Abstract
Parent-offspring interactions early in life can permanently shape the developmental path of those offspring. Manipulation of maternal care has long been used to alter the early-life environment of infants and impacts their later social behavior, aggression, and physiology. More recently, naturally occurring variation in maternal licking and grooming behavior has been shown to result in differences in social behavior and stress physiology in adult offspring. We have developed a model of natural variation in biparental care in the prairie vole (Microtus ochrogaster) and have demonstrated an association between the amount of early care received and later social behavior. In this study, we investigate the relationship between early life care and later aggression and neuroendocrine responses following chronic social isolation. Male and female offspring were reared by their high-contact (HC) or low-contact (LC) parents, then housed for 4 weeks post-weaning in social isolation. After 4 weeks, half of these offspring underwent an intrasexual aggression test. Brains and plasma were collected to measure corticotropin-releasing hormone (CRH) and vasopressin (AVP) immunoreactivity and plasma corticosterone (CORT). Male offspring of LC parents engaged in more aggressive behavior in the intrasexual aggression test compared to HC males. Female offspring of HC parents had higher plasma CORT levels after chronic social isolation and increases in the number and density of AVP-immunopositive cells in the supraoptic nucleus following an intrasexual aggression test. These findings show that the impact of early life biparental care on behavior and HPA activity following a social stressor is both sex-dependent and early experience-specific.
Keywords: Natural variation, Parental behavior, Aggression, Vasopressin, Corticotropin-releasing hormone, HPA activity
1. Introduction
Experiences early in an infant's life have the potential to create lasting changes in their developmental trajectories. Perhaps one of the most well-studied influences in early life is the mother-offspring relationship. Manipulations of the mother-offspring relationship have the ability to change the infant's social behavior, anxiety-like behavior and physiological reactivity to a stressor in adulthood. Short-term separation in the first weeks of life in rats leads to offspring that display greater amounts of exploratory behaviors in a novel environment [1-3] as well as a blunted hypothalamic-pituitary-adrenal (HPA) axis response to a physical stressor as adults [2, 4]. Conversely, long-term maternal separation results in increased anxiety-like behavior and decreased maternal behavior [5], increased depressive-like behavior [6], and an increased HPA response to a stressor [4, 6, 7]. Adverse early life environments, including conflict within the parent-offspring relationship, are also associated with alterations in HPA functioning in humans [8, 9] and several negative health outcomes, including increased risk for cardiovascular disease [10], increased inflammatory activity [11, 12], and increased risk for mood and anxiety disorders [13-16].
Adverse experiences, both those occurring during early development those happening in adolescence, can also have a profound effect on social behavior and displays of aggression. In humans, childhood neglect has been linked to increased amounts of social withdrawal in adolescence [17], increased amounts of aggressive behaviors [18, 19], and have higher rates of committing intimate partner violence [20]. It appears that the severity of many of these outcomes is dependent upon the age of the child when the neglect occurs [17, 18, 21]. In animal models, long-term social deprivation in infancy, a paradigm often used to model childhood neglect, leads to increased aggressive behavior in adulthood in several species, including non-human primates [22, 23] and rats [6] as well as increased maternal aggression in C57Bl/6 mice [24]. Chronic social isolation stress during the post-weaning adolescent period also results in excessive displays of aggression in several special of rats [25-28]. Data from humans as well as several animal models indicate that isolation and neglect within the parent-offspring relationship during early development and adolescence has lasting consequences for later interpersonal interactions and displays of aggressive behaviors.
Naturally occurring differences in maternal behavior have been observed in several species and can alter behavior and HPA function in similar ways to many experimental manipulations, where increased early care results in a more tightly-regulated HPA response. For example, maternal abuse of infant rhesus macaques is associated with increased anxiety-like behaviors and distress calling in the infants at 6 months of age [29] as well as higher cortisol levels and a decreased adrenocorticotropic hormone (ACTH) response to corticotropin releasing hormone (CRH) stimulation [30]. In rodents, variation in maternal licking/grooming (LG) care has been characterized in rats [31], as has its impact on behavior and HPA function. Offspring reared by high LG dams show a decreased corticosterone (CORT) response to an acute restraint stressor as well as increased glucocorticoid (GC) feedback sensitivity and decreased levels of CRH mRNA in the paraventricular nucleus of the hypothalamus (PVN) [32]. In addition, hippocampal GC receptor (GR) mRNA, important in the regulation of the negative feedback on the hypothalamus, is increased following rearing by high LG dams [32]. Cross-fostering studies indicate these differences in responsiveness are transmitted non-genomically – offspring resemble the behavioral and physiological phenotypes of their rearing rather than genetic dam [33].
Much of the work in rats has utilized non-social stressors to elicit responses. However, responses to varying types of stressors, including social stressors, are not always equal. For example, male mice reared under communal nesting conditions show an attenuated CORT response to a social stressor compared to standard-housed animals but do not show a reduction in CORT following a physical (non-social) stressor [34], indicating that responsiveness is dependent on the type of stressor. The consequences of social isolation have been extensively studied, particularly in relation to the effects on HPA activity. Social isolation housing in various strains of rats results in increases in anxiety-like [35, 36] and aggressive behaviors [28, 37], increases in plasma ACTH [37-39] and CORT levels [39, 40], as well as sex-specific increases in adrenal weights [38]. Chronic isolation housing in the prairie vole (Microtus ochrogaster) is associated with increases in anxiety-like behavior and aggression [41-43], increases in CORT levels [41, 42, 44-47], and increases in CRH-immunoreactive (CRH-ir) cell densities in the PVN [44-46] and CRH mRNA [42]. These results together indicate that long-term social isolation can alter HPA function and have impacts on associated behavioral outcomes in this species.
Just as the HPA response can vary following social compared to non-social stressors, it also shows a different pattern of activation and release in response to a short-term compared to long-term stressor. During an acute stress response, a physiological or psychological stressor triggers a response, prompting the PVN to release CRH as well as AVP into the hypophyseal blood portal system. These then act in tandem to stimulate the corticotroph cells in the anterior pituitary to release ACTH into the peripheral blood supply. This ACTH then travels to the adrenal glands, where it stimulates the release of glucocorticoids from the adrenal cortex. Following a long-term stressor or daily repeated acute stressors, the synergistic CRH and AVP release from the PVN shifts from a primarily CRH-driven effect to one that is principally controlled by AVP [48]. This increased AVP:CRH ratio in response to long-term stress provides an interesting component of the HPA response to chronic social isolation.
