Abstract
Human (HN) prevents stress-induced apoptosis in many cells/tissues. In this study we showed that HN ameliorated chemotherapy (Cyclophosphamide, CP and Doxorubicin, DOX)-induced male germ cell apoptosis both ex vivo in seminiferous tubule cultures and in vivo in the testis. HN acts by several putative mechanisms via binding to: an IL-12 like trimeric membrane receptor; BAX; or Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3, a proapoptotic factor). To understand the mechanisms of HN on male germ cell apoptosis, we studied five HN analogues including: HNG (HN-S14G, a potent agonist), HNG-F6A (no binding to IGFBP-3), HN-S7A (no self-dimerization), HN-C8P (no binding to BAX), and HN-L12A (a HN antagonist) on CP-induced male germ cell apoptosis in mice. CP-induced germ cell apoptosis was inhibited by HN, HNG, HNG-F6A, HN-S7A, and HN-C8P (less effective); but not by HN-L12A. HN-L12A, but not HN-S7A or HN-C8P, blocked the protective effect of HN against CP-induced male germ cell apoptosis. HN, HN-S7A, and HN-C8P restored CP-suppressed STAT3 phosphorylation. These results suggest that HN: 1) decreases DOX (ex vivo) and CP (in vivo) induced male germ cell apoptosis; 2) action is mediated by the membrane receptor/STAT3 with minor contribution by BAX-binding pathway; 3) self-dimerization or binding to IGFBP-3 may not be involved in HN’s effect in testis. HN is an important molecule in the regulation of germ cell homeostasis after injury and agonistic analogues may be developed for treating male infertility or protection against chemotherapy side effects.
INTRODUCTION
HN is a mitochondrial derived 24 amino acid peptide which prevents cell damage from stress/injury in many tissue/cells, including neuronal tissue [1–3], heart and blood vessel [4,5], bone [6], blood-derived cells [7,8], pancreas [9,10], and male germ cells [11,12]. We have previously shown that male germ cell apoptosis can be induced in rodents [13,14], monkeys [15,16], and men [17] by a variety of apoptotic stimuli including testicular hyperthermia, and administration of insulin-like growth factor binding protein 3 (IGFBP-3), gonadotropin-releasing hormone antagonist (GnRH-A), or testosterone (to suppress endogenous gonadotropins and subsequently intratesticular testosterone) [18]. Administration of HN prevents GnRH-A, heat, or IGFBP-3 induced male germ cell apoptosis in rodents [11,12]. The anti-apoptotic effect of HN may be mediated through binding to: 1) A membrane receptor composed of glycoprotein 130 (gp130) and WSX-1 (IL-27 receptor subunit α) via activation of the STAT3 pathway [2,19–21]; 2) A G protein-coupled receptor formyl peptide receptor-like-1,2 [8]; 3) BAX intracellularly to sequester BAX in the cytosol preventing its translocation to mitochondria to initiate apoptosis [12,22]; and 4) IGFBP-3 and restraining its pro-apoptotic actions without competing with IGFI/ IGFBP-3 association [23]. Using analogues of HN with different binding properties may help to dissect the mechanisms of anti-apoptotic actions of HN [22–25].
Advanced chemotherapies of cancer patients have increased survival and life expectancy, and also increased expectations for a more favorable adverse effect profile. Of the long term adverse effects of chemotherapies on cancer survivors, infertility has emerged as one of the chief complaints [26–28]. Accordingly, we explored the role of HN and HN analogues in preventing male germ cell apoptosis induced by chemotherapeutic drugs in mouse models. Doxorubicin (DOX) was chosen for the ex vivo study as an anthracycline antibiotic that acts by intercalating DNA to suppress proliferation and increase apoptosis and is active in vitro [29]. Cyclophosphamide (CP) was used in the in vivo animal experiments because it requires liver cytochrome P450 metabolism to become the activated form of the drug, 4-hydroxy-cyclophosphamide, which circulates to cancer cells and damages DNA leading to apoptosis [30].
MATERIALS AND METHODS
Materials
Cyclophosphamide monohydrate (CP) and doxorubicin hydrochloride (DOX) were obtained from Sigma (St. Louis, MO). HN peptide and the HN analogues were synthesized by CPC Scientific (Sunnyvale, CA). The brief description of the characteristics of each of the analog is provided in Table 1. In our in vivo experiments, HN and five HN analogues were studied by using a CP-induced male germ cell apoptosis mouse model. These analogues include HNG (HN with a substitution of serine 14 to glycine, HN-S14G) [31–33], HNG-F6A (HNG with a substitution of alanine for 6th phenylalanine, no binding to IGFBP-3)[23], HN-S7A (HN with a substitution of alanine for 7th serine, dimerization defective)[34], HN-C8P (HN with a substitution of proline for 8th cysteine, no binding to BAX)[1,22,34], and HN-L12A (HN with a substitution of alanine for 12th leucine, HN antagonist dimerizes with HN preventing HN binding to receptor)[34]. The rationale of using each of the analogues was to dissect the possible mechanisms of HN in preventing apoptosis.
Table 1.
HN analogues with their known properties and cytoprotective action
| HN Analogue |
Amino acid Substitution |
Characteristics | Cytoprotective Action |
|---|---|---|---|
| HN-S14G [31–33] | Ser14 to Gly | Same mechanisms of action as HN | 10 to 1000 more potent than HN in some cells |
| HNG-F6A [23] | Phe6 to Ala | Loss of IGFBP-3 binding | Similar/more effective than HN |
| HN-S7A [34] | Ser7 to Ala | Loss of membrane receptor binding | Not effective, prevents HN self-dimerization |
| HN-C8P [1,22,34] | Cys8 to Pro | Loss of BAX binding | Not effective, may act as dominant negative |
| HN-L12A [34] | Leu12 to Ala | Dimerizes with and inactivates HN | HN antagonist, forms inactive dimer with HN |
Mouse Seminiferous Tubule ex vivo Culture
A total of 15 mice were used for ex vivo studies. The animals were sacrificed and seminiferous tubules isolated from testes were cut into small segments under a dissecting microscope to identify light (early stages I–IV and late stages XI–XII) of the seminiferous epithelium) and dark (middle stages VII–VIII) segments. Ten to twelve light segments (2 mm in length) were placed per well on a six well plate with 2 ml serum free medium of F-10 Ham nutrient mixture (Gibco, Life Technologies, Grand Island, NY) following these treatments: control (Con, n=15 where n is the number of times the treatment was repeated with segments obtained from different donor animals), heat (43°C, 15min; used as a positive control, n=10), HN 10 µg/mL (HN, n=9), doxorubicin 1µM (Dox1, n=7), doxorubicin 1µM + HN 10µg/mL (Dox1+HN, n=7), doxorubicin 10µM (Dox10, n=10), and doxorubicin 10µM + HN 10µg/mL (Dox10+HN, n=10). After 12 hours of incubation at 34°C with 5% CO2, the seminiferous tubules from the seven treatment groups were used to make “squashed” seminiferous tubule preparations on a slide for TUNEL assay to detect germ cell apoptosis [35]. To quantify the rate of germ cell apoptosis, the squashed segments of seminiferous tubules were examined with an American Optical Microscope (Scientific Instruments, Buffalo, NY) with a 40× objective and a 10× eyepiece lens. A square grid fitted within the eyepiece provided a reference area of 62,500 µm2. The TUNEL positive apoptotic germ cells within the frame of grid were counted in 4 segments of seminiferous tubules in each group. The incidence of germ cell apoptosis was expressed as the number of apoptotic germ cells per mm2.
Animals and in vivo Experiments
Adult (12-week-old) male mice (C57BL/6J wild type, purchased from Jackson Laboratories, Bar Harbor, Maine) were used for animal experiments. All mice were housed in a standard animal facility under controlled temperature (22°C) and photoperiod of twelve hours of light and twelve hours of darkness with free access to food and water. Animal handling and experimentation were in accordance with the recommendation of American Veterinary Medical Association and were approved by the animal care and use review committee at the Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles (Harbor-UCLA) Medical Center.
