Abstract
Vocal learning underlies acquisition of both language in humans and vocal signals in some avian taxa. These bird groups and humans exhibit convergent developmental phases and associated brain pathways for vocal communication. The transcription factor FoxP2 plays critical roles in vocal learning in humans and songbirds. Another member of the forkhead box gene family, FoxP1 also shows high expression in brain areas involved in vocal learning and production. Here, we investigate FoxP2 and FoxP1 mRNA and protein in adult male budgerigars (Melopsittacus undulatus), a parrot species that exhibits vocal learning as both juveniles and adults. To examine these molecules in adult vocal learners, we compared their expression patterns in the budgerigar striatal nucleus involved in vocal learning, magnocellular nucleus of the medial striatum (MMSt), across birds with different vocal states, such as vocalizing to a female (directed), vocalizing alone (undirected), and non-vocalizing. We found that both FoxP2 mRNA and protein expressions were consistently lower in MMSt than in the adjacent striatum regardless of the vocal states, whereas previous work has shown that songbirds exhibit downregulation in the homologous region, Area X, only after singing alone. In contrast, FoxP1 levels were high in MMSt compared to the adjacent striatum in all groups. Taken together these results strengthen the general hypothesis that FoxP2 and FoxP1 have specialized expression in vocal nuclei across a range of taxa, and suggest that the adult vocal plasticity seen in budgerigars may be a product of persistent down-regulation of FoxP2 in MMSt.
Keywords: budgerigar; neural gene expression; FoxP1, FoxP2; open-ended vocal learning; vocal behavior
1. Introduction
Vocal learning is a phylogenetically rare trait found in relatively few evolutionary lineages including humans and some avian taxa [1,2]. These birds, which include songbirds and parrots, exhibit convergent developmental phases and brain pathways for learned vocal communication with humans [2], highlighting their value as models for investigating the neural and genetic basis of vocal learning.
The transcription factor FOXP2, a member of the forkhead box family, plays an important role in human speech. Mutations of this gene cause speech impairments due to poor coordination of orofacial movement [3], and structural and functional abnormalities in various brain regions including the basal ganglia and Broca’s area [4,5]. Interestingly, in songbirds, FoxP2 levels change both developmentally and acutely within the striatal (basal ganglia) vocal control nucleus, Area X, which is critical for vocal learning in songbirds [6–9]. In juvenile male zebra finches, FoxP2 mRNA expression increases in Area X during the sensorimotor song learning period, and disruption of the gene through shRNA-mediated knockdown disrupts song learning [7,10,11]. When adult males produce songs alone, known as undirected singing, FoxP2 mRNA expression decreases in Area X compared to baseline levels in non-singing birds [8,9]. Consistent with the mRNA data, both Western blot and immunohistochemistry reveals that FoxP2 protein decreases when birds produced undirected song relative to levels in non-singing birds. [8,12,13].
Another member of the forkhead box gene family, FoxP1, is also thought to play a role in brain regions involved in learning and producing vocalizations. FoxP1 is highly expressed in various song nuclei in songbirds, and the level of expression is similar across different ages and singing contexts [6–9]. Interestingly, along with general cognitive dysfunction, mutations in FOXP1 are also implicated in abnormal human speech development [14–19].
Song learning in the predominant songbird models is restricted to males and occurs only during a critical period early in life. In humans, however, both sexes maintain the capacity to learn new words or languages through adulthood. The budgerigar is a small parrot in which both males and females exhibit large vocal repertoires and the ability to learn new contact calls in adulthood [20–22]. FoxP1 and FoxP2 mRNAs are expressed in the striatal vocal learning nucleus, magnocellular nucleus of medial striatum (MMSt) of the budgerigar [6], however, it remains unclear whether vocal behavior acutely alters FoxP expression as it does in zebra and Bengalese finches [9].
Here we investigate the mRNA and protein expression of FoxP2 and FoxP1 in MMSt of budgerigars in different vocal states (vocalizing either in the presence of females or alone, and non-vocalizing) and compared these patterns to those in non-singing zebra finches. If FoxP2 expression in MMSt is behavior-driven as in Area X of the male zebra finch (low FoxP2 expression when they sing alone), then low expression is expected in MMSt when budgerigar males produce vocalizations alone. Alternatively, if the persistent vocal plasticity in budgerigars relies on continually lowered levels of FoxP2 in MMSt, then we expect low levels in all groups. Since there is no evidence from previous studies that the expression pattern of FoxP1 is behaviorally driven, we predict high FoxP1 expression in MMSt across vocal states as in other avian models.
2. Materials and Methods
2.1. Subjects
Eighteen adult male budgerigars (Melopsittacus undulatus) and four adult male zebra finches (Taeniopygia guttata) from our breeding colony or a local supplier were used in this experiment. Six adult female budgerigars were used to stimulate vocal behavior. They were group-housed with other adult conspecifics on a 12L:12D hour photoperiod with food and water ad libitum. All the experimental procedures were approved by New Mexico State University, Animal Care and Use Committee (protocols 2010-001 and 2013-030).
2.2. Behavior
Adult male budgerigars were randomly assigned to the following three different vocal states: i) female directed vocalizing (n=6), ii) undirected vocalizing (n=6), and iii) non-vocalizing (n=6). For the non-vocalizing group, we used birds that produced less than 8 total individual vocalizations, which included contact calls (0–2 calls) and other types of vocalizations (0–6 calls) during the recording sessions. Previous studies in zebra finches typically quantified only the amount of singing and did not include other calls (S.A. White, per obs), therefore their non-singing group also sometimes produced non-learned vocalizations. Therefore, our definition of “non-vocalizing group” is consistent with previous studies. As detailed below in the Results, some birds from each group produced “warble songs”, another type of learned vocalization noted for its complexity and variability [23]. We classified warble songs into bouts using previously established criteria [24]: a bout should i) consist of three different elements and ii) be more than 1 second long. If the warble is more than 10 seconds long, every 10 seconds counts as a separate warble bout. Since the duration of warble bouts classified in this way varies, we also counted the number of individual elements in each warble song [23]. For zebra finches, all of the males were non-singing (n=4); they did not produce any songs during the recording session. For the female directed vocalizing group, male budgerigars were moved to individual sound attenuation chambers with a microphone (23 × 25.5 × 48cm) on the morning of recording. Stimulant females were housed in other sound attenuation chambers, which were placed in front of each male assigned to the directed calling group. For undirected and non-vocalizing groups, male budgerigars were housed in individual recording chambers (75×27.5× 28.8cm) two days prior to the recording. On the third day, behavioral observation was performed in the morning. All the observation was between 90–120 minutes after the lights were turned on, and sounds were continuously recorded and digitized using Sound Analysis Pro [25]. All the animals had access to food and water ad libitum during the session.
