Abstract
Social isolation rearing (isolated condition, IC) is used as a model of early life stress in rodents. Rats raised in this condition are often compared to rats raised in an environmentally enriched condition (EC). However, EC rats are repeatedly exposed to forced novelty, another classic stressor in rodents. These studies explored the relationship between cocaine self-administration and glucocorticoid receptor (GR) activation and measured total levels of GR protein in reward-related brain regions (medial prefrontal cortex, orbitofrontal cortex, nucleus accumbens, amygdala) in rats chronically exposed to these conditions. For experiment 1, rats were housed in EC or IC and were then trained to self-administer cocaine. Rats raised in these housing conditions were tested for their cocaine responding after pretreatment with the GR antagonist, RU486, or the GR agonist, corticosterone (CORT). For experiment 2, levels of GR from EC and IC rats were measured in brain regions implicated in drug abuse using Western blot analysis. Pretreatment with RU486 (20 mg/kg) decreased responding for a low unit dose of cocaine (0.03 mg/kg/infusion) in EC rats only. IC rats were unaffected by RU486 pretreatment, but earned significantly more cocaine than EC rats after pretreatment with CORT (10 mg/kg). No difference in GR expression was found between EC and IC rats in any brain area examined. These results, along with previous literature, suggest that enrichment enhances responsivity of the HPA axis related to cocaine reinforcement, but this effect is unlikely due simply to differential baseline GR expression in areas implicated in drug abuse.
Keywords: Social isolation, environmental enrichment, cocaine self-administration, RU486, corticosterone, glucocorticoid receptor
1. Introduction
Early life stress is associated with negative mental health outcomes in humans, including increased risk of developing addiction. Predictive stressors are early life events that do not have to be traumatic [1-2]. Children raised in homes where one or more parents are unemployed, where parents have low levels of education, or children from households that have lower socioeconomic status are more likely to abuse stimulants during adulthood [3-5].
Abundant preclinical evidence indicates that adult rats acutely exposed to a variety of stressors demonstrate accelerated acquisition of stimulant intake [6], enhanced stimulant reward [7], and greater cocaine consumption [8]. However, it is less clear whether there is a differential effect of acute and chronic stress on stimulant reward in adolescent rats [reviewed in [9]]. Despite this, studies consistently report that rats isolated during adolescence (isolated condition, IC) self-administer low unit doses of stimulants at a greater rate compared to rats raised in social conditions [10] and compared to rats raised in enriched conditions (EC) [10-12].
In addition to isolation, however, exposure to novel objects is often used experimentally as a stressor [13-14]. Accordingly, short-term exposure to either isolation or novelty increases circulating levels of the stress hormone corticosterone (CORT) [15-16]. However, in contrast to isolation rearing, EC rats, which experience repeated exposure to novelty, self-administer stimulants at a lower rate, perform better on a battery of behavioral tasks [17-18], and also recover more rapidly from injury than IC rats [19]. This contrasts with other work showing that all of these outcomes are usually negatively affected by other stressors [20-22]. Thus, even though both isolation and novelty exposure cause CORT release acutely, lasting or repeated exposure to these stressors results in opposite stress-related behavioral outcomes.
There are several neurobiological targets implicated in both stress and drug abuse, including corticotrophin releasing factor (CRF), the dynorphin/kappa system, norepinephrine, and the hypothalamic-pituitary-adrenal (HPA) axis [reviewed in [23-25]]. Among these, the role of the HPA axis in drug abuse has been studied most extensively. CORT is a major end point of the HPA axis and it negatively regulates its own release via actions at the glucocorticoid receptor (GR) [26-27]. CORT level is correlated with cocaine self-administration, but only at low cocaine doses [28]. In fact, CORT is necessary for cocaine self-administration, as adrenalectomized rats do not acquire self-administration of cocaine [29] and do not undergo reinstatement [30]. Additionally, decreasing circulating CORT levels by inhibiting its synthesis with metyrapone [29] or ketoconazole [31] reduces maintenance of cocaine self-administration, although adrenalectomy does not affect cocaine self-administration once it has been acquired [32]. Regardless, once CORT levels have been increased to some threshold necessary for acquisition of stimulant self-administration, further increases in CORT do not amplify stimulant intake [reviewed in [33]].
The target receptor for CORT in these effects has not been defined, although GR might play some role in the maintenance of stimulant self-administration in rodents. Knockout of GR in the central nervous system attenuates cocaine self-administration in mice [34] and administration of the non-selective GR antagonist RU486 decreases stimulant self-administration in mice and rats [35-36].
Only one study has examined differences in HPA axis functioning between EC and IC rats in response to drugs of abuse. In that study, RU486 was found to decrease amphetamine self-administration to a greater extent in EC rats than IC rats [35]. Other studies have quantified aspects of the HPA axis in EC and IC rats, but the results have been mixed. Basal CORT was found to be lower in EC rats compared to IC rats in one study [35]. In contrast, other studies have found that EC rats have higher basal CORT compared to normal housed controls [37] and others have measured lower basal CORT after chronic isolation compared to rats in normal cage conditions [38-39]. Another study found no difference between IC rats and group-housed rats [40]. However, these discrepancies may be due, at least in part, to the type of cage IC rats are housed in, as rats housed in cages with wire grid floors have greater circulating CORT than rats raised in cages with sawdust bedding [41].
