Abstract
Female and male adult Wistar rats were fed standard chow or a simplified cafeteria diet for one month. Then, the rats were killed and the white adipose tissue (WAT) in four sites: perigonadal, retroperitoneal, mesenteric and subcutaneous (inguinal) were sampled and frozen. The complete WAT weight in each site was measured. Gene expression analysis of key lipid and glucose metabolism enzymes were analyzed, as well as tissue and plasma lactate and the activity of lactate dehydrogenase. Lactate gradients between WAT and plasma were estimated. The influence of sex and diet (and indirectly WAT mass) on lactate levels and their relationships with lactate dehydrogenase activity and gene expressions were also measured. A main conclusion is the high production of lactate by WAT, practically irrespective of site, diet or sex. Lactate production is a direct correlate of lactate dehydrogenase activity in the tissue. Furthermore, lactate dehydrogenase activity is again directly correlated with the expression of the genes Ldha and Ldhb for this enzyme. In sum, the ability to produce lactate by WAT is not directly dependent of WAT metabolic state. We postulate that, in WAT, a main function of the lactate dehydrogenase path may be that of converting excess available glucose to 3C fragments, as a way to limit tissue self-utilization as substrate, to help control glycaemia and/or providing short chain substrates for use as energy source elsewhere. More information must be gathered before a conclusive role of WAT in the control of glycaemia, and the full existence of a renewed glucose-lactate-fatty acid cycle is definitely established.
Introduction
Lactate is the main by-product of peripheral organ utilization of glucose under conditions of anaerobiosis / hypoxia [1] or when used by glycolytic obligatory cells such as mammalian erythrocytes [2]. Lactate is also an acidifying factor modulating oxygen release in hypoxic tissues (Bohr effect) [3]. Lactate is one of the main splanchnic (essentially liver) organs' substrate for gluconeogenesis [4], and helps transfer "oxygen debt" from glycolytic muscle during exercise to the liver, completing a Cori cycle [5]. Lactate is, also, a good substrate for lipogenesis in the liver [6] and other tissues [7,8].
Lactate is a main by-product of most tumour cells, which show high rates of glycolysis [9] due to the Warburg effect [10]. In addition, lactate may be used as energy substrate by a number of tissues: heart [11], brown and white [12] adipose tissues, muscle [13], glia [14] and others, including the biota [15], because of its rapid conversion to pyruvate. Lactate is the main circulating 3C substrate, and cell (cytoplasmic) reducing power exporter, reflecting NADH status, and acting as a marker and modulator of oxygen availability and utilization [16]. These multiple roles place lactate at the centre of a critical metabolic switch. The changes in the rate of synthesis, transport in the blood and final disposal deeply modulate (and/or are the consequence of) important metabolic substrate shifts.
The main form of adipose tissue, white adipose tissue (WAT) is usually associated with energy storage in the form of an enormous vacuole containing essentially triacylglycerols. However, a significant part of body fat reserves are not found in the main WAT sites, but present in other cell types, such as myocytes or hepatocytes [17] or, more commonly, as WAT interspersed between other cell types in a number of tissues [18–21]. Curiously, all body reserves' mass changes are correlated between the macroscopic and disperse triacylglycerol depots, which, this way, show a remarkable uniformity in its physiological function as reserve organ [22], irrespective of variation in additional specialised functions [23,24].
We have recently observed that cultured 3T3L1 adipocytes, in the absence of undifferentiated fibroblasts, use glucose at extremely high rates, releasing lactate under conditions of full normoxia [25]. This is in agreement with in vivo lactate production by WAT [26–28] parallel to its low consumption of oxygen [29,30]. The limiting factor for lactate production under high medium glucose seems to be the availability of ADP [25]. The WAT breakup of 6C glucose to two 3C units may be directed to decrease glucose availability [25] in order to help protect the tissue from excess substrate and the damaging hypertrophy caused by excess energy substrates [31]. This defence mechanism may help restrict the supply of substrates (mainly glucose), but also that of oxygen, possibly inducing hypoxia, which has been suggested to favour the development of inflammation [32,33] and the consequent WAT immune response [34]. However, the limited needs of oxygen of adipocytes [25], and the low WAT oxygen consumption [30] suggest that the development of inflammation in WAT may not be directly related to oxygen supply.
