Abstract
Background
Oxidative stress activates endothelial innate immunity and disrupts endothelial functions, including eNOS-derived NO bioavailability. Here, we postulated that oxidative stress induces sterol regulatory element binding protein 2 (SREBP2) and microRNA-92a (miR-92a), which in turn activate endothelial innate immune response, leading to dysfunctional endothelium.
Methods and Results
Using cultured endothelial cells (ECs) challenged by diverse oxidative stresses, hypercholesterolemic zebrafish, and Ang II-infused or aged mice, we demonstrated that SREBP2 transactivation of microRNA-92a (miR-92a) is oxidative stress-inducible. The SREBP2-induced miR-92a targets key molecules in endothelial homeostasis, including Sirtuin 1, Krüppel-like factor 2 (KLF2), and KLF4, leading to NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome activation and eNOS inhibition. In EC-specific SREBP2 transgenic mice, locked nucleic acid (LNA)-modified antisense miR-92a (LNA-92a) attenuates inflammasome, improves vasodilation, and ameliorates Ang II-induced and aging-related atherogenesis. In patients with coronary artery disease, the level of circulating miR-92a is inversely correlated with EC-dependent, flow-mediated vasodilation and is positively correlated with serum level of IL-1β.
Conclusions
Our findings suggest that SREBP2-miR-92a-inflammasome exacerbates endothelial dysfunction during oxidative stress. Identification of this mechanism may help in diagnosis and/or treatment of disorders associated with oxidative stress, innate immune activation, and endothelial dysfunction.
Keywords: endothelial cell, microRNA, sterol regulatory element binding proteins, inflammation, oxidative stress, inflammasome, endothelial dysfunction
Introduction
Oxidative stress, imposed by pathophysiological conditions such as hypertension, hyperlipidemia, and aging, triggers inflammatory responses of vascular endothelial cells (ECs). Although not viewed as typical immunogenic cells, ECs are suggested to be sentinel cells that detect danger signals, initiate innate immune responses, produce pro-inflammatory cytokines and chemokines, and recruit immune cells.1,2 Such increase in the endothelial innate immunity has emerged as an important mechanism underlying the interplay among oxidative stress, inflammation, and endothelial dysfunction.
Sterol regulatory element-binding proteins (SREBPs) are master regulators in cholesterol and lipid homeostasis.3 Decreases in the intracellular level of fatty acid or cholesterol activate SREBP1 or SREBP2 through a 2-step proteolytic cleavage, and the resulting mature N-terminal SREBPs transactivate genes involved in cholesterol and lipid synthesis. Recently, SREBPs have been implicated in innate immune responses in vascular cells, due to their regulation of inflammasomes, the intracellular multi-protein complexes mediating the activation of caspase-1 and subsequent maturation of interleukin 1β (IL-1β)/interleukin 18 (IL-18).4 Im et al. showed that SREBP1 activates the NOD-like receptor family, pyrin domain-containing 1 (NLRP1) inflammasome in LPS-stimulated macrophages5 and we reported that SREBP2 activates NLRP3 inflammasome in ECs under disturbed flow.6 Importantly, ectopic expression of SREBP2 in murine endothelium is sufficient to aggravate atherosclerosis, partially through the increased caspase-1-dependent IL-1β production, which suggests a primary role of EC innate immunity in atherogenesis.6 In human aortic sections, activated SREBPs are observed in macrophages and ECs in the atherosclerotic lesions.7 Although these studies suggest that SREBPs are critical regulators in vascular innate immunity, the upstream stress stimuli that induce or activate SREBPs and the SREBP downstream targets that disturb EC homeostasis are largely unexplored.
Stimuli such as disturbed flow and oxidized lipids that impose oxidative stress in ECs increase SREBP levels.6,8,9 They also induce microRNA-92a (miR-92a), a crucial miRNA that inhibits EC angiogenesis and impairs EC function.10–12 At the molecular level, miR-92a targets Krüppel-like factor 2 (KLF2), KLF4, and possibly Sirtuin 1 (SIRT1), all of which are tightly associated with redox balance, eNOS-derived NO bioavailability, and the inflammatory state.10,12–14 In regards to endothelial innate immune response, KLF2, KLF4, and SIRT1 suppress the production or antagonize the effect of IL-1β.15–17 In terms of translational implications, administration of locked nucleic acid (LNA)-modified antisense miR-92a (LNA-92a) prevents ischemic injury in pigs and ameliorates hyperlipidemia-induced atherosclerosis in mice.11,18 Despite the involvement of miR-92a in EC dysfunction, the molecular mechanism underlying stress-induction of miR-92a in ECs and its link to endothelial innate immunity is unclear.
Given the common stimuli (e.g. disturbed flow and oxidized lipids) and convergent functional consequences (i.e. endothelial inflammation and dysfunction) of SREBP2 and miR-92a, we postulated that oxidative stress induces SREBP2 and miR-92a, which in turn activate innate immune response, leading to dysfunctional endothelium. Here, we demonstrate that the SREBP2 transactivation of miR-92a is a ubiquitous response to oxidative stress. Downstream, this redox-sensitive pathway decreases the expression of anti-inflammatory genes, including SIRT1, KLF2, and KLF4, thus resulting in increased inflammasome and impaired eNOS-NO bioavailability. Furthermore, studies of zebrafish, mouse, and human samples suggest an inverse correlation between miR-92a level and functional endothelium.
Methods
Crosslinking immunoprecipitation (CLIP) and chromatin immunoprecipitation (ChIP)
For CLIP, HUVECs were irradiated with UV light at 400 mJ/cm2 to crosslink RNA and proteins. Cells were then lysed in a buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, 1 mM EDTA, and 100 U/μl RNase inhibitor. The lysates were incubated with protein G Dynabeads conjugated with anti-AGO2 (clone 4G8; Wako Chemicals) at 4°C overnight. Mouse IgG was used as an isotype control. The immunoprecipitated RNAs were then extracted with Trizol. For ChIP, HUVECs were treated with 0.75% formaldehyde for 20 minutes at room temperature. After sonication, the SREBP2-bound chromatin was immunoprecipitated by rabbit anti-SREBP2(N) (Abcam) conjugated to protein A Dynabeads. Protein and RNA were degraded by proteinase K and RNase A, respectively. The purified chromatin DNA was then used as the template for qPCR. As an isotype control, rabbit IgG was used in ChIP.
