Abstract
Tumor necrosis factor-alpha (TNF-α) inhibitors are effective treatment for juvenile idiopathic arthritis (JIA) but may increase infection rates. However, active JIA may also render patients vulnerable to infection. In this study, we prospectively assessed infection rates in JIA patients treated with and without TNF-α inhibitors and correlated disease activity with infection risk. TNF-α inhibitor-naïve JIA subjects were followed up for 12 months. Subjects initiated on TNF-α inhibitors after enrollment were analyzed in the TNF group. Subjects treated without TNF-α inhibitors were analyzed in the non-TNF group. Questionnaires captured mild or severe infections. JIA disease activity by Childhood Health Assessment Questionnaire (CHAQ) disability index/pain score and physician joint count/global assessment was recorded. Twenty TNF and 36 non-TNF subjects were analyzed. The total infection rate ratio for TNF versus non-TNF group subjects was 1.14 (95 % CI, 0.78–1.66; p=0.51). The average rate of infections per month was 0.29 for TNF and 0.24 for non-TNF subjects. No severe infections or hospitalizations occurred in either group. Secondary infectious outcomes were also similar between groups. Controlling for study group, an increase in CHAQ pain score correlated with an increase in several infectious outcome measures. Our results suggest no difference in infection rates between JIA subjects treated with and without TNF-α inhibitors. Additionally, JIA disease activity may have contributed to infection risk in our cohort, irrespective of immunosuppressive therapy. Future analysis of the relationship between treatment regimens, disease activity, and infection rates may help to further delineate predictors of infection risk in JIA patients.
Keywords: Infection, Juvenile idiopathic arthritis, Tumor necrosis factor inhibitors
Introduction
Tumor necrosis factor-alpha (TNF-α) inhibitors are effective in the treatment of juvenile idiopathic arthritis (JIA) but may increase infection rates due to immunosuppressive effects. However, evaluation of infection rates in JIA patients treated with these agents is relatively limited, and hypothetical risk is often inferred from adult studies of rheumatoid arthritis (RA) patients. Infection rates vary markedly between different studies investigating the use of TNF-α inhibitors in JIA patients [1-7]. Most documented infections are mild, the majority of which are upper respiratory tract infections (URTIs). In two recent reviews, reported rates of mild infections ranged from 8 to 97 % and reported rates of severe infections ranged from 0 to 9 % in pediatric patients treated with TNF-α inhibitors [8, 9]. However, previous studies assessing infection events in JIA patients on TNF-α inhibitors have several weaknesses, including study designs focused on drug efficacy rather than infection rates, limited comparisons of infection types and severity, and limited comparisons to patients on placebo therapy. Additionally, previous studies have not explored whether JIA patients are at increased risk for infection due to their underlying immune-mediated disease, and whether high JIA disease activity may therefore contribute to the risk of infection even in the absence of immunosuppressive therapy.
Adult RA patients may be at increased risk of mild and severe infections in comparison to the general population, even after accounting for treatment with immunosuppressive therapy. Increased rates of infection in RA patients may in part result from intrinsic immunologic disturbances related to RA activity itself [10, 11]. One study of adult RA patients showed that higher disease activity in RA (as measured by the clinical disease activity index [CDAI] and disease activity score 28 [DAS28]) independently increased infection risk [12].
Two recent observational retrospective cohort studies by Beukelman et al. demonstrated that JIA subjects had a twofold increase in rates of hospitalized bacterial infection and opportunistic infection (primarily herpes zoster), in comparison to a healthy cohort (children with attention deficit hyperactivity disorder). Additionally, infection rates in JIA subjects were not significantly higher during treatment with methotrexate or TNF-α inhibitors [13-15]. This suggests that JIA patients, even before treatment with immunosuppressive therapy, may have a higher rate of serious infections than healthy children, possibly due to underlying immune dysfunction. However, the potential contribution of uncontrolled disease activity to risk of infection in JIA patients has not yet been elucidated.
The purpose of this study was to prospectively assess infection rates in JIA patients treated with and without TNF-α inhibitors and to correlate disease activity with infection risk in JIA patients.