The present study examines the relationship between early life experience and later responsiveness to a social stressor using the socially monogamous prairie vole. We have previously shown that prairie voles display naturally occurring variation in the amount and type of biparental care directed toward offspring [49] and there is much evidence that this species responds to stressors in a sex-specific manner [45, 50, 51]. Here we use long-term isolation housing as a chronic social stressor, as well as an intrasexual aggression test as an acute social stressor to measure behavior toward a novel conspecific, plasma CORT, and CRH-ir and vasopressin-immunoreactivity (AVP-ir) in the central amygdala (CeAmy), PVN, and the supraoptic nucleus (SON) following high or low amounts of biparental care early in life. Based on our previous findings that high-contact (HC) parenting was associated with increased social behavior toward novel infants and juveniles [49] we expected adult offspring of HC parents to display lower levels of aggression and increased prosocial behavior toward a novel same-sex adult. As previously discussed, chronic social isolation in prairie voles results in an increase in several measures of HPA activity [42, 44-46]. In some cases, increased CORT levels are found only in females, suggesting that they may be more sensitive to the effects of social isolation [44, 45]. In addition, offspring of low LG rat dams show a delayed GC feedback system, leading to a prolonged rise in CORT levels and increased CRH mRNA in the PVN following a stressor [32]. We therefore predicted HC offspring, particularly females, would have lower CORT levels compared to LC offspring following a chronic social stressor as well as a combined chronic and acute social stress. Because of the differential regulation of the HPA response via AVP following chronic stress [48], AVP-ir levels in the PVN and SON were expected to be elevated in LC compared to HC offspring after chronic isolation, especially in females, while CRH-ir levels were expected to be elevated in LC compared to HC offspring following chronic isolation coupled with an intrasexual aggression test.
2. Methods and Materials
2.1 Subjects
Subjects included laboratory-bred prairie voles (Microtus ochrogaster), descendants from a stock wild-caught near Champaign, IL. Animals were housed on a 14:10 light dark cycle with lights on at 0600. Food (Purina high-fiber rabbit chow) and water were available in the home cage ad libitum. Breeder pairs and pre-weaning offspring were housed in large polycarbonate cages (44 × 22 × 16 cm) and were given cotton nestlets for bedding. Offspring were weaned on postnatal (PND) 20 and housed alone in a small polycarbonate cage (27 × 16 × 16 cm) with cotton nestlets for bedding until testing. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
2.2 Parental care quantification and ranking
Following methods previously described [49], the type and amount of parental care given to offspring was observed for two separate litters for 40 different breeder pairs. Each parent was observed for 20 minutes in the morning and again in the afternoon twice between PND 1-3 for a total of 4 maternal care and 4 paternal care observations per litter. Parents were distinguished from one another based on individual characteristics such as fur color and markings, body size, or the presence of pups visibly attached to the nipple. Observations were done with the animals in their home cage; animals were not disturbed during the observations. Behaviors were recorded in real time by a trained observer using Behavior Tracker software (www.behaviortracker.com). Behaviors recorded were based on an ethogram presented in Stone and Bales, 2010 [52] and included maternal and paternal huddling, non-huddling contact, licking/grooming, retrievals, and non-pup directed behaviors, such as nest building, eating and drinking. Maternal nursing postures were also recorded, including neutral, lateral, and active nursing.
In order to rank breeder pairs in relation to one another, total contact times for pup-directed behaviors were summed across each observation for the mother and the father. A mean was then calculated for the mother and the father, and these two means were then summed to produce an average total contact score for the breeder pair for a single litter. These scores for all 40 breeder pairs were then rank-ordered and split into quartiles. The top quartile breeder pairs were selected to produce HC offspring while the bottom quartile breeder pairs were selected to produce LC offspring for subsequent testing. Breeder pairs that fell in the middle quartiles were not used for this study.
2.3 Early parental care of subject offspring
After the selection of the HC and LC breeders, parental care observations were conducted upon the birth of subsequent litters to determine early life care directed toward subject offspring. Within 24 hours of birth, offspring were briefly removed from the home cage, at which time they were weighed, sexes checked, dyed for immediate identification using Nyanzol dye, and toe clipped for long-term identification – ear tagging was not possible due to the age of the pups. Offspring were also randomly assigned to adult behavioral testing conditions at this time. Only one animal per litter per sex was included in each condition. If necessary, litters were culled to 4 offspring, 2 males and 2 females. Only those litters with at least 3 pups were included in the study. Time out of the nest was kept under 15 minutes. Focal observations were conducted on each pup on PND 1-2 for 5 minutes in the morning and 5 minutes in the afternoon (4 total observations per subject) to characterize the type and amount of parental care each offspring received in the first days following birth. Observations were done in real time by a trained observer blind to parental ranking using Behavior Tracker. All observations were conducted while the animals were in their home cage and animals were not disturbed.
2.4 Behavioral testing
Offspring were weaned on PND 20, weighed, and were then placed into one of two testing conditions: chronic social stress or chronic + acute social stress. On PND 48 half of the offspring underwent behavioral testing (n = 38; 21 high-contact; 17 low-contact) while the other half remained undisturbed as isolation controls (n = 37; 19 high-contact; 18 low-contact), followed by plasma collection and brain removal. All behavioral testing occurred between 14:00-15:00.
2.4.1 Chronic social stress – Isolation housing
At weaning, 37 subjects (HC male: 10, LC male: 9; HC female: 9, LC female: 9) were placed in isolation housing, a chronic social stressor in the prairie vole [42, 44, 45]. Animals were housed singly in standard-size polycarbonate cages and were given a cotton nestlet to use as nesting material. Animals remained in isolation for a 4-week period.
