Abstract
DNMT3B encodes a DNA methyltransferase implicated in aberrant epigenetic changes contributing to leukemogenesis. We tested whether DNMT3B expression, measured by NanoString nCounter assay, associates with outcome, gene- and microRNA-expression and DNA methylation profiles in 210 older (≥60 years) adults with primary, cytogenetically normal AML (CN-AML). Patients were dichotomized into high versus low expressers using median cut. Outcomes were assessed in the context of known CN-AML prognosticators. Gene- and microRNA-expression, and DNA methylation profiles were analyzed using microarrays and MethylCap-sequencing, respectively. High DNMT3B expressers had fewer complete remissions (CR; P=0.002) and shorter disease-free (DFS; P=0.02) and overall (OS; P<0.001) survival. In multivariable analyses, high DNMT3B expression remained an independent predictor of lower CR rates (P=0.04) and shorter DFS (P=0.04) and OS (P=0.001). High DNMT3B expression associated with a gene-expression profile comprising 363 genes involved in differentiation, proliferation and survival pathways, but with only 4 differentially expressed microRNAs (miR-133b, miR-148a, miR-122, miR-409-3p) and no differential DNA methylation regions. We conclude that high DNMT3B expression independently associates with adverse outcome in older CN-AML patients. Gene-expression analyses suggest that DNMT3B is involved in the modulation of several genes, although the regulatory mechanisms remain to be investigated to devise therapeutic approaches specific for these patients.
Keywords: acute myeloid leukemia, DNMT3B expression, prognostication, gene-expression profiling
INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous disease presenting with a wide spectrum of prognostically relevant cytogenetic aberrations, gene mutations and abnormal expression of genes and microRNAs. Cytogenetically normal AML (CN-AML) patients, constituting 40 to 50% of all AML patients,1 are the largest and molecularly best characterized cytogenetic subset in primary (de novo) AML.1–3 Although leukemic blasts of these patients do not contain microscopically detectable chromosome abnormalities, they harbor prognostically relevant mutations and aberrantly expressed genes and microRNAs.2–16 In addition to these genetic alterations, epigenetic changes have recently been shown to participate in myeloid leukemogenesis and be pharmacologically targetable.17,18 Notably, some genes whose mutations are prognostic in CN-AML encode proteins that are implicated in epigenetic regulation of gene transcription, namely IDH2, ASXL1 and DNMT3A. The latter is among the most frequently mutated genes in primary CN-AML patients, being found mutated in 29 to 34% of the patients.9,19
DNMT3A encodes DNA methyltransferase 3A (DNMT3A), which is involved in epigenetic gene silencing through DNA hypermethylation.20 In addition to DNMT3A, DNMT1 and DNMT3B also mediate DNA methylation in normal and malignant cells, and may represent potential therapeutic targets in cancer and leukemia.21–24 However, in contrast to DNMT3A, no recurrent mutations of DNMT1 and DNMT3B genes have been reported in AML.25 Instead, one study has indicated that higher expression of DNMT3B is associated with worse outcome in AML.26 However, the patient cohort analyzed was cytogenetically diverse and heterogeneous for clinical features and treatment received. Thus, it is unknown whether DNMT3B expression is an independent prognostic factor and can be used for stratification guidance in CN-AML.
Thus, we analyzed the clinical significance of DNMT3B expression in the context of a comprehensive panel of molecular prognosticators in a relatively large cohort of older (aged ≥60 years) patients with CN-AML who were similarly treated on cytarabine/daunorubicin-based protocols. To gain biologic insights, we also derived genome-wide DNMT3B-associated gene- and microRNA-expression and DNA methylation profiles. We studied older patients because both the incidence of AML and the role of epigenetics increase with age. Moreover, we have recently reported a favorable clinical response to hypomethylating agents in this age group of AML patients.27
PATIENTS AND METHODS
Patients, treatment and cytogenetic studies
Pretreatment bone marrow (BM) or blood samples were obtained from 210 patients with primary CN-AML aged 60 to 83 years (median, 68 years) who received intensive first-line therapy on Cancer and Leukemia Group B (CALGB) trials.28–32 All patients received cytarabine-daunorubicin-based induction chemotherapy, and no patient received allogeneic hematopoietic stem cell transplantation (HSCT) during first complete remission (CR). For details regarding treatment protocols and sample collection, see Supplementary Information. All patients were enrolled on companion CALGB/Alliance protocols: 8461 (cytogenetic analyses), 9665 (tissue banking) and 20202 (molecular analyses).
Cytogenetic analyses were performed in institutional CALGB/Alliance cytogenetics laboratories. For the patient’s karyotype to be considered normal, ≥20 metaphase cells from short-term cultures of pretreatment BM specimens had to have been analyzed and the normal result confirmed by central karyotype review.33 All patients provided written informed consent for participation in these studies; study protocols were in accordance with the Declaration of Helsinki and approved by local Institutional Review Boards.
Single-gene expression analyses
The expression of DNMT3B transcript was assessed by NanoString nCounter assays (NanoString Technologies, Seattle, WA, USA; Supplementary Information).34 These assays measured global expression of the DNMT3B gene, and did not allow for quantification of isoform-specific expression of DNMT3B. DNMT3B expression levels were normalized using ABL as an internal control. We also used NanoString nCounter assays to measure expression of BAALC, ERG and miR-155, and real-time RT-PCR to measure miR-3151 expression, all of which have been previously shown to affect prognosis of older CN-AML patients.10,15,16
Mutational analyses
The presence or absence of FLT3 internal tandem duplication (FLT3-ITD),35,36 FLT3 tyrosine kinase domain mutations (FLT3-TKD),37 MLL partial tandem duplication (MLL-PTD),38 and mutations in the NPM1,5 CEBPA,39 WT1,40 IDH1 and IDH2,7 TET2,41 ASXL1,8 DNMT3A9 and RUNX142 genes were determined centrally as previously described.
Gene- and microRNA-expression profiling
The gene- and microRNA-expression profiling were assessed using the Affymetrix U133 plus 2.0 array (Affymetrix, Santa Clara, CA) and The Ohio State University (OSU) custom microRNA array (OSU_CCC Version 4.0), respectively, as previously reported,5,43 and detailed in the Supplementary Information. For DNMT3B, the Affymetrix U133 plus 2.0 arrays measured global DNMT3B expression levels, and did not quantify expression of the individual DNMT3B isoforms. For the gene- and microRNA-expression profiling, summary measures of gene and microRNA expression were computed, normalized, and filtered (Supplementary Information). A DNMT3B expression-associated signature (see Supplementary Information for details) was derived by comparing gene expression between high and low DNMT3B expressers in the Alliance cohort and in two additional sets of CN-AML patients with microarray and RNAseq gene expression data publicly available [German AML Cooperative Group (AMLCG)44 and The Cancer Genome Atlas (TCGA)25]. For comparison of the high DNMT3B expression signature with the FLT3-ITD signature we used gene set enrichment analysis (GSEA; for details see Supplementary Information). For the microRNA expression signature, only the CALGB/Alliance patients were used. Univariable significance levels of P<0.001 (false discovery rates (FDR) <0.01) were used to select genes and microRNAs that constituted the signatures. To assess enrichment of genes in the DNMT3B gene-expression-associated signature in distinct biologic processes, a Gene Ontology (GO) analysis was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID).45 We identified as statistically significant “annotation clusters” those clusters of GO terms with enrichment scores of >2.0, P-values ≤0.001 and Benjamini corrected P-values ≤0.05. All molecular analyses were performed centrally at OSU.