2.3. Vocal Counting
All vocalizations from the recordings were manually counted from spectrograms using Raven Pro 1.4 software (Cornell Lab of Ornithology, Ithaca, NY). Recording sessions varied from 90 to 120 minutes. Consequently, we used the rate of vocal element production (number of contact call elements or number of warble song elements divided by total minutes) to analyze the number of vocalizations in the given recording time for our analysis. We also counted the number of bouts of warble following a previous study [24], such that 10 seconds or less of continuous warble was counted as a single bout, while warbles lasting more than 10 second were classified as 1 bout for each 10 seconds of continuous warble. For budgerigars, we tallied the number of contact call elements, and the number of warble song bouts, and warble song elements in the recording session. No zebra finches produced songs, therefore we did not analyze song rate.
2.4. Tissue Preparation
Immediately after the recording session, birds were overdosed with isoflurane and decapitated to dissect their brains. Brains were flash frozen within five minutes on aluminum dishes floated on liquid nitrogen and then stored at −80° C until use. Brains were cryo-sectioned (Leica CM1850. Leica Microsystems, Buffalo Grove, IL) in the coronal plane at 20 µm thickness and thaw-mounted directly on positively charged slides (Fisher Scientific, Waltham, MA) and kept in an −80° C freezer. To enable visualization of key brain regions, some sections were Nissl stained using a series of thionin, alcohol, and xylene washes. Adjacent slides were assigned for in situ hybridizations and immunohistochemistry.
2.5. In situ hybridization
In situ hybridization was performed using riboprobes as described in Teramitsu et al. [7] except that the FoxP cDNA fragments were amplified by PCR from the pCR 4-TOPO vector (Invitrogen, Carlsbad, CA) using m13F and reverse primers. Briefly, sections were prepared for hybridization by fixation (4% paraformaldehyde), acetylation, and incubation of pre-hybridization buffer containing 50% formamide, 1× Denhardt’s, 0.2% SDS, 10 mM EDTA (pH 8.0), 200 mM Tris (pH=7.8), 1.5 mM NaCl, 250 µg/ml tRNA, and 25µg/ml polyA. Then sections were hybridized with 33P-UTP labeled RNA probes over night at 55°C in similar buffer that contain 10% dextran sulfate and 33P-UTP labeled RNA probes. On the next morning, we performed a series of SSC washes and slides were exposed to Biomax MR films (Eastman Kodak, Rochester, NY). The films were developed with Kodak developer and fixer (Eastman Kodak) for one week for FoxP1 and two weeks for FoxP2.
Zebra finch FoxP2 and FoxP1 clones were used in this experiment. We tested probes both from 3’ end and middle region of coding sequence and found similar expression patterns. For consistency we used the 3’ end probes for both FoxP2 and FoxP1 in all of our experiments. For the region of FoxP2 and FoxP1 coding sequences covered by these probes, zebra finch and budgerigar (GenBankAY466101.1 and NCBI RefSeq XM_005149417.1) have more than 97% sequence identity. In contrast, budgerigar FoxP1 and zebra finch FoxP2 have 63% identity over these regions while budgerigar FoxP2 and zebra finch FoxP1 also have only 63% identity. Therefore, cross-hybridization between FoxP1 probes and FoxP2 mRNA, and vice versa, is unlikely given our hybridization stringency. Sense probes were used for both FoxP1 and FoxP2 as negative controls.
The intensity of FoxP2 and FoxP1 expression was quantified from digitized photomicrographs of the x-ray films. Images were opened using Adobe Photoshop (Adobe Systems Inc. San Jose, CA) and were quantified by using the histogram tool to measure the level of signal intensity. Two sections from each hemispheres were quantified, the values averaged, and the average background intensity from outside of brain sections subtracted. To compare the expression of FoxP2 and FoxP1 among the groups, we used the ratio of MMSt intensity divided by intensity of the adjacent area within the striatum (adjacent striatum) to correct for differences in overall expression level from slide to slide or run to run. Since we used zebra finch clones for our probe, signals were expected to be stronger in zebra finch sections. Therefore, this internal control is critical for cross-species comparisons. During our initial data analysis we examined the distributions for our data, and found that most of them were not normal, nor could they be transformed to normality with the most common transformations. Therefore using JMP software, we performed non-parametric tests (Wilcoxon or Kruskal-Wallis tests), which are robust to deviations from normality and appropriate for small sample sizes. To examine the relationship between the call/warble element rate and gene expression, we ran Spearman’s Rho test using JMP software Version 11.0 (SAS Institute Inc., Cary NC).
2.6. Immunohistochemistry
Non-contact calling budgerigars (n = 4) and non-singing zebra finches (n = 4) were also examined for FoxP2 and FoxP1 labeled cells with immunohistochemistry. We used sections adjacent to those used for in situ hybridization. Sections were fixed with 4% paraformaldehyde for 10 minutes, rinsed three times with 1× PBS for 5 minutes each, incubated in 5% normal donkey serum (Jackson Immuno, West Grove, PA) solution with PBST (1× PBS with 0.3% Triton-X) for 1 hour at 4° C, and then incubated overnight at 4° C in a combination of FoxP1 (Rabbit, 1:500. ab16645. Abcam, Cambridge, MA) and FoxP2 (Goat, 1:1000. sc21069. Santa Cruz, Dulles TX) primary antibodies in humidified slide chambers. Both antibodies are successfully used in avian systems previously [26–28]. Sections were rinsed three times with 1× PBS for 5 minutes each, and incubated with a mixture of Alexa Fluor 488 (Donkey, 1:200. Life Technologies, Carlsbad, CA) and Alexa Fluor 594 (Donkey, 1:200) secondary antibodies for 2 hours at room temperature. Sections were rinsed three times, and coverslipped with Vectashield DAPI (Vector, Burlingame, CA). The same procedure without primary antibodies was performed as a negative control.
For quantification, we used confocal microscope (Leica TCS SP5 II. Leica, Solms, Germany) digital images taken from both left and right hemispheres from two sections. It should be noted that pictures of the adjacent striatum for the IHC analysis were taken from a more medial area than those for the in situ hybridization analysis. To count labeled cells for DAPI, FoxP2 and FoxP1, we used Image J (NIH, Bethesda, MD). Images were converted to 8-bit gray scale and made into a binary file that performed partial automatic counting. Cells that were three pixels or greater in size were automatically counted. We then manually adjusted to include labeled cells that were not automatically counted and noise that was incorrectly counted as a labeled cell. Cells were divided by the total number of cells (DAPI) and averaged for each individual animal because of the possible difference in cell density in the areas of interest. These averages then were used to determine the MMSt/Adjacent striatum ratio to correct for differences in florescent level from slide to slide or run to run. Values from budgerigars and zebra finches were compared using Wilcoxon unpaired tests.