Studies quantifying GR mRNA from EC and IC rats found no difference in prefrontal cortex [42]. GR protein has been reported to be increased in hippocampus in EC rats compared to normal housed controls in some studies [43-44], but not in all studies [45]. Notably, few studies have directly compared GR protein expression in EC to IC rats across more than one brain area. In addition, these studies have not examined the functional consequences of altered CORT or GR levels in differentially housed animals.
Given the observed decrease in stimulant self-administration following enrichment, it has been hypothesized that repeated exposure to novelty, experienced daily by EC rats, reduces sensitivity of the HPA axis in contrast to other traditional stressors. This produces a functional anti-stress effect [46]. However, the precise mechanisms underlying the functional adaptation are largely unknown. To address this hypothesis, the current experiments examined differences between EC and IC rats in cocaine self-administration after pretreatment with the GR antagonist RU486 or the GR agonist CORT, as well as total GR expression in various stress- and drug abuse-relevant brain regions. Rats were initially trained to lever press for food and then were trained to self-administer a high unit dose of cocaine (0.75 mg/kg/infusion). The high training dose of cocaine was selected to minimize initial EC/IC differences in response rate that typically occur at low unit doses of stimulant drugs [10-12]. Since baseline differences between EC and IC rats can complicate the interpretation of drug effects [47], engendering similar baseline response rates in EC and IC rats is advantageous for interpreting the potential differential effects of RU486 and CORT.
2. Materials and Methods
2.1. Subjects
Male Sprague Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and arrived in the colony at PND 21. Rats were immediately placed on a 12h light-dark cycle (lights on at 7:00AM) and were allowed food and water ad libitum. All procedures were approved by the University of Kentucky’s Institutional Animal Care and Use Committee and all procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Housing
Upon arrival to the colony, rats were randomly assigned to EC or IC cages. EC rats were placed in large stainless steel cages (122 × 61 × 45.5 cm) with 14 hard plastic objects (commercial toys) and 5-10 age-matched cohorts. Half of the objects in the cage were switched daily. Rats in the IC condition were placed singly in small stainless steel cages (17 × 24 × 20 cm) with wire grid floors and no objects. These small cages allowed food and water changes, as well as waste disposal, without human contact. Rats were returned to their housing conditions at the end of each session and remained in their environments for the duration of the experiment.
2.3. Experiment 1: Cocaine self-administration
2.3.1. Apparatus
All self-administration sessions were conducted in standard 2-lever operant conditioning chambers (28 × 24 × 21 cm; ENV-008CT; MED Associates, St. Albans VT) equipped with syringe pumps for drug delivery (PHM-100; MED Associates).
2.3.2. Pretraining
Seven days prior to surgery (~PND 48), all rats started training to lever press for food pellets (45 mg Dustless Precision Pellets, Bio-Serv, Frenchtown NJ). Training continued as follows: magazine shaping for 1 day, autoshaping for 3 days, and FR1 training for 3 days. During magazine shaping, food pellets were randomly delivered to the food hopper on a random time 36-sec interval. For autoshaping, both levers were extended and the cue light over the active lever was illuminated. One response on the active lever resulted in delivery of one sucrose pellet and retraction of both levers. If no response was made on the active lever, one pellet was delivered and the levers were retracted on a random time 60-sec interval. After magazine shaping and autoshaping, rats were trained to lever press for food pellets on a FR1 schedule of reinforcement for 3 days. For this procedure, both levers were extended but only responses on the active lever were reinforced. The position of the active lever was randomized across rats.
2.3.3. Surgical Procedures
Between PND 55-58, rats underwent surgery to implant a jugular catheter for self-administration. Briefly, rats were anesthetized with a mixture of ketamine (Butler Schein, Dublin OH) /xylazine (Akorn, Inc., Decatur IL) /acepromazine (Boehringer Ingelheim, St. Joseph MO) (75/7.5/0.75 mg/kg; 0.15ml/100g body weight; i.p.). A catheter was inserted into the right jugular vein, extended under the skin, and exited the body through an incision in the scalp. A cannula was attached to the end of the catheter and was secured to the skull using dental acrylic and four jeweler’s screws. Animals were allowed to recover for 7 days after surgery.
During self-administration sessions, rats were connected to the syringe pump (PHM-100; MED Associates) via tubing strung through a leash (PHM-120; MED Associates) and attached to a swivel (PHM-115; MED Associates) above the chamber. Immediately following daily self-administration sessions, rats were infused with 0.2 ml of a mixture containing 1% gentamicin (10.15 mg/ml, Abraxis BioScience, Los Angeles CA), 3% heparin (1000 USP units/ml, Abraxis BioScience, Los Angeles CA), and 96% sterile saline (0.9% NaCl).
2.3.4. Cocaine self-administration training
At ~PND 62, rats were allowed to lever press for cocaine on a FR1 schedule during 60-min sessions. Responses on the active lever resulted in a 0.1 ml infusion of cocaine (0.75 mg/kg/infusion) and illumination of two cue lights located above both the active and inactive levers. Cue lights remained illuminated for an additional 20 sec following the infusion. During this time, responses on the active lever were recorded but did not result in any consequence. Responses on the inactive lever at any time had no programmed consequence. The active lever remained the same during pretraining and cocaine self-administration. Sessions continued daily until responding was stable (no linear change in responding over 3 consecutive days). After this was achieved, the unit dose of cocaine was decreased to 0.3 mg/kg/infusion.
