolic Ca2+ levels were almost unaffected by MCU, showing a small decrease that was statistically significant for resting values (Figures 1E and 1F). The silencing experiments gave coherent results. FDB muscles were co-transfected in vivo with plasmids encoding either shluc (control) or shMCU and mtGCaMP6m. Ex vivo imaging experiments showed a marked reduction of both [Ca2+]mt resting values and caffeine-induced peaks in shMCU-transfected fibers (Figures 1G and 1H), while cytosolic Ca2+ values were virtually unaffected (Figures 1I and 1J). In order to mimic the physiological response of innervated muscles, we also measured Ca2+ transients upon K+-induced depolarization. Similarly to the caffeine experiments, higher and lower [Ca2+]mt peaks were detected in MCU overexpression and silencing respectively, although Ca2+ transients were smaller and shorter than caffeine-evoked transients. Interestingly, the opposite effect was detected in the [Ca2+]cyt peak, indicating a role of mitochondria as cytosolic Ca2+ buffers in vivo (Figures S1A-S1D). Importantly, neither MCU nor shMCU affected Δψ (Figures S1E and S1F), thus ruling out an indirect effect on the driving force for mitochondrial Ca2+ accumulation.
FIGURE 1. MCU is sufficient and required for mitochondrial Ca2+ uptake in skeletal muscle ex vivo.
A Flexor digitorum brevis (FDB) muscles were transfected with mtGCaMP6 and MCU-Cherry or shMCU. pmCherry-N1 or shluc were used as control, respectively. Seven days later single myofibers were isolated and placed in culture. B Immunofluorescence analysis shows colocalization of MCU-Cherry and mtGCaMP6 with the mitochondrial protein TOM20 in muscle fibers processed as in (A). Scale bar 5μm. C Left: representative traces of mitochondrial Ca2+ dynamics in a pmCherry-N1 (control, black trace) or MCU-Cherry (red trace) expressing fiber upon caffeine stimulation. Right: mean mitochondrial [Ca2+] increase. n=25. D Resting mitochondrial [Ca2+]. n=60. E Left: representative traces of cytosolic Ca2+ dynamics. Right: mean cytosolic [Ca2+] increase. n=18. F Resting cytosolic [Ca2+]. n=25. G Left: representative traces of mitochondrial Ca2+ dynamics in an shluc-Cherry (control, black trace) or shMCU-Cherry (shMCU, red trace) expressing fiber. Right: mean mitochondrial [Ca2+] increase. n=36. H Resting mitochondrial [Ca2+]. n=36. I Left: representative traces of cytosolic Ca2+ dynamics. Right: mean cytosolic [Ca2+] increase. n=23. J Resting cytosolic [Ca2+]. n=23. In each panel, data are represented as mean ± SEM. *p<0.05, ***p<0.001, t test (two-tailed, unpaired).
See also Figure S1.
MCU controls muscle size during post-natal growth
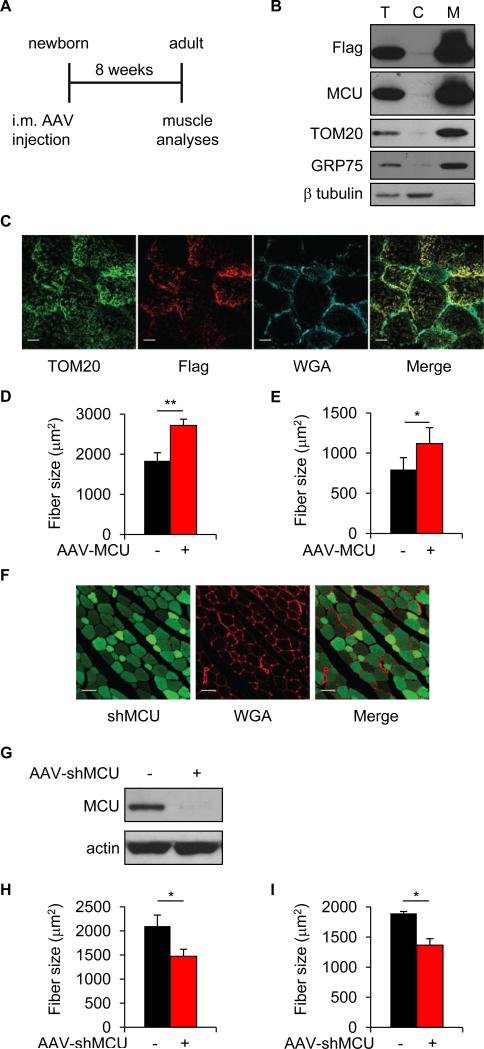
We thus investigated the role of MCU in both developing and adult muscle. We first focused on developing muscle, in which a greater plasticity could be expected. We injected hind limb muscles of newborn mice with AAV-MCU and analyzed the muscles eight weeks later (Figure 2A). A striking phenotype affecting muscle trophism was observed. MCU overexpression, confirmed by Western blotting of cytosolic and mitochondrial fractions (Figure 2B) and by immunofluorescence (Figure 2C), resulted in 47% increase in the average fiber area of MCU infected tibialis anterior (TA) compared to controls (Figure 2D). When measured one month after injection, TA muscle fiber size was 28% greater than control fibers (Figure S2), indicating a progressive event that starts early after injection and continues up to two month of age. To verify whether MCU-induced hypertrophy affected also different fiber types, we investigated soleus muscles, which are mitochondria-rich slow muscles. MCU triggered 41% hypertrophy compared to controls, suggesting that the effect of mitochondrial Ca2+ uptake in hypertrophy is independent of the number of mitochondria and of the overall metabolic properties (Figure 2E). Next, we analyzed the effect of MCU silencing. Newborn hind limb muscles were injected with AAV-shMCU and fiber size measured two months later. AAV-shMCU was efficiently delivered to TA muscle (Figure 2F) and decreased MCU protein expression (Figure 2G). Fiber size was markedly reduced both in TA and in soleus muscles (−30% and −28% respectively) (Figures 2H and 2I), highlighting the requirement of mitochondrial Ca2+ signals for the maintenance of skeletal muscle trophism.