Materials and methods
Study design
This prospective cohort study included JIA subjects between the ages of 1 and 21 years from a single center in the USA. Included subjects had a diagnosis of oligoarticular or polyarticular JIA, juvenile spondyloarthropathy, or unspecified juvenile arthritis. All included subjects were TNF-α inhibitor naïve at the time of enrollment. Subjects with systemic onset JIA, primary immunodeficiencies, and acquired immunodeficiencies (such as human immunodeficiency virus or malignancy) were excluded.
Baseline data were collected on all subjects. After enrollment, subjects who were initiated on a TNF-α inhibitor at the discretion of the treating physician were analyzed within the TNF group. Subjects who were treated without TNF-α inhibitors were analyzed in the non-TNF group. Subjects in either group could be treated with nonsteroidal anti-inflammatory drugs (NSAIDs), oral or intra-articular corticosteroids, methotrexate, or other disease-modifying antirheumatic drugs (DMARDs), as deemed necessary by the treating physician. Prior to initiation of a TNF-α inhibitor, all subjects in the TNF group were screened for Mycobacterium tuberculosis (by either purified protein derivative [PPD] test or quantiferon-tuberculosis gold test) and for chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) as clinically indicated. Upon enrollment in the study, all subjects were given an informational sheet explaining how to monitor for signs and symptoms of infection.
Subjects completed seven visits over 12 months. Demographic data, past medical history, and routine laboratory data and serologies (as ordered by the treating physician) were collected on all subjects at the baseline visit. Follow-up visits were then completed at 2–4 and 6–8 weeks, and 3–4, 6, 9, and 12 months after initiation of a TNF-α inhibitor for TNF group subjects. Visits were completed at the same time points after enrollment for non-TNF group subjects. At baseline and each follow-up visit, questionnaires captured infections diagnosed and treatments required. Severe infections were defined as those requiring hospitalization and/or intravenous antimicrobial therapy. Mild infections were identified by medical provider diagnosis or subjective caregiver report based on symptoms such as fever, rhinorrhea, etc. The following secondary infectious outcomes were also assessed: number of missed school days for infection, number of physician sick visits for infection, and number of antimicrobial agents prescribed.
Disease activity was recorded at each visit. Patient measures of disease activity included the Childhood Health Assessment Questionnaire (CHAQ) disability index (scored from 0 to 3.0) and pain score (visual analog scale scored from 0 to 100). Physician measures of disease activity included total joint count (out of 26 possible swollen, tender, or limited joints) and physician global assessment (visual analog scale scored from 0 to 10).
Research visits were completed in person during routine follow-ups with the subject’s treating physician whenever possible. For subjects who were not seen by their treating physician when follow-up was due, surveys were completed by phone or email in order to minimize missing data. For phone and email follow-ups, physician measures of disease activity were therefore not available.
Statistical analysis
Baseline demographics and clinical characteristics were compared using t tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. In order to account for differences in follow-up time between subjects, our primary outcome of total infections in each group was calculated as a rate based on total infection count over total follow-up time. Infection rate ratios and 95 % confidence intervals (CIs) between study groups were then calculated by Poisson regression, adjusting for age group and JIA subtype. All secondary infectious outcome rate ratios were calculated similarly. Longitudinal data, including rate of infection over time, as well as associations between infection and disease activity measures over time, were analyzed using Poisson regression and generalized estimating equations (GEE). Differences were considered significant at the p<0.05 level.
Results
Fifty-eight subjects were enrolled, out of 89 potential subjects originally approached. Initially, the TNF group was comprised of 20 subjects, and the non-TNF group was comprised of 38 subjects. One TNF subject self-discontinued TNF-α inhibitor therapy after 1 month and was therefore analyzed only through 3 months of follow-up in the TNF group. One non-TNF subject was diagnosed with a malignancy during the course of the study and was therefore excluded from all analysis. One non-TNF subject withdrew after the baseline visit and was therefore not included in the final outcome analysis. Our final analysis included 20 subjects in the TNF group and 36 subjects in the non-TNF group. Mean follow-up time was 8.6 months in the TNF group versus 9.4 months in the non-TNF group. Of the subjects included in the final analysis, 11 subjects (55 %) in the TNF group and 22 subjects (61 %) in the non-TNF group completed the full 12 months of follow-up. Within the TNF group, two subjects (10 %) withdrew from the study or were lost to follow-up and seven subjects (35 %) had not completed 12 months of follow-up at the close of the study. Within the non-TNF group, six subjects (17 %) withdrew from the study or were lost to follow-up and eight subjects (22 %) had not completed 12 months of follow-up at the close of the study.