3. Results
3.1. Vocal analysis
The number of contact calls and the number of individual elements and bouts of warble songs emitted by male budgerigars were counted and divided by the recording time to obtain vocalization rates. Birds that produced less than 8 total individual vocalizations during recording session were classified as non-vocalizing and retained for analysis. In vocalizing groups, contact call rates (contact calls/minute) varied from 0.03 to 7.89, and there was no significant difference in calling rates between directed and undirected groups when testing with a t-test. (d.f.=5.12, t ratio=−1.39, p=0.21). Three birds from the directed vocalizing group and one from non-vocalizing group produced a small number of warble songs (0.01 to 0.22 warble song bouts/minute, and 0.04 to 3.88 warble elements/min).
There was no association between contact call rates and gene expression patterns for either directed (FoxP1; Spearman’s rho=0.08 p=0.87, FoxP2; Spearman’s rho=0.2 p=0.70) or undirected vocalizing groups (FoxP1; Spearman’s rho=−0.37 p=0.47, FoxP2; Spearman’s rho=0.43 p=0.40). Moreover, neither the rate of warble song elements nor of song bouts was correlated with gene expression levels (Warble bout rate: FoxP1; Spearman’s rho=0.20 p=0.80, FoxP2; Spearman’s rho=−0.60 p=0.40. Warble element rate: FoxP1; Spearman’s rho=0.20 p=0.80, FoxP2; Spearman’s rho=−0.60 p=0.40.).
3.2. FoxP2 mRNA expression in budgerigar MMSt and zebra finch Area X
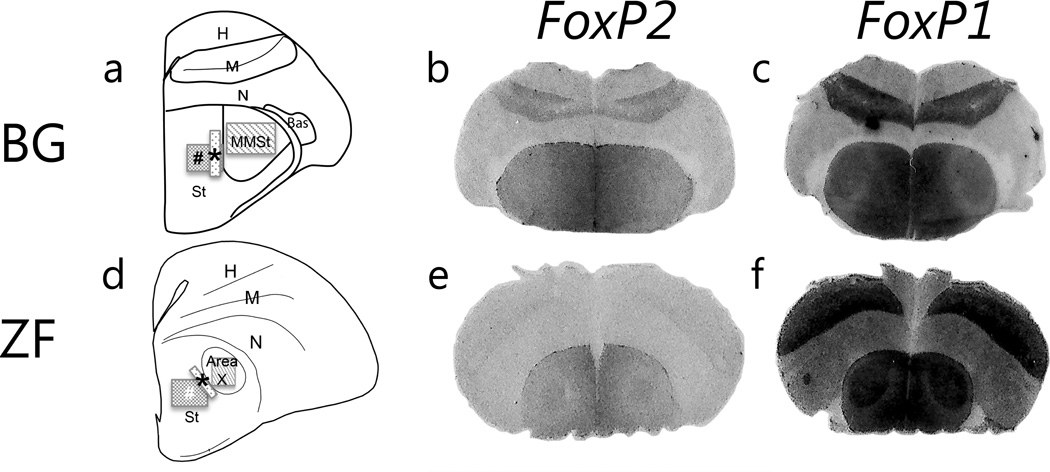
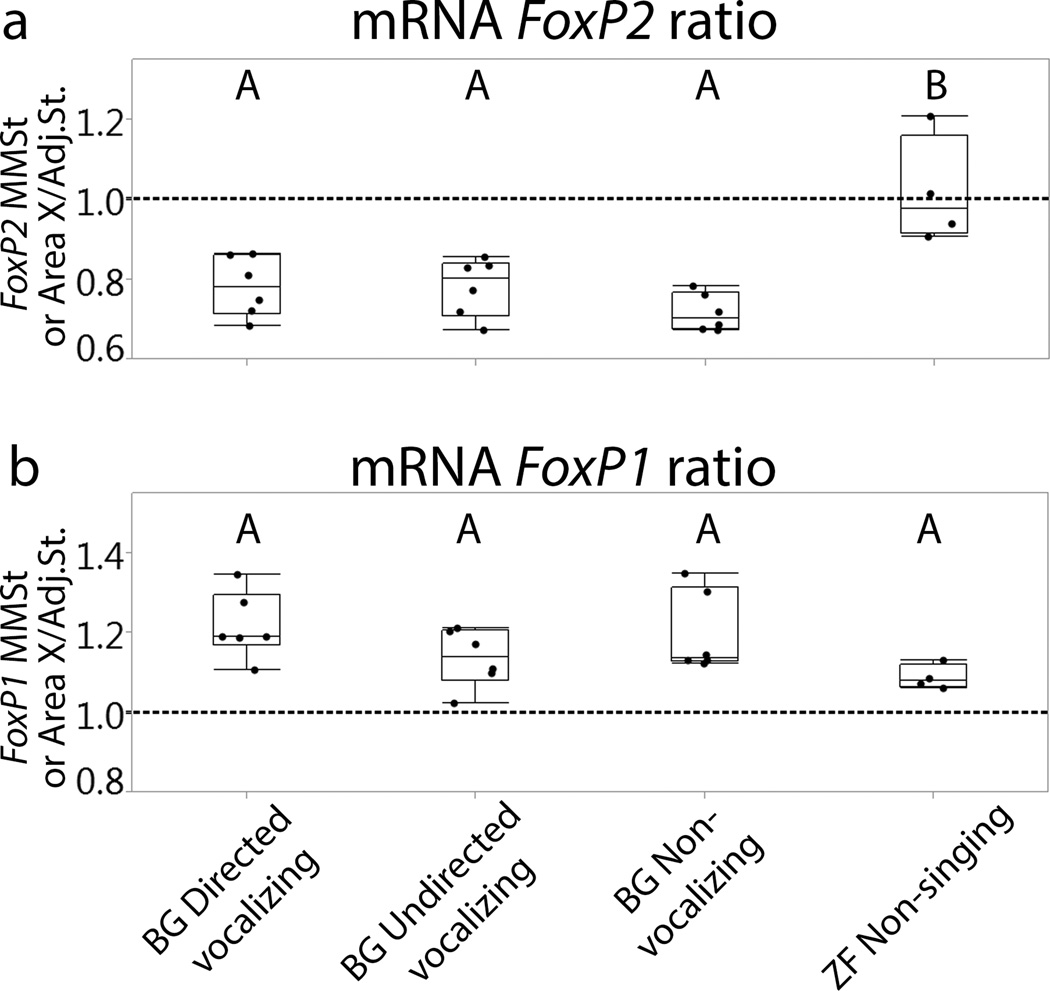
We observed a lower level of FoxP2 in MMSt compared to the adjacent striatum in all budgerigar groups (Fig.1). The mean ratio with standard error of mean (SEM) for budgerigar directed vocalizing = 0.78±0.03, budgerigar undirected vocalizing = 0.78±0.03, and budgerigar non-vocalizing = 0.72±0.03, whereas non-singing zebra finches exhibited equivalent levels across the striatum (zebra finch non-singing = 1.02±0.04). In budgerigars, the expression gradually increased from MMSt to medial striatum (Fig.1). Kruskal-Wallis tests revealed a significant difference among groups in the ratio of striatal vocal control nucleus to adjacent striatum (χ2 =11.58, d.f=3, p=0.01). We used Wilcoxon tests for posthoc pairwise comparisons. These tests revealed that FoxP2 ratios from zebra finches were higher than those from all budgerigar groups (Fig.2, zebra finch non-singing vs. budgerigar directed vocalizing, p=0.01; zebra finch non-singing vs. budgerigar undirected vocalizing, p=0.01; zebra finch non-singing vs. budgerigar non-vocalizing, p=0.01). There was no statistical difference among budgerigar groups (Fig.2). Thus expression patterns in the striatal vocal control nucleus differ between species, and budgerigars maintain low FoxP2 levels in MMSt regardless of the vocalization state.