FIGURE 2. MCU controls muscle size during post-natal growth.
A Hind limb muscles of newborn mice (4-6 days old) were injected with Flag-tagged AAV-MCU or AAV-shMCU. AAV-LacZ and AAV-shluc were used as negative controls, respectively. Two months later, muscles were isolated and processed for further analysis. B Immunoblotting of total protein lysates (T), cytosolic (C) and mitochondrial (M) fractions of TA muscles infected with AAV-MCU. Anti-Flag antibody was used to detect AAV-MCU, TOM20 was used as marker of outer mitochondrial membrane, GRP75 of mitochondrial matrix and β tubulin of cytosol. C TA muscle cryosections were immunostained with anti-TOM20 and anti-Flag antibodies. Wheat Germ Agglutinin (WGA) was used to label the sarcolemma. Scale bar 20 μm. D Mean fiber size of TA muscles. >600 fibers were measured for each muscle; n=3. E Mean fiber size of soleus muscles. >400 fibers per muscle; n=3. F Cryosection of AAV-shMCU infected TA muscle. shMCU was detected by ZsGreen fluorescence. Scale bar 50 μm. G Immunoblotting of TA muscles infected with AAV-shMCU. H Mean fiber size of TA muscles. >600 fibers per muscle; n=3. I Mean fiber size of soleus muscles. >500 fibers per muscle; n=3. In each panel, data are represented as mean ± SEM. *p<0.05, **p<0.01, t test (two-tailed, paired).
See also Figure S2.
Mitochondrial structure and function in MCU overexpression and silencing
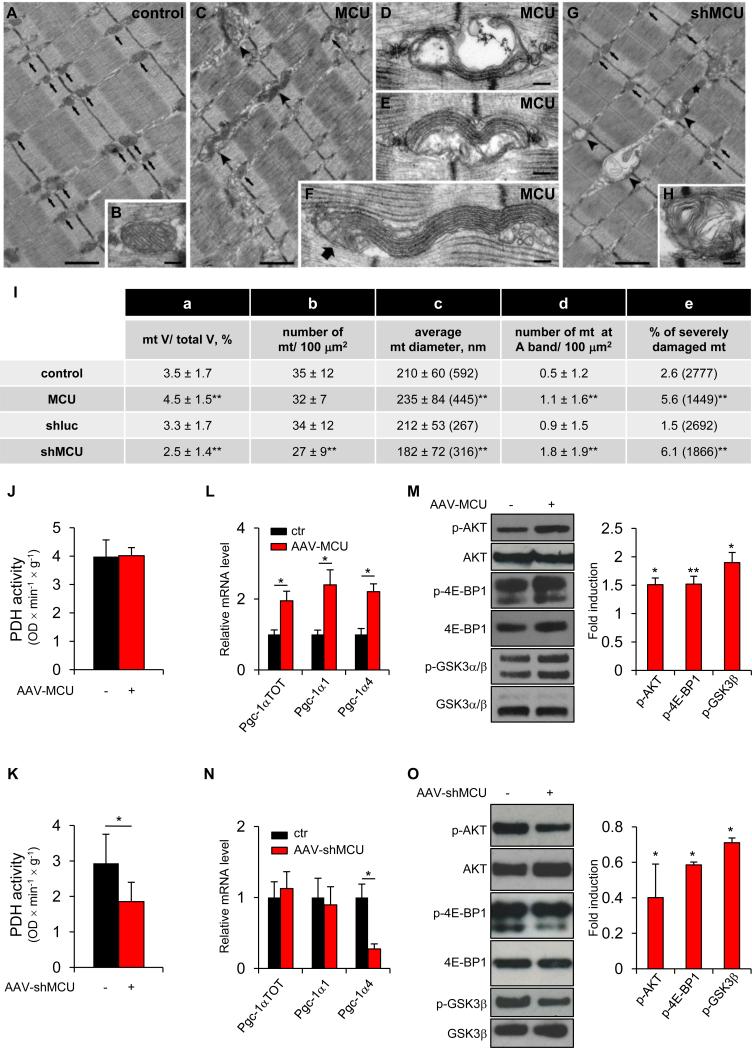
We then investigated the cellular changes that could underlie the trophic effect of MCU. We first focused on the effect on mitochondrial morphology and volume by electron microscopy (EM), and on their metabolic properties. In extensor digitorum longus (EDL) fibers from adult control mice mitochondria are positioned almost exclusively at the I band on both sides of the Z-lines (Boncompagni et al., 2009) (Figure 3A, arrows); in longitudinal sections their profiles appear round or oval, with parallel cristae within a dark/electron dense matrix (Figure 3B). In MCU-overexpressing EDL fibers, whereas many mitochondria appeared normal in shape and correctly localized with respect to the sarcomere (Figure 3C, arrows), we found some atypical mitochondria which forms wavy stacks of cristae protruding as long tentacles in inter-myofibrillar spaces (Figure 3C, arrowheads, and enlargements in Figures 3D-3F). These mitochondria, considered abnormal mitochondria in the quantitative analysis (Figure 3I, column e), accounted for <10% of total. Further quantitative investigation revealed that the fiber volume occupied by mitochondria increased from ≈3.5% to ≈4.5% (Figure 3I, column a), possibly due to the increase in the average mitochondrial diameter (Figure 3I, column c). In MCU-silenced fibers, the atypical wavy mitochondria observed in MCU-overexpressing fibers were never detected. In addition, the relative fiber volume occupied by mitochondria was significantly reduced, in parallel with a decreased number and size of these organelles (Figure 3G, arrows and 3I, columns a-c). The frequency of severely damaged mitochondria, i.e. presenting vacuoles and disrupted cristae (Figure 3G, arrowheads) or containing myelin-figures (Figure 3H), and longitudinally oriented organelles (Figure 3G, star) was also increased (6.1% vs. 1.5% of controls) (Figure 3I, columns d and e).
Figure 3. Effects of MCU modulation on mitochondrial structure and function and on hypertrophy-related pathways during muscle development.