Within the TNF group, 10 subjects were treated only with etanercept and 8 subjects were treated only with adalimumab. Two subjects were treated with different TNF-α inhibitors over the course of the study due to persistent uncontrolled disease (one with etanercept followed by adalimumab, one with adalimumab followed by certolizumab pegol).
There were no significant differences at baseline between the TNF and non-TNF group in regards to male to female ratio, age, race, JIA subtype, comorbidities, and immunization status (Table 1). All TNF subjects had negative screening tests for tuberculosis, and those TNF subjects who were screened for HBV or HCV had no evidence of chronic infection. Two subjects in the TNF group were also treated with methotrexate, while no subjects in the non-TNF group were treated with methotrexate (p=0.05). There were no other significant differences in baseline medications between the two groups, and no subjects in either group were treated with oral or intraarticular steroids. Prior to initiation of TNF-α inhibitors, subjects in the TNF group were noted to have significantly higher baseline disease activity (p<0.05 for all disease activity measures). Mean erythrocyte sedimentation rate (ESR) was 18 mm/h in the TNF group versus 7 mm/h in the non-TNF group (p=0.02). There were no other significant differences in baseline laboratory values or serologies between groups.
Table 1.
Baseline demographics and patient characteristics
| TNF group (n=20) | Non-TNF group (n=37) | p value | |
|---|---|---|---|
| Female | 13 (65 %) | 26 (70 %) | 0.68 |
| Mean age in years (range) | 12.8 (1–20) | 10.8 (3–19) | 0.13 |
| No. of patients age 1–7 | 3 (15 %) | 9 (24 %) | |
| No. of patients age 8–12 | 5 (25 %) | 12 (32 %) | 0.47 |
| No. of patients age 13–21 | 12 (60 %) | 16 (43 %) | |
| White | 17 (85 %) | 36 (97 %) | 0.08 |
| JIA subtype | |||
| Oligoarticular | 1 (5 %) | 7 (19 %) | 0.22 |
| Polyarticular | 4 (20 %) | 3 (8 %) | |
| Spondyloarthropathy | 14 (70 %) | 20 (54 %) | |
| Unspecified/other | 1 (5 %) | 7 (19 %) | |
| Mean length of disease at enrollment in months (SD) | 6.6 (8.4) | 25.1 (37.5) | 0.03 |
| Comorbidities | |||
| Uveitis | 0 (0 %) | 3 (8 %) | 0.19 |
| Inflammatory bowel disease | 0 (0 %) | 1 (3 %) | 0.46 |
| Psoriasis | 2 (10 %) | 2 (5 %) | 0.52 |
| Immunizations up to date | 20 (100 %) | 35 (95 %) | 0.45 |
| Yearly influenza vaccine received | 10 (50 %) | 17 (46 %) | 0.77 |
| Baseline medications | |||
| None | 0 (0 %) | 3 (8 %) | 0.19 |
| NSAIDs | 19 (95 %) | 33 (89 %) | 0.46 |
| Methotrexate | 2 (10 %) | 0 (0 %) | 0.05 |
| Sulfasalazine | 0 (0 %) | 1 (3 %) | 0.46 |
| Steroids (oral or intra-articular) | 0 (0 %) | 0 (0 %) | |
| Baseline disease activitya,b | |||
| CHAQ disability index | 0.8 (0.5) | 0.3 (0.4) | <0.001 |
| CHAQ pain visual analog scale | 43.1 (28.7) | 27.0 (26.8) | 0.04 |
| Total joint count | 6.2 (4.1) | 1.4 (1.8) | <0.001 |
| Physician global assessment | 5.5 (2.1) | 1.6 (1.5) | <0.001 |
| Baseline serologies | |||
| ANA+ | 9 (45 %) | 18 (49 %) | 0.79 |
| RF+ | 2 (10 %) | 7 (19 %) | 0.08 |
| Anti-CCP+ | 2 (10 %) | 3 (8 %) | 0.23 |
| HLA-B27+ | 5 (25 %) | 8 (22 %) | 0.75 |
| Baseline laboratory valuesa | |||
| WBC (count/nl) | 7.0 (1.5) | 7.5 (2.2) | 0.33 |
| Hemoglobin (gm/dl) | 12.6 (1.3) | 13.1 (0.9) | 0.12 |
| Platelets (count/nl) | 312 (64) | 294 (86) | 0.41 |
| ESR (mm/h) | 18 (26) | 7 (8) | 0.02 |
| IgA (mg/dl) | 141 (71) | 147 (68) | 0.77 |