Flow cytometry and NO bioavailability assay
Active caspase-1 was detected in living ECs with use of the Fluorescent Labeled Inhibitor of Caspases (FLICA) caspase-1 assay kit (ImmunoChemistry Technologies). FLICA-FAM-YVAD-FMK is a cell-permeable, non-toxic fluorochrome inhibitor of caspase-1 that interacts with the enzymatic-reactive center of activated caspase-1 via the YVAD recognition sequence, thus forming a covalent thioether adduct with the enzyme through the FMK moiety. The resulting green fluorescence is a direct measure of caspase-1 activity, which was analyzed by FACS with 488-nm excitation and 530-nm emission. NO was detected as the accumulated nitrite/nitrate, the stable breakdown product of NO, in cell culture media by use of a nitrate/nitrite fluorometric assay (Cayman Chemicals).
In vivo Ang II infusion and administration of LNA
All animal experiments were approved by UCSD IACUC. EC-SREBP2(N)-Tg and ApoE−/−/EC-SREBP2(N)-Tg mice were created as described.6 LNAs were designed and synthesized by Exiqon Inc, with the following sequences 5′-AGGCCGGGACAAGTGCAAT-3′ (LNA-92a) and 5′-TAACACGTCTATACGCCCA-3′ (LNA-control). Male ApoE−/−/EC-SREBP2(N)-Tg and their age- and gender-matched ApoE−/− littermates were used for the Ang II infusion and LNA injection. As illustrated in Supplemental Figure 1, one week before Ang II infusion, mice received tail-vein injections of LNA-control or LNA-92a at 16 mg/kg BW, a dose that effectively inhibits miR-92a expression.11 Osmotic minipumps (Alzet, Model 1004) filled with Ang II solution were implanted subcutaneously into the dorsal side of mice. Ang II was released at 1 μg/kg/min for 28-day. The second dose of LNA was given 10 days after the minipump implantation. The animals were sacrificed at the end of 28-day post minipump implantation.
IK17-EGFP Tg zebrafish
Tg(hsp70l:Hsa.IK17-EGFP) zebrafish with temperature-inducible expression of the EGFP-labeled single-chain human mAb IK17 (i.e. IK17-EGFP) was described.19 IK17-EGFP is driven by hsp70 and hence can be induced by heat shock at 37°C. Zebrafish larvae at 5-day post fertilization were fed a normal diet or high-cholesterol diet (HCD) containing 4% cholesterol for 4-week. One group of HCD-fed fish were subjected to heat shock (1 hour at 37°C) once every 3-day to maintain IK17-EGFP expression during the feeding period. The expression of IK17-EGFP was confirmed by microscopy (excitation at 488 nm).
Clinical samples, measurement of circulating miR-92a, IL-1β and flow-mediated dilation (FMD)
All clinical samples were obtained at Taipei Veterans General Hospital, with informed consent and IRB approval (protocol number: 2014-02-002A). The baseline characteristics of patients are summarized in Supplemental Table 1. Sera were collected after an 8-hour overnight fast. Circulating miR-92a level was measured as described.20 IL-1β level was assessed by ELISA (Human IL-1β Quantikine ELISA Kit, R&D Systems). Each standard and plasma sample was analyzed twice, and the mean value was used in all subsequent analyses. Endothelium-dependent FMD was assessed by use of a 7.5-MHz linear array transducer (Hewlett-Packard Sonos 5500) to scan the brachial artery in longitudinal sections, as described21 (Supplemental methods).
Statistical analysis
Data from in vitro experiments are expressed as mean±SD from at least 3 independent experiments, unless otherwise noted. Data from in vivo studies are expressed as mean±SEM. Two groups were compared by Student’s t test. Differences among multiple groups were evaluated by ANOVA followed by the Bonferroni post hoc test for equal sample sizes or Tukey-Kramer test for unequal sample sizes. Correlational analyses were performed using Spearman’s correlation after determining the (non-)normal distribution of the data. Values of p < 0.05 was considered statistically significant.
Supplemental methods
Additional experimental procedures are described in “Supplemental Methods”.
Results
SREBP2 and miR-92a are induced by oxidative stress in ECs
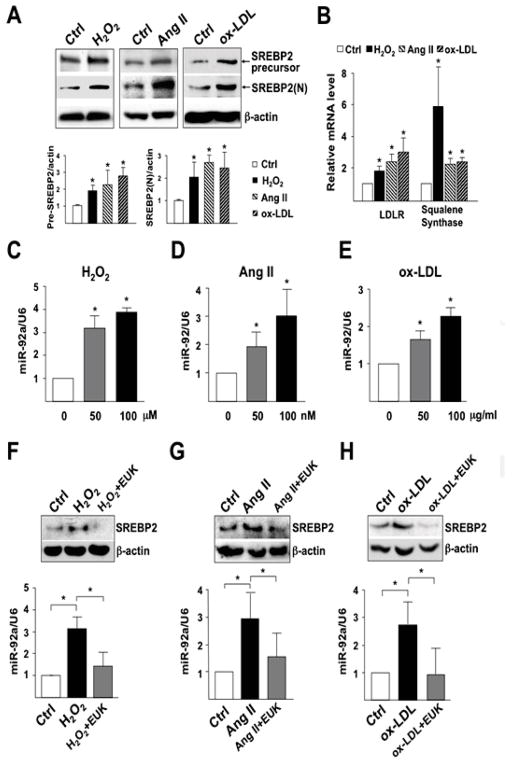
We first sought to test whether SREBP2 and miR-92a are induced by H2O2, Ang II, and oxidized LDL (ox-LDL), all of which directly or indirectly increase ROS in ECs to result in inflammatory responses. All 3 stimuli induced and activated SREBP2 in HUVECs, as evidenced by increased level of the SREBP2 precursor, as well as mature form of SREBP2 [i.e., SREBP2(N)] (Figure 1A). The activation of SREBP2 was associated with induction of SREBP2 transactivation targets such as LDLR and squalene synthase (Figure 1B). Notably, H2O2, Ang II, and ox-LDL also dose-dependently increased the level of miR-92a (Figure 1, C–E). Pretreatment with the ROS scavenger EUK-134, a catalase/SOD mimetic,22 attenuated the H2O2-, Ang II-, and ox-LDL-induced SREBP2 and miR-92a levels (Figure 1, F–H). These results indicate that SREBP2 and miR-92a are induced by oxidative stress in ECs.