A-H EM analysis of EDL muscles. Scale Bars: 1 μm (A, C, G), 0.1 μm (B, D-F, H). I Quantitative EM analysis. Values in columns a-d are shown as mean ± SD. In brackets: total number of mitochondrial profiles evaluated in the analysis. **p<0.01, t test (two-tailed, paired) of 3 muscles per group. J PDH activity of AAV-MCU infected TA muscles. n=4. K PDH activity of AAV-shMCU infected TA muscles. n=10. L Real-time RT-PCR analyses of AAV-MCU infected TA muscles. n=4. M Left: immunoblotting of AAV-MCU infected TA muscles. Right: quantification by densitometry. n=4. N Real-time RT-PCR analyses of AAV-shMCU TA infected muscles. n=4. O Left: immunoblotting of AAV-shMCU infected TA muscles. Right: quantification. n=4. In panels J-O data are represented as mean ± SEM. *p<0.05, **p<0.01, t test (two-tailed, paired).
See also Figures S3.
Next we investigated the effects on mitochondrial aerobic metabolism, focusing on the Ca2+-regulated enzymatic steps, such as pyruvate dehydrogenase (PDH). MCU overexpression neither affected the phosphorylation levels of PDH (Figure S3A) nor PDH activity (Figure 3J). A qualitative histochemical analysis of the activity of SDH, COX IV and NADH-TR in TA muscles did not show significant differences between MCU-overexpressing and control muscles. In addition, no difference was observed in glycogen content, as shown by PAS staining (Figure S3C). A comparative analysis of glycolitic (EDL) versus oxidative (soleus) muscles further confirmed that MCU overexpression does not qualitatively alter PAS and SDH activity (Figure S3D). Similar analyses were conducted on AAV-shMCU infected muscles. In agreement with data on MCU depleted muscles (Pan et al., 2013), MCU silencing increased PDH phosphorylation (Figure S3B) and decreased PDH activity (Figure 3K), although no significant changes in the histochemical pattern of SDH, COX IV and NADH-TR were observed (Figure S3E). Glycogen amount was also unaffected (Figure S3E). Overall, significant differences in mitochondrial volume were detected, but no obvious changes in structure and metabolic activity were observed that could be directly correlated with an effect on muscle size.
MCU regulates muscle hypertrophy signaling pathways
Since preliminary analysis showed no difference in autophagy (data not shown and Figure S3F), we focused our attention on the well-established hypertrophy pathways of skeletal muscle, PGC-1α4 and IGF1-AKT/PKB axis. PGC-1α is the master regulator of mitochondriogenesis, and a novel PGC-1α isoform (PGC-1α4) has been reported to trigger muscle hypertrophy (Ruas et al., 2012). Analysis of the mRNA expression of the Pgc-1α isoforms demonstrated that AAV-MCU triggers induction of both Pgc-1α1 and Pgc-1α4 (Figure 3L), thus revealing both an enhanced mitochondriogenesis (in agreement with the ultrastructural analysis) and a stimulation of the PGC-1α-related hypertrophy pathway. Activation of IGF1-AKT/PKB triggers hypertrophy, while its suppression determines muscle atrophy (Schiaffino and Mammucari, 2011). In addition, IGF1-AKT/PKB signaling is activated by PGC-1α4 (Ruas et al., 2012). Accordingly, AKT was phosphorylated, and thus activated, by AAV-MCU (Figure 3M). Specific AKT downstream targets were phosphorylated: in detail, 4E-BP1 and GSK3β, two inhibitors of protein translation (Schiaffino and Mammucari, 2011), were phosphorylated, and thus inhibited, in AAV-MCU muscles (Figure 3M). These data suggest that MCU-mediated hypertrophy is due to increased PGC-1α4 and IGF1-AKT/PKB-dependent signaling. Finally, satellite cells also contribute to normal muscle growth. Analysis of Pax7-positive nuclei demonstrated that MCU caused an increase in the average satellite cells number per fiber (Figure S3G).