For the HN analogue experiments, male mice were divided into seven groups (n=4–5 per group) and received one of the following treatments and sacrificed after 24 hours: 1) vehicle (control); 2) a single intra-peritoneal (IP) injection of HN peptide [HN, 40mg/Kg body weight (BW)]; 3) a single IP injection of CP (CP, 200mg/Kg BW) ; 4) IP injection of CP and HN (CP+HN); 5) a single IP injection of each HN analog (HNG 5mg/Kg BW, HNG-F6A 5mg/Kg BW, HN-S7A 40mg/Kg BW, HN-C8P 40mg/Kg BW, or HN-L12A 40mg/Kg BW) ; 6) IP injection of CP and each HN analog (CP+HNG, HNG-F6A, HN-S7A, HN-C8P, or HN-L12A); and 7) IP injection of CP+HN+HN analogue (HN-S7A, HN-C8P, or HN-L12A; to assess whether the analogues has enhancing or inhibitory effect on HN).
Tissue Preparation
To facilitate testicular fixation by using a whole-body perfusion technique, all animals were injected with heparin (130 IU/100g BW, IP) 15 min before a lethal injection of sodium pentobarbital (200 mg/kg BW, IP) [14]. One testis was removed and weighed after perfusion with saline. Portions of testicular parenchyma were snap frozen in liquid nitrogen, and stored at −80 C for Western blotting. The other testis was fixed by vascular perfusion with Bouin’s solution, and processed with routine paraffin embedding for in situ detection of apoptosis.
Western Blotting Analysis
Western blotting was performed as described previously [36]. In brief, proteins were denatured and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system (Invitrogen, Carlsbad, CA). After transferring, the Immuno-blot PVDF Membrane (Bio-Rad, Hercules, CA) was blocked for 1 h and then probed using anti-STAT3 or anti-pSer727 STAT3 (Cell signaling Technology, Inc., Beverly, MA) overnight at 4 C with constant shaking. After washing, membrane was then incubated with an anti-mouse (for STAT3 antibody, Santa Cruz Biotechnology, Santa Cruz, CA) or anti-rabbit (for pSer727 STAT3 antibody, Amersham Biosciences, Piscataway, NJ) IgG-HRP secondary antibody. All antibodies were diluted in blocking buffer. For immunodetection, membrane was incubated with enhanced chemiluminescence solutions per the manufacturer’s specifications (Amersham Biosciences, Piscataway, NJ), and exposed to Hyperfilm ECL (Denville Scientific Inc., Metuchen, NJ).
Assessment of apoptosis
Detection of apoptotic cell was performed in Bouin’s’-fixed, paraffin-embedded testicular sections by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxy-UTP nick end labeling (TUNEL) technique [37] using an ApopTag-peroxidase kit (Chemicon International, Inc., Temecula, CA). Enumeration of the Sertoli cell nuclei with distinct nucleoli and apoptotic germ cell population was quantified at stages I–IV (early stages), stages VII–VIII (middle stages) and stages XI–XII (late stages) of the seminiferous epithelial cycle using an Olympus BH-2 microscope (New Hyde Park, NY). Stages were identified according to the criteria proposed by Russell et al for paraffin sections [38]. The rate of germ cell apoptosis (apoptotic index, AI) was expressed as the number of apoptotic germ cells per Sertoli cells [37].
Statistical Analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Cooperation, San Rafael, CA). The Student-Newman-Keuls test after one-way repeated measures ANOVA was used for statistical significance. The seminiferous tubule culture experiments were replicated 7–15 times and for animal experiments each group has 4–5 mice. Differences were considered significant if P<0.05.
RESULTS
Effect of doxorubicin on germ cell apoptosis in seminiferous tubule cultures
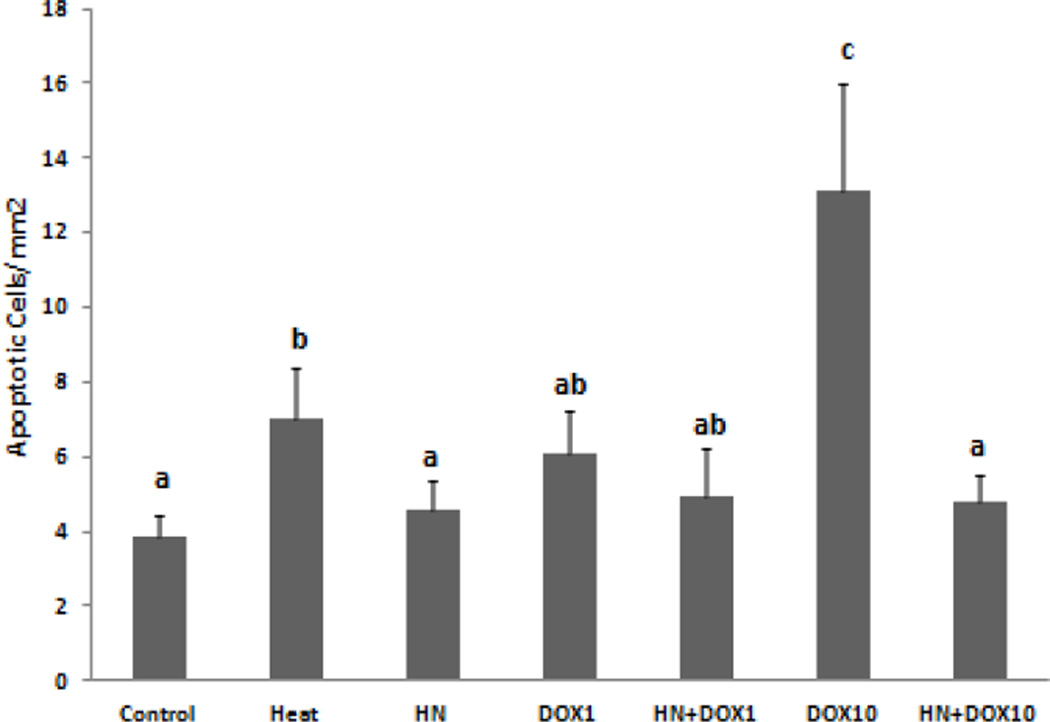
In short term (12 hours) ex vivo seminiferous tubule cultures HN (10µg/mL) treatment alone had no significant effect on germ cell apoptosis when compared with control. As expected, heat exposure (43°C, 15min) serving as a positive control significantly increased the rate of apoptosis compared to control and HN treated groups (p<0.05). We found in previous studies that HN (10ug/mL) prevented the heat induced germ cell apoptosis (data not shown). Low dose doxorubicin (1 µM) treatment increased apoptosis but did not reach statistical difference because of the large variations compared with control; addition of HN did not significantly decrease apoptosis (Fig. 1). High dose doxorubicin (10 µM) treatment significantly increased apoptosis compared to control (p<0.05); while HN (10 µg/mL) significantly reduced the number of apoptotic germ cells induced by high dose doxorubicin (p<0.05) (Fig. 1). In the squashed tubules, the germ cells undergoing apoptosis were mainly pachytene spermatocytes and round spermatids, no apoptotic spermatogonia were detected.
FIG. 1. Effect of HN on Doxorubicin-induced male germ cell apoptosis in seminiferous tubule culture.
Quantification of apoptotic cells in squashed seminiferous tubules after treatment ex vivo. The rate of germ cell apoptosis is similar between control and HN groups (p<0.05). High dose, but not low dose, of doxorubicin (DOX) significantly increased the number of apoptotic cells compared with control (p<0.05). Humanin treatment significantly attenuated germ cell apoptosis induced by high dose of DOX treatment (p<0.05). Values are the mean ± SEM. Means with unlike superscripts are significantly different (P<0.05).