JIA juvenile idiopathic arthritis, SD standard deviation, NSAIDs nonsteroidal anti-inflammatory drugs, CHAQ childhood health assessment questionnaire, ANA antinuclear antibody, RF rheumatoid factor, CCP cyclic citrullinated peptide, HLA human leukocyte antigen, WBC white blood cell, nl nanoliter, gm gram, dl deciliter, ESR erythrocyte sedimentation rate, mm millimeter, IgA immunoglobulin A, mg milligram
Measures reported as mean (SD)
CHAQ disease index range, 0–3.0; CHAQ pain visual analog scale range, 0–100; total joint count range, 0–26; physician global assessment range, 0–10
We found no difference in total infection rates between TNF and non-TNF group subjects over 12 months of follow-up. The total infection rate ratio for TNF versus non-TNF group subjects was 1.14 (95 % CI, 0.78–1.66, p=0.51) (Table 2). TNF group subjects had an average rate of 0.29 infections per month, and non-TNF group subjects had an average rate of 0.24 infections per month. Average infection counts at each visit and throughout total follow-up at 12 months did not differ significantly between TNF and non-TNF subjects (Fig. 1). No severe infections occurred in either group. Secondary infectious outcomes, including rates of physician sick visits, missed school days for infection, subjective infections reported by caregivers, and antimicrobial prescriptions, were also all similar between groups (Table 2). Within the TNF group, total infection rates were also stratified by treatment with adalimumab versus etanercept. The total infection rate ratio for TNF group subjects treated with adalimumab versus etanercept was 1.53 (95 % CI, 0.85–2.75, p=0.16).
Table 2.
Infection rate ratio outcomes in TNF versus non-TNF subjects
| Infection outcome | Rate ratio (TNF versus non-TNF group) | 95 % CI | p value |
|---|---|---|---|
| Total infections | 1.14 | 0.78–1.66 | 0.51 |
| Physician sick visits | 1.33 | 0.87–2.04 | 0.18 |
| Antimicrobial prescriptions | 1.85 | 0.95–3.57 | 0.07 |
| Missed school days for infection | 1.15 | 0.80–1.66 | 0.45 |
| Infections diagnosed subjectively by caregiver | 0.38 | 0.13–1.18 | 0.10 |
Fig. 1.
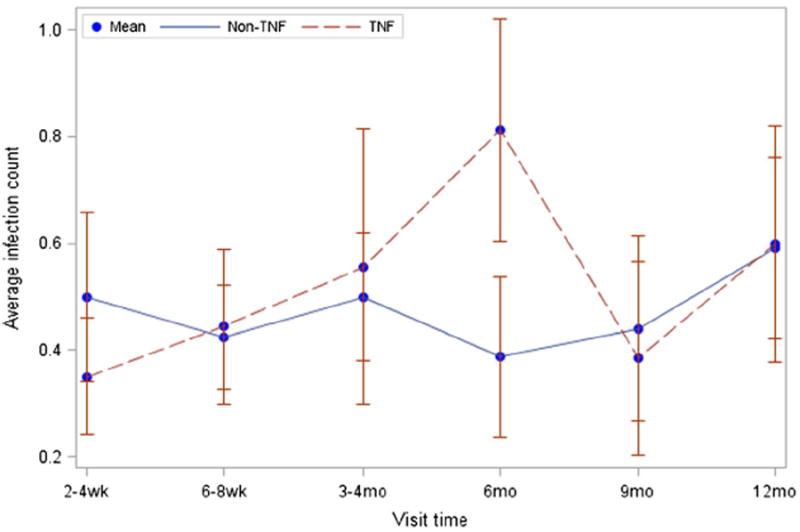
Visit time is time since start of TNF-α inhibition for the TNF group and time since enrollment for the non-TNF group. Each data point represents the average count of infections reported since the prior data point (not cumulative infections). p=0.61 comparing infection counts across visits between groups. p=0.11 at the 6 month visit
Types of infections reported were compared between TNF and non-TNF subjects (Table 3). Viral syndromes were the most commonly reported infections in both groups, affecting approximately 60 % of subjects in each group during the study period. Influenza was diagnosed in three non-TNF subjects (none of whom had received a yearly influenza vaccine), but no TNF subjects. One subject in the non-TNF group developed localized herpes zoster infection versus no subjects in the TNF group. Other infections reported included gastrointestinal infections, skin infections, URTIs (including acute otitis media, sinus infections, and throat infections), and lower respiratory tract infections (including bronchitis and pneumonia). No further microbiologic identification was available. No statistically significant differences in types of infections were noted between TNF and non-TNF subjects. Additionally, no statistically significant difference in the number of subjects affected by any type of infection over the course of the study was noted in the TNF versus non-TNF group.