DNA methylation
Genome-wide DNA methylation and levels of DNA methylation across the genome’s functional regions (i.e., genomic features) were measured using the MethylCap-seq assay as previously reported.18
Statistical analyses
The patients were dichotomized into high and low expressers using the median cut. This cut was supported by significant results of the trend test applied to outcome of patients divided into quartiles by DNMT3B expression (P≤0.001). We compared pretreatment features and outcome between patients with high and low DNMT3B expression. Definitions of clinical endpoints [i.e., CR rates, disease-free (DFS) and overall (OS) survival] are provided in the Supplementary Information. Baseline characteristics between high and low DNMT3B expressers were compared using the Fisher’s exact test for categorical and the Wilcoxon rank-sum test for continuous variables.46 The categorical variables included the European LeukemiaNet (ELN) Genetic Groups.47 The ELN guidelines classify CN-AML patients within the Favorable or Intermediate-I Genetic Groups based on CEBPA, NPM1 and FLT3 mutational status. The ELN Favorable Genetic Group consists of CN-AML patients with CEBPA mutation and/or NPM1 mutation without FLT3-ITD, whereas the Intermediate-I Genetic Group is comprised of patients with wild-type CEBPA and FLT3-ITD with or without NPM1 mutation, or wild-type NPM1 without FLT3-ITD.47
For time-to-event analyses, we calculated survival estimates using the Kaplan-Meier method, and compared groups by the log-rank test.46 In order to provide the odds ratios and hazard ratios and associated confidence intervals, logistic regression and Cox proportional hazards models were generated to compare outcomes between high and low DNMT3B expressers for CR and survival endpoints (DFS, OS), respectively, and P-values from the Wald test are reported. We constructed multivariable logistic regression models to analyze factors associated with the achievement of CR, and multivariable Cox proportional hazards models for factors associated with survival endpoints,46 the details of which are provided in the Supplementary Information. All analyses were performed by the Alliance for Clinical Trials in Oncology Statistics and Data Center.
RESULTS
Associations of DNMT3B expression with pretreatment clinical and molecular characteristics
At diagnosis, high DNMT3B expressers had higher white blood counts (WBC; P=0.004), and percentages of blood (P=0.004) and marrow (P=0.02) blasts than low DNMT3B expressers. Concerning molecular features, high DNMT3B expressers were more often FLT3-ITD-positive (P<0.001) and classified in the ELN Intermediate-I Genetic Group (P=0.02). IDH2-R140 mutations were less frequent in high DNMT3B expressers, whereas six of seven IDH2-R172 mutations were detected in this patient group. High DNMT3B expressers also had higher ERG (P<0.001), BAALC (P=0.002) and miR-155 (P=0.006) expression than low expressers (Table 1, Supplementary Figure S1).
Table 1.
Comparison of clinical and molecular characteristics of patients with cytogenetically normal acute myeloid leukemia with high versus low DNMT3B expression
| Characteristic | High DNMT3B (n=105) |
Low DNMT3B (n=105) |
P |
|---|---|---|---|
| Age, years | 0.82 | ||
| Median | 68 | 68 | |
| Range | 60–83 | 60–81 | |
| Sex, n (%) | 0.58 | ||
| Male | 58 (55) | 53 (50) | |
| Female | 47 (45) | 52 (50) | |
| Race, n (%) | 0.48 | ||
| White | 96 (92) | 92 (89) | |
| Nonwhite | 8 (8) | 11 (11) | |
| Hemoglobin, g/dl | 0.71 | ||
| Median | 9.4 | 9.3 | |
| Range | 6.5–12.4 | 5.4–15.0 | |
| Platelet count, × 109/l | 0.87 | ||
| Median | 68 | 71 | |
| Range | 4–850 | 11–510 | |
| WBC, × 109/l | 0.004 | ||
| Median | 43.7 | 21.8 | |
| Range | 1.0–450.0 | 0.8–249.3 | |
| Blood blasts, % | 0.004 | ||
| Median | 64 | 40 | |
| Range | 0–99 | 0–97 | |
| Bone marrow blasts, % | 0.02 | ||
| Median | 72 | 64 | |
| Range | 21–97 | 4–97 | |
| Extramedullary involvement, n (%) | 27 (27) | 24 (23) | 0.63 |
| NPM1, n (%) | 0.31 | ||
| Mutated | 67 (66) | 60 (59) | |
| Wild-type | 34 (34) | 42 (41) | |
| FLT3-ITD, n (%) | <0.001 | ||
| Present | 54 (53) | 21 (21) | |
| Absent | 48 (47) | 81 (79) | |
| CEBPA, n (%) | 0.83a | ||
| Mutated | 13 (13) | 12 (12) | |
| Single mutated | 10 | 5 | |
| Double mutated | 3 | 7 | |
| Wild-type | 88 (87) | 90 (88) | |
| ELN Genetic Groupb, n (%) | 0.02 | ||
| Modified Favorable | 38 (38) | 56 (55) | |
| Intermediate-I | 63 (62) | 45 (45) | |
| FLT3-TKD, n (%) | 0.83 | ||
| Present | 12 (12) | 11 (11) | |
| Absent | 89 (88) | 91 (89) | |
| WT1, n (%) | 0.41 | ||
| Mutated | 8 (8) | 5 (5) | |
| Wild-type | 93 (92) | 97 (95) | |
| TET2, n (%) | 1.00 | ||
| Mutated | 32 (32) | 31 (32) | |
| Wild-type | 68 (68) | 67 (68) | |
| MLL-PTD, n (%) | 1.00 | ||
| Present | 5 (6) | 5 (6) | |
| Absent | 74 (94) | 81 (94) | |
| IDH1, n (%) | 0.18 | ||
| Mutated | 14 (14) | 8 (8) | |
| Wild-type | 86 (86) | 94 (92) | |
| IDH2, n (%) | 0.05 | ||
| Mutated | 18 (18) | 31 (30) | |
| R140 | 12 | 30 | 0.005c |
| R172 | 6 | 1 | 0.13d |
| Wild-type | 82 (82) | 71 (70) | |
| RUNX1, n (%) | 0.31 | ||
| Mutated | 17 (18) | 11 (12) | |
| Wild-type | 79 (82) | 81 (88) | |
| ASXL1, n (%) | 0.69 | ||
| Mutated | 13 (13) | 15 (15) | |
| Wild-type | 87 (87) | 84 (85) | |
| DNMT3A | 0.65 | ||
| Mutated | 35 (35) | 31 (32) | |
| R882 | 19 | 20 | 1.00e |
| Non-R882 | 16 | 11 | 0.40f |
| Wild-type | 64 (65) | 66 (68) | |
| ERG expression group,g,h n (%) | <0.001 | ||
| High | 65 (62) | 40 (38) | |
| Low | 40 (38) | 65 (62) | |
| BAALC expression group,g,h n (%) | 0.002 | ||
| High | 64 (61) | 41 (39) | |
| Low | 41 (39) | 64 (61) | |
| miR-155 expression group,g,h n (%) | 0.006 | ||
| High | 63 (60) | 42 (40) | |
| Low | 42 (40) | 63 (60) | |
| miR-3151 expression group,g,i n (%) | 0.76 | ||
| High | 42 (49) | 39 (46) | |
| Low | 43 (51) | 46 (54) | |
Abbreviations: ELN, European LeukemiaNet; FLT3-ITD, internal tandem duplication of the FLT3 gene; FLT3-TKD, tyrosine kinase domain mutation in the FLT3 gene; MLL-PTD, partial tandem duplication of the MLL gene; n, number; WBC, white blood count.
