Abstract
Rett syndrome is a rare neurodevelopmental disorder usually affecting females and associated with a mutation in the MECP2 gene. Sleep problems occur commonly and we investigated the trajectories and influences of age, mutation and treatments. Data was collected at six time points over 12 years from 320 families registered with the Australian Rett Syndrome Database. Regression analysis was used to investigate relationships between sleep disturbances, age, mutation type and use of treatment, and latent class growth analysis was performed to identify sleep problem phenotypes and model the effect of mutation type. The age range of subjects was 2.0–35.8 years. The study showed that sleep problems occurred in more than 80% of individuals and the prevalence decreased with age. Night laughing and night screaming occurred in 77% and 49% respectively when younger. Those with a large deletion had a higher prevalence of night laughing which often occurred frequently. Treatment was associated with a 1.7% reduction in risk of further sleep problem. High and low baseline prevalence groups were identified. Approximately three quarters of girls and women with sleep disturbances were in the high baseline group and problems persisted into adulthood. On the contrary, 57% with night laughing and 42% with night screaming in the high baseline group exhibited mild improvement over time. Mutation type was not found to be a significant predictor of group membership. In conclusion, the evolution of sleep problems differed between subgroups of girls and women with Rett syndrome, in part explained by age and genotype. Treatment was not associated with improvement in sleep problems.
Keywords: Rett syndrome, sleep, MECP2
Introduction
Rett syndrome is a neurodevelopmental disorder usually associated with a mutation on the methyl CpG binding protein 2 (MECP2) gene (Amir et al., 1999), characterized initially by loss of communication and hand function skills and the development of hand stereotypies and impaired gait (Neul et al., 2010). Severity of symptoms and comorbidities, for example in relation to hand function, mobility, scoliosis and epilepsy, varies across individuals and certain mutations (p.R133C, p.R294X and p.R306C) are associated with milder phenotype whilst others (p.T158M, p.R168X, p.R255X, p.R270X and large deletion) are linked to more severe clinical presentation (Bebbington et al., 2008, Cuddapah et al., 2014). Early literature on Rett syndrome described the occurrence of sleep dysfunction, initially manifesting as screaming or night laughing in the young child (Hagberg, 1995, Hagberg, 2002, Hagberg, 2005, Naidu et al., 1986) and believed to be associated with immature sleep patterns (Nomura, 2005). As a consequence, impaired sleep was originally included (Hagberg et al., 2002) and has been retained as a supportive criterion in the current diagnostic criteria (Neul et al., 2010).
Sleep dysfunction in Rett syndrome was described in an early US clinic-based sample (n=20) published in 1990. Total sleep duration was longer than in age-matched typically developing peers (Piazza et al., 1990). Delayed sleep onset, shorter night sleep with night-waking and persisting daytime sleeps were also reported, and sleep patterns appeared to worsen with age. Using a sub-set of the Australian population-based data, persistence of daytime napping with increasing age was also confirmed as were delayed sleep onset and night waking but the proportion of total sleep at night did not decrease with age (Ellaway et al., 2001). In a more recent total Australian population-based study, sleep dysfunction was noted in approximately 80% of girls and women under 30 years of age, and specific symptoms such as night laughing and night screaming were reported to occur in approximately 60% and 35% respectively (Young et al., 2007). In the same study we also found that the prevalence of night laughter decreased with age and sleep problems were more likely in those with certain genotypes such as large deletion, p.R294X or p.R306C mutation. There is need for further longitudinal analyses to better understand the natural history of sleep dysfunction in Rett syndrome.
Management of sleep disturbance in children and adults with developmental disorders includes behavioral and pharmacological strategies but the evidence base in Rett syndrome is limited. One single case design study (n=3) tested behavioral strategies during short inpatient hospital stays and improvements in regularity of sleep pattern were achieved (Piazza et al., 1991). Shorter time to sleep onset has been observed with 5-Hydroxytryptophan, a metabolic intermediate in the synthesis of serotonin and melatonin, in five of seven cases when used over approximately 18 months (Nomura et al., 1985). In a randomized crossover trial of melatonin (n=9), time to sleep onset decreased but there was no change in the frequency of night waking (McArthur and Budden, 1998). Disrupted sleep is likely to have a large burden on the health and well-being of both the child and family affected by Rett syndrome (McDougall et al., 2005) yet our insight into how to manage sleep dysfunction is remarkably limited.
Using longitudinal data contained in the Australian Rett Syndrome Database, we determined the prevalence of sleep problems in Rett syndrome and investigated determinants of the trajectories of sleep problems in Rett syndrome including age, MECP2 mutation and use of treatment.
Methods
Study population and data ascertainment
Established in 1993, the Australian Rett Syndrome Database is a longitudinal and population-based registry of confirmed cases with Rett syndrome born since 1976 (Downs et al., 2008). Follow-up questionnaires have been distributed to parents/carers in 2000, 2002, 2004, 2006, 2009 and 2011 (Leonard et al., 2010). Females who were clinically (Neul et al., 2010) or genetically confirmed and whose parents/carer had completed at least one of the follow-up questionnaires were eligible for the study. Data were available from 320 cases.
Ethical approval of this study was obtained from the Human Research Ethics Committee of Princess Margaret Hospital for Children, Western Australia (1909/EP).
Sleep related variables
Parents or carers were asked at each time point if their daughter had any sleep problems since the last questionnaire. Data were available from 2000 on the presence and frequency of any sleep problems, night laughing and night screaming, and from 2006 for night waking. For each aspect of sleep, the frequency was described as “Did not occur”, “Sometimes” (recorded as “Less than once a month”, “Monthly” or “Twice a month”), or “Often” (recorded as “Weekly or more” or “Nightly). The data was supplemented where necessary with two items from the Rett Syndrome Behaviour Questionnaire (Mount et al., 2002): “Spells of laughter for no apparent reason during the night” (night laughing) and “Spells of screaming for no apparent reason during the night” (night screaming). For analyses, two binary variables were generated: “Presence of sleep problem” by combining the levels “Sometimes” and Often” and compared with “Did not occur”; and “Persistent sleep problem” using the level “Often” and compared with the combined levels “Did not occur” and “Sometimes”. Any and specific sleep problems were considered unresolved if the problem, as represented by the variables “Presence of sleep problem” and “Persistent sleep problem”, continued to the next time point. On the other hand, if no sleep problems were reported at the next time point, the conditions were considered resolved.
Respondents were also asked about the ongoing treatment(s) used for the individual’s sleep problems at each time point. The treatments were grouped into five categories: 1) Sleep medications: melatonin, nitrazepam, temazepam, oxazepam, clonidine, chloral hydrate and hypnotics; 2) Non-specific sleep medications: anti-epileptic drugs, risperidone, haloperidol, olanzapine, promethazine, trimeprazine, amitriptyline, fluoxetine, mirtazapine and medications that alleviated symptoms affecting sleep; 3) Non-pharmaceutical treatments; 4) More than one treatment types as described above; and 5) No treatment. For analyses, a binary variable, that indicated whether the child was treated or not treated, was created by combining the first four treatment groups.
Age at the time each follow-up questionnaire was returned was coded into four categories: younger than 7 years, 8 to younger than 13 years, 13 to younger than 18 years, and 18 years and older. Genotype of MECP2 was categorized as p.R168X, p.T158M, p.R294X, p.R270X, p.R255X, p.R133C, p.R306C, p.R106W, C-terminal deletions (CT), early truncating (ET), large deletions (LD), and other miscellaneous mutations (Other).