Table 3.
Types of infection by study group
| TNF group (n=20) | Non-TNF group (n=36) | p value | ||
|---|---|---|---|---|
| Viral infectionsa | Total no. of infections | 24 | 42 | |
| No. of subjects affected | 12 (60 %) | 22 (61 %) | 1.00 | |
| Gastrointestinal infections | Total no. of infections | 8 | 8 | |
| No. of subjects affected | 5 (25 %) | 5 (14 %) | 0.47 | |
| Skin and soft tissue infectionsb | Total no. of infections | 2 | 6 | |
| No. of subjects affected | 2 (10 %) | 5 (14 %) | 1.00 | |
| Upper respiratory tract infectionsc | Total no. of infections | 11 | 20 | |
| No. of subjects affected | 8 (40 %) | 12 (33 %) | 0.77 | |
| Lower respiratory tract infectionsd | Total no. of infections | 4 | 3 | |
| No. of subjects affected | 4 (20 %) | 3 (8 %) | 0.23 | |
| Total infections overall | 49 | 79 | ||
| Total no. of subjects with ANY infection overall | 15 (75 %) | 29 (81 %) | 0.74 |
Includes influenza in 3 non-TNF subjects (versus 0 TNF subjects)
Includes localized herpes zoster infection in 1 non-TNF subject (versus 0 TNF subjects)
Includes ear, sinus, and throat infections
Includes bronchitis and pneumonia
As expected, disease activity was higher at baseline in TNF versus non-TNF subjects. We therefore also sought to correlate disease activity with infection risk in our JIA cohort to evaluate the influence of disease activity upon infection rates in this cohort. While controlling for study group/treatment with a TNF-α inhibitor, for every increase of 1 unit in the CHAQ pain score, the total infection rate increased by 1.1 %, the rate of missed school for infection increased by 2.3 %, and the rate of physician sick visits increased by 1.7 % (p<0.001 for all outcomes). However, other measures of disease activity did not significantly correlate with our primary outcome of total infection rate or any of our secondary infectious outcomes.
Discussion
We observed no significant difference in the rate of infections for JIA patients treated with and without TNF-α inhibitors who were followed prospectively over 12 months. Importantly, there were no severe infections in any JIA subjects within our cohort. Our study suggests that TNF-α inhibitors may not significantly increase the risk of infection (either mild or severe) in JIA patients.
Risk of infection associated with TNF-α inhibitor use may differ between adult and pediatric patients. Some studies have suggested that rates of severe and opportunistic infections are increased in adult RA patients treated with TNF-α inhibitors. However, results are somewhat conflicting, and only a few studies have compared the risk of severe infection in RA patients treated with TNF-α inhibitors to RA patients on no immunosuppressive therapy. Rates of mild infections in these patients are also difficult to assess [16-23].
Studies of infection risk in JIA patients treated with TNF-α inhibitors have also yielded conflicting results. As mentioned above, reported rates of both mild and severe infections are inconsistent, ranging from 8 to 97 % and 0 to 9 %, respectively [8, 9]. In both adult RA and pediatric JIA patients, the use of other DMARDs in addition to TNF-α inhibitors may compound infection risk, making rates of infection attributable specifically to TNF-α inhibitors difficult to determine. However, some studies in JIA patients have shown that the risk of severe infections does not increase significantly with the combination of etanercept and methotrexate in comparison to methotrexate alone [24, 25]. Few studies in JIA patients have assessed the risk of infection associated with TNF-α inhibitor therapy with a comparator group not on immunosuppressive therapy. Our study primarily compared JIA patients on TNF-α inhibitors alone to JIA patients on no immunosuppressive therapy.