Effects of HN and HNG on CP-induced apoptosis in testis
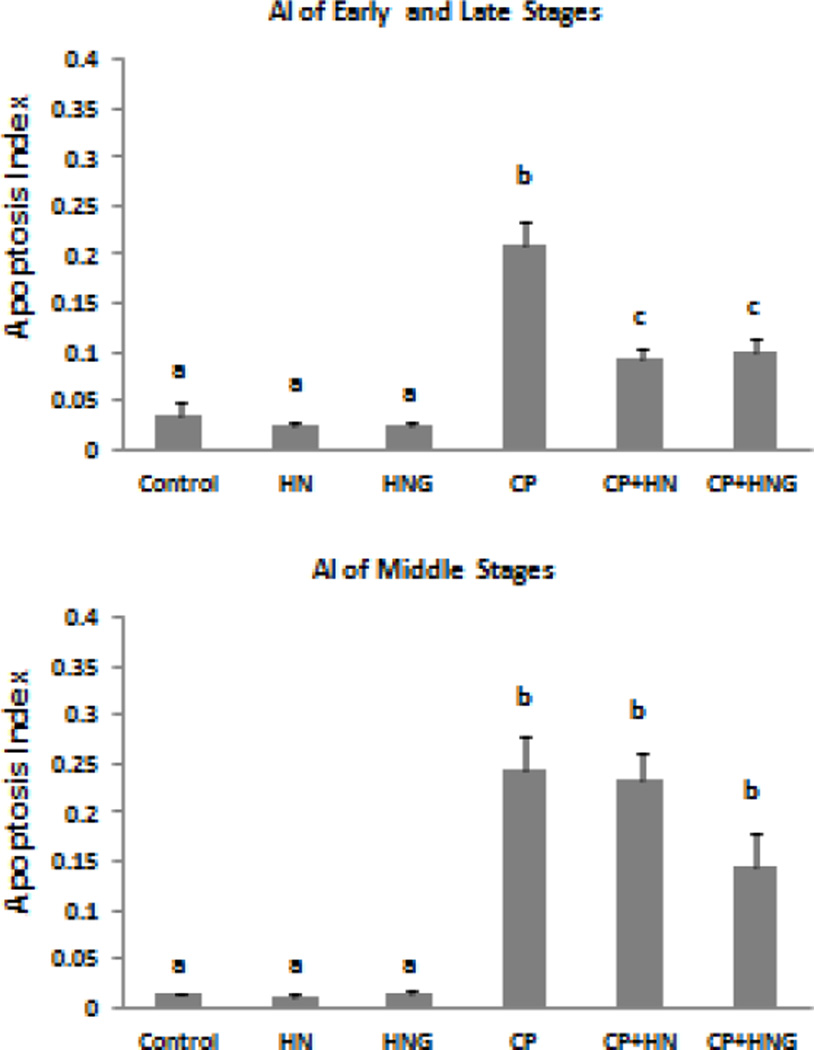
Synthetic HN or HNG peptide alone did not change the spontaneous germ cell apoptosis. CP treatment increased germ cell apoptosis at both early+late stages (I–IV and XI–XII) (Fig 2 upper panel, CP: 0.21±0.03, p<0.01 compared with control group, 0.04±0.01) and middle stages (VII–VIII) of seminiferous epithelial cycle in mice (bottom panel, CP: 0.25±0.03; p<0.01 compared with control group, 0.01±0.01). CP-induced germ cell apoptosis was significantly (~57%) inhibited by HN administration (40mg/kg BW) in early+late stages (I–IV and XI–XII) (Fig.2 upper panel, CP+HN: 0.09±0.01, p<0.05 compared with CP treated group), but not in middle stages (VII–VIII) (bottom panel, CP+HN: 0.24±0.03; p>0.05 compared with CP treated group). Similarly, HNG at 5 mg/kg BW was able to significantly inhibit (~52%) the CP-induced germ cell apoptosis in stages I–IV and XI–XII (Fig.2 upper panel, CP+HNG: 0.10±0.01, p<0.05 compared with CP treated group) which was similar to the effect of HN at 8-fold higher dose in early+late stages. HNG also prevented ~36% of CP-induced germ cell apoptosis in middle stages (VII–VIII) (Fig.2 bottom panel, CP+HNG: 0.14±0.04 compared with CP group), although this effect did not reach statistical significance (p>0.05). In this and all subsequent in vivo experiments mainly pachytene spermatocytes and round spermatids but not spermatogonia underwent apoptosis
FIG. 2. Effect of HN and HNG on Cyclophosphamide (CP)-induced male germ cell apoptosis.
Mice were treated with vehicle (Control), HN, HNG (Humanin-S14G), CP, CP + HN or CP+HNG as described in experimental procedures (n=4 each group). Apoptotic Index (AI) stands for the ratio of TUNEL positive germ cells per Sertoli cell (also in following figures). HN or HNG alone did not change apoptosis compared with control. CP significantly increased germ cell apoptosis both in early+late (I–IV and XI–XII) and middle (VII–VIII) stages as compared to control. HN significantly suppressed CP-induced apoptosis in early+late stages but not in middle stages. HNG prevented CP-induce apoptosis both in early+late and middle stages but fail to reach significance in the middle stages (P>0.05). Values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different.
Effect of HNG-F6A, a HN analogue that does not bind to IGFBP-3, on germ cell apoptosis
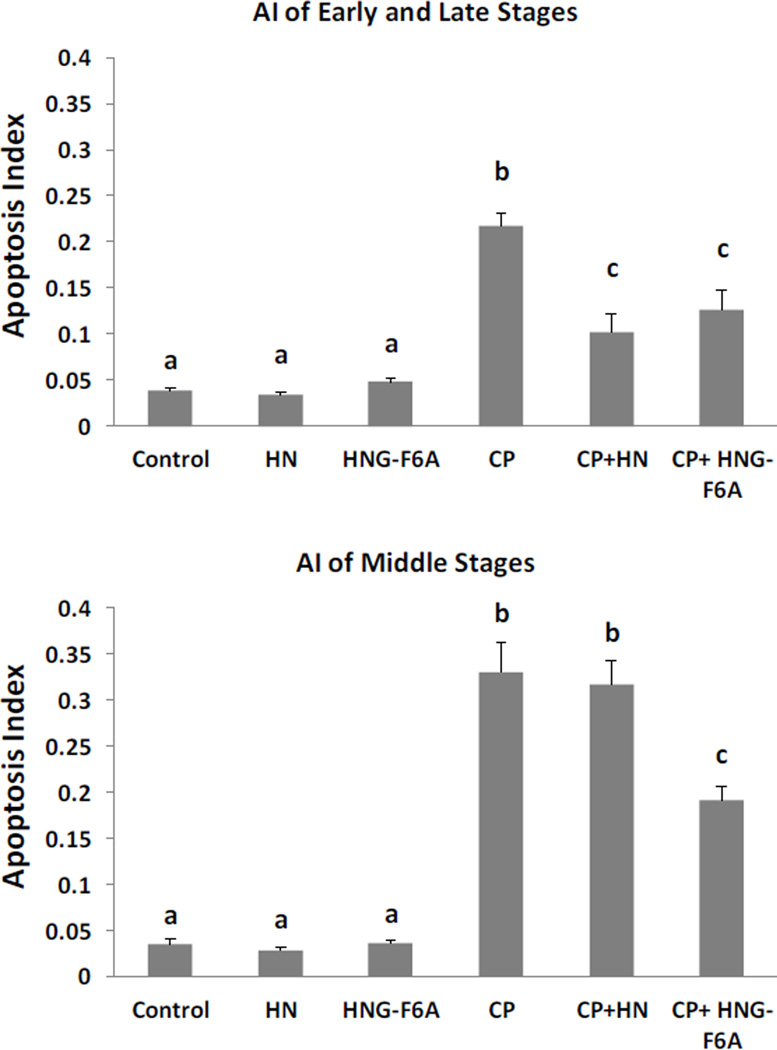
In cell culture systems synthetic HNG-F6A peptide does not bind with IGFBP-3 [23]. In present study, HNG-F6A alone did not change spontaneous germ cell apoptosis. Like HNG, HNG-F6A at 5 mg/kg BW dose was able to inhibit (~38%) the CP-induced germ cell apoptosis at stages I–IV and XI–XII (Fig.3 upper panel, CP+HNG-F6A: 0.13±0.02, p<0.05 compared with CP treated group: 0.21±0.01) which was similar to the effect of HN at 8-fold higher dose (40 mg/kg BW) in early+late stages (I–IV and XI–XII). In addition, HNG-F6A also prevented ~42% of CP-induced germ cell apoptosis in middle stages (VII–VIII) (Fig.3 bottom panel, CP+HNG-F6A: 0.19±0.02 vs CP: 0.33±0.03, p<0.05).
FIG. 3. Effect of HN and HNG-F6A on Cyclophosphamide (CP)-induced male germ cell apoptosis.
Mice were treated with vehicle (Control), HN, HNG-F6A (Humanin-S14G-F6A), CP, CP+HN or CP+HNG-F6A as described in experimental procedures (n=4 each group). HN or HNG-F6A alone did not change apoptosis compared with control. Compared with control, CP increased germ cell apoptosis both in early+late (I–IV and XI–XII) and middle (VII–VIII) stages. HN significantly suppressed CP-induced apoptosis in early+late stages but not in middle stages. HNG-F6A prevented CP-induce apoptosis both in early+late and middle stages remarkably. Values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different.