The P-value pertains to a comparison of frequencies of CEBPA mutations (single and double combined) versus CEBPA wild-type between high and low DNMT3B expressers.
The ELN modified Favorable Genetic Group is defined as CN-AML patients with mutated CEBPA and/or mutated NPM1 without FLT3-ITD. All remaining CN-AML patients (i.e., those with wild-type CEBPA and wild-type NPM1 with or without FLT3-ITD, or mutated NPM1 with FLT3-ITD) belong to the ELN Intermediate-I Genetic Group.47
The P-value pertains to a comparison of frequencies of IDH2-R140 mutations versus IDH2 wild-type between high and low DNMT3B expressers.
The P-value pertains to a comparison of frequencies of IDH2-R172 mutations versus IDH2 wild-type between high and low DNMT3B expressers.
The P-value pertains to a comparison of frequencies of DNMT3A-R882 mutations versus DNMT3A wild-type between high and low DNMT3B expressers.
The P-value pertains to a comparison of frequencies of DNMT3A non-R882 mutations versus DNMT3A wild-type between high and low DNMT3B expressers.
The median expression value was used as a cut point.
Data was assessed by the NanoString nCounter assay.
Data was assessed by real-time RT-PCR.
Associations of DNMT3B expression with clinical outcome in the entire patient cohort
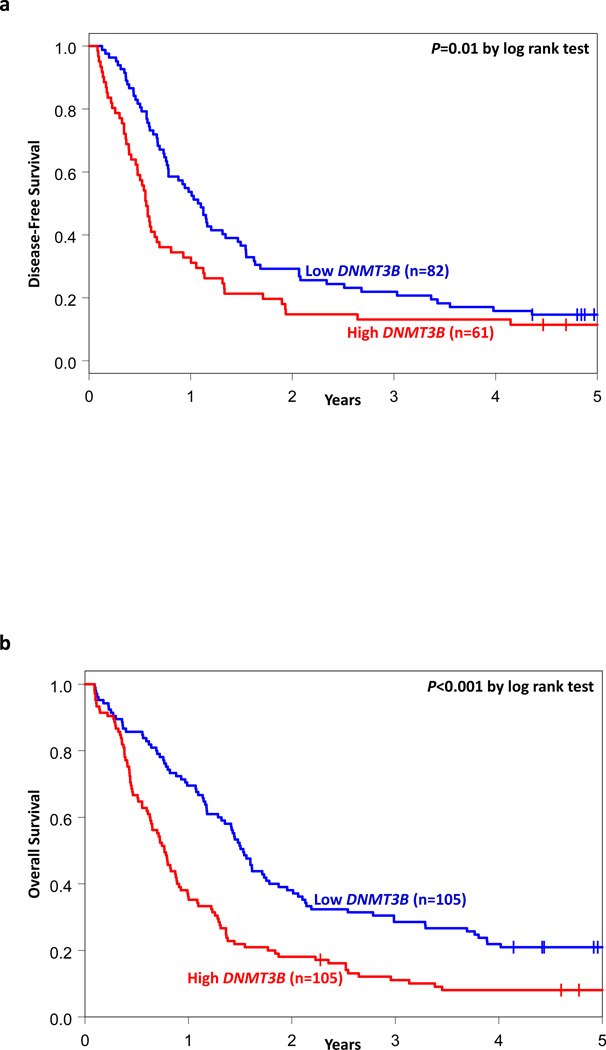
With a median follow-up for patients alive of 5.1 years (range, 2.3–11.6 years), high DNMT3B expressers had lower CR rates (P=0.002, Wald test; 58% vs 78%), and shorter DFS (P=0.02, Wald test) and OS (P<0.001, Wald test) than DNMT3B low expressers (Table 2, Figure 1).
Table 2.
Outcomes of patients with cytogenetically normal acute myeloid leukemia according to DNMT3B expression status
| Endpoint | High DNMT3B (I) |
Low DNMT3B (II) |
Pa | OR/HR (95% CI) I vs II |
|---|---|---|---|---|
| All patients | n=105 | n=105 | ||
| Complete remission, n (%) | 61 (58) | 82 (78) | 0.002 | 0.39 (0.21–0.71) |
| Disease-free survival | 0.02 | 1.55 (1.09–2.20) | ||
| Median, years | 0.6 | 1.1 | ||
| % Disease-free at 3 years (95% CI) | 13 (6–23) | 22 (14–31) | ||
| % Disease-free at 5 years (95% CI) | 11 (5–21) | 15 (8–23) | ||
| Overall survival | <0.001 | 1.85 (1.38–2.47) | ||
| Median, years | 0.8 | 1.5 | ||
| % Alive at 3 years (95% CI) | 11 (6–18) | 29 (20–37) | ||
| % Alive at 5 years (95% CI) | 8 (4–14) | 21 (14–29) | ||
| Patients in the ELN modified Favorable Genetic Groupb | n=38 | n=56 | ||
| Complete remission, n (%) | 27 (71) | 47 (84) | 0.14 | 0.47 (0.17–1.28) |
| Disease-free survival | 0.10 | 1.54 (0.92–2.57) | ||
| Median, years | 0.9 | 1.3 | ||
| % Disease-free at 3 years (95% CI) | 15 (5–30) | 30 (18–43) | ||
| % Disease-free at 5 years (95% CI) | 11 (3–26) | 21 (11–34) | ||
| Overall survival | 0.002 | 2.04 (1.29–3.22) | ||
| Median, years | 1.3 | 2.1 | ||
| % Alive at 3 years (95% CI) | 18 (7–31) | 39 (27–52) | ||
| % Alive at 5 years (95% CI) | 12 (4–24) | 30 (19–43) | ||
| Patients in the ELN Intermediate-I Genetic Groupb | n=63 | n=45 | ||
| Complete remission, n(%) | 31 (49) | 33 (73) | 0.01 | 0.35 (0.15–0.80) |
| Disease-free survival | 0.25 | 1.36 (0.81–2.27) | ||
| Median, years | 0.5 | 0.7 | ||
| % Disease-free at 3 years (95% CI) | 13 (4–27) | 9 (2–22) | ||
| % Disease-free at 5 years (95% CI) | 13 (4–27) | 3 (0–13) | ||
| Overall survival | 0.03 | 1.57 (1.06–2.33) | ||
| Median, years | 0.6 | 1.1 | ||
| % Alive at 3 years (95% CI) | 8 (3–16) | 16 (7–28) | ||
| % Alive at 5 years (95% CI) | 6 (2–14) | 9 (3–19) | ||
Abbreviations: CI, confidence interval; ELN, European Leukemia Net; HR, hazard ratio; n, number; OR, odds ratio.