As mentioned above, a recent retrospective cohort analysis by Beukelman et al. suggested that JIA patients have an increased risk of bacterial infection requiring hospitalization as compared to children without JIA. Additionally, this study found that the rate of hospitalized infections in JIA patients was not significantly increased during treatment with methotrexate or TNF-α inhibitors [13]. Our prospective study also suggests that overall infection rates may not be significantly different in JIA patients treated with or without TNF-α inhibitors.
Our results differ from many previous studies in that we observed no severe infections requiring intravenous antimicrobials or hospitalization in our JIA cohort. There are many potential explanations regarding the lack of severe infections in our cohort. Compared to previous studies, our sample size was small, and our population may have been healthier at enrollment, with shorter duration of disease prior to TNF-α inhibitor initiation. Unlike some previous studies, we excluded systemic onset JIA patients, and none of our patients were treated with corticosteroids during the course of the study.
Although we observed no severe infections in our cohort, we were able to assess rates of mild infections in JIA subjects treated both with and without TNF-α inhibitors. Many prior studies do not directly address the risk of mild infections in JIA patients, which may still significantly impact quality of life, contributing to missed school days and other undesirable outcomes. When comparing the mild infections reported in our cohort, we found no difference in mild infection rates between JIA subjects treated with and without TNF-α inhibitors.
Prior studies have not compared rates of infection between subjects treated with the different TNF-α inhibitors. Our total infection rate ratio of 1.53 for TNF group subjects treated with adalimumab versus etanercept was not significant (p=0.16). However, this may partially be due to our small sample size. Future studies within larger JIA cohorts should be conducted to more adequately determine the relative risks of infection during treatment with different TNF-α inhibitors.
There was no clear increased risk of a specific type of infection in either group within our cohort. Although our subjects reported primarily common infections, tuberculosis and other opportunistic infections must always be considered in patients being treated with TNF-α inhibitors. While tuberculosis has previously been observed in both adults and children being treated with these agents [26-30], there were no cases of tuberculosis in our study. Other opportunistic infections, such as herpes zoster, have also been described in JIA patients treated both with and without TNF-α inhibitors [15]. We observed one case of herpes zoster in a non-TNF subject. We observed no other opportunistic infections in our cohort. Our results suggest that the risk of opportunistic infections is low in JIA patients, regardless of treatment.
To our knowledge, this is the first prospective study designed to assess infection risk in juvenile idiopathic arthritis patients treated both with and without TNF-α inhibitors. We also investigated the potential association of disease activity with infection risk in children with inflammatory arthritis, which has previously been studied only in adults. In RA patients, disease activity may influence risk of infection, and infection rates may not be explained solely by treatment regimen [12]. JIA patients may have a higher rate of hospitalized bacterial infection than children without JIA, and varying treatment regimens may also not entirely explain this finding [13]. Disease activity may have contributed to risk of infection in our cohort. Subjects with higher CHAQ pain scores had higher total infection rates and higher rates of several secondary infectious outcomes over the course of our study. However, disease activity measures in our cohort were collected at the time of follow-up visits and reported infections typically occurred prior to follow-up visits. The association between high CHAQ pain scores and higher rates of infection outcomes is therefore difficult to interpret and could also simply indicate that JIA patients have increased pain during and after times of infection. None of our other measures of disease activity correlated with infection outcomes. However, our small sample size may have limited our detection of the influence of other disease activity measures upon infection risk. Nevertheless, the influence of JIA disease activity upon risk of infection remains unclear and should be studied further.
Our study had several limitations. Our cohort was from a single center, and our sample size, particularly for the TNF group, was small. Although our sample size was small, this is the first prospective study specifically examining infectious outcomes in children with JIA treated with and without TNF-α inhibitors. In a previous systematic review, we found that many prospective studies in the JIA population similarly included small numbers, yet provided meaningful information about the efficacy of biologics in this population [9]. However, no previous prospective studies have adequately addressed infectious complications. Our prospective study therefore advances the understanding of infection risk in patients with JIA treated both with and without TNF-α inhibitors.