Statistical analysis
Generalized estimating equations (GEE) (Zeger and Liang, 1986), a generalized linear model that accounts for within-subject correlation of responses on dependent variables, was used to estimate the occurrence of any sleep problem and specific sleep problems as binary variables for the explanatory variables age group and mutation type, with the latter adjusted for age. Logit link function, robust standard errors and exchangeable working correlation structures were used for parameter estimation. Fitted probabilities of sleep problems for each explanatory variable were then estimated based on the recycled predictions approach using the Stata margins command.
Using the log link function, the GEE model was applied to ascertain the effect (risk ratio) of treatment at one time point on unresolved sleep problems at subsequent time point. Relative risk adjusted for age for each of the six time points were also obtained using log binomial regression analysis.
Latent class growth analysis (Nagin, 2005), a discrete mixture model of longitudinal data grouping, was used to identify developmental trajectories of any sleep problem and specific sleep problems (except night waking as limited observations were available) as a continuous function of age. Temporal sequence of observation used in the analysis started from the first questionnaire returned. The number and the shape of sleep pattern was determined by selecting the model with a minimum value of Bayesian Information Criterion (BIC) (Andruff et al., 2009), a criterion for model selection that ensures goodness of fit and penalizes over-fitting. The probability of belonging to specific trajectory group by mutation type was determined using logistic regression with group membership as the outcome variable and individual mutation type as predicting variable.
The statistical analyses were conducted using Stata version 12 (StataCorp, 2011), SAS version 9.3 (SAS Institute Inc., 2002–2010), and both Stata and SAS version of the command, traj (Nagin, 2005, Jones et al., 2001, Jones and Nagin, 2007).
Results
There were 159, 189, 203, 208, 221 and 220 questionnaires returned in 2000, 2002, 2004, 2006, 2009 and 2011 respectively. The median age at the first (2000) and last (2011) follow-up year was 13.0 years (Interquartile Range (IQR) 8.3–17.5) and 18.1 years (IQR 11.3–24.6) respectively. Over the six time points, the prevalence of any sleep problem were generally very high (>91% except 85% in 2002) in the 7 years or younger age group and moderately high (73%–98%) in the older age groups (Table 1). In those aged 18 years and above the condition appeared to be slightly less common in recent surveys. For night laughing, 60–88% of girls in the youngest age group experienced the problem and it was more frequent in 2006 and 2009 when compared to other time points. Similar to that of any sleep problem, the prevalence of night laughing in the older age groups decreased in recent surveys. Night screaming, on average, was less common than night laughing, and for those in the 13 to 17 years age group the problem was reported less frequently over time (46% in 2000 vs. 27% in 2011). Night waking was common, especially in the 7 years or younger age group. For those in the older age group (13 year or above), there was a decline in prevalence of this condition in recent questionnaire years. The prevalence of having both night waking and laughing ranged from 35% in 2011 to 55% in 2006. Similarly, for night waking and screaming, the prevalence was 26% in 2011 and 36% in 2006. The frequency and prevalence of specific sleep problems by occurrence frequency and year of questionnaire are shown in Table 2.
Table 1.
Frequency and prevalence of sleep problems by age group and year of questionnaire*
| Year of questionnaire [n (%)] | ||||||
|---|---|---|---|---|---|---|
| Age group | 2000 (n=159) | 2002 (n=189) | 2004 (n=203) | 2006 (n=208) | 2009 (n=221) | 2011 (n=220) |
| Any sleep problem | ||||||
| 0–7 years | 33 (94.3) | 29 (85.3) | 34 (94.4) | 31 (93.9) | 22 (91.7) | 31 (93.9) |
| 8–12 years | 40 (93.0) | 43 (81.1) | 40 (87.0) | 36 (81.8) | 39 (92.9) | 30 (79.0) |
| 13–17 years | 42 (97.7) | 46 (95.8) | 37 (78.7) | 40 (87.0) | 36 (83.7) | 32 (84.2) |
| 18+ years | 32 (88.9) | 48 (90.6) | 61 (88.4) | 71 (88.8) | 91 (85.5) | 79 (73.2) |
| Night laughing | ||||||
| 0–7 years | 21 (60.0) | 27 (79.4) | 28 (77.8) | 29 (87.9) | 20 (87.0) | 24 (75.0) |
| 8–12 years | 30 (69.8) | 33 (63.5) | 35 (76.1) | 26 (63.4) | 27 (67.5) | 23 (62.2) |
| 13–17 years | 33 (78.6) | 42 (87.5) | 34 (73.9) | 32 (72.7) | 30 (69.8) | 22 (57.9) |
| 18+ years | 23 (63.9) | 39 (72.2) | 44 (62.9) | 53 (66.3) | 61 (59.8) | 50 (49.0) |
| Night screaming | ||||||
| 0–7 years | 8 (22.9) | 18 (52.9) | 23 (63.9) | 20 (60.6) | 10 (43.5) | 17 (53.1) |
| 8–12 years | 14 (32.6) | 25 (47.2) | 23 (50.0) | 16 (37.2) | 18 (46.2) | 15 (40.5) |
| 13–17 years | 19 (46.3) | 23 (47.9) | 20 (44.4) | 17 (37.8) | 12 (27.9) | 10 (27.0) |
| 18+ years | 9 (26.5) | 28 (53.9) | 28 (39.4) | 32 (40.5) | 44 (42.7) | 39 (37.1) |
| Night waking | ||||||
| 0–7 years | - | - | - | 28 (93.3) | 20 (87.0) | 25 (89.3) |
| 8–12 years | - | - | - | 30 (79.0) | 33 (91.7) | 21 (72.4) |
| 13–17 years | - | - | - | 34 (85.0) | 29 (78.4) | 20 (71.4) |
| 18+ years | - | - | - | 57 (85.1) | 76 (78.4) | 55 (64.0) |
Prevalence was calculated based on sample size that excluded missing data
Table 2.