Next, we checked whether the same hypertrophy pathways were also suppressed by MCU silencing. Pgc-1α4 expression was decreased by AAV-shMCU, while Pgc-1α1 was unaffected (Figure 3N). In addition, the AKT signaling pathway was inactivated by shMCU, as demonstrated by decreased phosphorylation of AKT, 4E-BP1 and GSK3β (Figure 3O). Finally, satellite cell number was decreased in shMCU infected muscles (Figure S3H). Overall, the above data indicate that MCU-mediated mitochondrial Ca2+ homeostasis regulates skeletal muscle size during post-natal growth by directly impinging on specific master regulators of hypertrophy.
MCU acutely controls muscle size in the adult
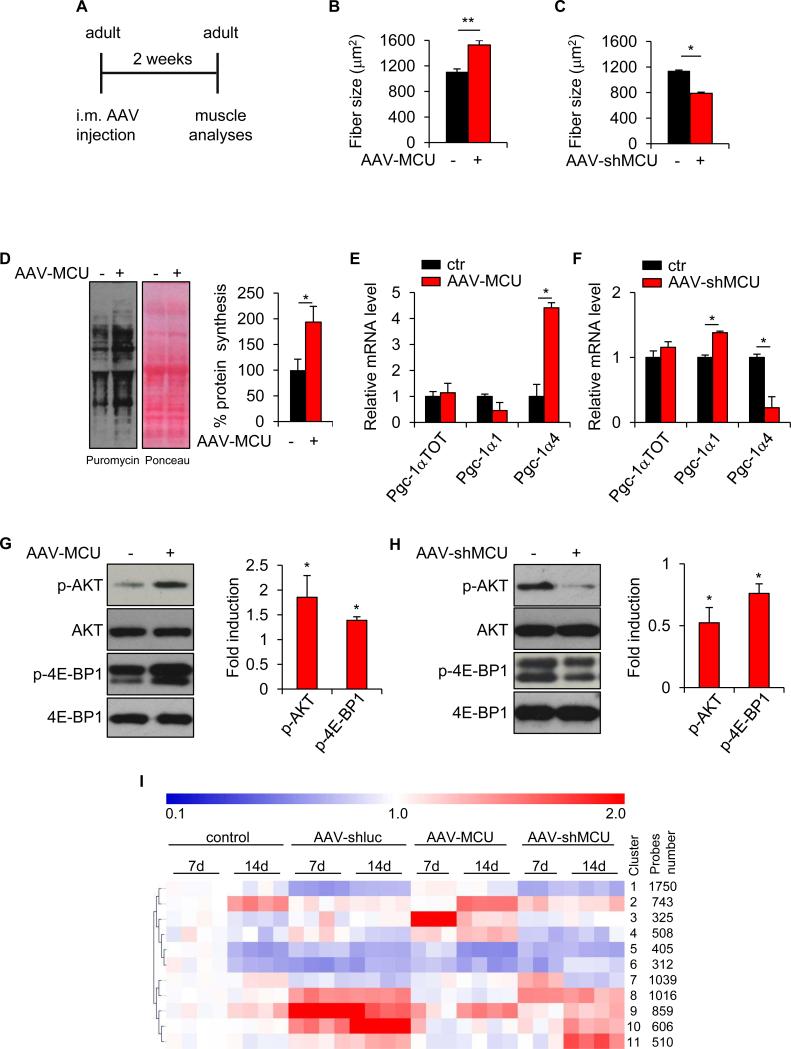
We proceeded to the analysis of adult muscle, where an effect on muscle trophism could have direct relevance for the understanding and potential targeting of age- and disease-related loss of muscle mass. For this purpose, adult EDL muscles were infected with AAV-MCU or AAV-shMCU and fiber size was measured two weeks later (Figure 4A). AAV-MCU infection triggered a 37% increase in fiber size (Figure 4B) while AAV-shMCU infection caused a 31% decrease, thus demonstrating that mitochondrial Ca2+ uptake is required for muscle trophism also in the adult (Figure 4C).
FIGURE 4. MCU acutely controls muscle size in the adult.
A EDL muscles of adult mice (2-3 month old) were infected with AAV-MCU or AAV-shMCU. AAV-LacZ or AAV-shluc were used as negative controls, respectively. Two weeks later muscles were isolated and processed for further analysis. B-C Mean fiber size of AAV-MCU and AAV-shMCU infected muscles. >300 fibers per muscle; n=3. D Protein synthesis analysis. EDL muscles were infected with AAV-MCU for two weeks. Puromycin was then i.p. injected and muscles were isolated 30 minutes later. Left: western blotting with anti-puromycin antibodies. Ponceau S staining was used as loading control. Right: quantification. n=4. E-F Real-time RT-PCR analyses of AAV-MCU and AAV-shMCU infected muscles. n=4. G-H Left: immunoblotting of AAV-MCU and AAV-shMCU infected muscles. Right: quantification. n=4. I Expression pattern clustering according to Self-Organising Tree Algorithm (SOTA). Gene expression values are relative to the average expression in control condition (7 days). In panels B-H, data are represented as mean ± SEM. *p<0.05, **p<0.01, t test (two-tailed, paired).