Effects of HN analogues that do not dimerize (HN-S7A) or bind to BAX (HN-C8P) on Male Germ Cell apoptosis
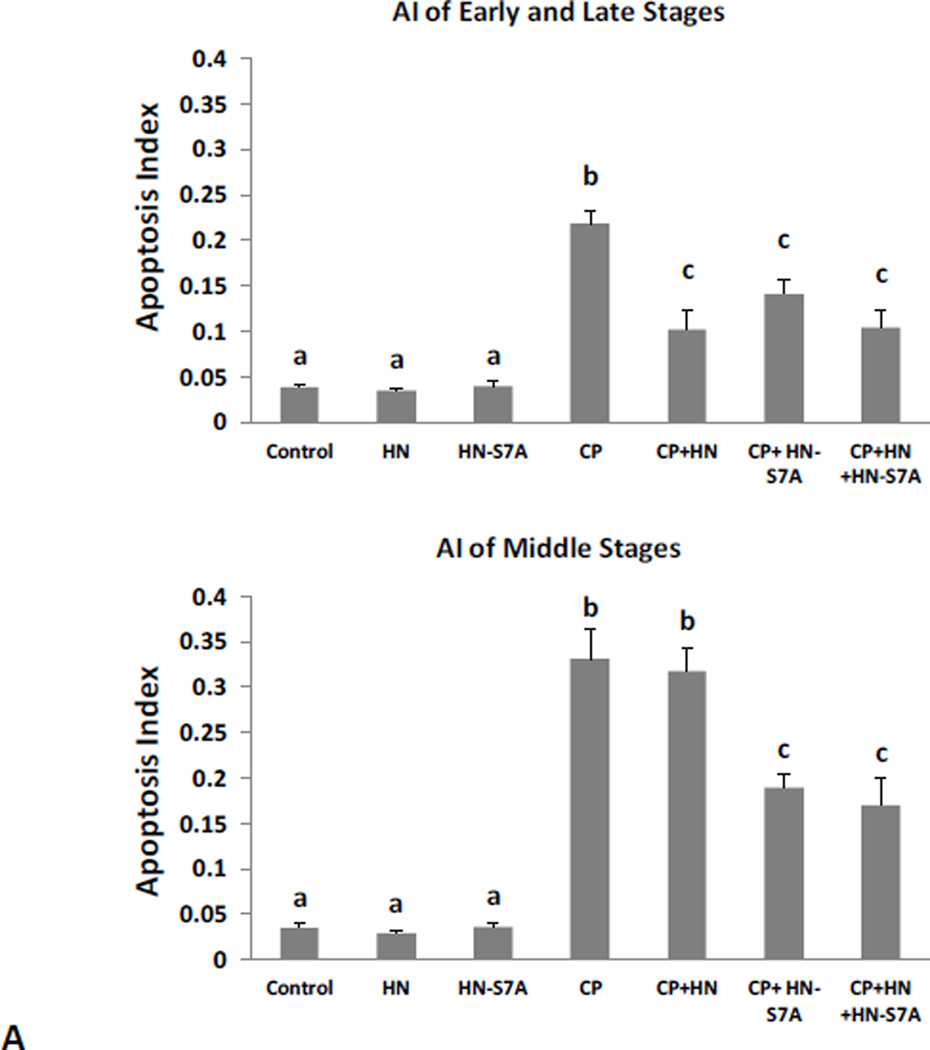
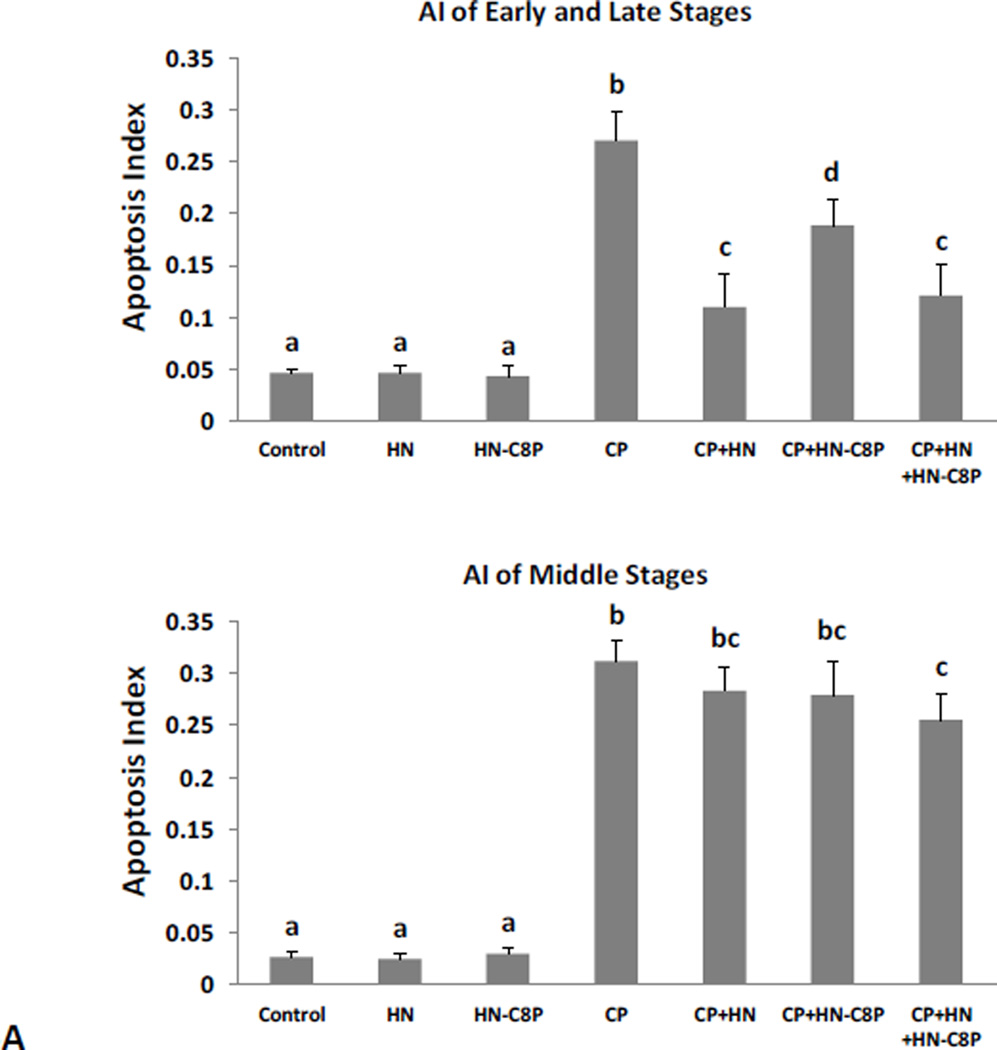
Synthetic HN-S7A or HN-C8P peptide alone did not change the spontaneous germ cell apoptosis. Similar to HN, CP-induced germ cell apoptosis in the early+late stages (I–IV and XI–XII) was inhibited by same dose (40mg/kg BW) of HN-S7A (Fig.4A upper panel, CP+HN: 0.10±0.02 vs CP+HN-S7A: 0.14±0.01, both p<0.05 when compared with CP group: 0.22±0.01). But in middle stages (VII–VIII), HN-S7A had better protective effect than HN (Fig.4A bottom panel, CP: 0.33±0.03, CP+HN: 0.32±0.03, CP+HN-S7A: 0.19±0.02, CP Vs CP+HN-S7A p<0.05). Adding HN to HN-S7A+CP had no additional effect both in early+late and middle stages (Fig.4A). HN-C8P on its own had no effect on germ cell apoptosis and showed less protection against CP-induced apoptosis (Fig.5A upper panel, HN-C8P ~30% protection, vs ~60% in HN, p<0.05), and no significant effect in middle (VII–VIII) stages which was similar to HN (Fig.5A bottom panel). Adding HN to HN-C8P+CP showed decreased CP-induced apoptosis as CP+HN both in early+late (I–IV and XI–XII) and middle stages (VII-VIII) (Fig.5A).