P-values provided are generated by logistic regression and Cox proportional hazards models to compare outcome of patients for CR and survival endpoints (DFS, OS), respectively, using the Wald test.
The ELN modified Favorable Genetic Group is defined as CN-AML patients with mutated CEBPA and/or mutated NPM1 without FLT3-ITD. All remaining CN-AML patients (i.e., those with wild-type CEBPA and wild-type NPM1 with or without FLT3-ITD, or mutated NPM1 with FLT3-ITD) belong to the ELN Intermediate-I Genetic Group.47
Figure 1.
Clinical outcome of CN-AML patients with high and low DNMT3B expression. Kaplan-Meier survival curves for (a) disease-free survival and (b) overall survival. P-values presented are from the log rank test.
In a multivariable model for CR, DNMT3B expression remained prognostic (P=0.04), after adjustment for BAALC expression status (P<0.001), WBC (P=0.007) and age (P=0.02) (Table 3). High DNMT3B expressers were half as likely to achieve a CR as low expressers. In multivariable analysis for DFS, high DNMT3B expression associated with shorter DFS (P=0.04), once adjusted for BAALC expression (P=0.004), DNMT3A-R882 mutation status (P=0.009) and ELN Genetic Groups (P=0.03). The risk of experiencing relapse or death was 46% higher for high DNMT3B expressers than for low expressers. DNMT3B expression also remained prognostic for OS (P=0.001), after adjustment for BAALC expression (P<0.001), miR-3151 (P=0.02) and miR-155 (P=0.02). The risk of death was 72% higher for high DNMT3B expressers compared with low expressers (Table 3).
Table 3.
Multivariable analyses of CN-AML patients according to DNMT3B expression status
| Variable | Complete remission | Disease-free survival | Overall survival | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| All patients | ||||||
| DNMT3B expression, high vs low | 0.49 (0.25–0.97) | 0.04 | 1.47 (1.01–2.13) | 0.04 | 1.72 (1.24–2.38) | 0.001 |
| BAALC expression, high vs low | 0.21 (0.10–0.43) | <0.001 | 1.79 (1.21–2.65) | 0.004 | 1.98 (1.39–2.80) | <0.001 |
| WBC, per 50-unit increase | 0.69 (0.52–0.90) | 0.007 | ||||
| Age, per 10-year increase | 0.49 (0.27–0.89) | 0.02 | ||||
| DNMT3Aa | ||||||
| R882 mutated vs wild-type | 1.85 (1.17–2.95) | 0.009 | ||||
| non-R882 mutated vs wild-type | 0.92 (0.52–1.61) | 0.76 | ||||
| ELN Genetic Group, Favorable vs Intermediate-Ib | 0.65 (0.45–0.96) | 0.03 | ||||
| miR-3151 expression, high vs low | 1.51 (1.07–2.13) | 0.02 | ||||
| miR-155 expression, high vs low | 1.47 (1.06–2.05) | 0.02 | ||||
| Patients in the ELN modified Favorable Genetic Groupb | ||||||
| DNMT3B expression, high vs low | No models including a significant term for DNMT3B expression were found | 1.99 (1.26–3.15) | 0.003 | |||
| BAALC expression, high vs low | 1.81 (1.13–2.92) | 0.01 | ||||
| Patients in the ELN Intermediate-I Genetic Groupb | ||||||
| DNMT3B expression, high vs low | No models including a significant term for DNMT3B expression were found | 1.73 (1.10–2.72) | 0.02 | |||
| BAALC expression, high vs low | 1.88 (1.05–3.35) | 0.03 | ||||
| miR-3151 expression, high vs low | 1.79 (1.05–3.07) | 0.03 | ||||
| WBC, per 50-unit increase | 1.16 (1.01–1.33) | 0.03 | ||||
Abbreviations: CI, confidence interval; ELN, European LeukemiaNet; HR, hazard ratio; OR, odds ratio; WBC, white blood count.
An odds ratio less than 1 means a lower CR rate for the higher values of the continuous variables and the first category listed for the categorical variables. A hazard ratio greater than 1 (less than 1) corresponds to a higher (lower) risk of an event for higher values of continuous variables and the first category listed of a dichotomous variable. Variables were considered for inclusion in the multivariable models if they had a univariable P-value of ≤0.20. See the Supplementary Information for a full list of variables evaluated in univariable analyses. Since NPM1, FLT3-ITD, and CEBPA mutations are integrated in the ELN genetic classification, they were not additionally considered as individual variables. In the entire patient cohort, variables considered for inclusion in the model for achievement of CR were DNMT3B, ERG, BAALC, miR-155 and miR-3151 expression, ELN Genetic Groups, WT1 and ASXL1 mutation status, WBC, age and extramedullary involvement. In the model for DFS, we considered DNMT3B, ERG, BAALC and miR-3151 expression, ELN Genetic Groups, FLT3-TKD, ASXL1, DNMT3A-R882 and DNMT3A non-R882 mutation status and extramedullary involvement; and in the model for OS, DNMT3B, ERG, BAALC, miR-155 and miR-3151 expression, ELN Genetic Groups, MLL-PTD, WT1, ASXL1, DNMT3A-R882 and DNMT3A non-R882 mutation status, WBC and extramedullary involvement. For patients in the modified Favorable ELN Genetic Group, variables considered for inclusion in the model for OS were DNMT3B, ERG, BAALC and miR-155 expression, ASXL1 and TET2 mutation status and extramedullary involvement. For patients in the Intermediate-I ELN Genetic Group, variables considered for inclusion in the model for OS were DNMT3B, ERG, BAALC, miR-155 and miR-3151 expression, RUNX1, IDH1, DNMT3A-R882 and DNMT3A non-R882 mutation status, and WBC and hemoglobin.
The types of DNMT3A mutations detected in our cohort are provided in Supplementary Table S2.