See also Figures S4 and Tables S1, S2, S3.
Adult muscle size is regulated by a fine equilibrium between protein synthesis and protein degradation of myofibrillar components. We analyzed protein synthesis by “surface sensing of translation” (SUnSET), a method based on the incorporation of puromycin into nascent peptide chains that allows accurate detection of protein synthesis rate in skeletal muscle in vivo (Goodman et al., 2011). Puromycin was injected to adult mice infected with AAV-MCU and 30 minutes later muscles were analyzed. Detection of puromycin with specific antibodies showed that protein synthesis was strongly induced by MCU (Figures 4D). As in developing muscle, also the experiments in adult muscle revealed a marked effect of MCU on PGC-1α4 and IGF1-AKT/PKB axis. In particular, Pgc-1α4 was drastically upregulated upon MCU overexpression, and downregulated upon MCU silencing (Figures 4E and 4F). In contrast to post-natal muscles, the effects on total Pgc1-α and Pgc1-α1 levels were very modest and did not correlate with the Pgc1-α4 change, thus suggesting a specific effect on the PGC1-α4-related hypertrophy pathway. Similarly, the analysis of the IGF1-AKT/PKB trophic pathway provided a coherent picture, with phosphorylation of AKT and downstream targets in MCU-overexpressing muscles (Figures 4G), and the opposite effect upon MCU silencing (Figures 4H). Finally, the number of Pax7-positive cells was unaffected by MCU, suggesting a marginal role of the satellite cell compartment in MCU-induced muscle hypertrophy in the adult (Figure S4A and S4B).
Finally, to get a broader view of the MCU-dependent transcriptional changes, and of the pathways involved in the trophic effect, we carried out RNA microarray analyses of single myofibers of AAV-MCU and AAV-shMCU infected muscles, with respective controls.
Cluster analysis, according to Self Organizing Tree Algorithm (SOTA) (Herrero et al., 2001), revealed that AAV infection per se affected most differentially expressed genes (clusters 1, 5, 6, 8, 9, 10) (Figure 4I). However, these genes do not play a role in muscle trophism, since infection with control AAV did not affect muscle size (not shown). The remaining clusters included genes induced by MCU overexpression (clusters 2, 3 and 4) or silencing (clusters 7 and 11). Interestingly, genes activated 14 days after AAV-MCU infection (clusters 2 and 4) were enriched for components of the cytoskeleton or genes involved in sarcomere organization and Ca2+ homeostasis (Table S1). Genes in clusters 2 and 4 were activated by AAV-MCU infection and inhibited by MCU silencing (Figure S4C). A GSEA analysis revealed that several pathways involved in hypertrophy were activated by MCU overexpression, including the insulin and mTOR signaling pathways (Table S2). It is interesting to note that most activated genes in response to MCU silencing (clusters 7 and 11) have mitochondrial functions (Table S3).
MCU protects from skeletal muscle atrophy
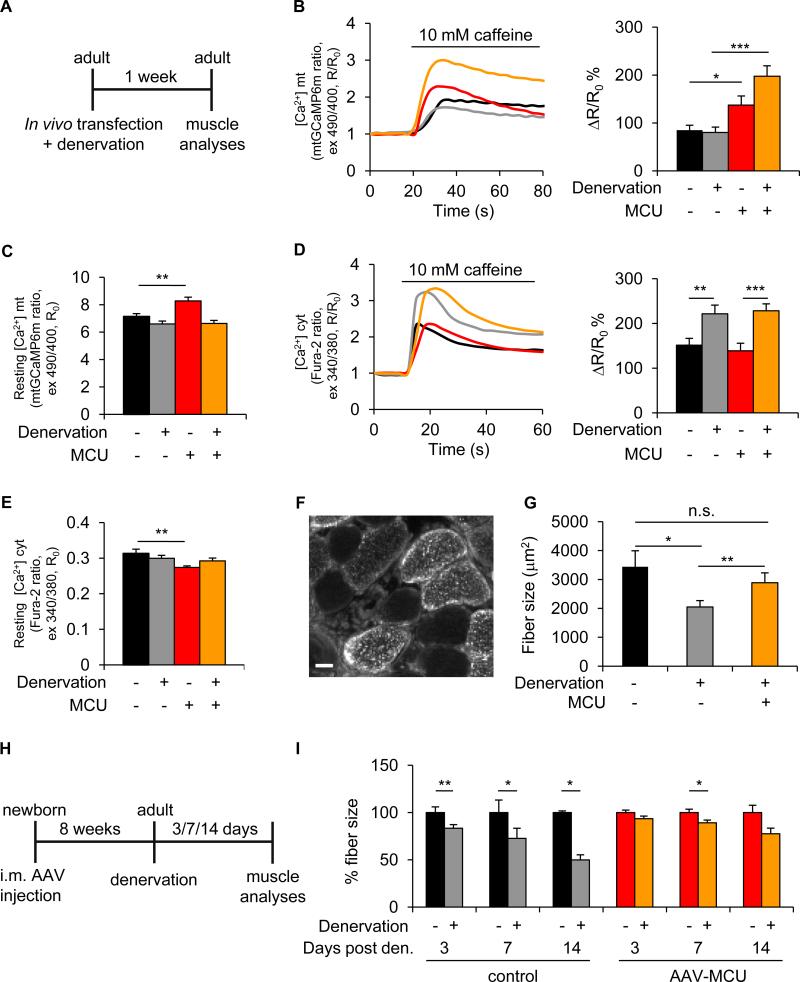
Finally, we investigated whether MCU overexpression could counteract conditions of disease-induced loss of muscle trophism. Denervation atrophy was triggered by sciatic nerve section, and Ca2+ signaling properties, together with muscle size, were evaluated (Figure 5A). Upon denervation, the cytosolic Ca2+ increase evoked by caffeine-induced SR release was markedly larger, although it did not evoke a larger Ca2+ uptake by mitochondria (Figures 5B and 5D), possibly due to the morphological remodeling of the fiber, alterations in the MCU complex assembly or in the SR/mitochondria coupling. Mitochondrial Δψ was unchanged (Figure S5A). When, however, MCU was overexpressed, mitochondrial Ca2+ uptake, induced by caffeine-evoked SR release, was greatly enhanced, reaching peak values that, due to the robust cytosolic rise, exceeded those of non-denervated fibers (Figures 5B and 5D). As to resting values, a significant difference (i.e. a higher [Ca2+]mt and lower [Ca2+]cyt resting value) was detected only in MCU-expressing non-denervated muscle, while denervated muscles exhibited a value similar to controls, irrespective of MCU expression (Figures 5C and 5E). The measurements of [Ca2+]mt and [Ca2+]cyt transients upon K+-induced depolarization gave similar results (Figures S5B and S5C).