Frequency and prevalence of specific sleep problems by occurrence frequency and year of questionnaire*
| Year of questionnaire [n (%)] | ||||||
|---|---|---|---|---|---|---|
| Frequency Scale | 2000 (n=159) | 2002 (n=189) | 2004 (n=203) | 2006 (n=208) | 2009 (n=221) | 2011 (n=220) |
| Night laughing | ||||||
| Did not occur | 49 (31.4) | 47 (25.0) | 57 (28.8) | 58 (29.3) | 70 (33.7) | 90 (43.1) |
| Sometimes | 61 (39.1) | 66 (35.1) | 72 (36.4) | 78 (39.4) | 83 (39.9) | 69 (33.0) |
| Often | 46 (29.5) | 75 (39.9) | 69 (34.9) | 62 (31.3) | 55 (26.4) | 50 (23.9) |
| Night screaming | ||||||
| Did not occur | 103 (67.3) | 93 (49.7) | 104 (52.5) | 115 (57.5) | 124 (59.6) | 130 (61.6) |
| Sometimes | 31 (20.3) | 49 (26.2) | 50 (25.3) | 51 (25.5) | 45 (21.6) | 43 (20.4) |
| Often | 19 (12.4) | 45 (24.1) | 44 (22.2) | 34 (17.0) | 39 (18.8) | 38 (18.0) |
| Night waking | ||||||
| Did not occur | - | - | - | 26 (14.9) | 35 (18.1) | 50 (29.2) |
| Sometimes | - | - | - | 27 (15.4) | 24 (12.4) | 26 (15.2) |
| Often | - | - | - | 122 (69.7) | 134 (69.4) | 95 (55.6) |
Prevalence was calculated based on sample size that excluded missing data
Age group
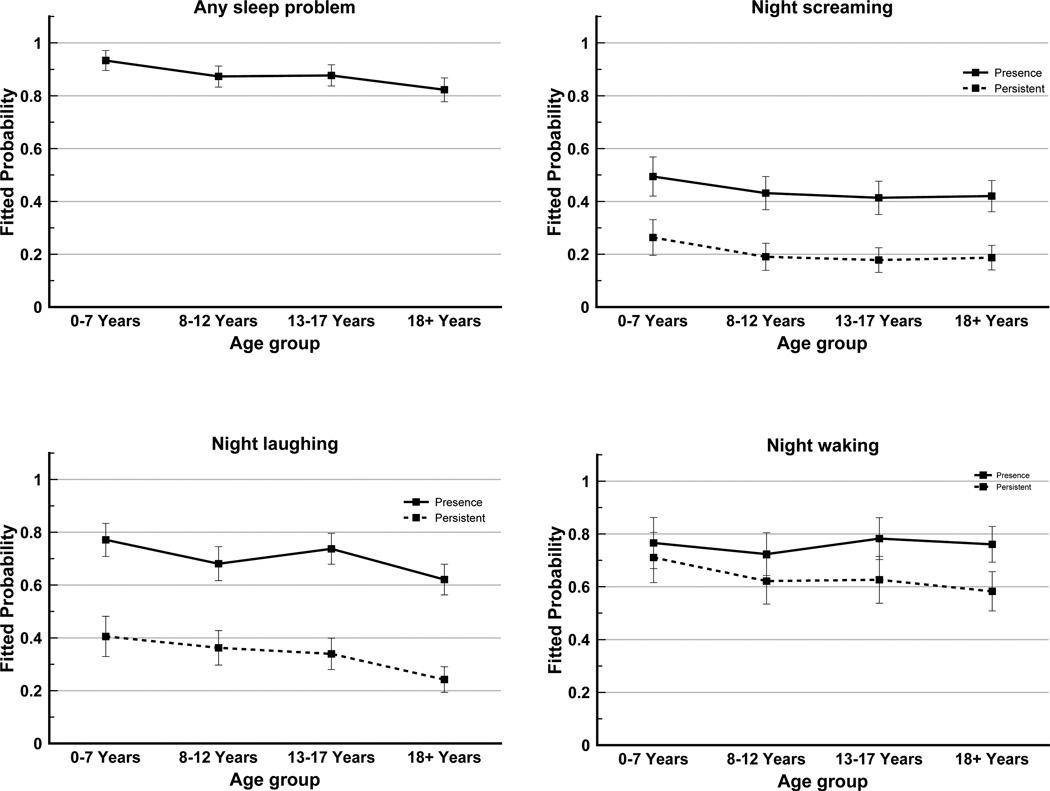
Taking into account repeated data from each case, the estimated probability of any sleep problem was highest in the 7 years or younger age group (93%) and decreased with age group (18 years and older: 82%) (Figure 1). Night laughing was more common in the 7 years or younger (77%) and 13 to 17 years (74%) age groups than the older age groups. Prevalence of night screaming was higher in the youngest age group (49%) and was between 41–43% in other age groups. Night waking was common (75–80%) and the likelihoods were similar across the four age groups. The probability of persistent night laughing, screaming and waking also diminished with age.
Figure 1.
Fitted probabilities of any sleep problem, night laughing, night screaming and night waking*, by age group
*Night waking: analysis based on 3 time points (2006, 2009 and 2011) as compared to 6 time points for any and specific sleep problems
Mutation type
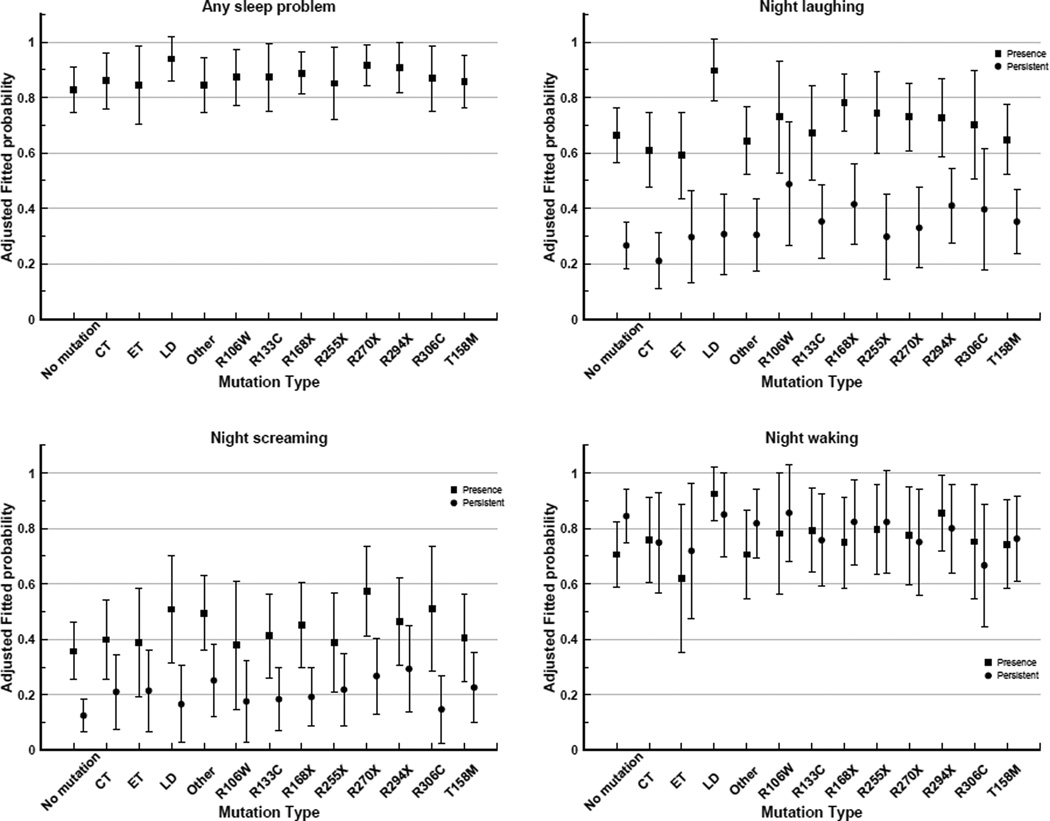
The frequency distribution of mutation type by questionnaire year is shown in Table 3. After adjusting for age, the highest probabilities of any sleep problem were for those with a large deletion (94%), p.R270X (92%) and p.R294X (91%) mutations (Figure 2). The presence of night laughing was reported for a high proportion of those with a large deletion (90%) and p.R168X (78%) mutation, whilst persistent night laughing was moderately common in those with p.R106W (49%) and p.R168X (42%) mutations. The highest probability of night screaming was estimated for those with p.R270X (57%), p.R306C (51%) and large deletion (51%) mutations. Interestingly, the likelihoods of night waking were lowest for early truncating (62%), p.R168X (75%) and p.R306C (75%). Similarly, persistent night waking was least likely in those with early truncating (50%), p.R306C (57%) and C-terminal deletions (58%) mutations.