2.3.5. Administration of RU486 and CORT
Once stability was reached at 0.3 mg/kg/infusion cocaine, all rats were randomly pretreated with either 20 mg/kg RU486 or vehicle (s.c.) 40 min before the beginning of the self-administration session; the RU486 dose was chosen based on a previous study with amphetamine [35]. Rats were given a minimum of two days between each pretreatment; during the intervening days, rats were maintained on cocaine self-administration in the absence of RU486 or vehicle. The unit dose of cocaine was then decreased for all rats from 0.3 to 0.1 mg/kg/infusion. Responding was stabilized and all rats were pretreated with 20 mg/kg RU486 as described above. The unit dose of cocaine was decreased again to 0.03 mg/kg/infusion and continued until stability was reached. All rats were again pretreated with 20 mg/kg RU486 as above. Following the RU486 test phase, the unit dose of cocaine was returned to 0.3 mg/kg/infusion and responding was allowed to stabilize once again. Rats then were administered 10 mg/kg CORT (i.p.) 10 min before the self-administration session.
2.3.6. Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse and was dissolved in sterile saline (0.9% NaCl). Cocaine was infused i.v. in a constant volume of 0.1 ml/infusion, with doses adjusted by varying the drug concentration. RU486 (mifepristone) was purchased from Sigma-Aldrich (St. Louis MO) and was suspended in warm 15% diluted Kolliphor EL (Sigma-Aldrich, St. Louis MO). Corticosterone (Sigma-Aldrich, St. Louis MO) was dissolved in 40% EtOH/saline.
2.4. Experiment 2: Western blot for GR protein levels
In a separate experiment, EC and IC rats were rapidly decapitated on PND 55 and medial prefrontal cortex (mPFC), orbital frontal cortex (OFC), nucleus accumbens (NAc), and amygdala (Amyg) were dissected on an ice-cold plate, flash frozen on dry ice, and stored at −80°C. Lysis buffer was added in a volume of 300 μl to each sample and homogenized with a Teflon pestle. Samples were centrifuged at 23,000g for 20 min and supernatant was collected. Levels of total protein were assessed using a BCA protein assay kit per assay instructions (ThermoFisher Scientific, Pittsburgh PA). Equal amounts of protein from samples (68 μg for NAc and Amyg, and 54.4 μg for mPFC and OFC) were boiled for 15 min at 65°C. Twenty μl of each prepared sample was loaded on a 12% Mini-PROTEAN SDS-polyacrylamide gel (Bio-Rad, Hercules CA) concurrent with a protein standard (Bio-Rad, Hercules CA). Proteins were separated by electrophoresis using a Bio-Rad PowerPac HC Mini-PROTEAN TetraSystem at 95V. Proteins were transferred to a nitrocellulose membrane (Bio-Rad, Hercules CA) at 100V for 60 min. Blots were blocked with 5% nonfat milk (w/v) in 1 × PBS for 60 min. Primary antibody for GR (sc-8992, Santa Cruz Biotechnology, Santa Cruz CA) was added to 5% nonfat milk (w/v) in PBS-Tween20 at 1:500 and was allowed to incubate at 4°C overnight with gentle agitation. On the following day, secondary antibody (926-32211 IR4Dye 800CW, LI-COR, Lincoln NE) was added to 5% nonfat milk (w/v) in PBS-Tween20 at 1:10,000 for 60 min, and optical density was determined using an Odyssey Infrared Imager (LI-COR, Lincoln NE). Antibodies were removed using warm stripping buffer (0.5% SDS/ 67.5% Tris-HCl/ 0.8% β-mercaptoethanol). The blot was then incubated using antibodies specific to the control protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:500; sc-25778, Santa Cruz Biotechnology, Santa Cruz CA) and secondary antibody (1:10,000, 926-32211 I4Dye 800CW; LI-COR, Lincoln NE) to check for loading differences between lanes.
2.5. Statistical Analysis
Data for total infusions and inactive lever presses during the acquisition phase of cocaine self-administration utilized separate 2 (environment; EC or IC) × 17 (session) mixed ANOVAs. Baseline responding, defined as the mean number of infusions earned over the last three days of acquisition at each dose of cocaine, were analyzed using a 2 (environment; EC or IC) × 3 (cocaine dose; 0.03, 0.1, and 0.3 mg/kg/infusion) mixed ANOVA. Baseline inactive lever presses, defined as the mean number of inactive lever presses over the last three days of acquisition at each dose of cocaine, were analyzed using a 2 (environment; EC or IC) × 3 (cocaine dose; 0.03, 0.1, and 0.3 mg/kg/infusion) mixed ANOVA. To analyze the effect of RU486 or vehicle on cocaine self-administration across cocaine doses, infusions earned at each separate dose of cocaine (or vehicle) were converted to percent baseline infusions earned using the formula: (number of infusions earned after pretreatment/mean number of infusions earned over the last 3 days of acquisition of that cocaine dose) × 100. Conversion to percent baseline was analyzed because the effect of RU486 is easier to interpret across multiple cocaine doses where the overall rate of self-administration varies at baseline. The data from RU486 pretreatment were analyzed using a 2 (environment; EC or IC) × 2 (session type; baseline and pretreatment) × 3 (cocaine dose; 0.03, 0.1, and 0.3 mg/kg/infusion) mixed ANOVA. An independent samples t-test was used to measure differences in cocaine self-administration after administration of vehicle. Inactive lever presses for RU486 pretreatment were analyzed using a 2 (environment; EC or IC) × 2 (session type; baseline and pretreatment) × 3 (cocaine dose; 0.03, 0.1, and 0.3 mg/kg/infusion) mixed ANOVA. Inactive lever presses after vehicle were analyzed using a 2 (environment; EC or IC) × 2 (session type; baseline and pretreatment) mixed ANOVA. To maintain consistency with the RU486 analysis, cocaine infusions earned after CORT pretreatment were also converted to percent baseline infusions earned using the formula described above and were analyzed using a 2 (environment; EC or IC) × 2 (session type; baseline and pretreatment) mixed ANOVA. Inactive lever presses after CORT were analyzed using a 2 (environment; EC or IC) × 2 (session type; baseline and pretreatment) mixed ANOVA.