The ELN modified Favorable Genetic Group is defined as CN-AML patients with mutated CEBPA and/or mutated NPM1 without FLT3-ITD. All remaining CN-AML patients (i.e., those with wild-type CEBPA and wild-type NPM1 with or without FLT3-ITD, or mutated NPM1 with FLT3-ITD) belong to the ELN Intermediate-I Genetic Group.47
Associations of DNMT3B expression with clinical outcome in ELN genetic groups
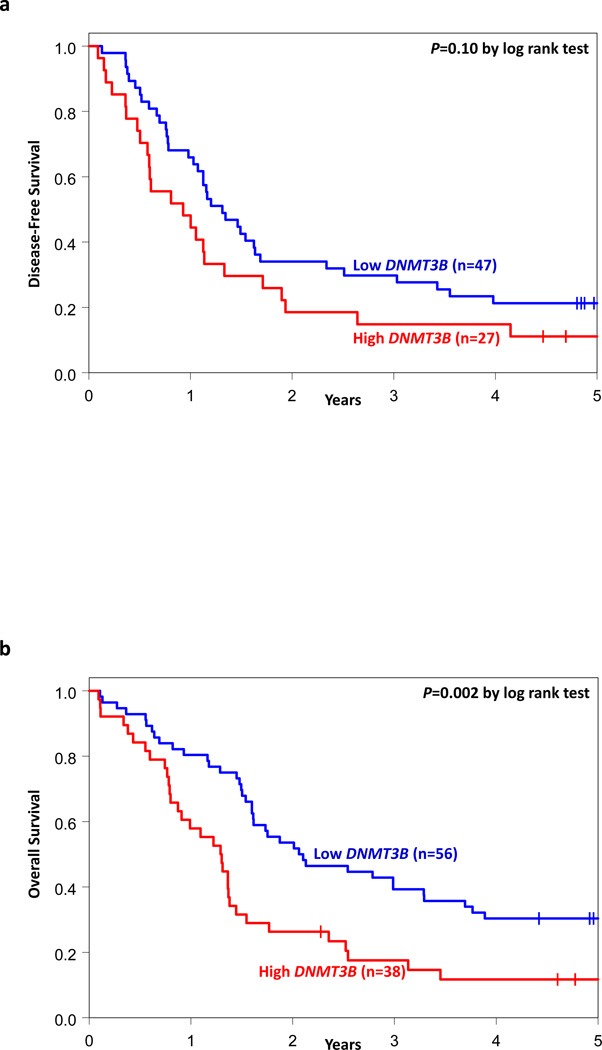
We analyzed the associations of DNMT3B expression with outcome separately within the ELN Favorable and Intermediate-I Genetic Groups. Within the Favorable Group (n=94), there was no significant difference in CR rates (71% vs 84%, P=0.14, Wald test) or DFS (P=0.10, Wald test) between high and low DNMT3B expressers. However, high expressers had shorter OS (P=0.002, Wald test) than low expressers (Table 2, Figures 2A and 2B). In multivariable analyses for the ELN Favorable Genetic Group (Table 3), DNMT3B expression remained significant for OS (P=0.003) after adjustment for BAALC expression (P=0.01). High DNMT3B expressers were twice as likely to die as low expressers.
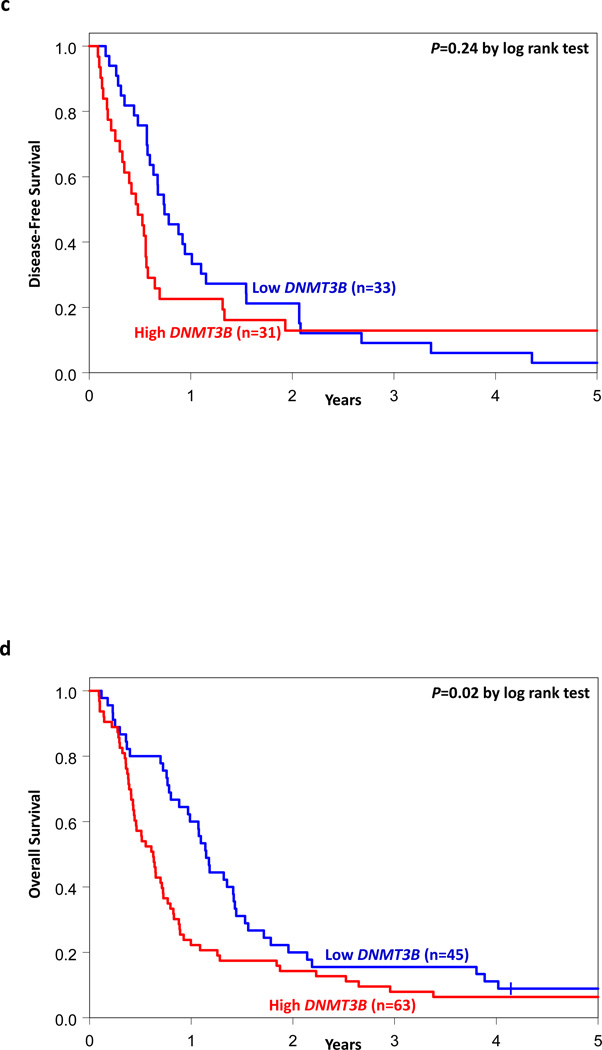
Figure 2.
Clinical outcome of CN-AML patients with high and low DNMT3B expression classified into European LeukemiaNet (ELN) Genetic Groups. Kaplan-Meier survival curves for (a) disease-free survival and (b) overall survival of patients in the ELN modified Favorable Genetic Group; (c) disease-free survival and (d) overall survival of patients in the ELN Intermediate-I Genetic Group. P-values presented are from the log rank test.
In the Intermediate-I Group (n=108), high DNMT3B expressers had a lower CR rate (49% vs 73%, P=0.01, Wald test) and shorter OS (P=0.03, Wald test) than low DNMT3B expressers, but there was no significant difference in DFS between the groups (Table 2, Figures 2C and 2D). In multivariable analyses within the ELN Intermediate-I Genetic Group (Table 3), DNMT3B expression was significant for OS (P=0.02), after adjustment for BAALC expression (P=0.03), miR-3151 expression (P=0.03) and WBC (P=0.03). High DNMT3B expressers were 1.7 times more likely to die than low expressers.
Genome-wide gene-expression profiles associated with DNMT3B expression
To gain biologic insights into the role of DNMT3B, we derived a DNMT3B-associated gene-expression profile using three independent sets of CN-AML patients, i.e., CALGB/Alliance (n=177), AMLCG (n=75) and TCGA (n=88). We identified 195 upregulated genes and 168 downregulated genes that were significantly associated with higher DNMT3B expression in each of the three cohorts (Supplementary Table S1). Since high DNMT3B expression was associated with the presence of FLT3-ITD (Table 1), we performed GSEA to test whether a set of 195 genes that are upregulated in high DNMT3B expressers is associated with a set of genes differentially expressed between patients who harbored FLT3-ITD versus those who did not (Supplementary Information). We found a significant correlation between the high DNMT3B expression and FLT3-ITD signatures (P=0.006; FDR=0.006; Supplementary Figure S2). Among the genes upregulated in high DNMT3B expressers, we noted a variety of genes previously involved in AML including CDK6 and WT1 that encode cyclin kinase and transcription factor proteins, respectively. Among the downregulated genes, we noted genes involved with both normal monocyte/macrophage differentiation and immune function including CD14, TLR4, CEBPB and TLR8.
Gene Ontology was used to assess the biologic features of the DNMT3B-expression profile (Table 4). For DNMT3B-associated upregulated genes, there were three GO terms comprising genes involved in nucleotide biosynthetic processes and metabolism and included in annotation cluster 1 that had a trend for statistical significance (Benjamini P-value <0.1). For DNMT3B-associated downregulated genes, cellular processes included lysosome biology, endocytosis and membrane signaling. These results may be interpreted as consistent with the previously noted dysregulated genes involved in monocyte/macrophage differentiation and activity.