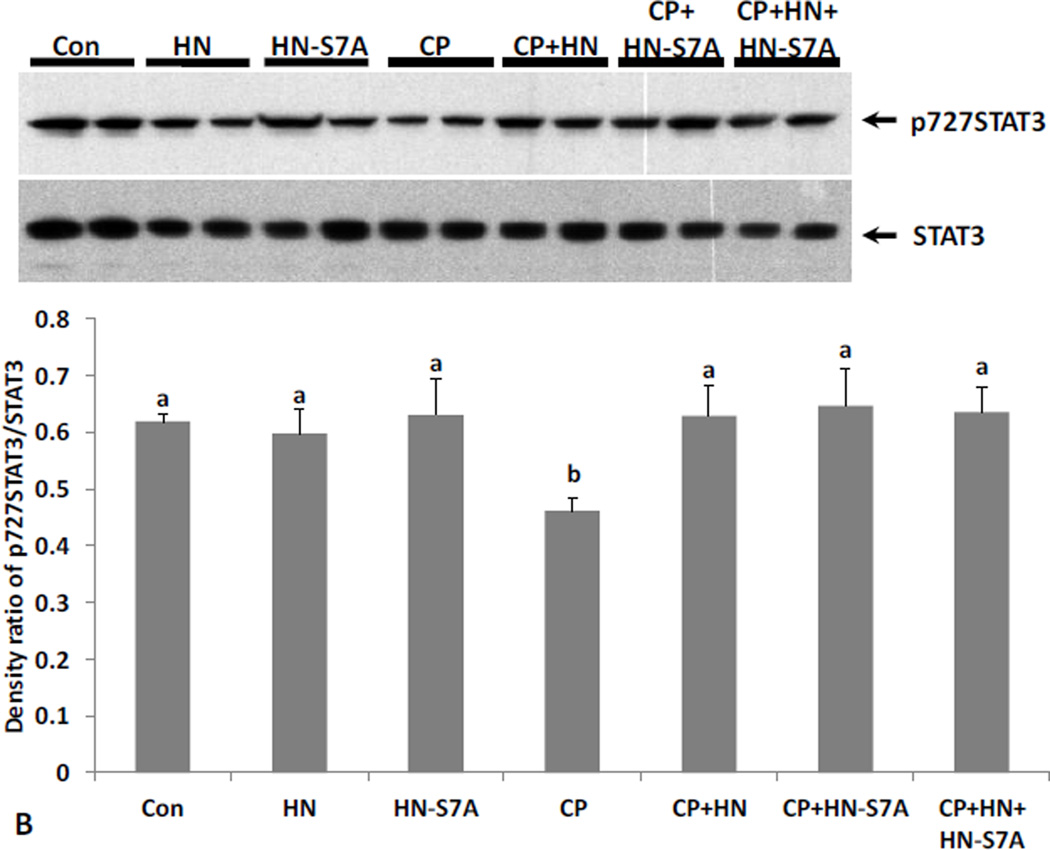
FIG. 4. Effect of HN and HN-S7A on Cyclophosphamide (CP)-induced male germ cell apoptosis and changes of STAT3 phosphorylation.
Mice were treated with vehicle (Control), HN, HN-S7A, CP, CP+HN, CP+HN-S7A, or CP+HN+HN-S7A as described in experimental procedures (n=5 each group). (A): Apoptotic cell numbers were determined by TUNEL staining method. HN or HN-S7A alone did not change apoptosis compared with control. Compared with control, CP increased germ cell apoptosis both in early+late (I–IV and XI–XII) and middle (VII–VIII) stages. HN significantly suppressed CP-induced apoptosis in early+late stages but not in middle stages. HN-S7A prevented CP-induce apoptosis both in early+late and middle stages remarkably. Addition of HN-S7A to HN did not change the effect of HN on CP-induced apoptosis. Values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different. (B): Change of STAT3 phosphorylation levels were detected by western blot and presented by density ratio of phosphorylated STAT3/STAT3. HN or HN-S7A alone did not change STAT3 phosphorylation compared with control. Compared with control, CP suppressed STAT3 phosphorylation. CP+HN, CP+HN-S7A, or CP+HN+HN-S7A all restored CP suppressed STAT3 phosphorylation. Density values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different.
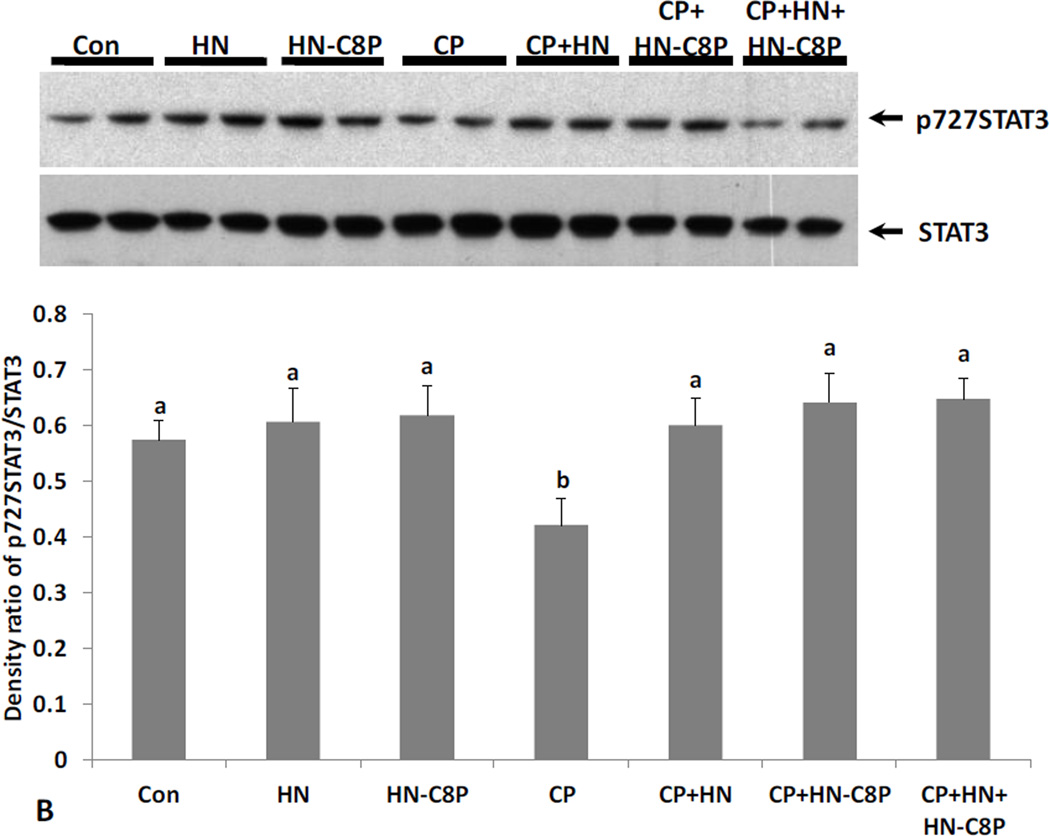
FIG. 5. Effect of HN and HN-C8P on Cyclophosphamide-induced male germ cell apoptosis and changes of STAT3 phosphorylation.
Mice were treated with vehicle (Control), HN, HN-C8P, CP, CP+HN, CP+HN-C8P or CP+HN+HN-C8P as described in experimental procedures (n=5 each group). (A): Apoptotic cell numbers were determined by TUNEL staining method. HN or HN-C8P alone did not change apoptosis compared with control. Compared with control, CP induced massive germ cell apoptosis both in early+late (I–IV and XI–XII) and middle (VII–VIII) stages. CP+HN significantly suppressed CP-induced apoptosis in early + late stages but not in middle stages. CP+HN-C8P showed weaker preventive effect against CP-induce apoptosis in early+late (p<0.05, compared with CP+HN) but no protective effect in middle stages. Addition of HN-C8P did not change the preventive effect of HN on CP-induced germ cell apoptosis. Values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different. (B): Changes of STAT3 phosphorylation levels were detected by western blot and presented by density ratio of phosphorylated STAT3/STAT3. HN or HN-C8P alone did not change STAT3 phosphorylation compared with control. Compared with control, CP suppressed STAT3 phosphorylation. CP+HN, CP+HN-C8P, or CP+HN+HN-C8P all restored CP suppressed STAT3 phosphorylation. Density values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different.