Figure 1.
FoxP2 and FoxP1 mRNA expressions. Schematic drawing of brain sections from adult male budgerigars (a) and zebra finch (d). Photomicrographs of brain sections from non-vocalizing adult male budgerigars (BG, top) and non-singing adult male zebra finches (ZF, bottom). Location of striatal vocal nuclei and adjacent areas in schematic sections adopted from the atlas at Reiner et al., 2004 [47]. (b and e) In situ signals for FoxP2. (c and f) In situ signals for FoxP1. Boxes indicate the approximate areas of measurement: striatal vocal control nucleus (MMSt for budgerigars and Area X for zebra finches) and adjacent striatum. * indicates the adjacent striatum area where mRNA was quantified, and # indicates that for protein expression. FoxP2 levels appear lower in the MMSt compared to the adjacent striatum while Area X exhibits similar or slightly higher expression level compared to adjacent area. In contrast, FoxP1 is highly expressed in the striatal vocal control nucleus in both species. Since zebra finch tissue produced stronger signals, the representative pictures for the two species were taken from different films with different exposure times. Abbreviations: H, Hyperpallium: M, Mesopallium; N, Nidopallium; Bas, Basorostral pallial nucleus; MMSt, Magnocellular nucleus of the medial striatum; St, striatum.
Figure 2.
FoxP2 and FoxP1 mRNA expression ratio (striatal vocal nucleus/adjacent striatum) in different groups. The ratio 1 on the Y-axis indicates the same expression levels in striatal vocal control nucleus and adjacent striatum. (a) There are significant differences between all budgerigar groups and zebra finches for FoxP2. (b) The expression ratio of FoxP1 demonstrates no significant difference among groups. Different letters above the box plots indicate significant differences (p-values in the text). BG=budgerigars, ZF=zebra finches.
3.3. FoxP1 mRNA expression in budgerigar MMSt and zebra finch Area X