Figure 1.
H2O2, Ang II, and ox-LDL induce SREBP2 and miR-92a in ECs. (A, B) HUVECs were treated with H2O2 (100 μM), Ang II (100 nM), or ox-LDL (100 μg/ml) for 16-hour. (A) Cellular proteins were collected for immunoblotting (IB) of SREBP2 precursor and mature form of SREBP2 [SREBP2(N)]. Bar graphs are densitometry quantifications of the ratios of SREBP2 precursor or mature SREBP2 to β-actin level. (B) RNA were collected for RT-qPCR analysis of mRNA encoding LDLR and squalene synthase. (C–E) Taqman miRNA qPCR analysis of miR-92a level in HUVECs treated with various concentrations of H2O2, Ang II, and ox-LDL for 16-hour. (F–H) HUVECs were pretreated with EUK-134 (1 μM) for 2-hour and then incubated with H2O2, Ang II or ox-LDL for 16-hour. SREBP2 was detected by IB and miR-92a level by Taqman miRNA qPCR. Data are mean±SD from at least 3 independent experiments. * p < 0.05 compared to respective control or between indicated groups.
SREBP2 transactivates miR-92a under oxidative stress
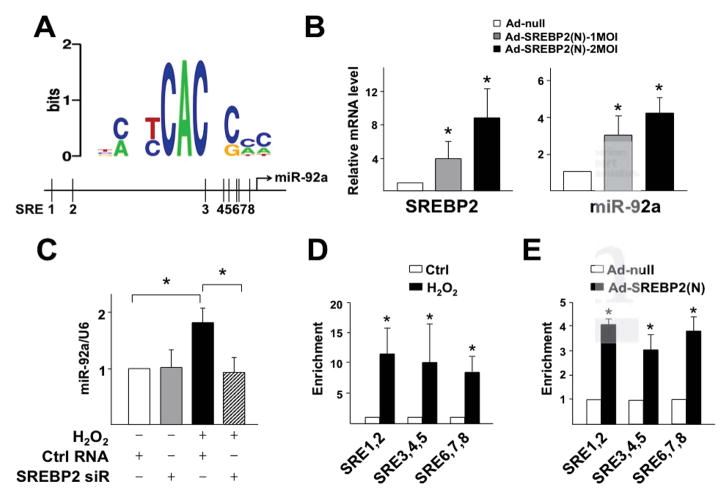
To examine whether SREBP2 transactivates miR-92a in response to oxidative stress, we performed bioinformatics analysis for putative sterol regulatory elements (SREs) in the promoter region of the miR-17-92 cluster. We found 8 SREs in the promoter region of the human miR-17-92 cluster, which are conserved in the mouse gene (Figure 2A; Supplemental Table 2). To validate this in silico prediction, we overexpressed the active/mature form of SREBP2, i.e. SREBP2(N) in ECs, which increased the level of miR-92a (Figure 2B). Conversely, knockdown of SREBP2 with siRNA inhibited H2O2-induced miR-92a (Figure 2C). To demonstrate enhanced transactivation of miR-92a by SREBP2 under oxidative stress, we performed ChIP assays to assess the direct binding of SREBP2 to SREs in the miR-17-92 promoter. H2O2 treatment of ECs or ectopic expression of SREBP2(N) in ECs, mimicking SREBP2 induction by oxidative stress, substantially enriched SREBP2(N) binding to segments of miR-92a promoter containing the predicted SREs (Figure 2, D and E). Thus, oxidative stress-activated/induced SREBP2 transactivates miR-92a.
Figure 2.
SREBP2 transactivates miR-92a under oxidative stress. (A) Bioinformatics analysis of SREs in the promoter region of human miR-17-92 cluster. (B) RT-qPCR and Taqman miRNA qPCR analyses of SREBP2 and miR-92a levels in HUVECs infected with Ad-null or Ad-SREBP2(N). (C) miR-92a level in HUVECs transfected with SREBP2 siRNA (10 nM) or control RNA and then treated with H2O2. (D,E) ChIP assays were performed with SREBP2 antibody or a nonspecific IgG in extracts from HUVECs treated with H2O2 (in D) or infected with Ad-SREBP2(N) (in E). The enrichment of SREBP2(N) binding to the putative SREs in the promoter region of miR-17-92 was quantified by qPCR, with non-treated group (in D) or Ad-null group (in E) set to 1. * p < 0.05 compared to respective control or between indicated groups.
Oxidative stress-induced miR-92a increases endothelial innate immunity
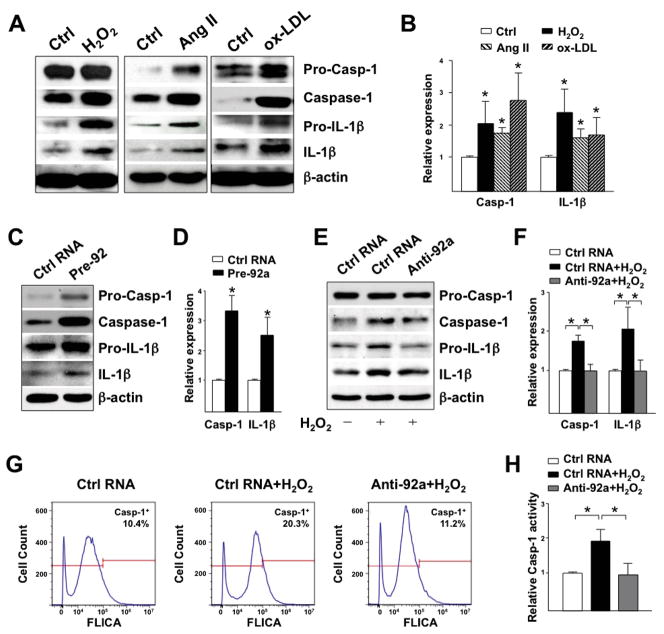
Recent findings suggest that SREBP induction or activation increases innate immunity, specifically, via NLRP3 inflammasome activation in ECs and macrophages.5,6 Because oxidative stress activates SREBP2-miR-92a (Figures 1 and 2), we examined whether oxidative stress activates inflammasome, and if so, the role of miR-92a in this innate immune response in ECs. H2O2, Ang II, and ox-LDL all increased the cleaved form of caspase-1 and IL-1β, hallmarks of inflammasome activation (Figure 3, A and B). Importantly, pre-miR-92a, mimicking miR-92a induction by SREBP2, also increased the cleavage of caspase-1 and IL-1β (Figure 3, C and D). Conversely, transfecting ECs with anti-miR-92a blocked these inflammasome-related events in H2O2-stimulated ECs (Figure 3, E and F). These results were further confirmed by using flow cytometry to detect the active caspase-1 in living cells (Figure 3, G and H). Therefore, the SREBP2-miR-92a axis contributes to oxidative stress-induction of the endothelial innate immune response, as evidenced by inflammasome activation.