Phosphorylation of Ser727 and Tyr705 leads to activation of STAT3. Immuno-blot analyses on testis homogenates showed that STAT3 phosphorylation had no significant change with HN or either HN analogue treatment under basal conditions in mice (Fig.4B and 5B). CP treatment suppressed Ser727-phosphorylated STAT3 in testes (p<0.05, Fig.4B and 5B). HN, HN-S7A, and HN-C8P treatment restored CP-induced Ser 727-phosphorylation of STAT3 (all p<0.05) (Fig.4B and 5B). Adding HN to the combination of CP+HN-S7A or CP+HN-C8P did not further enhance STAT3 phosphorylation.
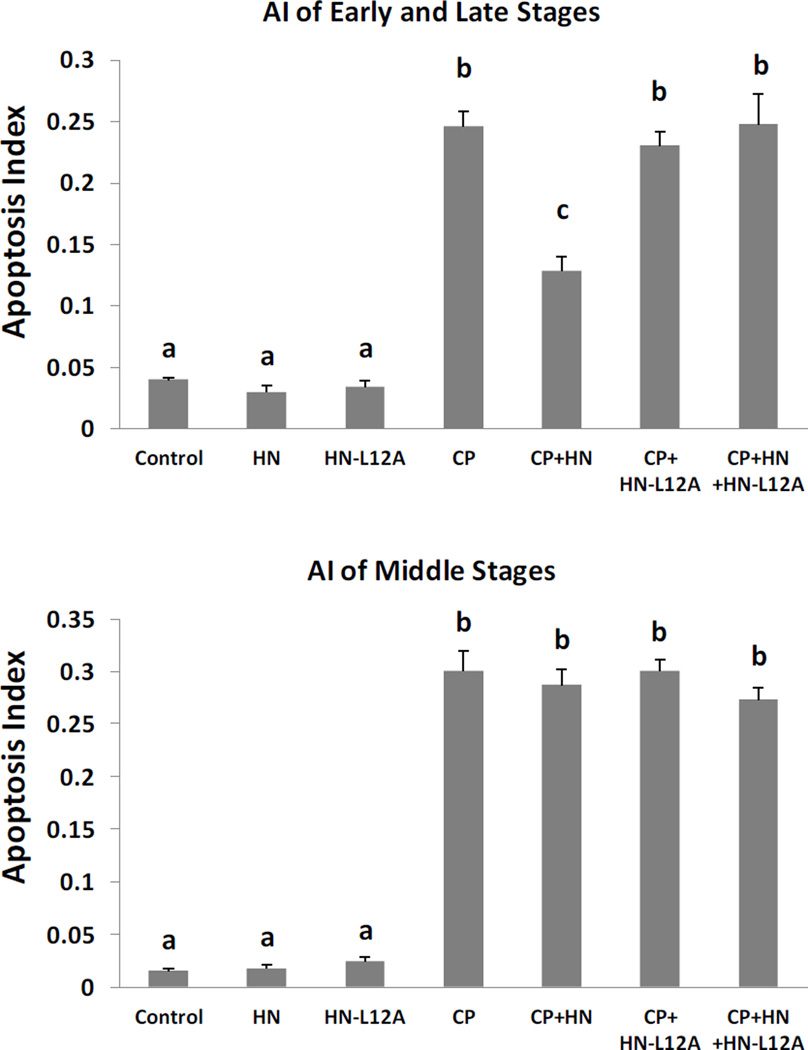
Effects of HN Antagonist, HN-L12A, on CP induced male germ cell apoptosis
Synthetic HN analogue HN-L12A alone did not change the spontaneous germ cell apoptosis. CP-induced germ cell apoptosis was significantly (~57%) inhibited by HN administration (40mg/kg BW) in early+late stages (I–IV and XI–XII) (Fig.6 upper panel, AI of CP+HN: 0.13±0.01, p<0.05 compared with AI of CP group: 0.25±0.01), but not in middle stages (VII–VIII). HN-L12A was unable to rescue the CP-induced germ cell apoptosis either in middle (VII–VIII) or early+late stages (I–IV and XI–XII) (Fig.6). Adding HN-L12A at the same dose as HN (40 mg/kg BW) completely blocked the preventive effect of HN against CP-induced germ cell apoptosis in early+late stages (I–IV and XI–XII) (Fig.6, CP: 0.25±0.01, CP+HN: 0.13±0.01, CP+HN+HN-L12A: 0.25±0.03). We were not able to show the blocking effect of HN-L12A on HN action in middle stages (VII–VIII) because HN and HN-L12A had no effect of apoptosis in the middle stages.
FIG. 6. Effect of HN and HN-L12A on Cyclophosphamide-induced male germ cell apoptosis.
Mice were treated with vehicle (Control), HN, HN-L12A, CP, CP+HN, CP+HN-L12A, or CP+HN+HN-L12A as described in experimental procedures (n=5 each group). Apoptotic cell numbers were determined by TUNEL staining method. HN or HN-L12A alone did not change apoptosis compared with control. Compared with control, CP increased germ cell apoptosis both in early+late (I–IV and XI–XII) and middle (VII–VIII) stages. CP+HN significantly suppressed CP-induced apoptosis in early + late stages but not in middle stages. CP+HN-L12A showed no preventive effect against CP-induced apoptosis in neither early+late or middle stages. CP+HN’s preventive effect was completely blocked by CP+HN+HN-L12A. Values are means ± SEM. Means with unlike superscripts are significantly (P<0.05) different.
DISCUSSION
Infertility and sub-fertility are among the most common long-term side effects of chemotherapy. Studies in men and rodents show that chemotherapy can result in decreased sperm count and reproductive capacity [26,27,39–41]. Increased survival with modern chemotherapeutics has elevated the expectation of fertility preservation in cancer survivors [28]. CP and DOX are two drugs which are commonly used in the treatment of cancers, either alone or in combination with other chemotherapeutic agents. These medications induce cancer cell apoptosis but also damage testicular germ cells [25]. By treating cultured mouse seminiferous tubule with DOX, we showed that HN significantly reduced doxorubicin induced germ cell apoptosis ex vivo. CP has been used in well characterized animal models to study the adverse effects on male germ cells [42]. We showed that HN significantly reduced CP-induced germ cell apoptosis in mice mainly in the early and late stages of the seminiferous tubule epithelium. Thus, HN prevents chemotherapeutic agents-induced male germ cell apoptosis.
Recently, a putative trimetric receptor (with three components: CNTFRα, IL-27 receptor WSX-1, and gp130) was found on the neuronal cell membrane that mediates the neuro-protective activity of HN via the STAT3 pathway [19–21]. Our unpublished data demonstrated that WSX-1 and gp130 (but not CNTFRα) and downstream activation of STAT3 is critical for HN-induced anti-apoptotic effects in male germ cells. In this study, we found that CP-induced male germ cell apoptosis in vivo by reducing pSTAT3 in the testis and HN restored the CP-suppressed STAT3 phosphorylation. This is consistent with our previous finding that HN reduced heat or GnRH-A-induced apoptosis by reversing the heat or GnRH-A induced suppression of STAT3 phosphorylation [12]. These data support that the STAT3 phosphorylation pathway is one of the mechanisms for anti-apoptotic effect of HN in male germ cells. The ubiquitous expression of STAT3 in the testis (data not shown) suggests that the action of HN might not only target germ cells. More studies are required to explore whether HN via STAT3 will act on Sertoli cells, Leydig cells or other cell types to exert its cytoprotective effect on germ cells.
In present study, we explored the mechanisms of the protective effect of HN by using different HN analogues. HN has 24 amino acids and one amino acid change modifies its function [33]. For example, HNG (change of the 14th amino acid from serine to glycine, HN-S14G) is more potent than HN in preventing cell damage [31–33]. Using 1/8 the dose of HN by injection, HNG showed similar cytoprotective effect against CP-induced germ cell apoptosis as HN, indicating that HNG is more potent than HN in vivo. This finding is consistent with the notion that HNG has greater protective effects on neuronal cells or tissues than HN. Double amino-acid substituted analogue HNG-F6A does not bind IGFBP-3 [23]. The present study showed HNG-F6A and HNG had similar protective effects against CP-induced germ cell apoptosis at equivalent doses, indicating that the cytoprotective effects of HN and its more potent analogues on male germ cells is unrelated to its binding to IGFBP3. This is similar to analogous experiments showing that HNG-F6A and HNG increases glucose stimulated insulin secretion to a similar extent in both isolated islets/cell culture and whole animals [43].