FIGURE 5. MCU protects from skeletal muscle atrophy.
A Adult mice muscles were transfected with plasmids encoding MCU-Cherry (for real-time imaging) or MCU-Flag (for fiber size analysis). At the same time, denervation was achieved by cutting the sciatic nerve high in the thigh. One week later muscles were isolated and processed for further analysis. B Mitochondrial Ca2+ uptake of denervated FDB muscles transfected with mCherry-N1 or MCU-Cherry (MCU) upon caffeine stimulation. Left: representative traces. Right: mean mitochondrial [Ca2+] increase. n=31. C Resting mitochondrial [Ca2+]. n=51. D Cytosolic Ca2+ transients. Left: representative traces. Right: mean cytosolic [Ca2+] increase. n=26. E Resting cytosolic [Ca2+]. n=32. In panels B-E data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, t test (two-tailed, unpaired). F Immunofluorescence image of a denervated MCU-Flag (MCU) transfected TA muscle section. Antibodies against Flag tag and dystrophin to mark the sarcolemma were used. Bar scale 100µm. G Fiber size analysis of TA muscles upon denervation and MCU-Flag (MCU) overexpression. >800 fibers per muscle; n=4. H-I AAV-MCU protects from atrophy in the adult when injected in the newborn. H Hind limb muscles of newborn mice were injected with AAV-MCU. Two months later, sciatic nerve was cut. TA muscle fiber size was analyzed 3, 7 and 14 days after denervation. I Fiber size analysis of AAV-MCU infected TA muscles upon denervation. >200 fibers per muscle; n=3. In panels G and I data are represented as mean ± SEM. *p<0.05, **p<0.01, t test (two-tailed, paired).
See also Figure S5.
We then evaluated muscle size. Denervation caused a 40% reduction in TA mean fiber size, as expected. When MCU was overexpressed, atrophy was reduced to about 16%, compared to innervated control fibers (Figures 5F and 5G). Similar results were obtained when denervation was induced in adult animals in which MCU overexpression was induced by perinatal AAV infection (i.e. the conditions of Figures 2 and 3). In this case, fiber size was measured 3, 7 and 14 days post denervation (Figures 5H and 5I). In control muscles, 17%, 30% and 50% atrophy was observed, respectively, while in AAV-MCU infected muscles, only 6%, 11% and 22% reduction in fiber area was measured, respectively. Overall, the above data indicate that MCU overexpression can strongly counteract pathological atrophy.
Discussion
The recent molecular identification of MCU (Baughman et al., 2011; De Stefani et al., 2011), and of its complex regulatory system (De Stefani and Rizzuto, 2014), is now allowing to molecularly validate the broad literature supporting the pleiotropic role of mitochondrial Ca2+ homeostasis in cell function and survival. MCU-dependent mitochondrial Ca2+ accumulation was shown to play a role in pancreatic β-cells (Alam et al., 2012; Tarasov et al., 2012), heart (Drago et al., 2012; Joiner et al., 2012), neurons (Qiu et al., 2013) and colon cancer (Marchi et al., 2013). In this scenario, the very mild phenotype of the Mcu−/− mouse was quite surprising (Pan et al., 2013). The observation that viable mice could be obtained only in a mixed genetic background, while MCU ablation was embryonically lethal in the inbred stains, points to yet unresolved compensatory mechanisms (Murphy et al., 2014). Interestingly, the Mcu−/− mice show clear metabolic and functional alterations of skeletal muscle, and a MICU1 mutation (with ensuing loss of MCU gatekeeping, and hence increase in resting [Ca2+]mt levels) was identified in subjects with a pathology comprising learning difficulties and early-onset proximal muscle weakness (Logan et al., 2014).