Figure 3.
Oxidative stress-induced miR-92a increases endothelial innate immunity. IB of caspase-1 and IL-1β in HUVECs (A) treated with H2O2 (100 μM), Ang II (100 nM) or ox-LDL (100 μg/ml) for 16-hour; (C) transfected with control RNA or pre-miR-92a (20 nM) for 72-hour; or (E) transfected with control RNA or anti-miR-92a for 48-hour before H2O2 treatment for 24-hour. Quantification in (B,D,F) is relative expression of caspase-1 and IL-1β, to that of β-actin. (G,H) Flow cytometry quantification of active caspase-1 in HUVECs transfected as in (E). Histograms in (G) are representative results and data in (H) are mean±SD from 5 independent experiments, with the percentage of caspase-1+ cell in control RNA group set to 1.
miR-92a targeting SIRT1, KLF2, and KLF4 increases endothelial innate immunity but decreases NO bioavailability
Oxidative stress-induced miR-92a should target KLF2, KLF4 (previously validated)12,14 and SIRT1 mRNAs (previously predicted)10, to result in increased IL-1β production but decreased NO bioavailability. Thus, we first verified the direct targeting of the SIRT1 3′ untranslated region (3′UTR) by miR-92a (Supplemental Figure 2A). Using a luciferase reporter containing SIRT1 3′UTR or its mutant, we confirmed that miR-92a directly targets SIRT1 mRNA (Supplemental Figure 2, B and C), which agrees with pre-miR-92a-decreased levels of SIRT1 mRNA, protein, and activity (revealed by increased acetylation of SIRT1 substrates p53 and NF-κB) (Supplemental Figure 2, D and E).
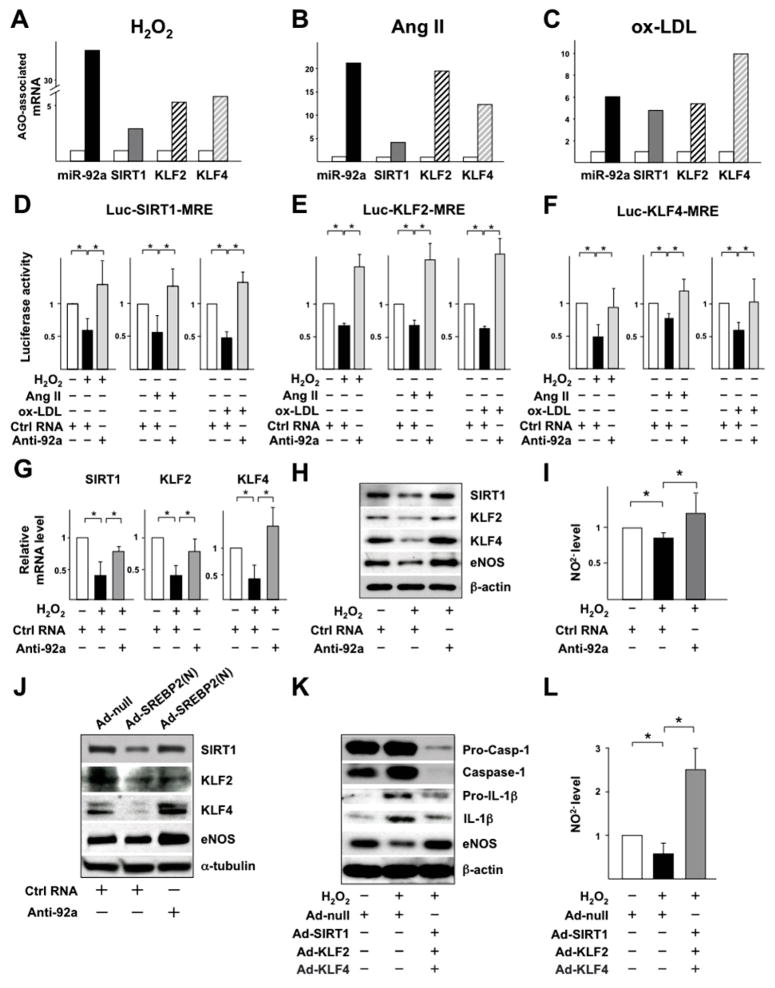
We next assessed whether oxidative stress increases the recruitment of miR-92a and its targeted SIRT1, KLF2, and KLF4 mRNAs to Argonaute (AGO)-mediated-miRNA-induced silencing complex (miRISC) in ECs. H2O2, Ang II, or ox-LDL treatment increased the association of miR-92a, SIRT1, KLF2, and KLF4 mRNAs with miRISC in ECs (Figure 4, A–C). As a control, H2O2 did not change the association of miR-92a with non-specific RNAs such as α-tubulin mRNA and 5S ribosomal RNA (5S rRNA) (data not shown). In agreement with the co-enrichment of miR-92a and its target mRNAs in miRISC, these stimuli decreased the luciferase activity in ECs transfected with luciferase reporters conjugated to the 3′UTR of SIRT1, KLF2, and KLF4 containing the miR-92a binding/responsive elements (MREs) as compared with untreated cells. This reduction of luciferase activity was restored by co-transfection of anti-miR-92a (Figure 4, D–F). Functionally, anti-miR-92a ameliorated the H2O2-decreased mRNA and protein levels of SIRT1, KLF2, and KLF4 as well as eNOS expression and activity (Figure 4, G–I). The suppression of SIRT1, KLF2, and KLF4 by H2O2 was mimicked by SREBP2(N) overexpression, whereas co-transfection with anti-miR-92a restored the levels of SIRT1, KLF2, and KLF4 (Figure 4J). Furthermore, the effect of ectopic expression of SIRT1, KLF2, and KLF4 was similar to anti-miR-92a in reversing the H2O2-induced EC inflammation (i.e., caspase-1 activation and IL-1β production) and H2O2-impaired NO bioavailability (Figure 4, K and L). Collectively, oxidative stress induction of SREBP2-miR-92a and the subsequent SIRT1, KLF2, and KLF4 targeting increases endothelial innate immunity and impairs eNOS-NO bioavailability, two key features of endothelial dysfunction.