In the present study we intended to find whether WAT lactate production in vivo may be in some way related to the excess energy supply of high-energy diets, but also to check whether sex [35] may also affect the WAT breakup of glucose to 3C units.
Materials and Methods
Animals, diet and experimental setup
All animal handling procedures and the experimental setup were in accordance with the animal handling guidelines of the corresponding European and Catalan Authorities. The Committee on Animal Experimentation of the University of Barcelona authorized the specific procedures used in the present study.
Nine week old female and male Wistar rats (Harlan Laboratory Models, Sant Feliu de Codines, Spain) were used. Six animals per group were housed in cages (two same-sex rats in each) with wood shards for bedding. They had free access to water and were kept in a controlled environment (lights on from 08:00 to 20:00; 21.5–22.5°C; 50–60% humidity). Two groups of animals for each sex were selected randomly, and were fed ad libitum, for 30 days, with either normal rat chow (type 2014, Harlan) or a simplified cafeteria diet as previously described [36].
The experimental setup consisted, thus, of four groups of 6 rats each: male-control, female-control, male-cafeteria and female-cafeteria. Rat weight and food consumption were measured, and we found that these parameters followed closely previous studies done using the same experimental setup [22]. Diet composition was (expressed as energy content): carbohydrate 67%, protein 20%, and lipid 13% for controls; the mean composition of the cafeteria diet ingested was: carbohydrate 47%, protein 12% and lipid 41%.
In a complementary study to analyse tissue blood flows, we used two additional groups of male rats, from the same supplier, stock, and age; they were subjected to the same feeding protocols (control or cafeteria diet) for 30 days. These animals were used exclusively for the measurement of tissue blood flow as described below.
Tissue sampling
At the end of the experiment, on day 30, the animals were anesthetized with isoflurane at the beginning of a light cycle, and blood was drawn from the exposed aorta using dry heparinized syringes, killing the animals by exsanguination. Blood was centrifuged 20 min at 2000xg, at 2–4°C. Plasma was frozen and kept at -20°C.
The rats were rapidly dissected, taking large samples of mesenteric (ME), perigonadal (periovaric in females and epididymal in males, PG), retroperitoneal (RP) and subcutaneous (inguinal fat pads, SC) WAT, which were frozen in liquid nitrogen. The samples were weighed, and stored at -80°C until processed. Later, the dissection of the dead rats continued, carefully extracting the remaining WAT in ME, PG and RP sites; the rats were skinned, and the subcutaneous WAT was dissected completely. The weights of WAT thus dissected were added to those of the frozen samples to know the mass of the four WAT sites.
WAT cellularity
Portions of frozen WAT were used for the estimation of total DNA with a classical chemical method [37]. Since a rat cell contains 5.6 pg of DNA [38], we were able to calculate the approximate number of cells per unit of WAT volume. We knew the weights and the density: ~0.9 g/mL [39] of WAT, and thus we determined the number of cells per g of tissue, and in the whole site, as well as their mean size, simply dividing the tissue volume by the number of cells.
Tissue protein content was estimated with a reliable biuret-Folin method [40]. After development of colour, turbidity was eliminated by adding to the tubes small amounts of finely powdered solid MgO, which adsorbed the suspended fat remnants, and centrifuging the tubes before reading their absorbance.
Estimation of tissue lactate
Samples of WAT tissue (in the range of 50 mg) were homogenized, still frozen, with a tissue disruptor (IKA-T10 basic Ultra-Turrax, IKA, Stauffen Germany) in 1 ml of chilled acetone:water mixture, to a final proportion (including the expected water content of the samples) of 1.25:1 [41]. The homogenate was centrifuged; all proteins removed with the precipitate, and floating lipids, not soluble in the diluted acetone, were discarded. The acetone tissue extract and plasma (10μL samples) were used for the estimation of lactate (kit 1001330 Spinreact, Sant Esteve d'en Bas, Spain), using sodium L-lactate (Sigma-Aldrich) as standard.