Table 3.
Frequency and prevalence of mutation type by questionnaire year*
| Year of questionnaire [n (%)] | ||||||
|---|---|---|---|---|---|---|
| Mutation type | 2000 (n=159) | 2002 (n=189) | 2004 (n=203) | 2006 (n=208) | 2009 (n=221) | 2011 (n=220) |
| No mutation | 33 (21.9) | 44 (24.3) | 41 (21.0) | 43 (21.5) | 39 (18.6) | 36 (17.5) |
| C-terminal | 12 (8.0) | 12 (6.6) | 15 (7.7) | 17 (8.5) | 20 (9.5) | 18 (8.7) |
| Early truncating | 7 (4.6) | 10 (5.5) | 8 (4.1) | 7 (3.5) | 10 (4.8) | 10 (4.9) |
| Large deletion | 5 (3.3) | 8 (4.4) | 10 (5.1) | 11 (5.5) | 12 (5.7) | 12 (5.8) |
| p.R106W | 3 (2.0) | 2 (1.1) | 4 (2.1) | 7 (3.5) | 7 (3.3) | 9 (4.4) |
| p.R133C | 8 (5.3) | 10 (5.5) | 11 (5.6) | 11 (5.5) | 18 (8.6) | 18 (8.7) |
| p.R168X | 15 (9.9) | 16 (8.8) | 19 (9.7) | 15 (7.5) | 14 (6.7) | 17 (8.3) |
| p.R255X | 8 (5.3) | 8 (4.4) | 11 (5.6) | 11 (5.5) | 15 (7.1) | 12 (5.8) |
| p.R270X | 12 (8.0) | 14 (7.7) | 14 (7.2) | 13 (6.5) | 15 (7.1) | 14 (6.8) |
| p.R294X | 10 (6.6) | 12 (6.6) | 15 (7.7) | 15 (7.5) | 15 (7.1) | 13 (6.3) |
| p.R306C | 7 (4.6) | 9 (5.0) | 10 (5.1) | 11 (5.5) | 9 (4.3) | 10 (4.9) |
| p.T158M | 14 (9.3) | 16 (8.8) | 16 (8.2) | 17 (8.5) | 18 (8.6) | 20 (9.7) |
| Other | 17 (11.3) | 20 (11.1) | 21 (10.8) | 22 (11.0) | 18 (8.6) | 17 (8.3) |
Prevalence was calculated based on sample size that excluded missing data
Figure 2.
Aged adjusted fitted probabilities of any sleep problem, night laughing, night screaming and night waking*, by mutation type
*Night waking: analysis based on 3 time points (2006, 2009 and 2011) as compared to 6 time points for any and specific sleep problems
CT, C-terminal deletions; ET, Early truncating; LD, Large deletion
Latent Class Growth Analysis
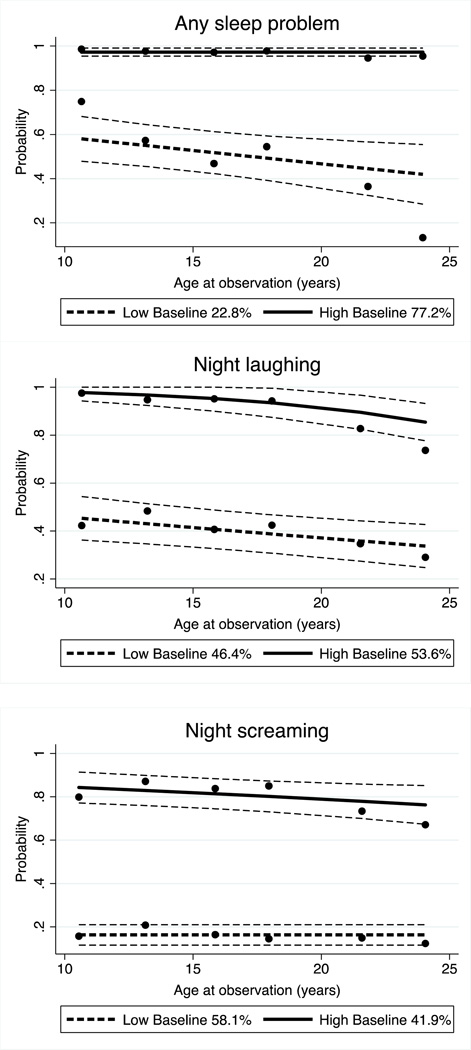
Two distinct developmental trajectories were found for the presence of any sleep problem, night laughing and night screaming, and the probability of these sleep problems was estimated as a linear function of age. The two trajectories were labeled as “High baseline” and “Low baseline”, reflecting the relative estimated level of prevalence of sleep problem at the first observation period in each group.
In 77% of individuals with high baseline prevalence, a sleep problem tended to persist into adulthood. In contrast, a smaller group (23%), with a low prevalence when first observed, appeared to improve through the course of follow up (Figure 3). For night laughing and night screaming, the distributions in the two trajectory groups were more similar (night laughing 57% high vs 43% low prevalence group; night screaming 42% high vs 58% low prevalence group). In contrast, those who had high prevalence of night laughing or screaming when younger improved with time whilst for their low prevalence counterparts the problem persisted when they grew older.
Figure 3.
Trajectory of any sleep problem, night laughing and night screaming by latent class and age at observation*
* Low and high baseline represents the relative estimated level of prevalence of sleep problem at the first observation period in each group
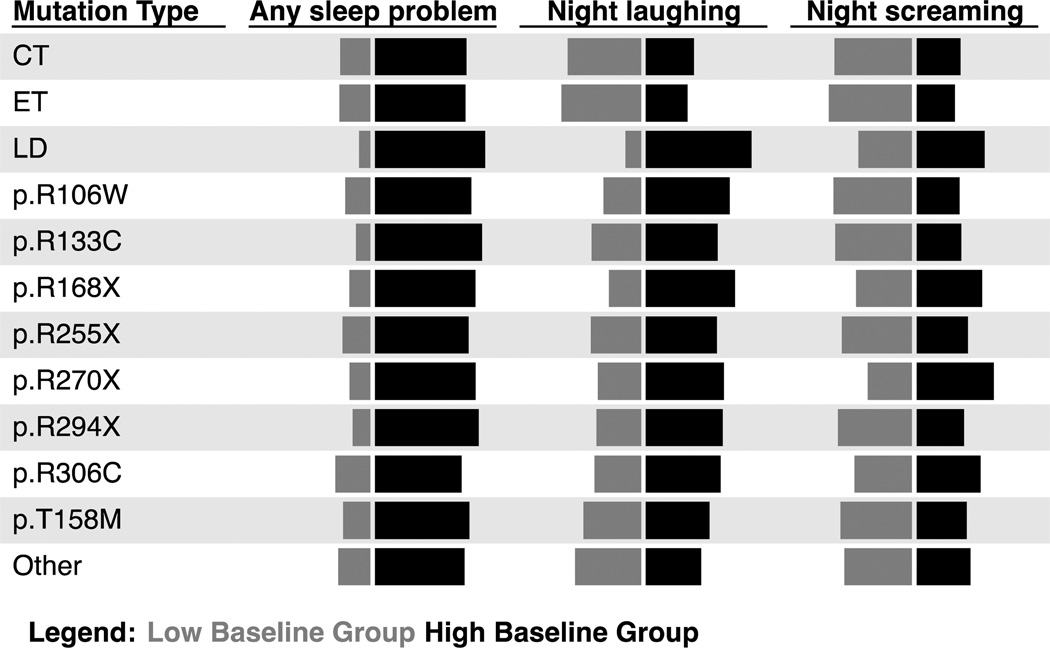
Mutation types, as time-invariant covariates, were tested for their predictability of any and specific sleep problems. The model indicated that the presence of any MECP2 mutation, in general, was more likely in those individuals with high baseline prevalence of any sleep problem compared with no MECP2 mutation (Odds ratio (OR) 2.65; 95% CI 1.30–5.41) (Figure 4). Some mutation types, notably large deletion, p.R168X and p.R270X, were more common in the high baseline prevalence group of individuals with night laughing and night screaming, although the differences between groups were not significant.