For Experiment 2, optical density of target bands was normalized to GAPDH. Normalized optical density for GR was converted to percent IC density in each brain area by using the formula: (optical density for each rat/mean optical density of IC rats) × 100. Percent IC GR expression was analyzed using separate independent sample t-tests for each brain region. This type of analysis was chosen instead of using a mixed model ANOVA since differences between brain regions could not be assessed given the Western blot design (i.e. different amounts of protein were loaded for different brain areas).
Experiment 1 included data from 6 EC rats and 7 IC rats. Data from 1 EC rat were excluded from Experiment 1 for failure to acquire greater lever pressing on the active (cocaine) lever than the inactive lever. Data from an additional 3 rats were excluded from the CORT portion of the experiment because of loose headmounts. Experiment 2 included data from 10 EC rats and 10 IC rats for mPFC, OFC, and NAc. Data from 1 EC rat was lost from Amyg during dissection; Amyg statistics included data from 9 EC rats and 10 IC rats. For all analyses, Bonferroni’s post hoc tests were used in the presence of significant interactions. P values less than 0.05 were deemed statistically significant.
3. Results
3.1. Experiment 1: Acquisition of Cocaine self-administration
For acquisition of cocaine self-administration, there was a main effect of session F(16, 176) = 15.39, p < 0.0001, but no main effect of environment F(1, 176) = 0.02, p > 0.05, and no interaction F(16, 176) = 1.08, p > 0.05. This indicates that the number of infusions increased across sessions as the dose was lowered from 0.75 to 0.3 mg/kg/infusion, but that there was no difference in the infusion rate between EC and IC rats using the current procedures (Fig. 1a). Analysis of inactive lever presses during the acquisition phase also revealed no significant main effects or interaction (data not shown).
Fig. 1. Baseline cocaine self-administration.
(a) Mean (± SEM) infusions earned during acquisition of cocaine self-administration in EC and IC rats using cocaine unit doses of 0.75 and 0.3 mg/kg/infusion. (b) Mean (± SEM) baseline infusions earned (left) and mean (± SEM) baseline inactive lever presses (right) at 0.03, 0.1, and 0.3 mg/kg/infusion cocaine in EC and IC rats.
3.2. Experiment 1: Effect of RU486 on Cocaine self-administration
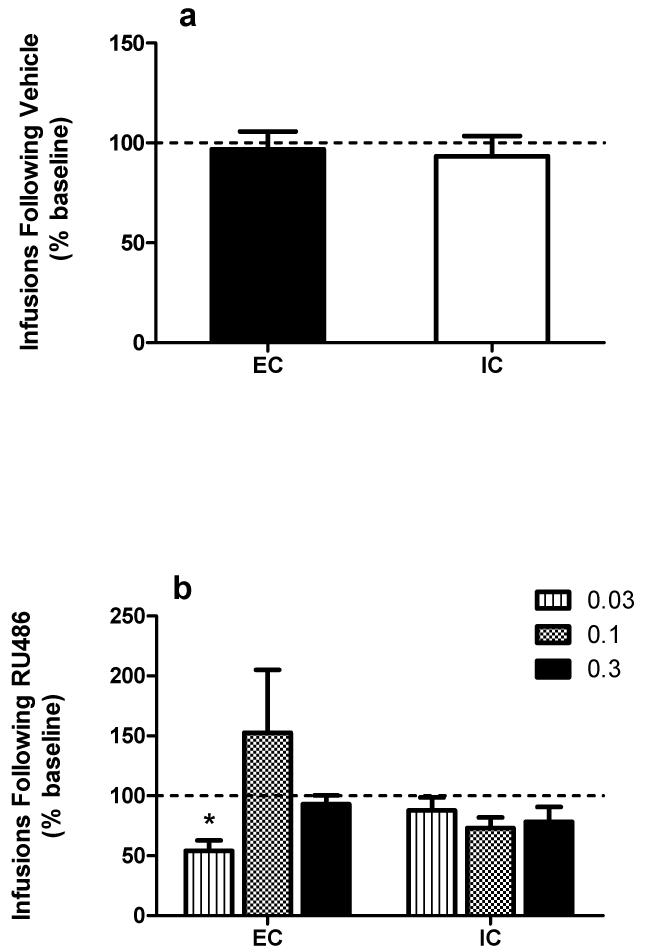
Analysis of the number of infusions at baseline stability in EC and IC rats across each test dose of cocaine revealed a main effect of cocaine dose only (F(2, 22) = 16.52, p < 0.001; Fig. 1b). Additionally, analysis of baseline inactive lever presses at stability revealed no significant main effects or interaction (Fig. 1b). Vehicle injection alone had no effect on the number of cocaine infusions earned, as there was no significant percent change from baseline (t(11) = 0.26, p > 0.05; Fig. 2a). Following RU486, analysis of percent change in infusions earned compared to baseline revealed no main effects of cocaine dose or environment, but revealed a significant interaction between cocaine dose and environment (F(2, 22) = 3.89, p < 0.05). Bonferroni’s post hoc analysis indicated a significant decrease in cocaine self-administration after RU486 pretreatment in EC rats compared to their baseline responding only at the lowest dose of cocaine (0.03 ug/kg/infusion). There were no other significant changes in responding after RU486 in any group at any of the cocaine doses tested (Fig. 2b).