Table 4.
Gene Ontology terms associated with differentially expressed genes in the high DNMT3B expression group
| Biologic process or cellular component | Number of genes |
Benjamini P-value |
|---|---|---|
| Associations with genes upregulated in the high DNMT3B expression group | ||
| Annotation Cluster 1. | ||
| GOTERM_BP: nucleotide biosynthetic process | 10 | 0.098 |
| GOTERM_BP: nucleobase, nucleoside and nucleotide biosynthetic process | 10 | 0.066 |
| GOTERM_BP: nucleobase, nucleoside, nucleotide and nucleic acid biosynthetic process | 10 | 0.066 |
| Associations with genes downregulated in the high DNMT3B expression group | ||
| Annotation Cluster 1 | ||
| GOTERM_CC: vacuole | 12 | 0.0052 |
| GOTERM_CC: lytic vacuole | 10 | 0.012 |
| GOTERM_CC: lysosome | 10 | 0.012 |
| Annotation Cluster 2 | ||
| GOTERM_BP: endocytosis | 11 | 0.0091 |
| GOTERM_BP: membrane invagination | 11 | 0.0091 |
| GOTERM_BP: membrane organization | 12 | 0.089 |
| Annotation Cluster 3 | ||
| GOTERM_CC: intrinsic to membrane | 74 | 0.028 |
Abbreviations: GO, Gene Ontology, see also Huang da et al.45; BP, biologic process; CC, cellular component.
Genome-wide microRNA profiles associated with DNMT3B expression
The influence of DNMT3B expression on microRNA genome-wide profiles could be evaluated in 162 patients. In contrast to coding genes, only four microRNAs were differentially expressed between high and low DNMT3B expressers (P≤0.001). High DNMT3B expression was associated with miR-133b upregulation, and miR-148a, miR-122 and miR-409-3p downregulation. miR-133b upregulation in high DNMT3B expressers was somewhat surprising as this microRNA was reported to have tumor suppressor activity in other cancers.48,49 However, consistent with the downregulated gene-expression profile discussed above, miR-133b has recently been shown to target GM-CSF, a cytokine involved in granulocyte-monocyte/macrophage differentiation.50 Among the downregulated microRNAs, miR-148a was reported to target DNMT3B and to be itself a target of aberrant hypermethylation in cancer.51,52 Lower expression of miR-122 has been associated with aggressive hepatocellular carcinoma and miR-409-3p with cell invasion and metastasis in gastric cancer53–56; however, a role for these microRNAs in AML is currently unknown.
Genome-wide methylation profiling associated with DNMT3B expression
Since DNMT3B encodes a methyltransferase that mediates de novo DNA methylation, we assessed whether high and low DNMT3B expressers differed in DNA methylation patterns. Surprisingly, we found no significant differences in genome-wide DNA methylation levels or in the numbers of differentially methylated regions (DMRs)18 in distinct functional genomic regions (e.g., gene promoters) when high versus low DNMT3B expressers were compared.
DISCUSSION
In this study, we report that high DNMT3B expression associates with lower CR rates and shorter DFS and OS in chemotherapy-treated CN-AML patients aged ≥60 years. High DNMT3B expression was associated with such adverse prognostic factors as FLT3-ITD, high ERG, BAALC and miR-155 expression and the ELN Intermediate-I Genetic Group; nevertheless the association of DNMT3B expression with clinical outcome is independent from the aforementioned and other established molecular and clinical prognosticators for all outcome endpoints studied.
Our findings are consistent to some extent with the only, to our knowledge, previous study that assessed the prognostic value of DNMT3B expression.26 Although in the subset of 93 CN-AML patients, Hayette et al.26 did not find significant differences in event-free survival (EFS) or OS between high and low DNMT3B expressers, high DNMT3B expressers had a shorter EFS than low DNMT3B expressers in the whole cytogenetically diverse cohort of 191 AML patients analyzed. It is difficult to directly compare their results with ours since approximately one-half of the patients analyzed by Hayette et al.26 had various abnormal karyotypes, more than two-thirds of the patients were younger than 60 years and a quarter underwent allogeneic HSCT in first CR. Thus, although the two studies are not comparable, they both conclude that higher DNMT3B expression is associated with worse outcome in AML.
Recently, the ELN Reporting System,47 which for CN-AML is based on only three molecular markers (i.e., FLT3-ITD, CEBPA and NPM1 mutations), was shown to provide important prognostic information in AML.57 However, we and others have shown that additional molecular markers, such as TET2,41 ASXL1,8 RUNX142 and DNMT3A58 mutations and expression of MN1,12 miR-15515 and miR-3151,16 may refine outcome prediction of CN-AML patients within the ELN Genetic Groups. Hence, in the current study, we investigated whether considering DNMT3B expression as a novel prognosticator could alter patient classification within the ELN Genetic Groups. In the Favorable Group, we found that low DNMT3B expression identified a subset of CN-AML patients with a significantly longer OS, thus making DNMT3B expression the third molecular marker, in addition to ASXL1 mutations8 and miR-155 expression,15 capable of refining prognostication of older patients in this ELN Genetic Group. We also observed a significant difference in OS between high and low DNMT3B expressers classified in the ELN Intermediate-I Genetic Group. Previously, RUNX1 mutations42 and expression levels of MN1,12 miR-15515 and miR-315116 were demonstrated to add prognostic information in this ELN Genetic Group.
We report the first, to our knowledge, DNMT3B-associated gene- and microRNA-expression and DNA methylation profiles in CN-AML. We were able to derive a strong gene expression profile comprising 363 genes by overlapping the microarray results from three independent sets of patients. The profile was quite heterogeneous, comprising genes encoding for proteins involved in multiple biologic processes that play a role in leukemia cell differentiation, proliferation and survival. Among the downregulated genes, we noted enrichment of genes involved in monocyte/macrophage differentiation and activity, suggesting a role of DNMT3B in impairing differentiation of the leukemic blasts into cells with normal innate immunity activity. Using GSEA, we found a significant correlation between the high DNMT3B expression and FLT3-ITD signatures (Supplementary Figure S2). This, along with the increased frequency of FLT3-ITD in high DNMT3B expressers (Table 1), suggests the existence of a functional association between high expression of the DNMT3B gene and FLT3-ITD.
In contrast, the DNMT3B-associated microRNA profile was relatively weak, comprising only four microRNAs that were differentially expressed in high versus low DNMT3B expressers. Nevertheless, the unique upregulation of expression of miR-133b, recently reported to target GM-CSF,50 in DNMT3B high expressers was somewhat consistent with the enrichment of the gene-expression profile in multiple downregulated genes involved in the differentiation and activity of hematopoietic cells participating in innate immunity.