Striatal vocal control nuclei (MMSt and Area X) exhibited a high expression level of FoxP1 compared to the adjacent striatum (Fig.1 and Fig.2 mean ratio with SEM for budgerigar directed vocalizing =1.22 ± 0.03, budgerigar undirected vocalizing = 1.14 ± 0.03, budgerigar non-vocalizing = 1.20 ± 0.03, and zebra finch non-singing = 1.20 ± 0.04). Although we did not quantify expression intensity in this study, we also observed high intensity of FoxP1 in ventral and medial striatum (Fig.1). We compared the striatal vocal control nucleus/adjacent striatum ratio among groups statistically. A Kruskal-Wallis test showed no statistical difference among groups (Fig.2, χ2 =6.74, d.f=3,p=0.08).
3.4. FoxP2 and FoxP1 protein expression in budgerigar MMSt and zebra finch Area X
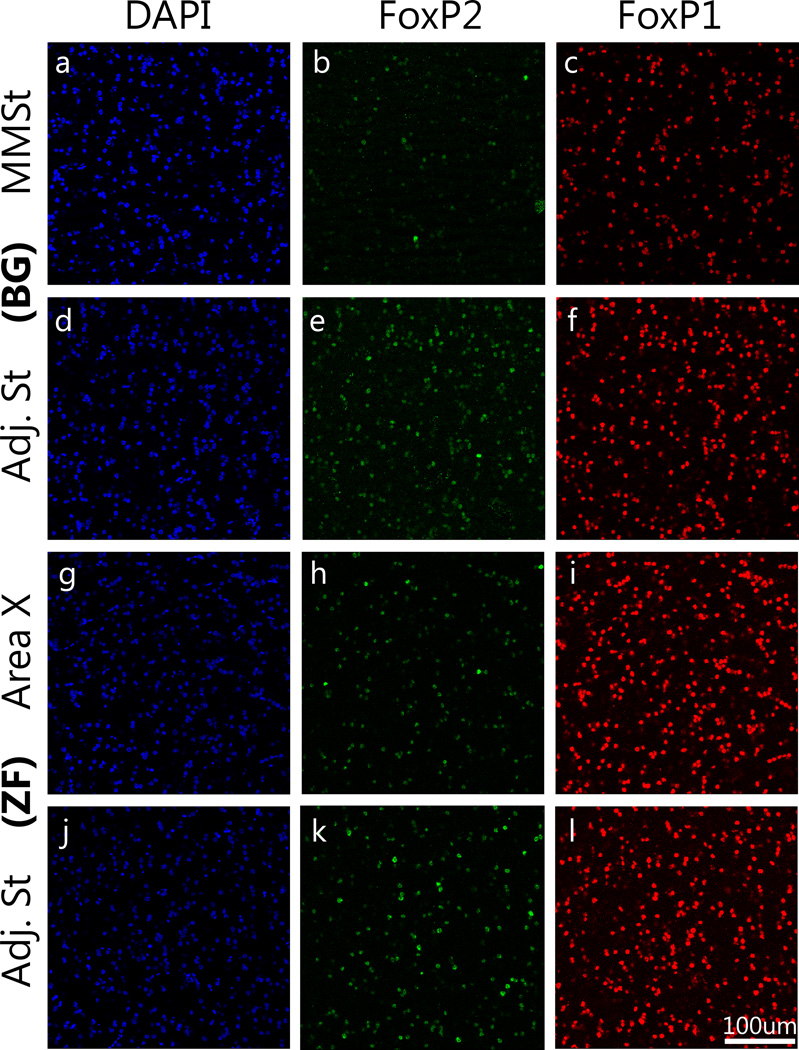
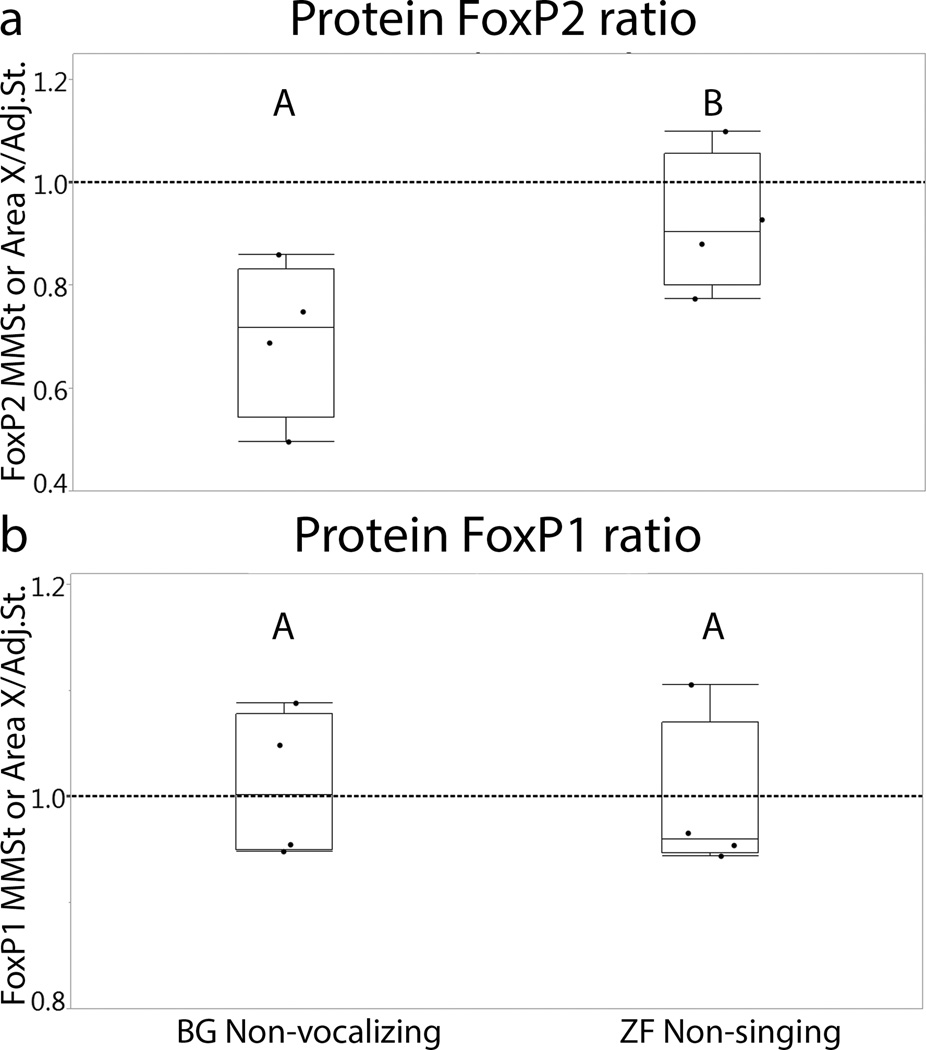
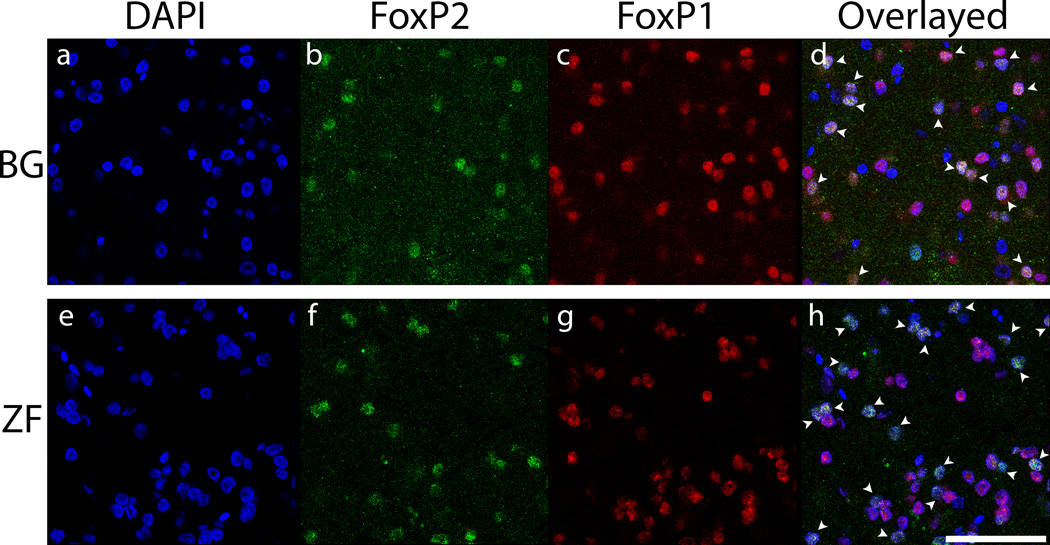
To investigate species differences at protein level, the number of FoxP2 and FoxP1 positive cells in non-vocalizing budgerigars and non-singing zebra finches were compared. To eliminate the effect of possible differences in cell density across regions, the number of FoxP2-positive or FoxP1-positive cells was normalized by dividing by the total number of DAPI-labeled. FoxP2 expression in the MMSt was lower compared to the adjacent striatum whereas a similar level of expression was found between Area X and the adjacent striatum in zebra finches (Fig.3). The mean ratio with SEM (striatal vocal control nucleus/adjacent striatum) of the budgerigar non-vocalizing group was 0.70±0.08, and that for zebra finch non-singing group was 0.92±0.07. There was a significant difference between the two species (Fig.4, Wilcoxon test, χ2=4.08, d.f.=1 p=0.04), with a higher ratio in zebra finches.
Figure 3.
Immunohistochemical detection of FoxP2 and FoxP1 proteins. Top two rows are budgerigars (BG: a–f) and bottom two rows are zebra finches (ZF: g–l). DAPI staining exposes all the cells in the area (Blue: a, d, g, j). FoxP2 (Green) reveals a lower expression in the MMSt compared to the adjacent striatum while the expression level is consistent throughout the striatum in zebra finches (b, e, h, k). Red signal indicates FoxP1-positive cells, which demonstrate constant expression levels throughout area and species (c, f, i, l). Scale bar = 100 µm.
Figure 4.