2.4.2 Chronic + acute social stress – Isolation housing + intrasexual aggression
At weaning, 38 subjects (HC male: 11, LC male: 9; HC female: 10, LC female: 8) were placed in isolation housing for 4 weeks as described above. After 4 weeks of isolation housing, animals underwent a 5-minute test of intrasexual aggression with a novel same-sex age-matched stimulus animal. This test is often used as a measure of mate-guarding behaviors following formation of a partner preference [53, 54]. Naïve prairie voles typically display low levels of aggression toward same-sex conspecifics, so for the purpose of this study this test is being used as a low-threat, low-aggression acute social stressor. The subject and stimulus animal were placed in a novel arena (27 × 16 × 16 cm) with clean bedding simultaneously. Behavior was video-recorded and scored later by an observer blind to rearing condition. Behaviors recorded included social behaviors such as sniffing, grooming and side-by-side contact, aggressive behaviors, including chasing, boxing, offensive rearing (initiating aggressive behavior toward the stimulus animal while standing on one's hind legs) and defensive rearing (defending against aggressive behavior from the stimulus animal while standing on one's hind legs), and a displacement behavior, rearing (standing on one's hind legs without engaging in any behavior with the stimulus animal). At least 24 hours prior to testing stimulus animals were screened for aggressive behavior toward a novel animal. Only those animals that did not display high levels of aggression were used for testing. During testing the stimulus animal was collared to aid in identification during behavior scoring. Stimulus animals were used for a maximum of 2 tests with at least 24 hours between tests. Stimulus animals were not reused if they experienced any aggressive behaviors from the subject animals. Tests were monitored for high levels of aggression and stopped if necessary. However, intense aggression was very rare between animals. Following behavioral testing animals were returned to their home cage.
2.5 Blood collection and brain extraction
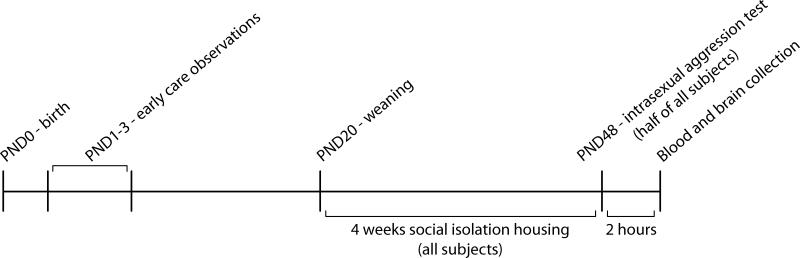
Two hours after behavioral testing a blood sample was collected and brains were extracted. Samples were taken from animals that underwent only chronic isolation at the same time. Animals were euthanized via cervical dislocation and rapid decapitation under deep anesthesia. Trunk blood was collected and immediately kept on ice. Samples were collected within 4 minutes of disturbing the home cage to avoid increases in plasma CORT due to disturbance. Brains were removed rapidly and fixed via passive perfusion in a 4% paraformaldehyde/acrolein solution. A timeline for all procedures occurring following initial breeder pair ranking is presented in Figure 1.
Figure 1.
Study timeline.
2.6 Corticosterone radioimmunoassay
Following collection, blood samples were centrifuged at 9000 rpm at 4° C for 12 minutes and plasma was extracted and stored at −20° C until assayed. Corticosterone was assayed using a radioimmunoassay (MP Biomedicals, Irvine, CA, USA) previously validated for use in the prairie vole [55]. Non-extracted samples were diluted 1:2,000 so that all values fell on the standard curve. Intra-assay coefficients of variation (CV) averaged 1.80%. There is no inter-assay CV to report as all samples were run in one assay.
2.7 Immunohistochemistry
Brains were sliced at 40 μm and stored in cryoprotectant at −20° C until assayed. Free-floating sections were washed in 0.01M KPBS and then pre-treated in 1% sodium borohydride for 20 minutes, followed by KPBS washes. Sections were incubated for 15 minutes in 0.014% phenylhydrazine, washed in KPBS, and then incubated in blocking solution (1% bovine serum albumin, 1% normal goat serum, and 0.3% Triton-X in KPBS) at room temperature for 1 hour. After another KPBS wash, sections were incubated in a primary antibody solution that contained blocking solution and rabbit anti-CRH antibody (1:40,000 dilution for sections containing PVN, 1:80,000 dilution for sections containing CeAmy; provided by Dr. Ann-Judith Silverman) or guinea pig anti-AVP antibody (1:50,000 dilution; Bachem, Torrance, CA, USA) for one hour at room temperature, then 65 hours at 4° C.
Following this incubation, sections were rinsed in KPBS, then incubated for 1 hour at room temperature in biotinylated goat anti-rabbit (CRH; Vector Laboratories, Burlingame, CA, USA) or biotinylated goat anti-guinea pig (AVP; Vector Laboratories) IgG in blocking solution (1:600 dilution). Sections were then washed in KPBS and incubated in avidin-biotin peroxidase solution (Vectastain Elite ABC Kit; Vector Laboratories; 5 μl solution A, 5 μl solution B, 0.4% Triton-X, and 1% bovine serum albumin in KPBS) for 1 hour at room temperature. Sections were next washed in KPBS, then in 0.175M sodium acetate before staining was developed using a diaminobenzidine solution (DAB Peroxidase Substrate Kit; Vector Laboratories), followed by rinsing with sodium acetate, then rinsing with KPBS. Sections were then mounted onto glass slides and allowed to dry overnight. Once dry, slides were dehydrated with ethanol, cleared with Histoclear (National Diagnostics, Atlanta, GA, USA), and coverslips mounted with Histomount (National Diagnostics).
2.8 Microscopy and Quantification of Immunoreactivity
Images of the PVN, SON and CeAmy were captured with a Zeiss Axioimager using an Axiocam MRC camera at 10X magnification. Analysis was done using NIH Image J software. When analyzing both AVP-ir and CRH-ir in the PVN, hand counts of labeled cell bodies were conducted for a single section of tissue at a caudal point in the PVN that takes a characteristic shape and fiber projection pattern described previously [56, 57]. This section is approximate to Figure 37 in the Franklin and Watson (2008) mouse brain atlas [58]. Cell counts for the SON (AVP-ir only) were conducted at the same level used for the PVN analysis. Because stained fiber density was very high and stained cells were so faint, cell counting was not possible in the CeAmy when analyzing CRH-ir and only density measurements were collected for this region.