In our work, we bypassed embryonic development by utilizing viral transduction and in vivo electroporation for directing an MCU expression system or MCU shRNAs to the muscle of living animals. Two stages (developing and adult skeletal muscle) which exhibit intrinsic differences in plasticity and signaling responses were independently assessed, and a clear coherent phenotype was apparent, with some differences that are worth of attention. Indeed, in both cases, mitochondrial Ca2+ accumulation via MCU positively correlated with the size of muscle fibers, i.e. a marked increase and reduction was observed in MCU-overexpressing and MCU-silenced fibers, respectively. In developing muscle, an increase in satellite cells was observed in MCU-overexpressers (and a reduction in MCU-silenced fibers), but this was not the case in adult muscle, possibly due to the quiescent state of satellite cells in the adult. This result indicates that an effect on the stem cell reservoir of muscle is not the key mechanism underlying the MCU-dependent increase in muscle mass.
We thus explored two different potential mechanisms for the increase in fiber size: a purely metabolic effect, and a regulation of the anabolic/catabolic balance of skeletal muscle. The first mechanism was unlikely for the following reasons: 1) PDH activity, albeit defective in MCU-silenced muscles (as in the Mcu−/− mouse), was unaffected by MCU overexpression; 2) the hypertrophic response was very similar in oxidative and glycolytic muscles, where the effect on mitochondrial metabolism should play a relatively minor role; 3) semi-quantitative analyses of aerobic metabolism revealed no major alteration. Nonetheless, EM analyses of MCU-silenced fibers showed an overall reduction in mitochondrial volume (and some mitochondrial damage), while MCU-overexpressing fibers showed increased mitochondrial volume and a peculiar proliferation of cristae, thus suggesting a role of mitochondrial Ca2+ homeostasis in the regulation of organelle biogenesis and morphology.
As to the anabolic/catabolic balance, we saw no difference in vivo in the autophagic rate, that we expected could be involved based on the induction of AMPK-dependent autophagy by inhibition of mitochondrial Ca2+ uptake (Cardenas et al., 2010). We then drew on anabolic pathways. PGC-1α4, a novel isoform of the transcriptional regulator of mitochondriogenesis PGC-1α, was shown to induce muscle hypertrophy, impinging on major anabolic routes, such as the IGF1-AKT/PKB axis (Ruas et al., 2012). Pgc1-α4 correlated with MCU expression, and with a cluster of genes involved in muscle hypertrophy. This was particularly clear in the adult muscle, where other Pgc1-α isoforms were not concomitantly modulated. As to the downstream effectors, we could demonstrate MCU-dependent phosphorylation of AKT and of its downstream targets 4E-BP1 and GSK3β. In agreement with these data, a marked increase in protein synthesis was measured in experiments of puromycin incorporation in nascent peptides.
Finally, MCU overexpression significantly counteracts denervation atrophy, by markedly increasing the [Ca2+]mt rises evoked by SR Ca2+ release and K+-induced depolarization. The clarification of this novel pathway will thus represent an important task for the future, with potential applications of utmost relevance for the pharmacological targeting of muscle loss in disease states and in aging.
Experimental Procedures
Expression plasmids
MCU-GFP, MCU-Flag, MCU-Cherry and mtGCaMP6m were already reported (De Stefani et al., 2011; Logan et al., 2014; Raffaello et al., 2013). pZac2.1, pZac2.1-LacZ and pZacf-U6-luc-ZsGreen (shluc-ZsGreen) were purchased from the University of Pennsylvania Vector Core.
For pZac2.1-MCU, MCU-Flag was amplified from MCU expression plasmid (De Stefani et al., 2011) with the following primers:
FOR: CTCGAGGCCACCATGGCGGCCGCCGCAGGTAG; REV: GAATTCTCACTTATCGTCGTCATCCTTGT and cloned into XhoI-EcoRI sites of pZac2.1.
For shMCU-ZsGreen, MCU targeting sequence was inserted into BamHI-EcoRI sites of shluc-ZsGreen with the following primers:
FOR: GATCGGATCCGAGATGACCGTGAATCTTCAAGAGAGATTCACGGTCATCTCGGATCTTTTTG
REV: AATTCAAAAAGATCCGAGATGACCGTGAATCTCTCTTGAAGATTCACGGTCATCTCGGATCC
For shMCU-Cherry and shluc-Cherry, ZsGreen cassettes of shMCU-ZsGreen and of shluc-ZsGreen were substituted with the mCherry cassette of pmCherry-N1 (Clontech Laboratories) at NheI-NotI sites.