FoxP2 and FoxP1 protein expression ratio of striatal vocal nucleus/adjacent striatum in non-vocalizing budgerigar and non-singing zebra finch groups. The value 1 on the Y-axis demonstrates the same level of expression between the striatal vocal nucleus and the adjacent striatum. (a) A significant difference in the FoxP2 expression ratio was found between the two species. (b) There is no difference between budgerigars and zebra finches in FoxP1 levels. Different letters above box plots indicate significant differences (p-values in the text). BG=budgerigars, ZF=zebra finches.
FoxP1 protein expression was observed in both budgerigar MMSt and zebra finch Area X, and its expression level was similar to that in the adjacent striatum (Fig.3). The mean ratio with SEM for budgerigar non-vocalizing group was 1.01±0.03, and non-singing zebra finch group was 1.00±0.04. No significant difference was found in the ratio (striatal vocal control nucleus/adjacent striatum) of FoxP1 expression between the groups (Fig.4, Wilcoxon test, χ2= 0.08,d.f=1, p=0.77).
Although quantification was not performed, we observed that FoxP2-labeled cells were usually co-localized with FoxP1-labeled cells (Fig.5, co-localized cells indicated with white arrows). While the intensity of FoxP1-labeled cells was uniform throughout the striatum, some variation in the intensity of FoxP2-labeled cells was observed. Strongly labeled FoxP2 cells were found along the ventricular zone in the striatum and the lamina between the striatum and the nidopallium (N), which is directly above the striatum. In contrast, the majority of FoxP2 labeled cells in the MMSt and Area X were weakly labeled (Fig. 5).
Figure 5.
High power image of DAPI, FoxP2, and FoxP1 protein signals in striatal vocal control nucleus of budgerigars (a–d) and zebra finches (e–h). Blue indicates DAPI (a and e), green indicates FoxP2 (b and f) and red indicates FoxP1 labeled cells (c and g). There are more FoxP2 expressing cells in zebra finches’ Area X than budgerigars’ MMSt. Most of FoxP2 labeled cells are co-localized with FoxP1 as indicated by the white arrows (d and h). Scale bar = 50 µm.
4. Discussion
4.1. Summary of findings
In this study, we examined expression patterns of both mRNA and protein of FoxP2 and FoxP1 in an adult vocal learner, the budgerigar. We focused on expression patterns in the striatal vocal control nucleus, MMSt, which is a key part of the parrot vocal learning pathway, and examined changes within the MMSt across different vocal states.
We discovered that, regardless of the vocal states (female directed vocalizing, undirected vocalizing and non-vocalizing), FoxP2 levels are lower in the MMSt relative to levels in the adjacent striatum in budgerigars. Previously, FoxP2 expression patterns in the songbird striatal vocal control nucleus Area X were found to be driven by the particular singing behavior of adult zebra finches, which are closed-ended vocal learners. In adult zebra finches, when males produce their songs alone, both the mRNA and protein decrease in Area X compared to baseline levels in non-singing birds [8,12,13]. In contrast, when male zebra finches sing to females, the level of FoxP2 mRNA in Area X remains similar to that in the adjacent striatum, whereas the Area X protein level decreases. In zebra finches, the effect of social context on FoxP2 mRNA is mediated by social regulation of a FoxP2-targeting miRNA [29]. In this experiment, we included a zebra finch non-singing group to provide a direct comparison with budgerigars. We found similar mRNA patterns to a previous study [12]: the expression level of FoxP2 was similar between Area X and adjacent striatum in non-singing zebra finches. Using immunohistochemistry, we also observed a similar number of FoxP2 labeled cells between these two areas in non-singing zebra finches. Previously, it has been reported that the amount of FoxP2 protein between these two areas is similar in zebra finches under the same behavioral conditions using Western blot [8]. We cannot compare protein levels directly between the two studies since protein levels were measured in different ways; however the different approaches highlight the same pattern of FoxP2 protein expression in non-singing zebra finches. In contrast, in the budgerigar we found lower levels of FoxP2 protein in MMSt than in adjacent striatum across all groups and this ratio was significantly lower in all budgerigar groups than in the non-singing zebra finches. Taken together, these studies suggest that down-regulation of FoxP2 is associated with vocal plasticity in both open-ended and closed-ended vocal learning avian models.
On the other hand, we found high mRNA and protein FoxP1 expression in the striatal vocal control nucleus of both budgerigars and zebra finches (MMSt and Area X) regardless of their vocal states. Using the ratio of striatal vocal nucleus and adjacent striatum, there were no significant differences among groups at either mRNA or protein levels. High level of FoxP1 was seen in previous studies in songbirds [6,7] and singing behavior did not affect expression level [9]. Therefore, our result strengthens the idea that FoxP1 expression in song nucleus is not vocal driven even in open-ended vocal learners.
We found no relationship between calling rates and levels of expression of either FoxP2 or Fox P1. We focused primarily on contact calls as these are the most commonly produced elements of the budgerigar repertoire. Further investigation of the effect of warble songs on expression of these genes would be worthwhile, though, as they have been shown to affect MMSt expression of the immediate early gene egr1 [24]. Budgerigars produce warble songs more consistently when they are housed together (E. Hara and T. Wright, pers obs). However, for consistency with previous studies examining FoxP2 expression, we recorded males either in isolation, or housed separately from females (for the directed group). Further study of the effect of warble song on FoxP1 and FoxP2 expression would require modification of this approach.
4.2. Role of FoxP2 and FoxP1
It has been suggested that FoxP2 down-regulation may play an important role in permitting adult song plasticity in zebra finches. Zebra finches that sang more variable undirected songs showed lower FoxP2 mRNA expression in Area X compared to adjacent striatal area while levels were similar between these areas when birds were either non-singing or sang less variable female-directed songs [8,12]. Knock-down of FoxP2 in Area X of juvenile zebra finches via viral-mediated shRNA manipulations prevented animals from copying tutor songs accurately [10,11], which might be due to decreased dendritic spine density in Area X [30]. Furthermore, disrupting FoxP2 in Area X in adult zebra finches altered song variability, possibly via dopamine receptor dependent modulation in the corticostriatal pathway [11]. In contrast to patterns in zebra finches, we found low levels of FoxP2 mRNA and protein in the MMSt regardless of the vocal status in adult budgerigars. Such persistent down-regulation is consistent with the fact that budgerigars are capable of modifying their contact calls as adults [21]. Previously it has been reported that FoxP2 mRNA expression in adult budgerigars is similar between MMSt and the surrounding striatum [6], a result that differs from ours here. This difference may be due to the use of sagittal sections in [6], as the gradual decrement from medial MMSt to lateral MMSt that we observed using coronal sections is not apparent on an individual sagittal section, or it may be due to the shorter behavior sessions before sacrifice used in the previous study. Our results suggest the novel hypothesis that a consistently low level of FoxP2 expression in MMSt permits the persistent vocal plasticity and open-ended learning observed in adult budgerigars.