Fig. 2. Effect of RU486 pretreatment on cocaine self-administration.
(a) Percent change in infusions earned (collapsed across cocaine unit dose) after vehicle pretreatment compared to baseline in EC and IC rats. (b) Percent change in infusions earned after RU486 pretreatment in EC and IC rats at 0.03, 0.1, and 0.3 mg/kg/infusion cocaine. * p < 0.05 from EC baseline
To insure that RU486 was not affecting lever pressing in general, number of responses on the inactive lever also were analyzed. Vehicle treatment did not alter inactive lever pressing and there were no main effects or interactions on inactive lever presses after pretreatment with RU486 (data not shown).
3.3. Experiment 1: Effect of CORT on Cocaine self-administration
Infusions earned after CORT pretreatment were converted to percent baseline infusions earned. At a cocaine unit dose of 0.3 mg/kg/infusion, a mixed ANOVA revealed a significant main effect of environment (F(1, 7) = 7.09, p < 0.05) and a significant interaction (F(1, 7) = 7.09, p < 0.05; Fig. 3). Analysis of inactive lever presses revealed no main effects or interactions after CORT pretreatment (data not shown).
Fig. 3. Effect of CORT pretreatment on cocaine self-administration.
Percent change in infusions earned after CORT pretreatment in EC and IC rats at 0.3 mg/kg/infusion cocaine. * p < 0.05 from IC.
3.4. Experiment 2: Western blot analysis of baseline levels of glucocorticoid receptor
Optical density of GR was normalized to the housekeeping protein GAPDH. Normalized GR density was converted to percent IC density for each brain area. There were no environment differences in GR expression in any brain region tested (NAc t(18) = −0.33, p > 0.05; Amyg t(17) = −0.06, p > 0.05; OFC t(18) = −0.06, p > 0.05; and mPFC t(18) = 1.48, p > 0.05; Fig. 4a).
Fig. 4. Total GR expression in stress- and reward-related brain areas.
(a) GR levels in EC rats expressed as percent difference from IC rats in medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), nucleus accumbens (NAc), and amygdala (Amyg). (b) Representative blot from nucleus accumbens
4. Discussion
These experiments revealed differences in cocaine self-administration between EC and IC rats after administration of the GR antagonist RU486 and the GR agonist CORT. Experiment 1 found that EC rats were more sensitive to RU486 than IC rats at a low unit dose of cocaine (0.03 mg/kg/infusion); EC rats self-administered significantly less cocaine after pretreatment with RU486, while IC rats did not significantly change their cocaine intake. CORT also affected cocaine self-administration differentially in EC and IC rats. While CORT did not significantly increase or decrease responding from baseline in either group, CORT pretreatment resulted in an enrichment-induced decrease in cocaine intake compared to isolation rearing, an effect that was not present in the absence of CORT. Experiment 2 found no significant difference between EC and IC rats in GR protein in mPFC, OFC, NAc or Amyg. Thus, taken together, these results indicate that enrichment enhances the response to GR-related signals associated with cocaine reinforcement without directly altering GR levels.
This study did not observe baseline differences in cocaine self-administration between EC and IC rats as observed previously at low unit doses [11]. However, the food pretraining procedure and initial high training dose used in this study (0.75 mg/kg/infusion) was specifically chosen to minimize differences between EC and IC rats. Other work has shown that higher training doses minimize the difference in cocaine self-administration between EC and IC rats [11]. Under these conditions, EC rats showed a reduction in cocaine self-administration following RU486, whereas IC rats did not. The findings with RU486 are consistent with a previous report showing that EC rats are more sensitive than IC rats to the rate decreasing effect of RU486 on amphetamine self-administration at low unit doses [35]. These results are also generally consistent with other work showing that the CORT synthesis inhibitor metyrapone is only effective at decreasing self-administration at low unit doses of cocaine [29]. While RU486 did not significantly alter cocaine self-administration in any group at 0.1 mg/kg cocaine, there was a trend for increased self-administration after RU486 pretreatment in EC rats. It is possible that RU486 might affect cocaine self-administration in a biphasic manner, with RU486 increasing self-administration at doses of cocaine between 0.03 and 0.3 mg/kg.
One limitation of this study is that RU486 is not completely selective for GR, even though it possesses greater affinity for GR than dexamethasone or cortisol. This drug also acts as an antagonist at both progesterone receptors (PR) and androgen receptors (AR) [48]. The behavioral consequences of RU486 are unlikely to be mediated by antagonism of PR, however, as the PR agonist progesterone either decreases self-administration of cocaine [49] or has no effect on cocaine reward using conditioned place preference [50]. Antagonism of AR is also unlikely to cause decreased self-administration of cocaine, as relative affinity of RU486 is lower for AR than for PR or GR [48]. In addition, available results suggest that the AR agonist testosterone does not affect cocaine self-administration in rats [51].