AAV production
AAV-MCU and AAV-LacZ were produced from pZac2.1-MCU and pZac2.1-LacZ respectively; AAV-shMCU and AAV-shluc were produced from pZacf-U6-MCU-ZsGreen and pZacf-U6-luc-ZsGreen respectively. AAV vectors were purchased by Vector Biolabs or prepared by the AAV Vector Unit at ICGEB Trieste (http://www.icgeb.org/avu-core-facility.html), as described previously (Arsic et al., 2004) with few modifications. The titer of recombinant AAVs was determined by quantifying vector genomes (vg) packaged into viral particles, by real-time PCR against a standard curve of a plasmid containing the vector genome (Zentilin et al., 2001); values obtained were in the range of 1×1012 to 1×1013 vg/ml.
In vivo AAV Infection, DNA transfection and denervation
In vivo experiments were performed in accordance with the Italian law D. L.vo n°26/2014.
AAV Infection
For experiments in the newborn, 10^10 vg were injected in hind limb of 4-6 days old male CD1 mice. Muscles were subsequently analyzed one or two months post-injection as reported in the “Results” section. An average of 64% of fibers were positive for the AAV infections. For experiments in the adult, male CD1 mice were used. EDL muscles were isolated through a small hindlimb incision and 10^10 vg were injected along the muscle length. Muscles were analyzed 15 days post-injection. An average of 72% of fibers were positive for the AAV infections.
DNA transfection and denervation
Tibialis anterior (TA) muscles and flexor digitorum brevis (FDB) muscles of adult male CD1 mice were transfected as previously reported (DiFranco et al., 2009; Sandri et al., 2004). Denervation was achieved by cutting the sciatic nerve high in the thigh.
Microarray data
Raw data are available in the GEO database (accession number GSE GSE60931). Detailed microarray methods are described in “Extended Experimental Procedures”.
See also Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
The authors are grateful to Stefano Schiaffino, Alessandra Zulian and Denis Vecellio Reane for helpful discussion. This research was supported by grants from European Union (ERC mitoCalcium, no. 294777 to R.R.), Italian Telethon Foundation (GPP10005A to R.R.; GGP13213 to F.P.), Italian Ministries of Health (Ricerca Finalizzata to R.R.), Italian Ministries of Education, University and Research (PRIN to R.R., FIRB to R.R., FIRB Futuro in Ricerca RBFR10EGVP_002 to C.M., and FIRB Futuro in Ricerca RBFR13A20K 2013 to S.B.), University of Padova (Progetto di Ateneo to C.M.), NIH (Grant #1P01AG025532-01A1 to R.R.; subcontract of RO1 AR059646 to F.P.), Cariparo and Cariplo Foundations (to R.R.), Italian Association for Cancer Research (AIRC) (to R.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
R.R. and C.M. conceived the project and wrote the manuscript. C.M., G.G., I.Z., A.R., A.B., S.Z., G.P., D.D.S. performed in vivo and ex vivo experiments. S.B. performed EM experiments and analyses and wrote relative text. F.C. and S.C. performed microarray experiments and analyses and wrote relative text. L.Z. prepared AAVs and assisted with AAVs experiments. M.S. co-supervised experiments on hypertrophy pathways. D.D.S. co-supervised Ca2+ measurements. F.P. supervised EM experiments and wrote relative text. G.L. supervised microarray experiments.
The authors declare no conflict of interest.
References
- Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. J Biol Chem. 2012;287:34445–34454. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, De Giorgi F, Murgia M, Marsault R, Massimino ML, Cantini M, Rizzuto R, Pozzan T. Subcellular analysis of Ca2+ homeostasis in primary cultures of skeletal muscle myotubes. Mol Biol Cell. 1997;8:129–143. doi: 10.1091/mbc.8.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Rizzuto R. Molecular control of mitochondrial calcium uptake. Biochem Biophys Res Commun. 2014;449:373–376. doi: 10.1016/j.bbrc.2014.04.142. [DOI] [PubMed] [Google Scholar]
- DiFranco M, Quinonez M, Capote J, Vergara J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J Vis Exp. 2009 doi: 10.3791/1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci U S A. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011;25:1028–1039. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero J, Valencia A, Dopazo J. A hierarchical unsupervised growing neural network for clustering gene expression patterns. Bioinformatics. 2001;17:126–136. doi: 10.1093/bioinformatics/17.2.126. [DOI] [PubMed] [Google Scholar]
- Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46:188–193. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corra F, Giorgi C, De Marchi E, et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Pan X, Nguyen T, Liu J, Holmstrom KM, Finkel T. Unresolved questions from the analysis of mice lacking MCU expression. Biochem Biophys Res Commun. 2014;449:384–385. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Tan YW, Hagenston AM, Martel MA, Kneisel N, Skehel PA, Wyllie DJ, Bading H, Hardingham GE. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nature communications. 2013;4:2034. doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic beta-cells. PLoS One. 2012;7:e39722. doi: 10.1371/journal.pone.0039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentilin L, Marcello A, Giacca M. Involvement of cellular double-stranded DNA break binding proteins in processing of the recombinant adeno-associated virus genome. J Virol. 2001;75:12279–12287. doi: 10.1128/JVI.75.24.12279-12287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.