The outer region of MMSt is thought to be involved in body movement in various avian models [31]. Humans have the ability to learn movements, such as dancing. Likewise, parrots have the ability to learn a complex movement by mimicking and performing rhythmic synchronizations like tapping to an audio-visual metronome [32,33]. Therefore, it is possible that the adjacent striatum is involved in other motor learning and FoxP2 also plays a crucial role in the area. Interestingly, this gradual down-regulation pattern in the striatal area of the budgerigar was also found for the calcium binding protein, calbindin in the budgerigar [34], whereas calbindin is highly expressed in Area X of male zebra finches [35]. Calbindin acts to buffer calcium, which may protect cells from otherwise harmful intracellular levels [36]. The degree of interaction between FoxP2 and calbindin is unclear. However, both molecules may play critical roles in differentiating open-ended from closed-ended vocal learners, and further investigation is warranted.
Our immunohistochemical results revealed variable intensity levels of staining for FoxP2 protein across individual cells in the MMSt. Since our immunohistochemistry was performed with fluorescent labeling, staining intensity varied between sections. Therefore, we did not quantify the intensity of labeled neurons in this study. However, most of the labeled neurons within the MMSt appeared to be of low intensity, with high intensity neurons present mainly at the lamina between the striatum and the nidopallium, and also at the ventricular zone. It has been reported that newly born neurons express high intensity FoxP2 signals in Area X of zebra finch [13]. Therefore, lamellar distribution in budgerigar may represent new neurons that will eventually migrate into MMSt. Moreover, in adult zebra finches singing behavior decreases the number of weakly stained FoxP2 neurons whereas strongly labeled FoxP2 neurons were not affected [13]. Budgerigars, however, mainly demonstrated weak staining in the MMSt regardless of their vocal states, which is consistent with ongoing vocal plasticity.
Some literature suggests that FOXP1 is also involved in human speech [14–19]. In addition, a mutation of this gene is found in some individuals with autism, for which one of the main characteristic is communication and language difficulties [16,37]. FOXP1 is also involved in organ development, including the heart, lungs, and esophagus [38,39]. In the central nervous system of mice, FoxP1 plays an important role in the definition of columnar identity of motor neurons in the spinal cords [40], and a recent report showed that it is involved in the development of medium spiny neurons in the striatum [41]. Taken together, these studies suggest that cellular differentiation is a primary function of FoxP1. In avian forebrains, high FoxP1 expression patterns are conserved in the striatum, dorsal and ventral mesopallium [42]. In vocal learning songbirds, FoxP1 is highly expressed in various vocal control nuclei, including the striatal vocal nucleus, but unlike FoxP2, the expression levels do not appear to be driven by age or singing states [9,12]. Therefore, the high expression of FoxP1 may be crucial for maintaining the organization of vocal nuclei in both open-ended and closed-ended vocal learners.
It is still unclear what upstream factors control FoxP2 and FoxP1 expression. However, recent study in rodents showed that when exogenous androgen was administered, both mRNA and protein expression of FoxP2 and FoxP1 increased in the striatum, and vocalizations were also altered [43]. Interestingly, androgen receptor expression is high in Area X of zebra finches [44], but low in MMSt in budgerigars [45]. Therefore, it is possible androgens play important role on vocal plasticity, which separate open-ended from closed-ended vocal learners.
4.3. Conclusion
There are some similarities between the development of human language and bird vocal repertoires including babbling-like vocalization at early development, an early critical period of rapid learning, and the importance of auditory feedback [46]. Like humans, budgerigars have the ability to learn vocalizations throughout their lifetime. Consequently, further investigations of molecular mechanisms for vocal learning in this species may offer insight into the maintenance of adult vocal plasticity in humans. In this study, we documented for the first time expression patterns of FoxP2 and FoxP1 at mRNA and protein levels in different vocal states in the striatal vocal nucleus of budgerigars. Manipulative studies of gene expression will be necessary to test the mechanism of action of these molecules in adult vocal learning. It has been established that viral manipulations of these molecules are effective in songbirds [10,11], therefore, both overexpression and knock-down of these genes should be feasible using similar approaches in budgerigars. Such experiments in open-ended vocal learners like the budgerigars will offer new insights into the neural and molecular mechanisms of adult vocal learning ability in humans.
Highlights.
The budgerigar is an open-ended vocal learner.
FoxP2 expression patterns were examined in a striatal vocal nucleus.
Relative expression was low and not dependent on vocal state.
These FoxP2 patterns differ from those in a closed-ended vocal learner, the zebra finch.
Expression patterns in another learning-related gene, FoxP1 were similar in the two species.
Acknowledgements
We thank Alfredo Montoya and staff of the NMSU Animal Care Facility for bird care. Special thanks to Patricia Hash-Duarte, Esteban Lucero, Tawni Voyles, Keely Brown, Breanne Cordier, Jon Heston, Dr. Georg Striedter, Dr. Anna Young, Dr. Julie Miller, and Dr. Peter Cook for their contributions. Material was prepared on equipment supported by NSF MRI-DBI-095817, preliminary studies by S. White and T. Wright were supported by the Grass Foundation, and primary research was supported by NIH NICHD grant SC1HD068128 (PI T. Wright).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflicts of interests.