Density measurements were taken for AVP-ir in the PVN and SON and for CRH-ir in the PVN and CeAmy. The percentage of immunoreactive staining for each region was determined using the threshold function in Image J to separate stained cell bodies and fiber projections from background. For all measurements, a standardized sampling area was used for each tissue section to ensure that any differences seen were not the result of changes in defining borders for each region. This method has been used previously for both AVP-ir and CRH-ir [46, 57, 59]. All density measurements were taken bilaterally and the two measurements were then averaged to produce a single percentage of staining for each region. In the PVN and SON, measurements were taken from the same single section as was used for cell body counts. Three density measurements were collected within each section of PVN: one including the region of both cells and fibers (sampling area: 40 × 70 μm), and two measuring projecting fibers (vertical sampling area: 50 × 90 μm; horizontal sampling area: 40 × 20 μm). Two areas were used to ensure the fornix was not included in the sampling area and the density of the fiber projections was the sum of the two measurement areas. A single box was used in the SON region that encompassed both cell bodies and fiber projections (sampling area: 90 × 40 μm). The image was rotated approximately 45° in Image J prior to taking the density measurements to avoid inclusion of the optic tract in the sampling area. A rounded sampling area was used for the CeAmy (sampling area diameter: 125 μm). For this region, density measurements were taken bilaterally for 4 sections, corresponding to Figures 41-43 in the mouse brain atlas [58]. In some cases, only 3 sections were available for analysis. Percentages were then averaged across all sections to produce a single percentage of immunoreactive staining for the region.
2.9 Data analysis
Analyses were conducted using SAS 9.2. All significance levels were set at p < 0.05 and residuals were checked for normality. Early parental care was compared between male and female offspring using an analysis of variance (ANOVA) with Breeder Pair included as a random variable to determine if parents cared for male and female offspring differently. A false discovery rate (FDR) correction was used to correct for multiple comparisons [60]. Because there were no statistical differences in the amount of care directed toward male offspring compared to female offspring, sexes were combined to analyze early parental care directed toward HC compared to LC offspring. Early care was then analyzed for HC and LC offspring using an ANOVA with Breeder Pair included as a random variable. A false discovery rate (FDR) correction was again used to correct for multiple comparisons. Body weights were analyzed with independent t-tests for birth and weaning weights and with a 2-way Group × Condition ANOVA for weights at adult testing. Behavior in an intrasexual aggression test was analyzed using an ANOVA. Physiological measures, including plasma corticosterone and CRH and AVP immunoreactivity, were analyzed using an ANOVA for the chronic stress as well as the chronic + acute stress groups. Analyses for all physiological measures compared the chronic stress group and the chronic + acute stress group in parallel. Because the interest was in the effects of early experience on adult physiology and behavior for each sex, analyses of stress physiology and behavior in an intrasexual aggression test were analyzed separately for both sexes.
3. Results
3.1 Early parental care and body weight of subject offspring
Parental care directed toward offspring in the first days following birth differed between HC and LC litters but not between male and female offspring. There were no sex differences between the care directed toward male and female offspring in any individual parental behavior or in total maternal or paternal behavior or overall total care (see Table 1 for means and standard errors of total care measures). Total care summed between mothers and fathers was significantly greater in HC compared to LC offspring (F(1,108) = 19.17, adjusted p = 0.0006; Table 1). There was no difference between groups in total maternal care, while total paternal care was significantly increased in HC compared to LC offspring (F(1,108) = 45.94, adjusted p = 0.0006). When examining individual parental behaviors, HC parents tended to spend more time quiescent in the nest while LC parents spent a greater amount of time engaging in active behaviors, particularly in relation to paternal behavior. HC fathers spent more time huddling (F(1,108) = 11.32, adjusted p = 0.0040) and in non-huddling contact (F(1,108) = 32.65, adjusted p = 0.0006) with pups while LC fathers spent more time nest building (F(1,108) = 9.46, adjusted p = 0.0072) and retrieving infants (F(1,108) = 5.35, adjusted p = 0.0460). HC mothers spent a greater amount of time in a neutral nursing posture (F(1,108) = 10.15, adjusted p = 0.0060) while LC mothers spent more time retrieving pups (F(1,108) = 11.72, adjusted p = 0.0040) and huddling over pups (F(1,108) = 8.76, adjusted p = 0.0090) and tended to spend a greater amount of time nest building (F(1,108) = 4.82, adjusted p = 0.0554).
Table 1.
Total maternal, paternal, and overall care (s) by early rearing group and by sex; mean (SE)
| High contact offspring | Low contact offspring | |
|---|---|---|
| Total parental care | 1346.04 (24.44)*** | 1167.62 (35.64) |
| Total maternal care | 878.62 (34.28) | 885.96 (38.81) |
| Huddling | 325.05 (41.67) | 480.43 (52.85)** |
| Non-huddling contact | 4.23 (1.49) | 4.66 (1.15) |
| Licking/grooming | 18.21 (2.63) | 18.66 (3.54) |
| Retrievals | 4.36 (0.85) | 11.69 (3.31)** |
| Nest building | 6.08 (2.70) | 24.46 (8.00)^ |
| Active nursing | 44.59 (8.48) | 40.46 (9.10) |
| Lateral nursing | 73.67 (19.26) | 86.66 (30.03) |
| Neutral nursing | 350.05 (46.15)** | 244.83 (28.90) |
| Total paternal care | 412.42 (32.38)*** | 206.74 (30.36) |
| Huddling | 235.15 (44.08)** | 83.63 (25.60) |
| Non-huddling contact | 209.67 (26.19)*** | 92.94 (18.95) |
| Licking/grooming | 9.31 (2.37) | 8.46 (2.51) |
| Retrievals | 1.83 (1.22) | 9.09 (3.58)* |
| Nest building | 2.74 (0.81) | 8.89 (3.31)** |
| Male offspring | Female offspring | |
| Total parental care | 1230.01 (33.91) | 1293.28 (29.05) |
| Total maternal care | 820.35 (37.99) | 941.31 (33.45) |
| Total paternal care | 333.49 (35.05) | 298.97 (32.46) |
adjusted p < 0.001
adjusted p < 0.01
adjusted p < 0.05
adjusted p = 0.05
There were also no differences in body weight between HC and LC offspring on PND 1 or at weaning on PND 20. A 2-way Group × Condition ANOVA showed no significant effect of either early care group or adult testing condition and no significant interaction between group and condition on body weight at behavioral testing on PND 48 (see Table 2 for means and standard errors).