The sequence motif from Pro3 to Pro19 forms the core domain of the 24 amino acid sequence of HN. Within the core domain, each amino acid, Pro3, Cys8, Leu9, Leu12, Thr13, Ser14, and Pro19, but not Ser7, have been found to be essential for the neuro-protective function of HN [1,44]. HN-S7A is a dimerization-defective mutant, i.e. HN-S7A does not self-dimerize and does not dimerize with HN. HN-S7A has no cytoprotective effect in neuronal cells in vitro indicating that the self-dimerization process is essential for HN’s neuro-protective function culture [34]. In our in vivo experiment, HN-S7A was effective in preventing CP-induced germ cell apoptosis but did not affect the ability of HN to protect CP-induced male germ cells apoptosis suggesting that the self-dimerization process may not be critical for HN’s cytoprotective effect in testis in vivo. Thus the loss of the cytoprotective effect of HN-S7A in neuron cell culture experiments were not replicated in vivo mouse experiments. The action of HN-S7A may be different in vitro versus in vivo where interactions with HN-S7A may enhance its activity. Three components (CNTFRα, WSX-1, and gp130) of a humanin receptor were found on the neuronal cell membrane that mediates the neuro-protective activity of HN. Our unpublished work demonstrated that only WSX-1 and gp130 (but not CNTFRα) are critical for HN-induced anti-apoptotic effects in male germ cells (data not shown). The assembly of different subunits of membrane receptor of HN in testis may be one of the reasons that self-dimerization may not be necessary for HN’s effect in testis as that in neuron. We also found that HN-S7A suppressed CP-induced male germ cell apoptosis both in early+late stages (I–IV and XI–XII) as well as middle stages (VII–VIII), while HN only suppressed CP-induced apoptosis in early+late but not in middle stages. This suggests that HN-S7A may have additional mechanism for protecting male germ cells which require further identification. Western blot analyses showed both HN and HN-S7A restored the CP-suppressed STAT3 phosphorylation supporting that the mechanism of the cyto-protective effects of HN and HN-S7A is mediated through the IL6-like trimeric membrane receptor.
Cysteine at position 8 is an amino acid essential for HN’s neuro-protective function [1,22,44–48]. HN-C8P does not bind with BAX and shows no anti-apoptotic effects in cell culture [22,34]. Our experiments showed that in vivo HN-C8P partially prevented CP-induced germ cell apoptosis but was less potent than HN. This finding indicates the intracellular binding to BAX may play a role, but not a major part, in protective effect of HN in CP-induced germ cell death which is consistent to our previous observations [12]. Western blot showed that HN-C8P restored the CP-suppressed STAT3 phosphorylation which suggests but does not prove that the mechanism of HN-C8P’s protective effect may be through membrane receptor.
The sequence motif from Leucine 9 to Leucine 12 plays a functional role as a hydrophobic core of the HN peptide [1,34,44]. Although replacement of Leu12 with Alanine does not attenuate HN’s secretory activity, HN-L12A lacks the ability to bind with membrane receptor and has no anti-apoptotic effect in cell culture [34]. HN-L12A dimerizes with HN prevents its binding to the receptors acting as a HN antagonist. In the present study, HN-L12A did not prevent CP-induced germ cell apoptosis but completely blocked the cytoprotective effect of HN against CP-induced male germ cell apoptosis at similar dose of HN. These results indicated that membrane receptor binding ability is important for HN and HN analogues cytoprotective activity. Published data from other investigators showed that HN-S7A and HNC8P had no cytoprotective effect in vitro [1,34,44], while we found that these two HN analogues partially protected male germ cell from CP-induced apoptosis in vivo. The contrasting effects of HN analogues (such as HN-S7A and HN-C8P) in vitro and in vivo suggest that HN and HN analogues may be modified in vivo or may act through different pathways or systems (e.g. IGF-1 pathway, [49]) instead of directly affecting the target cells or organs. Arakawa et al studied the biological activity and structural stabilities of HN analogues. Theses investigators also concluded that different biological activities of HN analogues could not be explained only by one simple factor (e.g. the structure stabilities) [48]. Our published data showed that HN prevents male germ cell apoptosis induced by different stress mainly by binding to the putative membrane receptor and STAT3 pathway but it is known that HN also binds extra-cellularly to IGFBP-3 [32,50] or intra-cellularly to BAX [22,46,50], we conclude that mechanism of HN’s anti-apoptotic effect in male germ cell is complex and may act through multiple pathways including in vivo modification of HN and its analogues. Understanding of the mechanisms of HN action may help to design specific targets that may be used for drug development, e.g. agonists that protect male germ cells from stress-induced death (e.g. testicular hyperthermia, hormonal deprivation, or chemotherapy). In present study, the germ cells undergoing apoptosis were mainly pachytene spermatocytes and round spermatids. We did not observe apoptotic spermatogonia likely due to the short duration of exposure to CP (12 hours ex vivo and 24 hours in vivo). Male germ cells at different stages of the seminiferous epithelium have different mechanisms regulating the balance between apoptosis/survival homeostasis. We have shown that germ cells in early+late stages in acute experiments are more susceptible to heat stress than those middle stage germ cells; while middle stages germ cell apoptosis could be induced by testosterone deprivation [14–16]. In the present study, HN showed preventive effects against CP induced apoptosis in early+late but not in middle stages most likely because of the protective effect of intratesticular testosterone present in the middle stages. If we examined the testis after longer exposure to CP we expect that the early+late stage apoptosis will progress to involve middle stage germ cells.
In summary, we have demonstrated that 1) HN significantly prevents male germ cell apoptosis induced by chemotherapeutic agents both ex vivo and in vivo; 2) the anti-apoptotic effect of HN, HN-S7A, and HN-C8P on CP-induced male germ cell apoptosis is mainly mediated through the cell membrane receptor and downstream STAT3 pathway; 3) lack of IGFBP-3 binding ability does not neutralize HNG’s cytoprotective effect in testis; 4) self-dimerization may not be necessary for HN preventing CP-induced germ cell apoptosis in vivo; 5) BAX-binding pathway may also play a role in HN’s protective effect; 6) HN-L12A is an antagonist to HN preventing its cytoprotective effect in germ cell apoptosis in vivo. HN may be an important molecule in the regulation of germ cell homeostasis and may have roles in male infertility and prevention of chemotherapy-induced onco-infertility [51].
ACKNOWLEDGEMENTS
This study supported by the Endocrinology, Metabolism and Nutrition training Grant (T32 DK007571) and UCLA Clinical and Translational Science Institute (UL1TR000124) at Los Angeles Biomedical Research Institute and Harbor-UCLA Medical Center and Grants to P.C. (1R01AG034430, 1R01GM090311, P01AG034906 and 1R01ES020812). We thank Vince Atienza for technical assistance.
Footnotes
The authors disclose no potential conflicts of interest.