Figure 4.
Predicted group membership probability by mutation type*
*Length of the horizontal bar represents the likelihood of sleep problems in girls and women with specific mutation type
CT, C-terminal deletions; ET, Early truncating; LD, Large deletion
Treatment
The prevalence of receiving any treatment for sleep problems was, on average across the six time points, 16.1%. In terms of treatment type, non-specific sleep medications were most commonly used (43.4%), followed by sleep medications (28.3%), multiple treatment types (16.4%) and non-pharmaceutical treatments (12.0%). The most commonly used medication was melatonin, which accounted for 17.6% of the responses (Table 4). Anti-epileptic medications and variants of benzodiazepine used for seizure control were used for sleep management in 12.5% and 10.8% of responses respectively.
Table 4.
Frequency and prevalence of sleep medication use in girls and women with a sleep problem by medication type and questionnaire year
| Year of questionnaire [n (%)] | |||||||
|---|---|---|---|---|---|---|---|
| Medication | 2000 (n=147) | 2002 (n=166) | 2004 (n=172) | 2006 (n=178) | 2009 (n=188) | 2011 (n=172) | Total |
| Melatonin | 1 (0.7) | 2 (1.2) | 3 (1.7) | 6 (3.4) | 10 (5.3) | 9 (5.2) | 31 (3.0) |
| Antiepileptic drug | 11 (7.5) | 5 (3.0) | 1 (0.6) | 2 (1.1) | 1 (0.5) | 2 (1.2) | 22 (2.2) |
| Benzodiazepine^ | 3 (2.0) | 3 (1.8) | 3 (1.7) | 4 (2.3) | 2 (1.1) | 4 (2.3) | 19 (1.9) |
| Benzodiazepine# | 3 (2.0) | 3 (1.8) | 1 (0.6) | 4 (2.3) | 3 (1.6) | 4 (2.3) | 18 (1.8) |
| Clonidine | 0 | 2 (1.2) | 2 (1.2) | 5 (2.8) | 4 (2.1) | 4 (2.3) | 17 (1.7) |
| Trimeprazine | 1 (0.7) | 0 | 4 (2.3) | 5 (2.8) | 3 (1.6) | 3 (1.7) | 16 (1.6) |
| Amitriptyline | 1 (0.7) | 2 (1.2) | 2 (1.2) | 2 (1.1) | 3 (1.6) | 2 (1.2) | 12 (1.2) |
| Risperidone | 1 (0.7) | 4 (2.4) | 1 (0.6) | 3 (1.7) | 1 (0.5) | 0 | 10 (1.0) |
| Promethazine | 2 (1.4) | 0 | 3 (1.7) | 1 (0.6) | 0 | 1 (0.6) | 7 (0.7) |
| Miscellaneous | 2 (1.4) | 0 | 1 (0.6) | 0 | 1 (0.5) | 2 (1.2) | 6 (0.6) |