Table 2.
Offspring body weights (g) on PND 1, 20, and 48; mean (SE)
| PND 1 (g) | PND 20 (g) | PND 48 (g) | |
|---|---|---|---|
| HC Offspring | 3.71 (0.07) | 22.09 (0.36) | 39.98 (0.93) |
| LC Offspring | 3.66 (0.07) | 22.81 (0.31) | 40.83 (0.81) |
3.2 Intrasexual Aggression
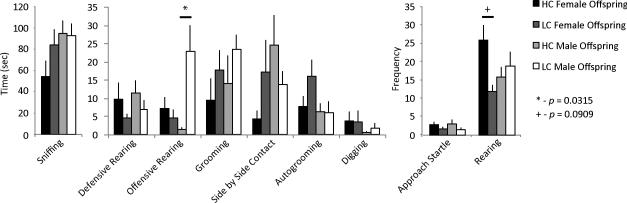
The early rearing environment was related to behavior in an intrasexual aggression test in a sex-dependent manner. Males reared by LC parents initiated aggression toward a novel non-aggressive stimulus animal in a test of intrasexual aggression significantly more than did males reared by HC parents (F(1,19) = 11.30, adjusted p = 0.0315; Figure 2). Females reared by HC parents tended to display more rearing, a displacement behavior, in a test of intrasexual aggression compared to females reared by LC parents (F(1,16) = 8.67, adjusted p = 0.0909).
Figure 2.
Intrasexual aggression behavior. Initiation of aggression via offensive rearing was increased in LC compared to HC male offspring (F(1,19) = 11.30, adjusted p = 0.0315) in a test of intrasexual aggression while rearing behavior tended to be increased in HC compared to LC female offspring (F(1,16) = 8.67, adjusted p = 0.0909).
3.3 Physiological measures
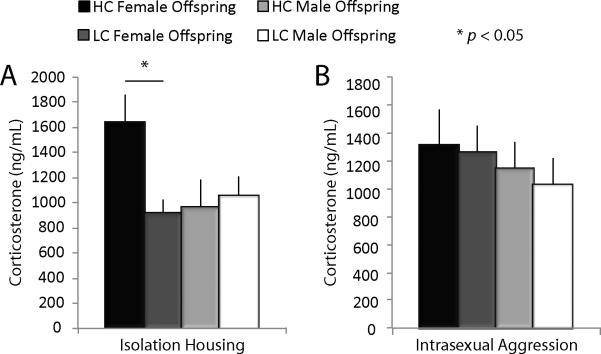
Measures of plasma CORT, CRH-ir, and AVP-ir show effects of rearing environment for female but not male offspring following chronic isolation and chronic isolation plus intrasexual aggression stress conditions as an adult. Following 4 weeks of chronic isolation stress, females reared by HC parents had significantly elevated levels of plasma CORT compared to females reared by LC parents (F(1,15) = 7.30, p = 0.0172; Figure 3A). There were no differences in males after 4 weeks of chronic isolation housing and no differences in either males or females after 4 weeks of chronic isolation plus an intrasexual aggression test.
Figure 3.
Plasma corticosterone levels. (A) Elevated plasma CORT levels were seen in HC compared to LC female (F(1,15) = 7.30, p = 0.0172) but not male offspring following isolation housing. (B) No changes were seen in plasma CORT levels after an intrasexual aggression test.
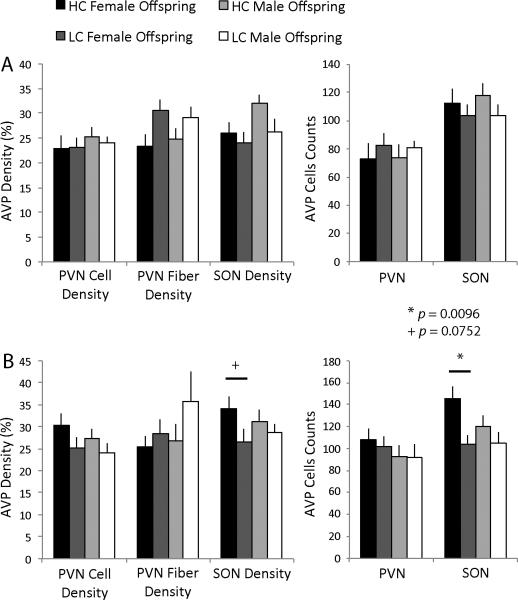
There were no differences between HC and LC early care groups for males or females in either adult stress condition in the number of CRH-immunopositive cells in the PVN, the density of CRH-immunopositive cells and fibers in the PVN, or in the density of CRH-immunopositive cells and fibers in the CeAmy (see Table 3 for means and standard errors). In the chronic isolation plus intrasexual aggression test condition, females of HC parents had significantly more AVP-immunopositive cells in the SON compared to LC females (F(1,15) = 9.00, p = 0.0096; Figure 4B) and also tended to have a greater density of AVP-immunopositive cells and fibers in the SON (F(1,15) = 3.69, p = 0.0752). Meanwhile, there were no differences between HC and LC males in AVP-ir in the SON in this testing condition. There were also no differences in AVP-ir cell number, cell density, or fiber density in the PVN between HC and LC males and females in the chronic isolation plus intrasexual aggression test condition. There were no differences in any measures of AVP-ir in any animals who underwent only chronic social isolation.
Table 3.
CRH-ir in the CeAmy and PVN; mean (SE)
| CeAmy CRH-ir Fiber Density | PVN CRH-ir Fiber Density | PVN CRH-ir Cell Count | |
|---|---|---|---|
| Isolation housing | |||
| HC Female offspring | 31.38 (1.61) | 6.52 (1.25) | 101.22 (8.49) |
| LC Female offspring | 34.50 (1.82) | 5.01 (0.74) | 95.89 (10.09) |
| HC Male offspring | 30.57 (1.58) | 5.95 (0.75) | 103.60 (4.49) |
| LC Male offspring | 31.58 (1.95) | 4.73 (0.82) | 106.22 (14.75) |
| Isolation housing + Intrasexual aggression | |||
| HC Female offspring | 19.64 (1.07) | 7.39 (1.27) | 106.25 (11.11) |
| LC Female offspring | 20.58 (1.83) | 6.36 (1.07) | 121.14 (11.42) |
| HC Male offspring | 22.06 (1.26) | 6.37 (1.26) | 114.78 (8.34) |
| LC Male offspring | 21.62 (1.61) | 7.06 (1.50) | 111.75 (11.42) |
Figure 4.