The differential response of EC and IC rats to CORT pretreatment was somewhat unexpected. Since antagonism of GR by RU486 decreased cocaine self-administration in EC rats, we hypothesized that activation of GR by CORT would increase cocaine self-administration in EC rats. This hypothesis was not confirmed. Instead, neither EC nor IC rats decreased their self-administration from baseline after CORT pretreatment. However, CORT lead to a significant reduction in cocaine intake in EC rats compared to IC rats. This environment-induced difference in response to CORT could be due to different amounts of circulating endogenous CORT or different amounts of GR in regulatory brain regions between EC and IC rats. However, the results from Experiment 2 suggest that the latter is not likely, at least not in the brain regions examined (mPFC, OFC, NAc or Amyg).
The dose of CORT used in this study (10 mg/kg) is higher than that used to reproduce physiological stress in standard-caged rats (~3 mg/kg) [52]. However, this dose has been used to increase levels of c-fos in prefrontal cortex [53], a brain region implicated in stress and reward. A higher dose was not tested because repeated injections of 20 mg/kg CORT induces depressive-like behaviors in rat models [54]. Nonetheless, using the current procedures, the difference in EC and IC cocaine intake found after CORT treatment is likely due to IC rats having lower circulating levels of CORT, as shown by others [38-39]. Because their endogenous levels are low, IC rats may require a higher dose of exogenous CORT to mimic physiological stress levels, which are the levels needed to increase cocaine intake [30]. In contrast, because EC rats have higher circulating levels of CORT than standard-caged rats [37], higher doses of CORT could trigger negative feedback of CORT synthesis. This could result in decreased cocaine self-administration, as observed in rats after repeated administration of the GR agonist dexamethasone [55]. One limitation of this study is that the effects of CORT on cocaine self-administration were assessed in the absence of a vehicle control (40% ethanol). However, the amount of ethanol received by each animal in the vehicle injection was low (each rat received ~0.32 g/kg ethanol), a dose known to be below the threshold for producing any decrease in psychomotor performance [56-57] so the effects of CORT injection are likely to have been mediated by CORT.
While this study did not measure levels of endogenous CORT, our laboratory has previously measured CORT in EC and IC rats using an indwelling i.v. catheter and it was found that EC rats have lower baseline CORT compared to IC rats when measured acutely during the early light phase [35]. However, this method of blood extraction raises questions about the results’ validity. Since IC rats remain in their environments with no handling, their CORT response to blood extraction while being restrained by hand might be greater than EC rats’ response to the same procedure. Thus, the CORT response measured by [35] might not accurately reflect baseline levels of CORT, but might instead reflect these animals’ CORT response to handling and blood extraction. To accurately measure baseline levels of CORT in these groups of rats, without any confounding by the blood draw procedure, a different CORT detection method would have to be employed.
While Experiment 2 indicated that there were no significant differences in total baseline GR between EC and IC rats, cellular localization of this receptor was not examined. GR function can differ as a result of its location. Cell surface GR can act on second messengers and induce rapid changes in cell function [58], whereas nuclear GR has actions on a genomic level [59]. While total GR did not differ between EC and IC, the distributions of GR between cytoplasm, cell surface, and nucleus could differ between these groups, which could explain the observed behavioral responses to RU486 and CORT. Further, it is worth noting that GR is not the only target of CORT that is relevant to cocaine reward. CORT activation of the organic cation transporter 3 in NAc enhances cocaine-induced dopamine release in this brain area [60], indicating that this target could contribute to stress-potentiated stimulant reward. Additional studies are needed to measure GR totals in membrane fractions compared to levels in cytoplasm and nucleus and also to measure GR in additional regions implicated in stress, such as the hypothalamus.
5. Conclusions
Together, these studies confirm that EC and IC rats differentially alter their cocaine intake after pharmacological manipulation of GR. While these behavioral differences are not mediated by differences in total GR expression, it is likely that the HPA axis is functioning differently in EC and IC rats. As proposed previously, exposure to novelty, as experienced daily by EC rats, is thought to have an anti-stress effect, thus decreasing drug abuse vulnerability [46]. The current results suggest that the anti-stress effect of enrichment may relate to a more sensitive and adaptive HPA axis in EC rats. In any case, understanding the neurobiological mechanisms underlying environment-dependent differences in drug abuse vulnerability is crucial for the development of more effective prevention and treatment programs.
Highlights.