References
- 1.Janik V, Slater PB. Vocal Learning in Mammals. Advances in the Study of Behavior. Advances in the Study of Behavior. 1997;26:59–99. [Google Scholar]
- 2.Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai C, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- 4.Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 2003;18:194–200. doi: 10.1002/hbm.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liégeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6:1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- 6.Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 Expression in Avian Vocal Learners and Non-Learners. Journal of Neuroscience. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. Journal of Neuroscience. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JE, Spiteri E, Condro MC, Dosumu-Johnson RT, Geschwind DH, White SA. Birdsong Decreases Protein Levels of FoxP2, a Molecule Required for Human Speech. Journal of Neurophysiology. 2008;100:2015–2025. doi: 10.1152/jn.90415.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Heston JB, Burkett ZD, White SA. Expression analysis of the speech-related genes FoxP1 and FoxP2 and their relation to singing behavior in two songbird species. Journal of Experimental Biology. 2013;216:3682–3692. doi: 10.1242/jeb.085886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murugan M, Harward S, Scharff C, Mooney R. Diminished FoxP2 Levels Affect Dopaminergic Modulation of Corticostriatal Signaling Important to Song Variability. Neuron. 2013;80:1464–1476. doi: 10.1016/j.neuron.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teramitsu I, White SA. FoxP2 Regulation during Undirected Singing in Adult Songbirds. Journal of Neuroscience. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson CK, Schwabe F, Schoof A, Mendoza E, Gampe J, Rochefort C, et al. Young and intense: FoxP2 immonoreactivity in Area X varies with age, song stereotypy, and singing in male zebra finches. Frontires in Neural Circuits. 2013;7:1–17. doi: 10.3389/fncir.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worthey EA, Raca G, Laffin JJ, Wilk BM, Harris JM, Jakielski KJ, et al. Whole-exome sequencing sopports genetic heterogeneity in childhood apraxia of speech. Journal of Neurodevelopmental Disorders. 2013;5:1–16. doi: 10.1186/1866-1955-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horn D, Kapeller J, Rivera-Brugués N, Moog U, Lorenz-Depiereux B, Eck S, et al. Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum Mutat. 2010;31:E1851–E1860. doi: 10.1002/humu.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, Langlois M, et al. The American Journal of Human Genetics: De Novo Mutations in FOXP1 in Cases with Intellectual Disability, Autism, and Language Impairment. The American Journal of Human Genetics. 2010;87:671–678. doi: 10.1016/j.ajhg.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr CW, Moreno-De-Luca D, Parker C, Zimmerman HH, Ledbetter N, Martin CL, et al. Chiari I malformation, delayed gross motor skills, severe speech delay, and epileptiform discharges in a child with FOXP1 haploinsufficiency. Eur J Hum Genet. 2010;18:1216–1220. doi: 10.1038/ejhg.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pariani MJ, Spencer A, Graham JM, Rimoin DL. A 785kb deletion of 3p14.1p13, including the FOXP1 gene, associated with speech delay, contractures, hypertonia and blepharophimosis. Eur J Med Genet. 2009;52:123–127. doi: 10.1016/j.ejmg.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Fevre AK, Taylor S, Malek NH, Horn D, Carr CW, Abdul-Rahman OA, et al. FOXP1 mutations cause intellectual disability and a recognizable phenotype. Am J Med Genet A. 2013;161A:3166–3175. doi: 10.1002/ajmg.a.36174. [DOI] [PubMed] [Google Scholar]
- 20.Farabaugh SM, Linzenbold A, Dooling RJ. Vocal plasticity in budgerigars (Melopsittacus undulatus): Evidence for social factors in the learning of contact calls. J Comp Psychol. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Dahlin CR, Young AM, Cordier B, Mundry R, Wright TF. A test of multiple hypotheses for the function of call sharing in female budgerigars, Melopsittacus undulatus. Behav Ecol Sociobiol. 2013;68:145–161. doi: 10.1007/s00265-013-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hile AG, Striedter GF. Call Convergence within Groups of Female Budgerigars (Melopsittacus undulatus) Ethology. 2000;106:1105–1114. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- 23.Tu HW, Osmanski MS, Dooling RJ. Learned vocalizations in budgerigars (Melopsittacus undulatus): The relationship between contact calls and warble song. J Acoust Soc Am. 2011;129:2289–2297. doi: 10.1121/1.3557035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis ED, Mello CV. Molecular mapping of brain areas involved in parrot vocal communication. J Comp Neurol. 2000;419:1–31. doi: 10.1002/(sici)1096-9861(20000327)419:1<1::aid-cne1>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchernichovski O, Nottebohm F, Ho C, Pesaran B, Mitra P. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 26.Soundararajan P, Fawcett JP, Rafuse VF. Guidance of postural motoneurons requires MAPK/ERK signaling downstream of fibroblast growth factor receptor 1. Journal of Neuroscience. 2010;30:6595–6606. doi: 10.1523/JNEUROSCI.4932-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soderstrom K, Luo B. Late-postnatal cannabinoid exposure persistently increases FoxP2 expression within zebra finch striatum. Dev Neurobiol. 2010;70:195–203. doi: 10.1002/dneu.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitney O, Voyles T, Hara E, Chen Q, White SA, Wright TF. Differential FoxP2 and FoxP1 expression in a vocal learning nucleus of the developing budgerigar. Dev Neurobiol. 2014 doi: 10.1002/dneu.22247. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z, Luo G, Fu L, Fang Z, Wang X, Li X. miR-9 and miR-140-5p target FoxP2 and are regulated as a function of the social context of singing behavior in zebra finches. Journal of Neuroscience. 2013;33:16510–16521. doi: 10.1523/JNEUROSCI.0838-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010;9:732–740. doi: 10.1111/j.1601-183X.2010.00607.x. [DOI] [PubMed] [Google Scholar]
- 31.Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, et al. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE. 2008;3:e1768. doi: 10.1371/journal.pone.0001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore BR. Avain Movement Imitation and a New Form of Mimicry: Tracing the Evolution of a Complex Form of Learning. Behaviour. 1992;122:231–263. [Google Scholar]
- 33.Hasegawa A, Okanoya K, Hasegawa T, Seki Y. Rhythmic synchronization tapping to an audio–visual metronome in budgerigars. Sci Rep. 2011;1:1–8. doi: 10.1038/srep00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Calero E, Bahamonde O, Martinez S. Differences in number and distribution of striatal calbindin medium spiny neurons between a vocal-learner (Melopsittacus undulatus) and a non-vocal learner bird (Colinus virginianus) Front Neuroanat. 2013;7:1–10. doi: 10.3389/fnana.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Calero E, Scharff C. Calbindin expression in developing striatum of zebra finches and its relation to the formation of area X. J Comp Neurol. 2012;521:326–341. doi: 10.1002/cne.23174. [DOI] [PubMed] [Google Scholar]
- 36.Rintoul GL, Raymond LA, Baimbridge KG. Calcium buffering and protection from excitotoxic cell death by exogenous calbindin-D28k in HEK 293 cells. Cell Calcium. 2001;29:277–287. doi: 10.1054/ceca.2000.0190. [DOI] [PubMed] [Google Scholar]
- 37.O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- 39.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- 40.Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated Actions of the Forkhead Protein Foxp1 and Hox Proteins in the Columnar Organization of Spinal Motor Neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carri AD, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development. 2012;140:301–312. doi: 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- 42.Jarvis ED, Yu J, Rivas MV, Horita H, Feenders G, Whitney O, et al. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J Comp Neurol. 2013;521:3614–3665. doi: 10.1002/cne.23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowers JM, Perez-Pouchoulen M, Roby CR, Ryan TE, McCarthy MM. Androgen Modulation of Foxp1 and Foxp2 in the Developing Rat Brain: Impact on Sex Specific Vocalization. Endocrinology. 2014 doi: 10.1210/en.2014-1486. en20141486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim Y-H, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- 45.Matsunaga E, Okanoya K. Vocal area-related expression of the androgen receptor in the budgerigar (Melopsittacus undulatus) brain. Brain Research. 2008;1208:87–94. doi: 10.1016/j.brainres.2008.02.076. [DOI] [PubMed] [Google Scholar]
- 46.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 47.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]