Figure 4.
Oxidative stress-induced miR-92a targets SIRT1, KLF2, and KLF4 to increase endothelial innate immunity. (A–C) Following 16-hour treatment of H2O2, Ang II, or ox-LDL, AGO-miRISC was immunoprecipitated and the AGO-bound miR-92a and SIRT1, KLF2 and KLF4 mRNAs were quantified by RT-qPCR. Mouse IgG was used as an isotype control. (D–F) BAECs were transfected with luciferase reporter containing 5 tandem miR-92a binding/responsive elements (MREs) in SIRT1 3′UTR, 2 tandem MREs in KLF2 3′-UTR, or KLF4 3′-UTR together with anti-miR-92a or control RNA for 24-hour and then treated with H2O2, Ang II, or ox-LDL for 16-hour. Luciferase activity was measured and normalized to that of Renilla. (G–I) HUVECs were transfected with anti-miR-92a or control RNA for 48-hour before H2O2 treatment. (J) HUVECs were infected with Ad-null or Ad-SREBP2(N) before transfection with control RNA or anti-miR-92a. (K, L) HUVECs were infected with Ad-null or Ad-SIRT1, Ad-KLF2, and Ad-KLF4 together for 48 h, and then treated with H2O2 for 24-hour. The mRNA levels of SIRT1, KLF2 and KLF4 were assessed by RT-qPCR (G). The levels of proteins shown in (H, J, K) were revealed by IB. (I, L) The NO level in the medium was measured by a fluorometric assay (I, L).
SREBP2 induction of miR-92a leads to impaired EC functions in the vessel wall
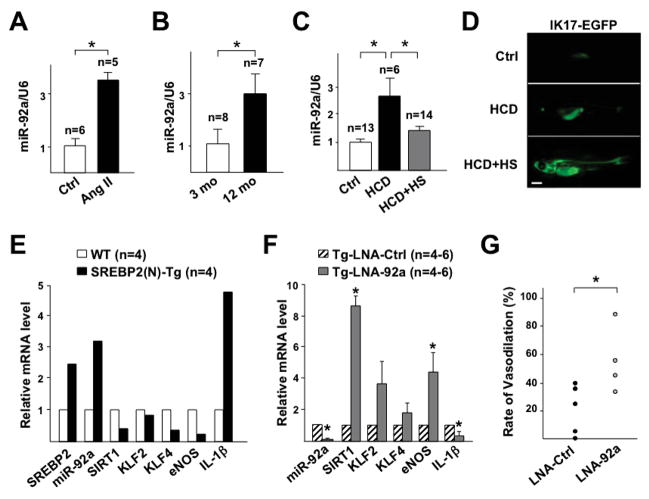
We next examined whether miR-92a is oxidative stress-inducible in the vessel wall in vivo in mouse and zebrafish models. The aortic miR-92a level was higher in the Ang II-infused C57BL6 mice than normotensive controls (Figure 5A). Consistent with increased oxidative stress in the aging vessel,23,24 the miR-92a level in the aortas was also higher in 12-than 3-month-old C57BL6 mice (Figure 5B). We also used a hypercholesterolemic zebrafish model with accelerated vascular accumulation of oxidized lipids with enhanced oxidation-specific epitopes (OSEs).19,25 Four-week HCD significantly increased miR-92a level in the trunks, where major blood vessels are located. This induction was substantially inhibited in zebrafish expressing IK17, a human mAb against malondialdehyde-LDL and ox-LDL19,26 (Figure 5, C and D). Thus, miR-92a was also induced in the zebrafish in an oxidative stress-dependent manner. To examine whether SREBP2 increases miR-92a in the endothelium in vivo, we assessed the miR-92a level in intima isolated from EC-SREBP2(N)-Tg mice with the active form of SREBP2 overexpressed only in the endothelium.6 Ectopic expression of SREBP2(N) increased the miR-92a level and decreased mRNA level of SIRT1, KLF2, KLF4, and eNOS in the intima (Figure 5E). Furthermore, an endothelial innate immune response was induced, as evidenced by increased IL-1β mRNAs level in EC-SREBP2(N)-Tg mice (Figure 5E). Consistent with the reduced eNOS expression, flow-mediated vasodilation, a hallmark of endothelial dysfunction was attenuated (Supplemental Figure 3). To address whether miR-92a mediates the detrimental effects of SREBP2 in the vessel wall, we inhibited miR-92a with LNA in the carotid arteries of EC-SREBP2(N)-Tg mice. While miR-92a was significantly inhibited by LNA, the mRNA levels of SIRT1, KLF2, KLF4, and eNOS were substantially increased and that of IL-1β decreased in mice receiving LNA-92a as compared with control LNA (Figure 5F). Functionally, LNA-92a administration improved the flow-mediated vasoreactivity (Figure 5G).
Figure 5.
Oxidative stress induction of miR-92a and inflammasome in vivo. (A,B) Taqman miRNA qPCR of miR-92a level in whole aortas isolated from 8-week-old C57BL6 with or without Ang II infusion (A) and C57BL6 at 3 month- and 12 month-old (B). (C,D) hsp70:IK17-EGFP Tg zebrafish were fed a normal diet or HCD. IK17-EGFP was heat shock-induced (HS) in one group of HCD-fed fish. The trunk regions were collected for miR-92a qPCR (C). IK17 induction was confirmed by visualization of EGFP (D). Bar = 0.1 cm. (E,F) RT-qPCR analysis of miR-92a and mRNA levels of various genes in intima isolated from EC-SREBP2(N)-Tg and that from wild-type littermates (WT) pooled from 4 animals in each group (E) and carotid arteries of EC-SREBP2(N)-Tg with local delivery of control LNA (LNA-Ctrl) or LNA-92a (F). (G) Vasodilatory function of carotid arteries from EC-SREBP2(N) Tg mice treated as in (F). n denotes number of animals used and * indicates p < 0.05 compared to respective control or between indicated groups.