AVP-ir in the PVN and SON. (A) There were no differences in AVP-ir in the PVN or SON of the hypothalamus following chronic social isolation between HC and LC offspring for either males or females. (B) HC female offspring had a greater number of AVP-labeled cells in the SON compared to LC offspring (F(1,15) = 9.00, p = 0.0096) and tended to also have a greater density of AVP-positive cells and fibers in the SON (F(1,15) = 3.69, p = 0.0752) after an intrasexual aggression test. There were no differences in males in AVP-ir following varying early care.
4. Discussion
The results presented here provide evidence for sex-dependent effects of early experience on reactivity to a chronic isolation stress in adulthood. Male offspring showed increases in aggressive behavior but no changes in HPA activity to an acute social stressor after chronic isolation while female offspring displayed differences in CORT levels as well as AVP-ir but no change in aggression. The differential HPA response between the sexes, with females being more reactive than males to social stress, matches other data in the prairie vole suggesting females are more susceptible to social stressors [44, 45] but differences between HC and LC offspring are not necessarily consistent with findings in rat models of early experience [32, 33]. This is the first study, to our knowledge, demonstrating relationships with stress reactivity following naturally occurring variation in early biparental care in a mammalian species.
Offspring of HC parents received significantly more care and, in particular, greater amounts of specific components of paternal care while offspring of LC parents received more active parenting behaviors. This confirms our previous findings that HC and LC parents differ not only in the amount of care directed toward their offspring, but also in the type of care given [49]. One key difference between the current experiment and our previous study is the amount of huddling behavior directed toward offspring. Here, HC fathers displayed significantly more huddling behavior compared to LC fathers while LC mothers huddled over pups more than their HC counterparts. One potential explanation is that, because HC mothers are engaging in a decreased amount of huddling with pups, HC fathers have more access to the infants, thereby increasing the amount of time they are able to spend in a huddling posture over the pups. What remains unclear and warrants further research is if increased paternal access to offspring is opportunistic – the mother is spending more time away from the nest, leading to more pup access for the father – or whether it is due to the father displacing the mother from the nest, thereby gaining access to the infants.
In an acute social stressor, LC male offspring initiated aggressive interactions with a non-aggressive cagemate at a higher rate than HC male offspring. The relatively high level of aggression in these animals is of note because the subject animals were sexually naïve, a point at which they would typically have shown very low levels of aggression. In addition, they were paired in a neutral cage with a same-sex size-matched animal pre-screened to be non-aggressive, further reducing the likelihood of aggressive interactions. That an increase in aggressive behavior was observed in LC males suggests that early rearing environment is related to not just pro-social behavior, as we have previously demonstrated [49], but also to aggression. Indeed, previous research has shown a link between early experience and later aggressive behaviors that appears to be both sex- and species-dependent. For example, long-term maternal separation decreases maternal aggression in rat dams [5] and decreases intermale aggression in adult C57Bl/6 mice [24] yet increases intermale aggression in adult rats [6]. Post-weaning social isolation stress has also been shown to increase aggression toward novel infants in the prairie vole [41] and toward social interaction partners in adolescent rats [61] as well as alter the types of aggressive behaviors displayed [62]. Aggression was increased here following a combination of decreased early parental care in the LC offspring coupled with chronic isolation housing, supporting the idea that decreased social stimulation in early life as well as post-weaning may increase aggressive social interactions in adulthood. Indeed, there is a great deal of evidence in humans establishing a link between childhood neglect and later aggression [18, 19].
The stress response is controlled by both the HPA and hypothalamic-neurohypophyseal systems, where parvocellular neurons in the PVN respond to a stressor by releasing both CRH and AVP into the hypophyseal portal blood supply. This in turn stimulates corticotroph cells in the anterior pituitary via the Zona externa of the median eminence to release ACTH into the blood stream, which eventually acts on the adrenal cortex to produce a GC response to the stressor. After chronic stress, however, the regulation of this pathway shifts from a joint CRH/AVP release to a predominantly AVP-driven response [48, 63]. Results here fit with this concept; no differences were seen in CRH-ir in the PVN or CeAmy, a region important in the limbic control of the stress response, while there were differences in the number of AVP-labeled cells in the SON but not the PVN. These differences in AVP production from the SON, however, were seen only in females that had undergone both a chronic and an acute social stressor. As mentioned previously, parvocellular neurons in the PVN synthesize and release AVP to promote the release of ACTH. While the magnocellular and parvocellular neuronal organization has not been thoroughly characterized in the prairie vole, in the rat, these parvocellular neurons are not thought to be present in the SON [64]. Instead, AVP, as well as the related neuropeptide oxytocin, are produced by magnocellular neurons in the SON and released via the Zona interna of the median eminence from the posterior pituitary. It is likely these magnocellular neurons that are responsible for the AVP response to acute stressors (see [65] for review). The increase in AVP-labeled cells in only the SON of HC females here suggests that early life care does not result in differential AVP reactivity to a chronic stressor but does have impacts on AVP response to an acute social stress. This is further supported by the lack of a difference in measures of CRH-ir. The AVP response to these stressors could also potentially be linked to dendritic peptide release or to actions of axonal projections to regions other than the previously mentioned hypophyseal portal system. There is evidence for dendritic release of AVP and the closely related neuropeptide OT [66-68]. In addition, axonal OT projections from the hypothalamus have been found in the nucleus accumbens in the prairie vole [69] and the CeAmy in rats [70]. It may be that AVP release from the hypophyseal portal system into the peripheral blood supply works not only synergistically with CRH but also works together with dendritic AVP release and a more extensive network of axonal projections to influence stress responsivity.