Our dropout/non-completion rate was 41 % due to subjects moving away or changing physicians during the course of the study as well as not completing 12 months of follow-up prior to the end of the study period. Despite this limitation, the mean follow-up time for subjects was still 8.6 months in the TNF group and 9.4 months in the non-TNF group. Analysis of our infectious outcomes, both primary and secondary, took into account varying follow-up times by evaluating these outcomes as rates (events per month).
This study was also observational. Treatment regimens were not controlled, and patients and physicians were certainly not blinded to their treatments. In addition, the self-reporting of infections may have been influenced by recall bias. Importantly, we did not obtain objective measures of infection such as positive cultures or serologic testing to confirm diagnoses but instead relied on self-report by the subject and family. Nonetheless, there was equal reporting of infection in both groups.
In conclusion, although JIA patients treated with TNF-α inhibitors must always be diligently monitored for infection, rates of infection in JIA patients treated with and without TNF-α inhibitors were not significantly different in our cohort. Our results suggest that the presumed increased risk of infection in JIA patients treated with TNF-α inhibitors may in fact not be evident in clinical practice. We must also consider JIA disease activity as a potential contributor to risk of infection, which may influence therapeutic decisions for these patients. Future analysis of the relationship between treatment regimens, disease activity, and infection rates may help to further delineate potential mechanisms and predictors of infection risk in JIA patients.
Acknowledgments
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Weill Cornell Medical College. REDCap is a secure, web-based application designed to support data capture for research studies. Grant support (CTSC GRANT UL1-RR024996) has been used to fund REDCap at this institution. We acknowledge Dr. Zhengming Chen for his contribution to parts of our statistical analysis. We also acknowledge Dr. Farzana Nuruzzaman, Dr. Sarah Taber, and Chahait Singh for their contributions to subject recruitment and data collection.
Footnotes
Disclosures None
Ethical standards This study was approved by the Institutional Review Board at the Hospital for Special Surgery and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study. Informed consent was provided by legal guardians for all minors under the age of 18 years. All persons between the ages of 7 and 17 years gave their assent prior to their inclusion in the study.
Contributor Information
Heather M. Walters, Email: heather.marie.walters@gmail.com, Department of Pediatric Rheumatology, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021, USA.
Nancy Pan, Department of Pediatric Rheumatology, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021, USA.
Thomas J. A. Lehman, Department of Pediatric Rheumatology, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021, USA
Alexa Adams, Department of Pediatric Rheumatology, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021, USA.
Wei-Ti Huang, Department of Epidemiology and Biostatistics, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021, USA.
Lemonia Sitaras, Department of Pediatrics, Weill Cornell Medical College/New York Presbyterian Hospital, 525 East 68th Street, New York, NY 10065, USA.
Susanna Cunningham-Rundles, Department of Pediatrics, Weill Cornell Medical College/New York Presbyterian Hospital, 525 East 68th Street, New York, NY 10065, USA.
Thomas J. Walsh, Department of Pediatrics, Weill Cornell Medical College/New York Presbyterian Hospital, 525 East 68th Street, New York, NY 10065, USA Department of Medicine, Weill Cornell Medical College/New York Presbyterian Hospital, 525 East 68th Street, New York, NY 10065, USA.