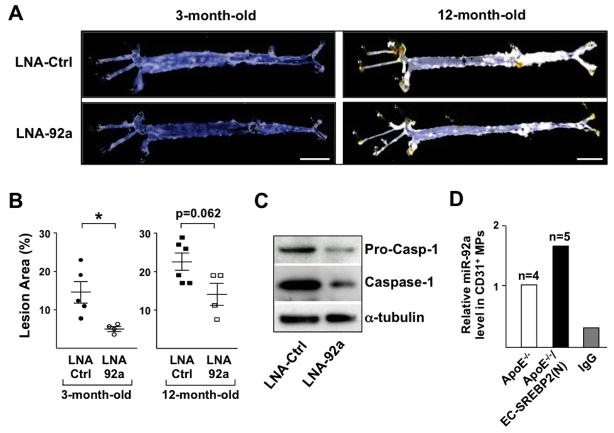
To evaluate the detrimental effect of the SREBP2-miR-92a pathway at the disease level, we used a model of EC-SREBP2(N)-Tg in an ApoE−/− background treated with Ang II (1 μg/min/kg). With a regular diet, Ang II infusion accelerates atherosclerosis.27 Thus, the etiology of atherosclerosis in this model is due mainly to the oxidative stress-induced endothelial dysfunction rather than diet-induced hyperlipidemia. The experimental design is outlined in Supplemental Figure 1, and the efficacy of LNA-92a was confirmed by the decreased miR-92a but increased mRNA levels of miR-92a targets, i.e., SIRT1, KLF2, and KLF4, as well as eNOS (Supplemental Figure 4A). Noticeably, atherosclerosis, including overall lesion size and that in the aortic arch, was reduced in mice treated with LNA-92a as compared with control LNA. Furthermore, the inhibitory effect of LNA-92a on atherosclerosis was apparent in both 3- and 12-month-old animals (Figure 6, A and B, Supplemental Figure 4, B and C). Lung ECs from LNA-92a-treated mice showed decreased inflammasome activity, as indicated by the decreased expression and cleavage of caspase-1 (Figure 6C). The miR-92a level in CD31+ microparticles (MPs) collected from sera was higher in Ang II-challenged ApoE−/−/EC-SREBP2(N) mice than their ApoE−/− littermates (Figure 6D), which suggests that the SREBP2-induced miR-92a in the endothelium was released into the circulation.
Figure 6.
Inhibition of miR-92a mitigates oxidative stress-induced atherosclerosis in mice. (A, B) En face imaging of mouse aorta and quantification of lesion area in ApoE−/−/EC-SREBP2(N) mice fed normal chow and infused with Ang II (1 μg/min/kg) for 28-day. The age of mice and tail-vein injection of LNA are as indicated. Bar = 0.5 cm. (C) Caspase-1 in lung ECs isolated from 3-month-old ApoE−/−/EC-SREBP2(N) mice receiving LNA-Ctrl or LNA-92a was detected by IB. (D) miR-92a was detected in CD31+ microparticles (MPs) isolated from sera of 3-month-old ApoE−/− or ApoE−/−/EC-SREBP2(N) mice treated with Ang II. IgG was used as an isotype control in MP isolation from sera of ApoE−/−/EC-SREBP2(N) mice.
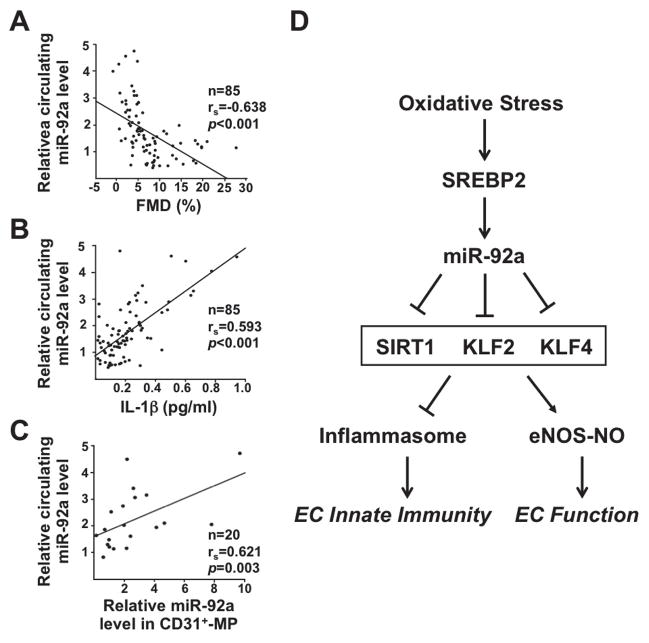
miR-92a level is inversely correlated with EC function in patients
To investigate whether circulating miR-92a level is associated with EC function in human patients, we assessed the serum miR-92a level in relation to FMD, the clinical readout for endothelial function.28 The circulating level of miR-92a was indeed inversely correlated with FMD in 85 patients with diagnosed stable coronary artery disease (CAD) (Figure 7A). Conversely, the levels of miR-92a and IL-1β were positively correlated in these patients (Figure 7B). Furthermore, the level of circulating miR-92a was positively correlated with miR-92a abundance in the CD31+ MPs (Figure 7C), which suggests that miR-92a in circulation in part originats from the endothelium. Therefore, the serum level of miR-92a could indicate endothelial dysfunction and innate immunity under certain cardiovascular events.
Figure 7.
miR-92a inversely correlates with EC function in human patients. (A–C) Correlations between circulating miR-92a level and (A) FMD; (B) serum IL-1β concentration; and (C) miR-92a level in serum CD31+ MPs from patients with stable CAD. While n denotes number of patients, rs is Spearman’s correlation coefficient. (D) Schematic illustration of the hypothesis “oxidative stress activates EC innate immune response and impairs EC function through induction of SREBP2-miR-92a”. SREBP2 transactivation of miR-92a, through targeting SIRT1, KLF2, and KLF4, activates inflammasome and inhibits eNOS-derived NO bioavailability. Therefore, SREBP2-miR-92a-inflammasome may be a crucial pathway linking oxidative stress, inflammation, and endothelial dysfunction.