| Olanzapine | 0 | 1 (0.6) | 0 | 1 (0.6) | 1 (0.5) | 2 (1.2) | 5 (0.5) |
| Chloral hydrate | 0 | 1 (0.6) | 1 (0.6) | 2 (1.1) | 0 | 1 (0.6) | 5 (0.5) |
| Haloperidol | 0 | 1 (0.6) | 0 | 0 | 1 (0.5) | 1 (0.6) | 3 (0.3) |
| Hypnotics | 2 (1.4) | 0 | 0 | 0 | 1 (0.5) | 0 | 3 (0.3) |
| Mirtazapine | 0 | 0 | 1 (0.6) | 0 | 0 | 0 | 1 (0.6) |
| Fluoxetine | 0 | 1 (0.6) | 0 | 0 | 0 | 0 | 1 (0.6) |
Clobazam, clonazepam and diazepam
Nitrazepam, lorazepam, temazepam and oxazepam
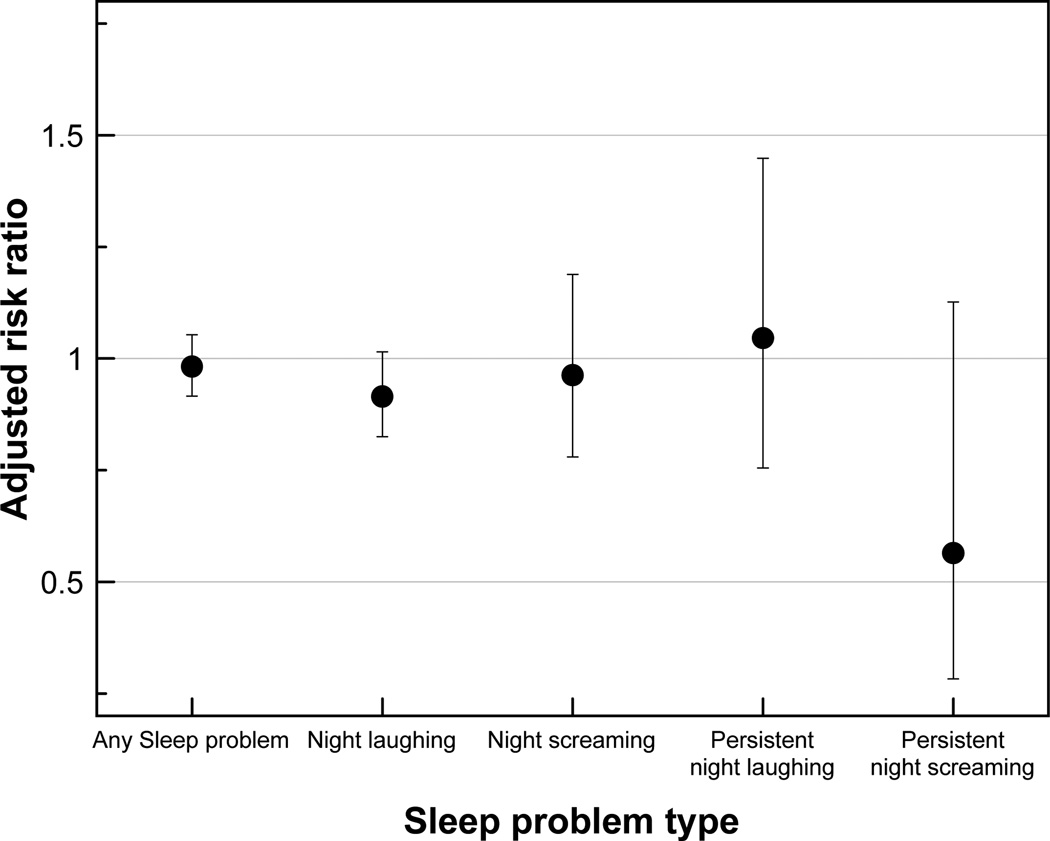
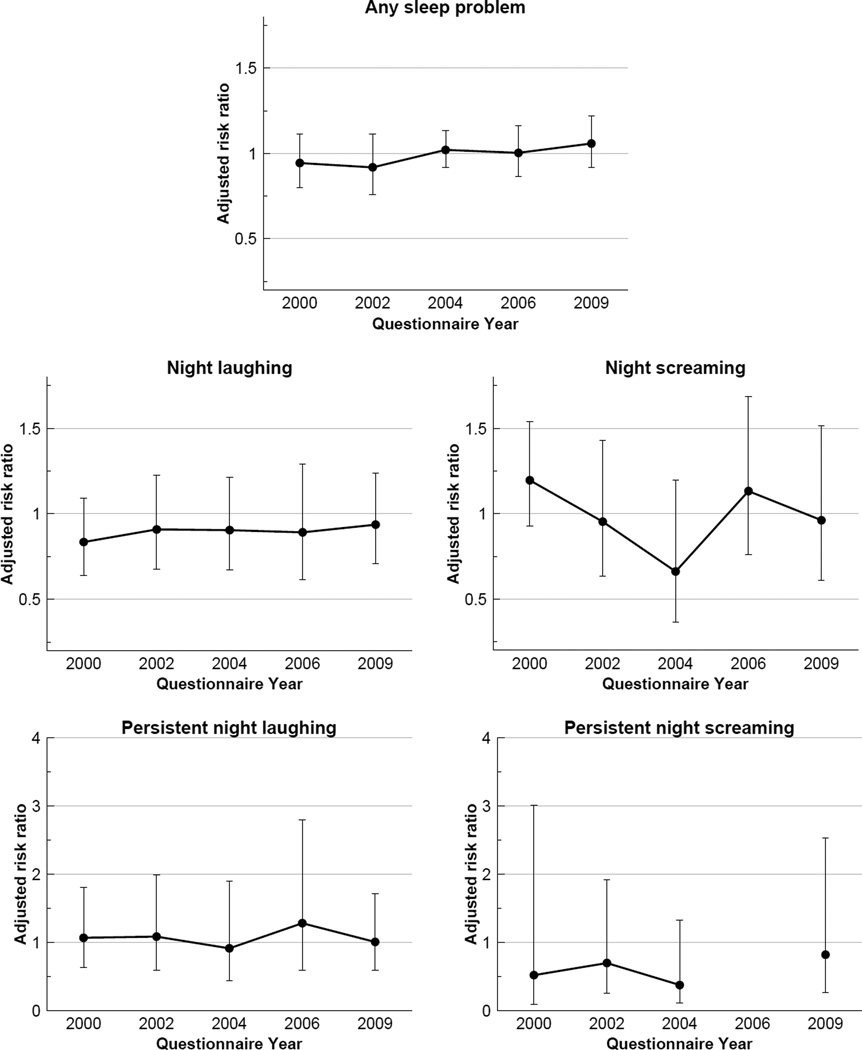
Treatment, on average reduced the risk of any sleep problem from one time point to the next by around 1.7% (Risk ratio (RR) 0.98; 95% CI 0.92, 1.05) (Figure 5). By questionnaire year, the effect was highest in 2000 (RR 0.94; 95% CI 0.80, 1.11) and 2002 (RR 0.92; 95% CI 0.76, 1.11) but it gradually shifted to the null and in 2009 treatment was associated with a small increase in risk (RR 1.06; 95% CI 0.92,1.22) of sleep problem (Figure 6). For night laughing and screaming, treatment has little effect on resolving the problems, but the risk of experiencing persistent night screaming appeared to be reduced (RR 0.56; 95% CI 0.28, 1.13). No obvious pattern was observed for the risks of night laughing and screaming.
Figure 5.
Effect of any treatment (compared to no treatment) on the likelihood of unresolved sleep problem by sleep problem type
Figure 6.
Effect of any treatment (compared to no treatment) on unresolved sleep problems by sleep problem type and questionnaire year
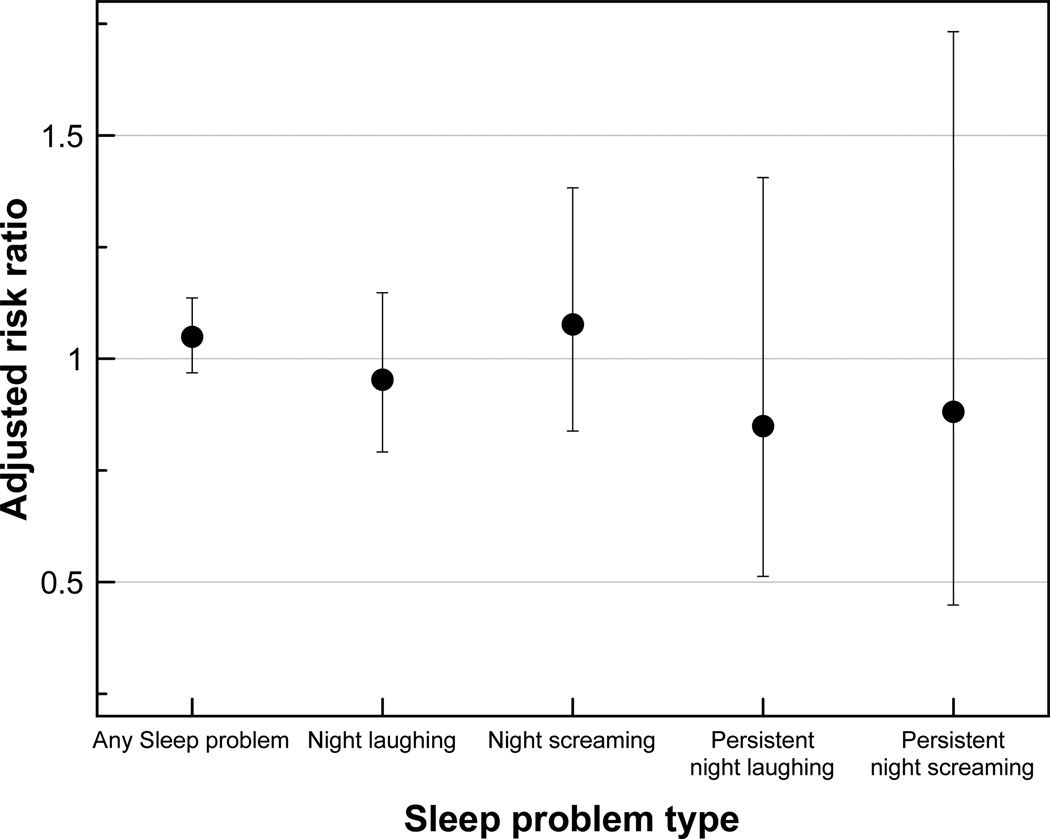
For individuals who were treated with medications that were used specifically for sleep problem, including melatonin, the likelihood of any sleep problem persisting to subsequent time point increased slightly (RR 1.05; 95% CI 0.97,1.14) when compared with those who did not receive treatment for their sleep problem (Figure 7). Specific sleep medications also appeared to reduce the risk of further night laughing and persistent night screaming, but not the occurrence of night screaming.