Sima S. Toussi, Department of Pediatrics, Weill Cornell Medical College/New York Presbyterian Hospital, 525 East 68th Street, New York, NY 10065, USA
References
- 1.Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–3106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 2.Gerloni V, Pontikaki I, Gattinara M, Fantini F. Focus on adverse events of tumour necrosis factor alpha blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum Dis. 2008;67:1145–1152. doi: 10.1136/ard.2007.069484. [DOI] [PubMed] [Google Scholar]
- 3.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342:763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 4.Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin S, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–1504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 5.Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–820. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 6.Bracaglia C, Buonuomo PS, Tozzi AE, Pardeo M, Nicolai R, Campana A, et al. Safety and efficacy of etanercept in a cohort of patients with juvenile idiopathic arthritis under 4 years of age. J Rheumatol. 2012;39:1287–1290. doi: 10.3899/jrheum.111555. [DOI] [PubMed] [Google Scholar]
- 7.Mori M, Takei S, Imagawa T, Imanaka H, Nerome Y, Higuchi R, et al. Safety and efficacy of long-term etanercept in the treatment of methotrexate-refractory polyarticular-course juvenile idiopathic arthritis in Japan. Mod Rheumatol. 2012;22:720–726. doi: 10.1007/s10165-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 8.Hashkes PJ, Uziel Y, Laxer RM. The safety profile of biologic therapies for juvenile idiopathic arthritis. Nat Rev Rheumatol. 2010;6:561–571. doi: 10.1038/nrrheum.2010.142. [DOI] [PubMed] [Google Scholar]
- 9.Toussi SS, Pan N, Walters HM, Walsh TJ. Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-α inhibitors: systematic review of the literature. Clin Infect Dis. 2013;57:1318–1330. doi: 10.1093/cid/cit489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon T, Testa M, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35:387–393. [PubMed] [Google Scholar]
- 11.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 12.Au K, Reed G, Curtis JR, Kremer J, Jeffrey G, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 13.Beukelman T, Xie F, Chen L, Baddley JW, Delzell E, Grijalva CG, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64:2773–2780. doi: 10.1002/art.34458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurd A, Beukelman T. Infectious complications in juvenile idiopathic arthritis. Curr Rheumatol Rep. 2013;15:327. doi: 10.1007/s11926-013-0327-1. [DOI] [PubMed] [Google Scholar]
- 15.Beukelman T, Xie F, Baddley JW, Chen L, Delzell E, Grijalva CG, et al. Brief report: incidence of selected opportunistic infections among children with juvenile idiopathic arthritis. Arthritis Rheum. 2013;65:1384–1389. doi: 10.1002/art.37866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010;39:327–346. doi: 10.1016/j.semarthrit.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Crum NF, Lederman ER, Wallace MR. Infections associated with tumor necrosis factor-alpha antagonists. Medicine (Baltimore) 2005;84:291–302. doi: 10.1097/01.md.0000180044.19285.9a. [DOI] [PubMed] [Google Scholar]
- 18.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 19.Askling J, Dixon W. The safety of anti-tumour necrosis factor therapy in rheumatoid arthritis. Curr Opin Rheumatol. 2008;20:138–144. doi: 10.1097/BOR.0b013e3282f4b392. [DOI] [PubMed] [Google Scholar]
- 20.Patkar NM, Teng GG, Curtis JR, Saag KG. Association of infections and tuberculosis with antitumor necrosis factor alpha therapy. Curr Opin Rheumatol. 2008;20:320–326. doi: 10.1097/BOR.0b013e3282fa74f7. [DOI] [PubMed] [Google Scholar]
- 21.Salliot C, Gossec L, Ruyssen-Witrand A, Luc M, Duclos M, Guignard S, et al. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology (Oxford) 2007;46:327–334. doi: 10.1093/rheumatology/kel236. [DOI] [PubMed] [Google Scholar]
- 22.Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology (Oxford) 2003;42:617–621. doi: 10.1093/rheumatology/keg263. [DOI] [PubMed] [Google Scholar]
- 23.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 24.Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, et al. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2794–2804. doi: 10.1002/art.24777. [DOI] [PubMed] [Google Scholar]
- 25.Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis. 2009;68:519–525. doi: 10.1136/ard.2007.087593. [DOI] [PubMed] [Google Scholar]
- 26.Kilic O, Kasapcopur O, Camcioglu Y, Cokugras H, Arisoy N, Akcakaya N. Is it safe to use anti-TNF-α agents for tuberculosis in children suffering with chronic rheumatic disease? Rheumatol Int. 2012;32:2675–2679. doi: 10.1007/s00296-011-2030-8. [DOI] [PubMed] [Google Scholar]
- 27.Mohan AK, Coté TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004;39:295–299. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- 28.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 29.Elbek O, Uyar M, Aydin N, Borekci S, Bayram N, Bayram H, et al. Increased risk of tuberculosis in patients treated with antitumor necrosis factor alpha. Clin Rheumatol. 2009;28:421–426. doi: 10.1007/s10067-008-1067-x. [DOI] [PubMed] [Google Scholar]
- 30.Armbrust W, Kamphuis SSM, Wolfs TWF, Fiselier TJW, Nikkels PG, Kuis W, et al. Tuberculosis in a nine-year-old girl treated with infliximab for systemic juvenile idiopathic arthritis. Rheumatology (Oxford) 2004;43:527–529. doi: 10.1093/rheumatology/keh074. [DOI] [PubMed] [Google Scholar]