In addition to differences in AVP-ir, female, but not male, offspring also displayed differences in plasma CORT levels following a chronic social isolation stressor. HC females had elevated levels of CORT after 4 weeks of isolation housing compared to LC female offspring. Previous research has shown that female prairie voles show a greater CORT response to a resident-intruder test following chronic isolation housing stress than do males [45], suggesting females may be particularly sensitive to social isolation. Based on studies of variation in maternal care in rat dams [32, 33] as well as studies of early rearing influences on social stress responses in mice [34], we had expected that LC offspring would show an elevated HPA response to a chronic social isolation compared to HC offspring. Given the role of social interaction in this species, including adult pair-bond formation, we alternately predicted that HC offspring would display greater HPA activity following long-term social isolation compared to LC offspring. In this case, decreased early care from LC parents may prepare offspring for lower amount of social interaction later in life. In fact, this second hypothesis was true both for CORT and for AVP-ir. It may be that lower amounts of care and different styles of early care of these LC offspring provide some sort of protection against chronic social isolation stress, particularly in female offspring. In addition, there was no CORT elevation in LC offspring following the intrasexual aggression test as has been seen after a stressor in a rat model of varying early life experience. Following a stressor, CORT levels in the prairie vole peak at 30 minutes post-stress and then begin to return to baseline [55]. Blood samples here were taken 2 hours following behavior testing to look for an extended rise in GC in response to a stressor, as has been observed in rats reared by low LG dams after a restraint stress [32]. This prolonged increase in circulating GC following decreased early care seen in rats was not present in LC prairie vole offspring, suggesting that HPA regulation in this species does not necessarily follow that found in rats following varying early care. While not directly compared, the data suggest that plasma CORT levels were increased in animals following the intrasexual aggression test compared to plasma CORT levels in animals who experienced only isolation. This supports the use of an intrasexual aggression test, even with a non-aggressive stimulus animal, as an acute social stressor.
While LC rearing experiences may in some way be protective against later long-term social isolation, an alternative explanation is that the HPA axis in LC females underwent an early-life reprogramming in response to decreased early care, thereby leaving them unable to mount an appropriate HPA response to a social challenge as an adult. In this regard, the lack of a CORT response in these females following chronic social isolation, coupled with a CORT response to a novel acute stressor that was indistinguishable from the response of HC females, mimics patterns seen in individuals diagnosed with post-traumatic stress disorder (PTSD). One physiological component of PTSD is a decreased GC release in response to a stressor [71]. In addition, individuals with PTSD display enhanced GC feedback sensitivity [72]. A potential mechanism for this enhanced negative feedback and associated lower GC levels is that chronic stress, such as is present in several animal models of adverse early experience, leads to lasting changes in the GR system. Indeed, early handling in rats results in an increased density of hippocampal GR [73], suggesting that differences in the early environment resulted in differential functioning of GR. It may be that female prairie voles reared by LC parents have altered HPA function and GR systems so that long-term social isolation stress in adulthood results in outcomes that resemble components of PTSD, an idea that warrants further investigation. It would also be worthwhile to add a medium-contact group in future studies to determine whether this and other findings occur on a continuum or if LC rearing is, in fact, a distinct style of infant care.
It is worth noting the differences in stressor types used in this model of varying early care in the prairie vole compared to those often used in other rodents. Research in models of maternal behavior in rats uses primarily physical or non-social stressors, such as restraint stress or forced swim testing. Given the nature of social bonding and attachment in the prairie vole, they provide a unique chance to study the consequences of early life experience on reactivity to psychological or social stressors. Enriching early environments can be protective against social stressors while not impacting reactivity to physical stressors [34], although it appears that increased amounts of tactile stimulation during early rearing here actually increased the CORT and AVP-ir response to long-term social isolation. An additional question raised is whether differences seen here are the result of epigenetic changes due to the early environment or are caused by some genetic difference between HC and LC parents that is passed to offspring. Evidence in rats suggests that early rearing experience shapes neuropeptide receptor systems in offspring [74, 75]. We have previously used cross-fostering methods and found in the prairie vole, however, that OTR and V1aR binding distributions are transmitted from genetic parent to offspring in a sex-dependent manner while behavior was transmitted to offspring via non-genomic mechanisms (Perkeybile et al, in preparation). These findings suggest that aggressive behaviors may be due to the early rearing experience while AVP-ir changes may have a stronger genetic basis. It is clear, however, that additional cross-fostering work needs to be done to better understand this point.
In conclusion, the present findings indicate that early parental care is associated with changes in behavior and HPA response to chronic and acute social stressors in a sex-dependent and experience-specific manner. Males reared by LC parents showed increases in aggressive behavior toward a novel non-aggressive conspecific while females of HC parents had increased levels of plasma CORT and an increased number of AVP-immunopositive cells in the SON after long-term social isolation. These results suggest that increased amounts of parental care may not always lead to a more tightly-regulated HPA response to social stress and extend our understanding of the consequences of naturally occurring variation in early infant care in a biparental species.
Highlights.
We examine the relationship between early experience and social stress responses.
Behavior and HPA responses to social stress were sex- and early care-dependent.
Low-contact male offspring displayed increased aggression toward a novel conspecific.
High-contact female offspring had elevated CORT and AVP-ir after long-term isolation.
Acknowledgements
The authors thank Meredith Lee and Julie VanWesterhuyzen for technical assistance and Drs. Cindy Clayton and Rhonda Oates-O'Brien for veterinary care.
Role of the funding sources
This work was supported by HD060117 to K.L.B. and by the University of California, Davis. Funding sources had no role in the design of experiments, in the collection, analysis, and interpretation of data, in the writing of this report, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report that they have no conflicts of interest.