Surprisingly, despite the fact that DNMT3B encodes a DNA methyltransferase, we observed no significant association of high DNMT3B levels and DNA methylation changes. No difference in global DNA methylation levels and number of DMRs could be identified between DNMT3B high and low expressers. Our results are reminiscent of a recent report showing that changes in DNMT3B expression did not affect methylation levels of putative DNMT3B target genes.59 Moreover, Russler-Germain et al.60 have recently demonstrated that DNA methylation levels in leukemic blasts from CN-AML patients are not influenced by DNMT3B expression since mainly inactive splice variants of DNMT3B are expressed in these cells. Overall, therefore, these data may suggest that although overexpressed DNMT3B is a potentially valuable predictive marker for response to conventional chemotherapy, it does not necessarily identify subsets of older AML patients characterized by aberrant DNA methylation who might be responsive to hypomethylating azanucleosides.
In summary, we have demonstrated that DNMT3B expression constitutes an independent prognostic factor in older CN-AML patients treated intensively, and could also refine the ELN classification. Furthermore, we have provided some insights into the biologic activity of DNMT3B in CN-AML, which is seemingly independent from mechanisms of DNA hypermethylation and/or microRNA-dependent gene repression. Further studies focused on gaining more clinical and mechanistic insights into the leukemogenic role of DNMT3B expression are warranted to design active therapeutic strategies for high DNMT3B expressers in CN-AML.
Supplementary Material
ACKNOWLEDGEMENTS
The Cancer and Leukemia Group B institutions, and their principal investigators participating in this study are provided in the Supplementary Information. We thank Donna Bucci and the CALGB/Alliance Leukemia Tissue Bank at The Ohio State University Comprehensive Cancer Center, Columbus, OH, for sample processing and storage services; Lisa J. Sterling and Chris Finks for data management; and The Ohio State University Comprehensive Cancer Center’s Nucleic Acid and Microarray Shared Resources for technical support. This work was supported in part by the National Cancer Institute (grants CA101140, CA114725, CA140158, CA31946, CA33601, CA16058, CA77658, and CA129657), the Coleman Leukemia Research Foundation, the Pelotonia Fellowship Program (A.-K.E.), the Conquer Cancer Foundation (J.H.M.) and the Deutsche Krebshilfe–Dr Mildred Scheel Cancer Foundation (H.B.).
Footnotes
Presented in part at the 18th Congress of the European Hematology Association, Stockholm, Sweden, June 13–16, 2013, and published in abstract form: Niederwieser C, Kohlschmidt J, Maharry K, Mrózek K, Metzeler K, Volinia S et al. High expression of DNMT3B negatively impacts on clinical outcome of older patients (pts) with primary cytogenetically normal (CN) acute myeloid leukemia (AML) [CALGB 20202 (ALLIANCE)]. Haematologica 2013; 98(suppl 1): 484 (abstract S1168).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
CN, JK, SV, K Mrózek, GM and CDB designed the study, analyzed the data and wrote the manuscript, and all authors agreed on the final version; SPW, KHM, A-KE, PY, DF HB, SS, JHM, JPC, Y-ZW, and RB carried out laboratory-based research; JK, K Maharry, SV, and DN performed statistical analyses; and AJC, MRB, BLP, JEK, JOM, THC, RAL, RMS, K Mrózek, GM and CDB were involved directly or indirectly in the care of patients and/or sample procurement.
Supplementary Information is available at Leukemia’s website (http://www.nature.com/leu)
REFERENCES
- 1.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 2.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker A, Marcucci G. Molecular prognostic factors in cytogenetically normal acute myeloid leukemia. Expert Rev Hematol. 2012;5:547–558. doi: 10.1586/ehm.12.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitman SP, Maharry K, Radmacher MD, Becker H, Mrózek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–3626. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker H, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Margeson D, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker H, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Margeson D, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:788–792. doi: 10.1182/blood-2010-01-262543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcucci G, Maharry K, Wu Y-Z, Radmacher MD, Mrózek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzeler KH, Becker H, Maharry K, Radmacher MD, Kohlschmidt J, Mrózek K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN Favorable genetic category. Blood. 2011;118:6920–6929. doi: 10.1182/blood-2011-08-368225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcucci G, Metzeler KH, Schwind S, Becker H, Maharry K, Mrózek K, et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2012;30:742–750. doi: 10.1200/JCO.2011.39.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwind S, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Holland KB, et al. BAALC and ERG expression levels are associated with outcome and distinct gene- and microRNA expression profiles in older patients with de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:5660–5669. doi: 10.1182/blood-2010-06-290536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heuser M, Beutel G, Krauter J, Döhner K, von Neuhoff N, Schlegelberger B, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–3905. doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 12.Schwind S, Marcucci G, Kohlschmidt J, Radmacher MD, Mrózek K, Maharry K, et al. Low expression of MN1 associates with better treatment response in older patients with de novo cytogenetically normal acute myeloid leukemia. Blood. 2011;118:4188–4198. doi: 10.1182/blood-2011-06-357764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcucci G, Mrózek K, Radmacher MD, Garzon R, Bloomfield CD. The prognostic and functional role of microRNAs in acute myeloid leukemia. Blood. 2011;117:1121–1129. doi: 10.1182/blood-2010-09-191312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwind S, Maharry K, Radmacher MD, Mrózek K, Holland KB, Margeson D, et al. Prognostic significance of expression of a single microRNA, miR-181a, in cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:5257–5264. doi: 10.1200/JCO.2010.29.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcucci G, Maharry KS, Metzeler KH, Volinia S, Wu Y-Z, Mrózek K, et al. Clinical role of microRNAs in cytogenetically normal acute myeloid leukemia: miR-155 upregulation independently identifies high-risk patients. J Clin Oncol. 2013;31:2086–2093. doi: 10.1200/JCO.2012.45.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisfeld AK, Marcucci G, Maharry K, Schwind S, Radmacher MD, Nicolet D, et al. miR-3151 interplays with its host gene BAALC and independently affects outcome of patients with cytogenetically normal acute myeloid leukemia. Blood. 2012;120:249–258. doi: 10.1182/blood-2012-02-408492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcucci G, Yan P, Maharry K, Frankhouser D, Nicolet D, Metzeler KH, et al. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J Clin Oncol. 2014;32:548–556. doi: 10.1200/JCO.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan P, Frankhouser D, Murphy M, Tam HH, Rodriguez B, Curfman J, et al. Genome-wide methylation profiling in decitabine-treated patients with acute myeloid leukemia. Blood. 2012;120:2466–2474. doi: 10.1182/blood-2012-05-429175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renneville A, Boissel N, Nibourel O, Berthon C, Helevaut N, Gardin C, et al. Prognostic significance of DNA methyltransferase 3A mutations in cytogenetically normal acute myeloid leukemia: a study by the Acute Leukemia French Association. Leukemia. 2012;26:1247–1254. doi: 10.1038/leu.2011.382. [DOI] [PubMed] [Google Scholar]