Figure 7.
Effect of specific sleep medication (compared to no treatment) on the likelihood of unresolved sleep problem by sleep problem type
Discussion
This is the first study to examine the trajectory of sleep problems in Rett syndrome, using the largest population-based sample to date. The majority experienced sleep problems and the prevalence reduced slightly with age, in particular night screaming. There was some effect of MECP2 mutation type: night laughing and screaming were common in those with a large deletion and night waking was less frequent for those with p.R306C, early truncating and C-terminal deletion mutations. By examining individual trajectories in response to treatment we were unable to identify any benefit. Latent class analyses indicated two longitudinal trajectories for sleep problems and membership with each group was not related to mutation type.
The prevalence of sleep problems in Rett syndrome was high (>80%), consistent with our previous population-based findings (Young et al., 2007). Parents reported that approximately three quarters experienced night laughing and 40% screaming. Other than a case series undertaken almost three decades ago (Naidu et al., 1986), there is limited literature on the prevalence of sleep problems in Rett syndrome and therefore the Australian longitudinal population-based source has proven to be a particularly valuable resource in this respect (Young et al., 2007). Sleep dysfunction occurs in other populations with intellectual disability (Didden et al., 2002) and autism (Wiggs and Stores, 2004) but may be particularly common in genetically caused syndromes associated with intellectual disability e.g. Sanfilippo and Angelman syndromes (Dorris et al., 2008). A qualitative study of nine families with a daughter with Rett syndrome found that poor sleep affected the mood, energy levels and general performance of both the child and parents, with additional impact on parental relationships and social activities (McDougall et al., 2005).
Sleep problems in Rett syndrome may develop before or around the period of regression (Lee et al., 2013, Nomura, 2005), a time when the hallmark signs of Rett syndrome become apparent often also accompanied by autistic signs such as social withdrawal and inconsolable crying (Lee et al., 2013, Nomura and Segawa, 1990, Witt-Engerström, 1993). We previously reported that the prevalence of sleep problems was higher during childhood and slightly lower in adulthood (Young et al., 2007). Using population data now collected over 20 year and trajectory analyses, we have demonstrated in the present study that reduction in any of the symptoms of sleep dysfunction with time occurs in only a proportion of individuals. However, it was difficult to predict what factors were associated with this improvement. Whereas some symptoms decrease with age for some, the burden of sleep dysfunction continues for many girls and their families.
The type of MECP2 mutation has been associated with many aspects of the clinical phenotype in Rett syndrome including early development (Fehr et al., 2011), clinical severity (Bebbington et al., 2008), and the prevalence of comorbidities such as epilepsy (Bao et al., 2013) and scoliosis (Ager et al., 2006). Sleep dysfunction occurred very commonly and overall, we did not identify relationships with type of MECP2 mutation. However, we did find that there were some associations between specific aspects of sleep dysfunction and the type of mutation. For example, night laughing was reported more commonly for those with a large deletion and was more frequent in those with the p.R106W mutation, and night screaming was more likely in those with the p.R270X mutation. On the other hand, persistent night waking was less common with the p.R306C, early truncating or C-terminal deletion mutations. We have now updated the findings of our previous study (Young et al., 2007) as our cohort has now increased in size and followed over a longer period. Our more powerful analyses suggest that mutation has some role to play in the types of sleep dysfunction which individually may well be working through different mechanisms. Thus, investigations of the biology of sleep dysfunction in Rett syndrome should be cognizant of potential for the phenotype “sleep dysfunction” to be heterogeneous in its underlying pathological mechanisms.
Managing sleep disturbance in Rett syndrome could potentially benefit both the child and the family and available treatments include attention to sleep hygiene, associated behavioral strategies (Piazza et al., 1991), use of specific sleep medications such as melatonin (McArthur and Budden, 1998, Nomura et al., 1985) and chloryl hydrate, and use of non-specific sleep medications such as anti-epileptic drugs, risperidone and haloperidol. The evidence base for treatment in Rett syndrome is extremely limited and we were only able to identify three small studies investigating behavioral strategies (Piazza et al., 1991) or use of melatonin (or related) medications (McArthur and Budden, 1998, Nomura et al., 1985). Effectiveness in each study was limited to shorter time to sleep onset, but night waking persisted and there was decreasing effectiveness of melatonin with time (McArthur and Budden, 1998). Melatonin remains the most assessed sleep medication overall if one takes account of other literatures on treating sleep dysfunction for developmental disabilities (Hollway and Aman, 2011). With a limited body of evidence on how to manage sleep dysfunction in Rett syndrome and few demonstrated benefits, clinicians can only be guided by previous experience, anecdote and trial and error. The Australian experience would suggest a heterogeneous approach to management with melatonin only being used in less than 5% of cases and little overall evidence of benefit to the patient of the approaches being used over more than a decade of observations. While sleep problems overall persisted despite treatment, there was a reduced relative risk of persistent night screaming indicating that for some, the severity of this aspect of sleep was reduced. However, the results from this study in combination with the lack of existing literature illustrate that the optimal types of management best suited to Rett syndrome are not yet known.
The Australian Rett Syndrome Database is population-based and longitudinal and has achieved high response fractions over its lifetime (Downs et al., 2008). Thus, it is a valuable and comprehensive resource for tracking symptoms in Rett syndrome. Our current study has investigated prevalence over time in the largest sample to date and we have also been able to explore the effectiveness of treatments using a cohort study design. Over time the questions on sleep dysfunction have been slightly modified and improved making some adjustment or compensatory mechanisms necessary when comparing data from different waves. For instance, we referred to responses to the Rett Syndrome Behaviour Questionnaire (Mount et al., 2002) to determine the prevalence of night waking prior to 2009.
We acknowledge that our dataset does not provide specific information on additional sleep disorders such as persistence of daytime napping (Ellaway et al., 2001), nighttime seizures (McDougall et al., 2005), sleep disordered breathing such as apnea and bruxism, which also have implications for health and wellbeing. Missing data is problematic in many studies. Those in this study with incomplete responses may not have had sleep problems and thus it is possible that we have overestimated the prevalence. We also acknowledge that our assessment of treatment effectiveness has limitations including the broadness of our data in terms of the ordinal scale and the biennial observations. However, our analyses provides important new information in an area that is lacking and have the advantage of a 12 year lens through which to observe effects. We have identified a strong platform of need that strongly justifies research efforts to identify and test an effective treatment for sleep dysfunction in Rett syndrome.
Our study has important implications for clinicians. Sleep problems are extremely common in Rett syndrome with significant burden for health and wellbeing of both those with Rett syndrome and their families. Clinical management is heterogeneous and does not appear to be associated with clear and consistent improvement. Therefore, clinicians can only be guided by their previous experience and careful assessment and follow-up in order to judge effectiveness on a case-by-case basis. The organization of access to respite options may be critically important for family health and wellbeing. There is an urgent need to understand the mechanisms of sleep dysfunction in Rett syndrome and thereafter identify and test treatments for its amelioration.