REFERENCE
- 1.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Aβ. Proc Natl Acad Sci USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuoka M. Humanin: a defender against Alzheimer’s disease? Recent Pat CNS Drug Discov. 2009;4:37–42. doi: 10.2174/157488909787002609. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 4.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, Nemkal A, Atzmon G, Klein L, Gundewar S, Ji SY, Lavu M, Predmore BL, Lefer DJ. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson E, Wickström M, Perup LS, Johnsen JI, Eksborg S, Kogner P, Sävendahl L. Protective role of humanin on bortezomib-induced bone growth impairment in anticancer treatment. J Natl Cancer Inst. 2014;106(3):djt459. doi: 10.1093/jnci/djt459. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Li H, Yuan H, Zheng M, Bai C, Chen L, Pei X. Humanin delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–971. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- 8.Ying G, Iribarren P, Zhou Y, Gong W, Zhang N, Yu ZX, Le Y, Cui Y, Wang JM. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- 9.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4(7):e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P. The neurosurvival factor humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, Jia Y, Hwang D, Cobb LJ, Cohen P, Wang C. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- 12.Jia Y, Lue YH, Swerdloff R, Lee KW, Cobb LJ, Cohen P, Wang C. The cytoprotective peptide humanin is induced and neutralizes Bax after pro-apoptotic stress in the rat testis. Andrology. 2013;1(4):651–659. doi: 10.1111/j.2047-2927.2013.00091.x. Epub 2013 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1995;136:2770–2775. doi: 10.1210/endo.136.6.7750502. [DOI] [PubMed] [Google Scholar]
- 14.Lue YH, Sinha Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone (T) on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- 15.Lue Y, Wang C, Liu YX, Hikim AP, Zhang XS, Ng CM, Hu ZY, Li YC, Leung A, Swerdloff RS. Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2006;91:539–545. doi: 10.1210/jc.2005-1808. [DOI] [PubMed] [Google Scholar]
- 16.Jia Y, Sinha Hikim AP, Swerdloff RS, Lue YH, Vera Y, Zhang XS, Hu ZY, Li YC, Liu YX, Wang C. Signaling pathways for germ cell death in adult Cynomolgus monkeys (Macaca fascicularis) induced by mild testicular hyperthermia and exogenous testosterone treatment. Biol Reprod. 2007;77:83–92. doi: 10.1095/biolreprod.106.058594. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Cui YG, Wang XH, Jia Y, Sinha HA, Lue YH, Tong JS, Qian LX, Sha JH, Zhou ZM, Hull L, Leung A, Swerdloff RS. Transient Scrotal Hyperthermia and Levonorgestrel Enhance Testosterone-Induced Spermatogenesis Suppression in Men through Increased Germ Cell Apoptosis. J Clin Endocrinol Metab. 2007;92:3292–3304. doi: 10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- 18.Sinha Hikim AP, Swerdloff RS. Temporal and stage-specific changes in spermatogenesis of rat after gonadotropin deprivation by a potent gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1993;133:2161–2170. doi: 10.1210/endo.133.5.8404667. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka M, Hashimoto Y. Humanin and the receptors for humanin. Mol Neurobiol. 2010;41:22–28. doi: 10.1007/s12035-009-8090-z. [DOI] [PubMed] [Google Scholar]
- 22.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 23.Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer's survival peptide Humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terashita K, Hashimoto Y, Niikura T, Tajima H, Yamagishi Y, Ishizaka M, Kawasumi M, Chiba T, Kanekura K, Yamada M, Nawa M, Kita Y, Aiso S, Nishimoto I. Two serine residues distinctly regulate the rescue function of Humanin, an inhibiting factor of Alzheimer's disease-related neurotoxicity: functional potentiation by isomerization and dimerization. J Neurochem. 2003;85(6):1521–1538. doi: 10.1046/j.1471-4159.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto Y, Terashita K, Niikura T, Yamagishi Y, Ishizaka M, Kanekura K, Chiba T, Yamada M, Kita Y, Aiso S, Matsuoka M, Nishimoto I. Humanin antagonists: mutants that interfere with dimerization inhibit neuroprotection by Humanin. Eur J Neurosci. 2004;19:2356–2364. doi: 10.1111/j.0953-816X.2004.03298.x. [DOI] [PubMed] [Google Scholar]
- 26.Marcon L, Hales BF, Robaire B. Reversibility of the effects of subchronic exposure to the cancer chemotherapeutics bleomycin, etoposide, and cisplatin on spermatogenesis, fertility, and progeny outcome in the male rat. J Andrology. 2008;29:4. doi: 10.2164/jandrol.107.004218. [DOI] [PubMed] [Google Scholar]
- 27.Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer. 2009;53:261–266. doi: 10.1002/pbc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SH, Shin CH. Reduced male fertility in childhood cancer survivors. Ann Pediatr Endocrinol Metab. 2013;18(4):168–712. doi: 10.6065/apem.2013.18.4.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watring WG, Byfield JE, Lagasse LD, Lee YD, Juillard G, Jacobs M, Smith ML. Combination Adriamycin and radiation therapy in gynecologic cancers. Gynecol Oncol. 1974;2(4):518–526. doi: 10.1016/0090-8258(74)90062-6. [DOI] [PubMed] [Google Scholar]
- 30.Gor PP, Su HI, Gray RJ, Gimotty PA, Horn M, Aplenc R, Vaughan WP, Tallman MS, Rebbeck TR, DeMichele A. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast cancer research. 2010;12:R26. doi: 10.1186/bcr2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunesová G, Hlavácek J, Patocka J, Evangelou A, Zikos C, Benaki D, Paravatou-Petsotas M, Pelecanou M, Livaniou E, Slaninova J. The multiple T-maze in vivo testing of the neuroprotective effect of humanin analogues. Peptides. 2008;29:1982–1987. doi: 10.1016/j.peptides.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Miao J, Zhang W, Yin R, Liu R, Su C, Lei G, Li Z. S14G HN ameliorates Abeta25–35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42:557–567. doi: 10.1016/j.npep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Yamada M, Chiba T, Sasabe J, Terashita K, Aiso S, Matsuoka M. Nasal colivelin treatment ameliorates memory impairment related to Alzheimer’s disease. Neuropsychopharmaco. 2008;33:2020–2032. doi: 10.1038/sj.npp.1301591. [DOI] [PubMed] [Google Scholar]
- 34.Yamagishi Y, Hashimoto Y, Niikura T, Nishimoto I. Identification of essential amino acids in Humanin, a neuroprotective factor against Alzheimer's disease-relevant insults. Peptides. 2003;24(4):585–595. doi: 10.1016/s0196-9781(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 35.Erkkila K, Henriksen K, Hirvonen V, Rannikko S, Salo J, Parvinen M, Dunkel L. Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J Clin Endocrinol Metab. 1997;82:2314–2321. doi: 10.1210/jcem.82.7.4044. [DOI] [PubMed] [Google Scholar]
- 36.Jia Y, Castellanos J, Wang C, Sinha-Hikim I, Lue Y, Swerdloff RS, Sinha-Hikim AP. Mitogen-Activated Protein Kinase Signaling in Male Germ Cell Apoptosis in the Rat. Biol.Reprod. 2009;80:771–780. doi: 10.1095/biolreprod.108.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha Hikim AP, Rajavashisth TB, Sinha Hikim I, Lue Y, Bonavera JJ, Leung A, Wang C, Swerdloff RS. Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod. 1997;57:1193–1201. doi: 10.1095/biolreprod57.5.1193. [DOI] [PubMed] [Google Scholar]
- 38.Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testes. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 39.Delbes G, Vaisheva F, Luu T, Marcon L, Hales BF, Robaire B. Reversibility of the effects of the chemotherapeutic regimen for non-Hodgkin lymphoma, cyclophosphamide, doxorubicin, vincristine, and prednisone, on the male rat reproductive system and progeny outcome. Reproductive Toxicology. 2010;29:332–338. doi: 10.1016/j.reprotox.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Dohle GR. Male infertility in cancer patients: Review of the literature. Int J Urol. 2010;17(4):327–331. doi: 10.1111/j.1442-2042.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- 41.Trost LW, Brannigan RE. Oncofertility and the male cancer patient. Curr Treat Options Oncol. 2012;13(2):146–160. doi: 10.1007/s11864-012-0191-7. [DOI] [PubMed] [Google Scholar]
- 42.Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56(6):1490–1497. doi: 10.1095/biolreprod56.6.1490. [DOI] [PubMed] [Google Scholar]
- 43.Kuliawat R1, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, Bastie C, Su K, Huffman D, Surana M, Barzilai N, Fleischer N, Muzumdar R. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J. 2013;27(12):4890–4898. doi: 10.1096/fj.13-231092. Epub 2013 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J Neurosci. 2001;21(23):9235–9245. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T. Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Mol Cell Neurosci. 2004;25(1):95–102. doi: 10.1016/j.mcn.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- 47.Arakawa T, Kita Y, Niikura T. A rescue factor for Alzheimer's diseases: discovery, activity, structure, and mechanism. Curr Med Chem. 2008;15(21):2086–2098. doi: 10.2174/092986708785747616. [DOI] [PubMed] [Google Scholar]
- 48.Arakawa T1, Niikura T, Kita Y. The biological activity of Humanin analogs correlates with structure stabilities in solution. Int J Biol Macromol. 2011;49(1):93–97. doi: 10.1016/j.ijbiomac.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50(1):R11–R19. doi: 10.1530/JME-12-0203. Print 2013 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia Y, Lee KW, Swerdloff R, Hwang D, Cobb LJ, Sinha Hikim A, Lue YH, Cohen P, Wang C. Interaction of insulin-like growth factor-binding protein-3 and BAX in mitochondria promotes male germ cell apoptosis. J Biol Chem. 2010;285:1726–1732. doi: 10.1074/jbc.M109.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen P. New Role for the Mitochondrial Peptide Humanin: Protective Agent Against Chemotherapy-Induced Side Effects. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju006. dju006. [DOI] [PMC free article] [PubMed] [Google Scholar]