- 20.Hervouet E, Vallette FM, Cartron PF. Dnmt3/transcription factor interactions as crucial players in targeted DNA methylation. Epigenetics. 2009;4:487–499. doi: 10.4161/epi.4.7.9883. [DOI] [PubMed] [Google Scholar]
- 21.Trowbridge JJ, Sinha AU, Zhu N, Li M, Armstrong SA, Orkin SH. Haploinsufficiency of Dnmt1 impairs leukemia stem cell function through derepression of bivalent chromatin domains. Genes Dev. 2012;26:344–349. doi: 10.1101/gad.184341.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li KK, Luo LF, Shen Y, Xu J, Chen Z, Chen SJ. DNA methyltransferases in hematologic malignancies. Semin Hematol. 2013;50:48–60. doi: 10.1053/j.seminhematol.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Walsh G, Liu DD, Lee JJ, Mao L. Expression of ΔDNMT3B variants and its association with promoter methylation of p16 and RASSF1A in primary non-small cell lung cancer. Cancer Res. 2006;66:8361–8366. doi: 10.1158/0008-5472.CAN-06-2031. [DOI] [PubMed] [Google Scholar]
- 24.Hlady RA, Novakova S, Opavska J, Klinkebiel D, Peters SL, Bies J, et al. Loss of Dnmt3b function upregulates the tumor modifier Ment and accelerates mouse lymphomagenesis. J Clin Invest. 2012;122:163–177. doi: 10.1172/JCI57292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayette S, Thomas X, Jallades L, Chabane K, Charlot C, Tigaud I, et al. High DNA methyltransferase DNMT3B levels: a poor prognostic marker in acute myeloid leukemia. PLoS One. 2012;7:e51527. doi: 10.1371/journal.pone.0051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 29.Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman P, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. N Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 30.Lee EJ, George SL, Caligiuri M, Szatrowski TP, Powell BL, Lemke S, et al. Parallel phase I studies of daunorubicin given with cytarabine and etoposide with or without the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age or older with acute myeloid leukemia: results of Cancer and Leukemia Group B study 9420. J Clin Oncol. 1999;17:2831–2839. doi: 10.1200/JCO.1999.17.9.2831. [DOI] [PubMed] [Google Scholar]
- 31.Baer MR, George SL, Sanford BL, Mrózek K, Kolitz JE, Moore JO, et al. Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: Cancer and Leukemia Group B study 9720. Leukemia. 2011;25:800–807. doi: 10.1038/leu.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcucci G, Moser B, Blum W, Stock W, Wetzler M, Kolitz JE, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. J Clin Oncol. 2007;25(suppl):360s. (abstract 7012). [Google Scholar]
- 33.Mrózek K, Carroll AJ, Maharry K, Rao KW, Patil SR, Pettenati MJ, et al. Central review of cytogenetics is necessary for cooperative group correlative and clinical studies of adult acute leukemia: the Cancer and Leukemia Group B experience. Int J Oncol. 2008;33:239–244. [PMC free article] [PubMed] [Google Scholar]
- 34.Payton JE, Grieselhuber NR, Chang LW, Murakami M, Geiss GK, Link DC, et al. High throughput digital quantification of mRNA abundance in primary human acute myeloid leukemia samples. J Clin Invest. 2009;119:1714–1726. doi: 10.1172/JCI38248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 36.Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 38.Whitman SP, Ruppert AS, Marcucci G, Mrózek K, Paschka P, Langer C, et al. Long-term disease-free survivors with cytogenetically normal acute myeloid leukemia and MLL partial tandem duplication: a Cancer and Leukemia Group B study. Blood. 2007;109:5164–5167. doi: 10.1182/blood-2007-01-069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcucci G, Maharry K, Radmacher MD, Mrózek K, Vukosavljevic T, Paschka P, et al. Prognostic significance of gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: a Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:5078–5087. doi: 10.1200/JCO.2008.17.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paschka P, Marcucci G, Ruppert AS, Whitman SP, Mrózek K, Maharry K, et al. Wilms' tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:4595–4602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzeler KH, Maharry K, Radmacher MD, Mrózek K, Margeson D, Becker H, et al. TET2 mutations improve the new European LeukemiaNet risk classification of acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2011;29:1373–1381. doi: 10.1200/JCO.2010.32.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendler JH, Maharry K, Radmacher MD, Mrózek K, Becker H, Metzeler KH, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and microRNA expression signatures. J Clin Oncol. 2012;30:3109–3118. doi: 10.1200/JCO.2011.40.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcucci G, Radmacher MD, Maharry K, Mrózek K, Ruppert AS, Paschka P, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 44.Metzeler KH, Hummel M, Bloomfield CD, Spiekermann K, Braess J, Sauerland MC, et al. An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood. 2008;112:4193–4201. doi: 10.1182/blood-2008-02-134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 46.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models. New York, NY, USA: Springer; 2005. [Google Scholar]
- 47.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Li M, Li L, Yang X, Lan G, Zhang Y. MiR-133b is down-regulated in human osteosarcoma and inhibits osteosarcoma cells proliferation, migration and invasion, and promotes apoptosis. PLoS One. 2013;8:e83571. doi: 10.1371/journal.pone.0083571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan F-T, Qian F, Fang K, Lin K-Y, Wang W-T, Chen Y-Q. miR-133b, a muscle-specific microRNA, is a novel prognostic marker that participates in the progression of human colorectal cancer via regulation of CXCR4 expression. Mol Cancer. 2013;12:164. doi: 10.1186/1476-4598-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturrock A, Mir-Kasimov M, Baker J, Rowley J, Paine R., 3rd Key role of microRNA in the regulation of granulocyte macrophage colony-stimulating factor expression in murine alveolar epithelial cells during oxidative stress. J Biol Chem. 2014;289:4095–4105. doi: 10.1074/jbc.M113.535922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14:872–877. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu A, Xia J, Zuo J, Jin S, Zhou H, Yao L, et al. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med Oncol. 2012;29:2701–2709. doi: 10.1007/s12032-011-0134-3. [DOI] [PubMed] [Google Scholar]
- 53.Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köberle V, Kronenberger B, Pleli T, Trojan J, Imelmann E, Peveling-Oberhag J, et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013;49:3442–3449. doi: 10.1016/j.ejca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Li A, Song W, Qian J, Li Y, He J, Zhang Q, et al. MiR-122 modulates type I interferon expression through blocking suppressor of cytokine signaling 1. Int J Biochem Cell Biol. 2013;45:858–865. doi: 10.1016/j.biocel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Zheng B, Liang L, Huang S, Zha R, Liu L, Jia D, et al. MicroRNA-409 suppresses tumour cell invasion and metastasis by directly targeting radixin in gastric cancers. Oncogene. 2012;31:4509–4516. doi: 10.1038/onc.2011.581. [DOI] [PubMed] [Google Scholar]
- 57.Mrózek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaidzik VI, Schlenk RF, Paschka P, Stölzle A, Späth D, Kuendgen A, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG) Blood. 2013;121:4769–4777. doi: 10.1182/blood-2012-10-461624. [DOI] [PubMed] [Google Scholar]
- 59.Hagemann S, Kuck D, Stresemann C, Prinz F, Brueckner B, Mund C, et al. Antiproliferative effects of DNA methyltransferase 3B depletion are not associated with DNA demethylation. PLoS One. 2012;7:e36125. doi: 10.1371/journal.pone.0036125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russler-Germain DA, Spencer DH, Young MA, Lamprecht TL, Miller CA, Fulton R, et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell. 2014;25:442–454. doi: 10.1016/j.ccr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.