References
- 1.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 2.Levine S, Haltmeyer GC, Kargs GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiol Behav. 1967;2:5. [Google Scholar]
- 3.Padoin MJ, Cadore LP, Gomes CM, Barros HMT, Lucion AB. Long-lasting effects of neonatal stimulation on the behavior of rats. Behavioral Neuroscience. 2001;115:1332–40. doi: 10.1037//0735-7044.115.6.1332. [DOI] [PubMed] [Google Scholar]
- 4.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) messenger-RNA, median-eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 5.Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–72. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 6.Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. European Journal of Neuroscience. 2006;24:1711–20. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Kajantie E, Raikkonen K. Early life predictors of the physiological stress response later in life. Neuroscience and Biobehavioral Reviews. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Goodman E, McEwen BS, Huang B, Dolan LM, Adler NE. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosomatic Medicine. 2005;67:9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- 11.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDade TW. Early environments and the ecology of inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17281–8. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon HJ, Kang ES, Lee EH, Jeong EG, Jeon JR, Mischoulon D, et al. Childhood trauma and platelet brain-derived neurotrophic factor (BDNF) after a three month follow-up in patients with major depressive disorder. J Psychiatr Res. 2012;46:966–72. doi: 10.1016/j.jpsychires.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: Role of neurotrophins from rodents to non-human primates. Neuroscience and Biobehavioral Reviews. 2009;33:573–85. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19119–24. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 17.Hildyard KL, Wolfe DA. Child neglect: developmental issues and outcomes. Child Abuse Negl. 2002;26:679–95. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- 18.Kotch JB, Lewis T, Hussey JM, English D, Thompson R, Litrownik AJ, et al. Importance of early neglect for childhood aggression. Pediatrics. 2008;121:725–31. doi: 10.1542/peds.2006-3622. [DOI] [PubMed] [Google Scholar]
- 19.Prino CT, Peyrot M. The effect of child physical abuse and neglect on aggressive, withdrawn, and prosocial behavior. Child Abuse Negl. 1994;18:871–84. doi: 10.1016/0145-2134(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 20.Widom CS, Czaja S, Dutton MA. Child abuse and neglect and intimate partner violence victimization and perpetration: A prospective investigation. Child Abuse Negl. 2014;38:650–63. doi: 10.1016/j.chiabu.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children's adjustment: Contributions of developmental timing and subtype. Dev Psychopathol. 2001;13:759–82. [PubMed] [Google Scholar]
- 22.Mitchell GD, Raymond EJ, Ruppenth Gc, Harlow HF. Long-term effects of total social isolation upon behavior of rhesus monkeys. Psychol Rep. 1966;18:567–&. [Google Scholar]
- 23.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–8. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 24.Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–50. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Day HD, Seay BM, Hale P, Hendricks D. Early social deprivation and the ontogeny of unrestricted social behavior in the laboratory rat. Developmental Psychobiology. 1982;15:47–59. doi: 10.1002/dev.420150108. [DOI] [PubMed] [Google Scholar]
- 26.Luciano D, Lore R. Aggression and social experience in domesticated rats. Journal of Comparative and Physiological Psychology. 1975;88:917–23. doi: 10.1037/h0076439. [DOI] [PubMed] [Google Scholar]
- 27.Toth M, Mikics E, Tulogdi A, Aliczki M, Haller J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Hormones and Behavior. 2011;60:28–36. doi: 10.1016/j.yhbeh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- 29.McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Developmental Psychobiology. 2006;48:537–50. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev Psychopathol. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 33.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 34.Branchi I, Santarelli S, D'Andrea I, Alleva E. Not all stressors are equal: early social enrichment favors resilience to social but not physical stress in male mice. Horm Behav. 2013;63:503–9. doi: 10.1016/j.yhbeh.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Parker V, Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology. 1986;25:663–4. [Google Scholar]
- 36.Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. European Journal of Neuroscience. 2013;37:1022–31. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malkesman O, Maayan R, Weizman A, Weller A. Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav Brain Res. 2006;175:408–14. doi: 10.1016/j.bbr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Hatch AM, Wiberg GS, Zawidzka Z, Cann M, Airth JM, Grice HC. Isolation syndrome in the rat. Toxicol Appl Pharmacol. 1965;7:737–45. doi: 10.1016/0041-008x(65)90132-8. [DOI] [PubMed] [Google Scholar]
- 39.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behav Brain Res. 2005;156:289–96. doi: 10.1016/j.bbr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 41.Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan YL, Liu Y, Young KA, Zhang ZB, Wang ZX. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neuroscience Letters. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–53. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and Behavior. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biological Psychiatry. 1996;40:918–22. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- 48.Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880–4. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 49.Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Front Behav Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiol Behav. 2006;87:424–9. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 51.DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11980–4. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone AI, Bales KL. Intergenerational transmission of the behavioral consequences of early experience in prairie voles. Behavioural Processes. 2010;84:732–8. doi: 10.1016/j.beproc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Hormones and Behavior. 2003;44:178–84. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 54.Hostetler CM, Harkey SL, Krzywosinski TB, Aragona BJ, Bales KL. Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole. Behav Pharmacol. 2011;22:703–10. doi: 10.1097/FBP.0b013e32834affd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taymans SE, DeVries AC, DeVries MB, Nelson RJ, Friedman TC, Castro M, et al. The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): Evidence for target tissue glucocorticoid resistance. General and Comparative Endocrinology. 1997;106:48–61. doi: 10.1006/gcen.1996.6849. [DOI] [PubMed] [Google Scholar]
- 56.Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24:874–86. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang ZX, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. Journal of Comparative Neurology. 1996;366:726–37. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 58.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotxic Coordinates. 3rd ed. Academic Press; New York: 2008. [Google Scholar]
- 59.Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin? Front Psychiatry. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamini Y, Hochberg Y. Controlling the false discovery rate - A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 61.Meng QX, Li NX, Han XA, Shao F, Wang WW. Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neuroscience Letters. 2010;480:25–9. doi: 10.1016/j.neulet.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 62.Toth M, Halasz J, Mikics E, Barsy B, Haller J. Early social deprivation induces disturdbed social communication and violent aggression in adulthood. Behavioral Neuroscience. 2008;122:849–54. doi: 10.1037/0735-7044.122.4.849. [DOI] [PubMed] [Google Scholar]
- 63.Dallman MF. Stress update - Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4:62–9. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- 64.Swanson LW, Sawchenko PE. Hypothalamic integration - Organization of the paraventricular and supraoptic nuclei. Annual Review of Neuroscience. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 65.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Frontiers in Neuroendocrinology. 2004;25:132–49. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 67.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophyseal peptides by exocytosis. Neuroscience. 1989;32:435–9. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 68.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, et al. Dendritic Peptide Release Mediates Interpopulation Crosstalk between Neurosecretory and Preautonomic Networks. Neuron. 2013;78:1036–49. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 71.Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biological Psychiatry. 1991;30:1031–48. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 72.Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic-stress-disorder. American Journal of Psychiatry. 1993;150:83–6. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 73.Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 74.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 75.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J Neuroendocrinol. 2002;14:349–53. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]