-
*
Rats were raised in environmental enrichment (EC) or isolation (IC)
-
*
EC rats decreased low-dose cocaine self-administration after pretreatment with RU486
-
*
EC and IC rats significantly differed in responding when pretreated with CORT
-
*
Glucocorticoid receptor protein did not differ between EC and IC in any area tested
Acknowledgements
Funding provided by NIH grants: R01 DA012964, T32 DA016176, T32 DA035200 and F32 DA036291.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Clark DB, Lesnick L, Hegedus AM. Traumas and Other Adverse Life Events in Adolescents With Alcohol Abuse and Dependence. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1744–51. doi: 10.1097/00004583-199712000-00023. [DOI] [PubMed] [Google Scholar]
- [2].Wills TA, Vaccaro D, McNamara G. The role of life events, family support, and competence in adolescent substance use: a test of vulnerability and protective factors. Am J Community Psychol. 1992;20:349–74. doi: 10.1007/BF00937914. [DOI] [PubMed] [Google Scholar]
- [3].SAMHSA . In: Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Administration SAaMHS, editor. Rockville, MD: 2013. [Google Scholar]
- [4].Gruenewald PJ, Johnson FW, Ponicki WR, Remer LG, Lascala EA. Assessing Correlates of the Growth and Extent of Methamphetamine Abuse and Dependence in California. Substance Use & Misuse. 2010;45:1948–70. doi: 10.3109/10826081003682867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hayes-Smith J, Whaley RB. Community Characteristics and Methamphetamine Use: A Social Disorganization Perspective. Journal of Drug Issues. 2009;39:547–76. [Google Scholar]
- [6].Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- [7].Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-Induced Potentiation of Cocaine Reward: A Role for CRFR1 and CREB. Neuropsychopharmacology. 2009;34:2609–17. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goeders N, Guerin G. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- [9].Burke A, Miczek K. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–80. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- [11].Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DEH, Birnbaum SG, et al. Environmental Enrichment Produces a Behavioral Phenotype Mediated by Low Cyclic Adenosine Monophosphate Response Element Binding (CREB) Activity in the Nucleus Accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–7. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–46. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- [14].Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- [15].Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain, Behavior, and Immunity. 2008;22:105–13. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beerling W, Koolhaas JM, Ahnaou A, Bouwknecht JA, de Boer SF, Meerlo P, et al. Physiological and hormonal responses to novelty exposure in rats are mainly related to ongoing behavioral activity. Physiology & Behavior. 2011;103:412–20. doi: 10.1016/j.physbeh.2011.03.014. [DOI] [PubMed] [Google Scholar]
- [17].Jones GH, Marsden CA, Robbins TW. Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behavioural Brain Research. 1991;43:35–50. doi: 10.1016/s0166-4328(05)80050-6. [DOI] [PubMed] [Google Scholar]
- [18].Quan MN, Tian YT, Xu KH, Zhang T, Yang Z. Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in Wistar rats. Neuroscience. 2010;169:214–22. doi: 10.1016/j.neuroscience.2010.04.048. [DOI] [PubMed] [Google Scholar]
- [19].Berrocal Y, Pearse DD, Singh A, Andrade CM, McBroom JS, Puentes R, et al. Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J Neurotrauma. 2007;24:1761–72. doi: 10.1089/neu.2007.0327. [DOI] [PubMed] [Google Scholar]
- [20].Maldonado Bouchard S, Hook M. Psychological stress as a modulator of functional recovery following spinal cord injury. Frontiers in Neurology. 2014;5 doi: 10.3389/fneur.2014.00044. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Raio CM, Brignoni-Perez E, Goldman R, Phelps EA. Acute stress impairs the retrieval of extinction memory in humans. Neurobiology of Learning and Memory. 2014;112:212–21. doi: 10.1016/j.nlm.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Naegeli KJ, O’Connor JA, Banerjee P, Morilak DA. Effects of milnacipran on cognitive flexibility following chronic stress in rats. European Journal of Pharmacology. 2013;703:62–6. doi: 10.1016/j.ejphar.2013.02.006. [DOI] [PubMed] [Google Scholar]
- [23].Fitzgerald PJ. Elevated Norepinephrine May Be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Substance Abuse: Research and Treatment. 2013;7:171–83. doi: 10.4137/SART.S13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Research. 2010;1314:235–52. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gysling K. Relevance of both type-1 and type-2 corticotropin releasing factor receptors in stress-induced relapse to cocaine seeking behaviour. Biochemical Pharmacology. 2012;83:1–5. doi: 10.1016/j.bcp.2011.07.101. [DOI] [PubMed] [Google Scholar]
- [26].Karanth S, Linthorst AC, Stalla GK, Barden N, Holsboer F, Reul JM. Hypothalamic-Pituitary-Adrenocortical Axis Changes in a Transgenic Mouse with Impaired Glucocorticoid Receptor Function. Endocrinology. 1997;138:3476–85. doi: 10.1210/endo.138.8.5331. [DOI] [PubMed] [Google Scholar]
- [27].Kendall JW, Matsuda K, Duyck C, Greer MA. Studies of the Location of the Receptor Site for Negative Feedback Control of ACTH Release. Endocrinology. 1964;74:279–83. doi: 10.1210/endo-74-2-279. [DOI] [PubMed] [Google Scholar]
- [28].Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–48. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- [29].Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Research. 1996;722:145–52. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- [30].Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and Behavioral Effects of Psychostimulants. II: Cocaine Intravenous Self-administration and Reinstatement Depend on Glucocorticoid Levels. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1401–7. [PubMed] [Google Scholar]
- [31].Goeders NE, Peltier RL, Guerin GF. Ketoconazole reduces low dose cocaine self-administration in rats. Drug and Alcohol Dependence. 1998;53:67–77. doi: 10.1016/s0376-8716(98)00108-2. [DOI] [PubMed] [Google Scholar]
- [32].Guerin GF, Schmoutz CD, Goeders NE. The extra-adrenal effects of metyrapone and oxazepam on ongoing cocaine self-administration. Brain Research. 2014;1575:45–54. doi: 10.1016/j.brainres.2014.05.039. [DOI] [PubMed] [Google Scholar]