Discussion
Here, we demonstrate that oxidative stress activates the endothelial innate immune response by inducing SREBP2-miR-92a. This finding presents a novel function of SREBP2 in addition to its canonical role in cholesterol synthesis and uptake. The induction of this pathway is a common response of ECs to disturbed flow, H2O2, Ang II, ox-LDL, and oxidized palmitoyl-arachidonylphosphatidyl choline (ox-PAPC), all of which cause oxidative stress6,12 (Figure 1, and Supplemental Figure 5). This response is attenuated when ECs are pretreated with the ROS scavengers SOD/catalase mimetic EUK-134 or cell-permeable SOD (Figure 1, Supplemental Figure 5), which suggests that the SREBP2-miR-92a pathway is redox-sensitive. In vivo, miR-92a is induced in the mouse aorta with increased oxidative stress from Ang II infusion or aging (Figure 5, A and B). Similarly, miR-92a is induced in zebrafish by HCD (Figure 5C). Because IK17 antagonizes ox-LDL19,29, the IK17 inhibition of miR-92a further indicates that oxidized lipids and lipoproteins likely induce miR-92a in zebrafish. Together, these in vitro and in vivo findings strongly suggest that oxidative stress-induced miR-92a is a conserved mechanism among vertebrates. Consistent with this conclusion is the observation that miR-17-92 promoters from human, mouse, and zebrafish all contain multiple putative SREs (Supplemental Tables 2 and 3). Intriguingly, when ECs are supplemented with 25-hydroxycholesterol (25-HC) which blocks the canonical cholesterol-sensing pathway, ox-PAPC and disturbed flow are still able to activate SREBP23 (Supplemental Figure 5C). Thus, oxidative stress induction of miR-92a via SREBP2 differs from miR-33 induction, which responds to sterol depletion.30 As an SREBP2 intronic miRNA, miR-33a targets the cholesterol transporters ABCA1 and ABCG1 for restoration of cellular cholesterol homeostasis.30 However, miR-92a downregulates KLF2, KLF4, and SIRT1 to affect endothelial innate immunity and function. Thus, the redox-sensitive SREBP2, by transactivating miR-92a, mediates the innate immune response in ECs under oxidative stress (Summarized in Figure 7D). Because the endothelium does not readily accumulate lipid and cholesterol, these results suggest that the major functional relevance of oxidative stress-induction of SREBP2 in ECs is increased innate immunity rather than cholesterol synthesis, uptake, and storage.
The miR-17-92 cluster is upregulated by c-Myc and NF-κB in cancer cells, fibroblasts, and epithelial cells.31,32 Although STAT3 regulates miR-92a in ECs,11 whether STAT3 directly transactivates miR-92a is unknown. Given that (a) NF-κB upregulates SREBP2,33 (b) STAT3 crosstalks with NF-κB,34 and (c) SREBP2 and c-Myc are both basic helix-loop-helix transcription factors and likely share transactivating targets that contain E-box in their promoter regions,35 SREBP2 is likely involved in a network regulating miR-92a. Indeed, the H2O2-induced miR-92a is attenuated in ECs if RelA (i.e., NF-κB p65 subunit) or c-Myc has been knocked down (Supplemental Figure 6A). Because toll-like receptors (TLRs) are key mediators in innate immunity and most, if not all TLRs are expressed in ECs, we have also examined the TLR pathway involved in the oxidative stress-induced miR-92a. When we inhibited myeloid differentiation factor 88 (Myd88), a common signaling adaptor downstream of TLRs except TLR3, the ox-LDL-induced miR-92a was suppressed (Supplemental Figure 6B). In line with this, Myd88 inhibition also attenuated the activation and induction of SREBP2 in LPS-stimulated macrophages and the consequent foam cell formation.33 Therefore, SREBP2-miR-92a may act as a critical nexus linking oxidative stress and endothelial innate immune response and subsequent inflammation.
Most, if not all, oxidative insults suppress SIRT1, KLF2, and KLF4, as well as uncouple eNOS in ECs.14,36–38 Because of the positive effect of SIRT1, KLF2, and KLF4 on the NO bioavailability, the finding that SREBP2-miR-92a suppresses the expression of SIRT1, KLF2, and KLF4 (Figures 4, 5) reveals a post-transcriptional mechanism by which oxidative stress diminishes NO bioavailability, thus resulting in endothelial dysfunction. Another new finding is the SREBP2-miR-92a induction of inflammasome in ECs (Figure 3). Given that SIRT1 deacetylates and inhibits NF-κB39 (Supplemental Figure 2) and that KLF2 and KLF4 suppress both IL-1β expression and IL-1β-mediated inflammatory responses,15,16 miR-92a-induced innate immune responses (i.e., inflammasome and IL-1β induction) likely also involve targeting SIRT1, KLF2, and KLF4. Indeed, ectopic expression of these genes reversed H2O2-stimulated inflammasome activation (Figure 4K). Notably, SIRT1 can deacetylate and inhibit SREBP2.40 Hence, the miR-92a suppression of SIRT1 would further potentiate SREBP2 and inflammasome under oxidative stress. Interestingly, activated caspase-1 proteolytically cleaves not only pro-IL-1β but also SIRT1.41 While increasing mature IL-1β production and decreasing the level of SIRT1, the oxidative stress-induced miR-92a may provoke a vicious loop of innate immune responses in ECs via inflammasome-dependent activation of caspase-1.
Within the pathophysiologic context, 3 factors would contribute to increased vascular oxidative stress in ApoE−/−/EC-SREBP2(N)-Tg mice receiving Ang II (Figure 6). First, Ang II accelerates atherogenesis in ApoE−/− mice with increased oxidative stress without altering the lipid profile.27 Second, ectopic expression of SREBP2(N) in the endothelium increases miR-92a level (Figure 5C), which enhances the innate immune response in the intima,3 independent of other immuno-sensitive cells. Third, aging is an independent factor contributing to increased oxidative stress in the vessel wall.23 Noticeably, either in LNA-control or LNA-92a group, aging still aggravates atherosclerotic lesion area (Figure 6, A and B; Supplemental Figure 4B). This observation suggests that part of the atherosclerotic process in adulthood is less dependent on miR-92a. Because atherogenesis is a complex and multi-factorial process, the aging-induced SREBP2-miR-92a may still be involved in atherosclerosis. Indeed, both SREBP2 and miR-92a levels are increased in the aortas of 12-month-old mice relative to 3-month-old mice (Figure 5B, Supplemental Figure 7). Thus, we could not rule out the possibility that Ang II may induce higher level of miR-92a in 12 month-old mice, which may require higher degree of miR-92a inhibition to ameliorate the lesion development. In terms of the therapeutic potential of targeting miR-92a in treating cardiovascular impairments, Bonauer et al demonstrated that antagomiR-92a significantly improves reperfusion in an ischemic hindlimb model;10 Hinkel et al showed that LNA-92a reduces infarct size and preserves cardiac function in a porcine ischemia/reperfusion model;18 and Loyer et al. found that LNA-92a attenuates atherogenesis in hypercholesterolemic LDLR−/− mice.11 These therapeutic effects involving miR-92a suppression are probably associated with ameliorated endothelial inflammation and dysfunction. To this end, we observed a pleiotropic effect of LNA-miR-92a in decreasing blood pressure and incidence of aneurysms (data not shown) for which endothelial dysfunction is an independent risk factor.