Acknowledgements
The authors acknowledge the National Institute of Child Health and Human Development (US) for its current funding of the Australian Rett Syndrome Study under NIH Grant No. 5R01HD043100-05 and the National Health and Medical Research Council (NHMRC) under project grant #303189 and #1004384. Helen Leonard is funded by a NHMRC program grant #572568. We thank the Australian clinicians who have reported cases and the families for their ongoing participation in our study. We acknowledge the Australian Paediatric Surveillance Unit and the Rett Syndrome Association of Australia for their support.
Footnotes
Disclosure of financial support and conflicts of interest
There are no potential conflicts of interest or commercial support for the authors.
| Study design | Data collection | Data analysis | Interpretation of results | Preparation of the manuscript | |
|---|---|---|---|---|---|
| Kingsley Wong | ✓ | ✓ | ✓ | ✓ | |
| Helen Leonard | ✓ | ✓ | ✓ | ✓ | |
| Peter Jacoby | ✓ | ✓ | |||
| Carolyn Ellaway | ✓ | ✓ | |||
| Jenny Downs | ✓ | ✓ | ✓ | ✓ |
References
- Ager S, Fyfe S, Christodoulou J, Jacoby P, Schmitt L, Leonard H. Predictors of scoliosis in Rett syndrome. J. Child Neurol. 2006;21:809–813. doi: 10.1177/08830738060210091501. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van Den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andruff H, Carraro N, Thompson A, Gaudreau P. Latent Class Growth Modelling: A Tutorial. Tutorials Quant Meth Psych. 2009;5:11–24. [Google Scholar]
- Bao X, Downs J, Wong K, Williams S, Leonard H. Using a large international sample to investigate epilepsy in Rett syndrome. Dev. Med. Child Neurol. 2013;55:553–558. doi: 10.1111/dmcn.12093. [DOI] [PubMed] [Google Scholar]
- Bebbington A, Anderson A, Ravine D, et al. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology. 2008;70:868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- Cuddapah VA, Pillai RB, Shekar KV, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J. Med. Genet. 2014 doi: 10.1136/jmedgenet-2013-102113. Epub January 7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didden R, Curfs LM, Van Driel S, De Moor JM. Sleep problems in children and young adults with developmental disabilities: home-based functional assessment and treatment. J. Behav. Ther. Exp. Psychiatry. 2002;33:49–58. doi: 10.1016/s0005-7916(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Dorris L, Scott N, Zuberi S, Gibson N, Espie C. Sleep problems in children with neurological disorders. Dev Neurorehabil. 2008;11:95–114. doi: 10.1080/17518420701860149. [DOI] [PubMed] [Google Scholar]
- Downs J, Bebbington A, Woodhead H, et al. Early determinants of fractures in Rett syndrome. Pediatrics. 2008;121:540–546. doi: 10.1542/peds.2007-1641. [DOI] [PubMed] [Google Scholar]
- Ellaway C, Peat J, Leonard H, Christodoulou J. Sleep dysfunction in Rett syndrome: lack of age related decrease in sleep duration. Brain Dev. 2001;23(Suppl 1):S101–S103. doi: 10.1016/s0387-7604(01)00356-4. [DOI] [PubMed] [Google Scholar]
- Fehr S, Bebbington A, Ellaway C, Rowe P, Leonard H, Downs J. Altered attainment of developmental milestones influences the age of diagnosis of rett syndrome. J. Child Neurol. 2011;26:980–987. doi: 10.1177/0883073811401396. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Rett syndrome: clinical peculiarities and biological mysteries. Acta Paediatr. 1995;84:971–976. doi: 10.1111/j.1651-2227.1995.tb13809.x. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:61–65. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Rett syndrome: long-term clinical follow-up experiences over four decades. J. Child Neurol. 2005;20:722–727. doi: 10.1177/08830738050200090401. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6:293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- Hollway JA, Aman MG. Pharmacological treatment of sleep disturbance in developmental disabilities: a review of the literature. Res. Dev. Disabil. 2011;32:939–962. doi: 10.1016/j.ridd.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Method Res. 2007;35:542–571. [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2001;29:374–393. [Google Scholar]
- Lee J, Leonard H, Piek J, Downs J. Early development and regression in Rett syndrome. Clin. Genet. 2013 doi: 10.1111/cge.12110. [DOI] [PubMed] [Google Scholar]
- Leonard H, Downs J, Jian L, et al. Valproate and risk of fracture in Rett syndrome. Arch. Dis. Child. 2010;95:444–448. doi: 10.1136/adc.2008.148932. [DOI] [PubMed] [Google Scholar]
- Mcarthur AJ, Budden SS. Sleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatment. Dev. Med. Child Neurol. 1998;40:186–192. doi: 10.1111/j.1469-8749.1998.tb15445.x. [DOI] [PubMed] [Google Scholar]
- Mcdougall A, Kerr AM, Espie CA. Sleep disturbance in children with Rett syndrome: A qualitative investigation of the parental experience. J Appl Res Intellect Disabil. 2005;18:201–215. [Google Scholar]
- Mount RH, Charman T, Hastings RP, Reilly S, Cass H. The Rett Syndrome Behaviour Questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. J. Child Psychol. Psychiatry. 2002;43:1099–1110. doi: 10.1111/1469-7610.00236. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Naidu S, Murphy M, Moser HW, Rett A. Rett syndrome--natural history in 70 cases. Am. J. Med. Genet. Suppl. 1986;1:61–72. doi: 10.1002/ajmg.1320250507. [DOI] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y. Early behavior characteristics and sleep disturbance in Rett syndrome. Brain Dev. 2005;27(Suppl 1):S35–S42. doi: 10.1016/j.braindev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Segawa M. Clinical features of the early stage of the Rett syndrome. Brain Dev. 1990;12:16–19. doi: 10.1016/s0387-7604(12)80167-7. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Segawa M, Higurashi M. Rett syndrome--an early catecholamine and indolamine deficient disorder? Brain Dev. 1985;7:334–341. doi: 10.1016/s0387-7604(85)80040-1. [DOI] [PubMed] [Google Scholar]
- Piazza CC, Fisher W, Kiesewetter K, Bowman L, Moser H. Aberrant sleep patterns in chuldren with rett syndrome. Brain Dev. 1990;12:488–493. doi: 10.1016/s0387-7604(12)80213-0. [DOI] [PubMed] [Google Scholar]
- Piazza CC, Fisher W, Moser H. Behavioral treatment of sleep dysfunction in patients with the Rett syndrome. Brain Dev. 1991;13:232–237. doi: 10.1016/s0387-7604(12)80055-6. [DOI] [PubMed] [Google Scholar]
- Sas Institute Inc. SAS (r) Proprietary Software 9.3. Cary, NC: SAS Institute Inc; 2002–2010. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev. Med. Child Neurol. 2004;46:372–380. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]
- Witt-Engerström I. Evolution of Clinical Signs. In: Hagberg B, editor. Rett Syndrome-Clinical and Biological Aspects. London: Mac Keith Press; 1993. [Google Scholar]
- Young D, Nagarajan L, De Klerk N, Jacoby P, Ellaway C, Leonard H. Sleep problems in Rett syndrome. Brain Dev. 2007;29:609–616. doi: 10.1016/j.braindev.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]