- [33].Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- [34].Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, et al. The Glucocorticoid Receptor as a Potential Target to Reduce Cocaine Abuse. The Journal of Neuroscience. 2003;23:4785–90. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stairs D, Prendergast M, Bardo M. Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology. 2011;218:293–301. doi: 10.1007/s00213-011-2448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fiancette J-F, Balado E, Piazza P-V, Deroche-Gamonet V. Mifepristone and spironolactone differently alter cocaine intravenous self-administration and cocaine-induced locomotion in C57BL/6J mice. Addiction Biology. 2010;15:81–7. doi: 10.1111/j.1369-1600.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- [37].Moncek F, Duncko R, Johansson BB, Jezova D. Effect of Environmental Enrichment on Stress Related Systems in Rats. Journal of Neuroendocrinology. 2004;16:423–31. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- [38].Djordjevic A, Adzic M, Djordjevic J, Radojcic MB. Stress Type Dependence of Expression and Cytoplasmic-Nuclear Partitioning of Glucocorticoid Receptor, Hsp90 and Hsp70 in Wistar Rat Brain. Neuropsychobiology. 2009;59:213–21. doi: 10.1159/000223733. [DOI] [PubMed] [Google Scholar]
- [39].Sánchez MM, Aguado F, Sánchez-Toscano F, Saphier D. Neuroendocrine and Immunocytochemical Demonstrations of Decreased Hypothalamo-Pituitary-Adrenal Axis Responsiveness to Restraint Stress after Long-Term Social Isolation. Endocrinology. 1998;139:579–87. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- [40].Butler TR, Ariwodola O, Weiner J. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Frontiers in Integrative Neuroscience. 2014;38:2199–207. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- [42].Garrido P, De Blas M, Ronzoni G, Cordero I, Antón M, Giné E, et al. Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: relationship to working and emotional memories. J Neural Transm. 2013;120:829–43. doi: 10.1007/s00702-012-0935-3. [DOI] [PubMed] [Google Scholar]
- [43].Sampedro-Piquero P, Begega A, Arias JL. Increase of glucocorticoid receptor expression after environmental enrichment: Relations to spatial memory, exploration and anxiety-related behaviors. Physiology & Behavior. 2014;129:118–29. doi: 10.1016/j.physbeh.2014.02.048. [DOI] [PubMed] [Google Scholar]
- [44].Olsson T, Mohammed AH, Donaldson LF, Henriksson BG, Seckl JR. Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res Mol Brain Res. 1994;23:349–53. doi: 10.1016/0169-328x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- [45].Lin E-JD, Choi E, Liu X, Martin A, During MJ. Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice. Behavioural Brain Research. 2011;216:349–57. doi: 10.1016/j.bbr.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Progress in Neurobiology. 2010;92:572–92. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [47].Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, et al. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav Pharmacol. 2009;20:312–21. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sitruk-Ware R, Spitz IM. Pharmacological properties of mifepristone: toxicology and safety in animal and human studies. Contraception. 2003;68:409–20. doi: 10.1016/s0010-7824(03)00171-9. [DOI] [PubMed] [Google Scholar]
- [49].Zlebnik N, Saykao A, Carroll M. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology. 2014;231:3787–98. doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Russo SJ, Sun WL, Minerley AC, Weierstall K, Nazarian A, Festa ED, et al. Progesterone does not affect cocaine-induced conditioned place preference or locomotor activity in male rats. Ethn Dis. 2010;20:73–7. [PubMed] [Google Scholar]
- [51].Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of Gonadectomy and Gonadal Hormone Replacement on Cocaine Self-Administration in Female and Male Rats. Neuropsychopharmacology. 2004;29:929–42. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- [52].Scherer IJ, Holmes PV, Harris RBS. The importance of corticosterone in mediating restraint-induced weight loss in rats. Physiology & Behavior. 2011;102:225–33. doi: 10.1016/j.physbeh.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Szakács R, Fazekas I, Mihály A, Krisztin-Péva B, Juhász A, Janka Z. Single-dose and chronic corticosterone treatment alters c-Fos or FosB immunoreactivity in the rat cerebral cortex. Acta Histochemica. 2010;112:147–60. doi: 10.1016/j.acthis.2008.10.001. [DOI] [PubMed] [Google Scholar]
- [54].Iijima M, Ito A, Kurosu S, Chaki S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Research. 2010;1359:75–80. doi: 10.1016/j.brainres.2010.08.078. [DOI] [PubMed] [Google Scholar]
- [55].Mantsch JR, Saphier D, Goeders NE. Corticosterone Facilitates the Acquisition of Cocaine Self-Administration in Rats: Opposite Effects of the Type II Glucocorticoid Receptor Agonist Dexamethasone. Journal of Pharmacology and Experimental Therapeutics. 1998;287:72–80. [PubMed] [Google Scholar]
- [56].Hagues G, Costentin J, Duterte-Boucher D. Modulation of morphine and alcohol motor stimulant effects by cannabinoid receptors ligands. Behavioural Brain Research. 2007;178:274–82. doi: 10.1016/j.bbr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- [57].Steiner MA, Lecourt H, Strasser DS, Brisbare-Roch C, Jenck F. Differential Effects of the Dual Orexin Receptor Antagonist Almorexant and the GABAA-[alpha]1 Receptor Modulator Zolpidem, Alone or Combined with Ethanol, on Motor Performance in the Rat. Neuropsychopharmacology. 2011;36:848–56. doi: 10.1038/npp.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Samarasinghe RA, Witchell SF, DeFranco DB. Cooperativity and complementarity: synergies in non-classical and classical glucocorticoid signaling. Cell Cycle. 2012;11:2819–27. doi: 10.4161/cc.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nishi M, Kawata M. Dynamics of Glucocorticoid Receptor and Mineralocorticoid Receptor: Implications from Live Cell Imaging Studies. Neuroendocrinology. 2007;85:186–92. doi: 10.1159/000101917. [DOI] [PubMed] [Google Scholar]
- [60].Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, et al. Corticosterone Acts in the Nucleus Accumbens to Enhance Dopamine Signaling and Potentiate Reinstatement of Cocaine Seeking. J Neurosci. 2013;33:11800–10. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]