The increase in miR-92a level in CD31+ MPs from ApoE−/−/EC-SREBP2(N) mouse sera suggests that miR-92a is released from the endothelium into the circulation under SREBP2 activation (Figure 6D). This mechanism is likely common to the human vasculature and is supported by the positive correlation between the miR-92a level in CD31+ MPs and circulation (Figure 7C). Such an increase could be due, in part, to the increase in EC-derived MPs under pathophysiological conditions,42 and/or to the augmentation of miR-92a packaged into MPs resulting from increased miR-92a level in ECs. Although the mechanism for EC miR-92a secretion into the circulation via MPs remains to be determined, the translational relevance of this data is demonstrated by the finding that the circulating miR-92a level is inversely correlated with FMD, a clinical readout of EC-dependent NO bioavailability, and positively correlated with IL-1β, the end-product of inflammasome activation (Figure 7, A and B). Because IL-1β can be largely produced by myeloid cells, we propose that oxidative stress-induced miR-92a increases endothelial innate immune response, which predisposes the endothelium to other detrimental factors, including the macrophages-mediated inflammatory response. Thus, the positive correlation between circulating miR-92a and serum IL-1β levels in patients with stable CAD suggests that miR-92a is indicative of vascular inflammation. In line with this, we also observed a significant positive correlation between the circulating miR-92a level with that of high-sensitivity C-reactive protein (hs-CRP), the marker of systemic inflammation in the same group of patients (R=0.502, p<0.001, data not shown). Because of the endothelium-derived nature of miR-92a,11,43 assessment of circulating miR-92a, in conjunction with CRP may provide further insight into cardiovascular diseases that are initiated from or highly associated with endothelial dysfunction.
Supplementary Material
Acknowledgments
We thank Dr. Yun Fang (Department of Medicine, University of Chicago) for generously sharing the Luc-KLF4-MRE plasmid, Dr. Sotirios Tsimikas (Department of Medicine, UCSD) for his useful comments, Drs. Jian Kang and Soo Mun Ngoi (Department of Medicine, UCSD) for technical assistance, and Dr. Traci Marin (Department of Cardiopulmonary Sciences, Loma Linda University) for consultation on statistics.
Funding Sources: This work was supported in part by NIH research grants R01HL89940 and R01HL105318 (JS); R01HL106579 and R01HL108735 (JS, SC); K99HL122368 (ZC); R01HL093767 (to YM); and Taiwan Ministry of Science and Technology I-RiCE Program NSC 102-2911-I-009-101 (HH, SL, JC, PH).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Bell E. Innate Immunity Endothelial Cells as Sentinels. Nat Rev Immunol. 2009;9:532–532. [Google Scholar]
- 2.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells. J Hematol Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 5.Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, Raffatellu M, Osborne TF. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JY. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Xu S, Jiang B, Cohen RA, Zang M. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS One. 2013;8:e67532. doi: 10.1371/journal.pone.0067532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, Chien S, Shyy JY. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002;22:76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 9.Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JY, Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 10.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 11.Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Yang T, Liu Y, Zhang H, Wang K, Liu M, Chen G, Xiao X. Kruppel-like factor 4 inhibits the expression of interleukin-1 beta in lipopolysaccharide-induced RAW264.7 macrophages. FEBS Lett. 2012;586:834–840. doi: 10.1016/j.febslet.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany NY) 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinkel R, Penzkofer D, Zuhlke S, Fischer A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C, Dimmeler S. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128:1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Green SR, Baek JS, Lee SH, Ellett F, Deer E, Lieschke GJ, Witztum JL, Tsimikas S, Miller YI. In vivo visualization and attenuation of oxidized lipid accumulation in hypercholesterolemic zebrafish. J Clin Invest. 2011;121:4861–4869. doi: 10.1172/JCI57755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 21.Huang PH, Chen YH, Chen YL, Wu TC, Chen JW, Lin SJ. Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome X. Heart. 2007;93:1064–1070. doi: 10.1136/hrt.2006.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 24.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res. 2009;104:952–960. doi: 10.1161/CIRCRESAHA.108.189803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 27.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R International Brachial Artery Reactivity Task F. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 29.Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, Pattison J, Torzewski M, Sollors J, Friedmann T, Lai NC, Hammond HK, Getz GS, Reardon CA, Li AC, Banka CL, Witztum JL. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58:1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 32.Zhou R, Hu G, Gong AY, Chen XM. Binding of NF-kappaB p65 subunit to the promoter elements is involved in LPS-induced transactivation of miRNA genes in human biliary epithelial cells. Nucleic Acids Res. 2010;38:3222–3232. doi: 10.1093/nar/gkq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li LC, Varghese Z, Moorhead JF, Lee CT, Chen JB, Ruan XZ. Cross-talk between TLR4-MyD88-NF-kappaB and SCAP-SREBP2 pathways mediates macrophage foam cell formation. Am J Physiol Heart Circ Physiol. 2013;304:H874–884. doi: 10.1152/ajpheart.00096.2012. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Shentu TP, Wen L, Johnson DA, Shyy JY. Regulation of SIRT1 by oxidative stress-responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxid Redox Signal. 2013;19:1522–1538. doi: 10.1089/ars.2012.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Hoffman TA, Dericco J, Naqvi A, Jain MK, Irani K. Transcriptional repression of Kruppel like factor-2 by the adaptor protein p66shc. FASEB J. 2009;23:4344–4352. doi: 10.1096/fj.09-138743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, Israelian K, Westphal CH, Rodgers JT, Shioda T, Elson SL, Mulligan P, Najafi-Shoushtari H, Black JC, Thakur JK, Kadyk LC, Whetstine JR, Mostoslavsky R, Puigserver P, Li X, Dyson NJ, Hart AC, Naar AM. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 43.de Winther MP, Lutgens E. MiR-92a: at the heart of lipid-driven endothelial dysfunction. Circ Res. 2014;114:399–401. doi: 10.1161/CIRCRESAHA.114.303125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.