The proportion of lipid per g of tissue in rats treated with the same experimental setup is known [22]. By discounting the mass of fat and protein from that of tissue we were able to obtain an estimate of the amount of water present in the WAT samples (in the range of 20%). The moles of lactate per mL of tissue or plasma and the amount of water in that same volume allowed us to calculate the molal concentrations of lactate in plasma and tissues, and to establish a molal concentrations ratio for each individual rat WAT site.
Measurement of lactate dehydrogenase activity
A modified UV method was used [42]. Frozen tissue samples were homogenized, using the tissue disruptor, in 10 volumes of chilled Krebs-Ringer bicarbonate solution, pH 7.8 containing 5 mM dithiothreitol, 0.5% bovine serum albumin, 1% dextran (MW 200,000), 0.1% Triton X-100, and 1 mM EDTA. Freshly (i.e. not later than 3 h after thawing/homogenisation in the buffer) prepared homogenates were used for all enzyme activity measurement incubations, carried out at 25°C. Homogenates were centrifuged at 4°C and 5,000xg for 10 minutes to obtain a clear intermediate phase (precipitated debris and floating lipid were discarded). The reaction mixture contained 150 μM NADH, 125 mg/L bovine serum albumin and 1 mM sodium pyruvate (all products were obtained from Sigma-Aldrich). The proportion of original tissue in each measuring well was in the range of 2.0–2.5 mg, in a volume of 0.02 mL of homogenate, diluted to the adequate proportions with homogenization medium. Absorbance at 340 nm was measured at intervals of 30 s for up to 10 min. In each case, the decrease in absorbance due to the formation of NAD+ was plotted, and initial (V0) activities were determined from the course of the reaction at different times. V0 was assumed to correspond to Vmax under the described conditions of analysis. Protein content was measured in each of the homogenates used for enzyme activity estimation, and used for the presentation of enzyme activities, expressed in nkat/g of protein to allow comparisons between samples with different fat content.
Gene expression analysis
Total tissue RNA was extracted from the frozen tissue samples using the Tripure reagent (Roche Applied Science, Indianapolis IN USA), and were quantified in a ND-100 spectrophotometer (Nanodrop Technologies, Wilmington DE USA). RNA samples were reverse transcribed using the MMLV reverse transcriptase (Promega, Madison, WI USA) system and oligo-dT primers.
Real-time PCR (RT-PCR) amplification was carried out using 10 μL amplification mixtures containing Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA USA), 4 ng of reverse-transcribed RNA and 150 nM of primers. Reactions were run on an ABI PRISM 7900 HT detection system (Applied Biosystems) using a fluorescent threshold manually set to 0.15 for all runs.
A semi-quantitative approach for the estimation of the concentration of specific gene mRNAs per unit of tissue weight was used [43]. Rplp0 was the charge control gene [44,45]. We expressed the data as the number of transcript copies per gram of protein in order to obtain comparable data between the groups. The genes analysed and a list of primers used is presented in Table 1.
Table 1. Primers used for the analysis of gene expression in WAT of control and cafeteria diet-fed rats.
| gene | Protein | 5' > 3' | 3' > 5' | bp |
|---|---|---|---|---|
| Ldha | lactic acid dehydrogenase (muscle type) | CACTGGGTTTGAGACGATGA | GTCAGCAAGAGGGAGAGAGC | 125 |
| Ldhb | lactic acid dehydrogenase (heart type) | CCAGGAACTGAACCCAGAGA | TCATAGGCACTGTCCACCAC | 131 |
| Glut4 | glucose transporter 4 | CTTGATGACGGTGGCTCTGC | CACAATGAACCAGGGGATGG | 127 |
| Hk2 | hexokinase 3 | ATTCACCACGGCAACCACAT | GGACAAAGGGATTCAAGGCATC | 113 |
| G6pd | glucose-6P dehydrogenase | GACTGTGGGCAAGCTCCTCAA | GCTAGTGTGGCTATGGGCAGGT | 77 |
| Pdk4 | pyruvate dehydrogenase kinase type 4 | GTCAGGCTATGGGACAGATGC | TTGGGATACACCAGTCATCAGC | 137 |
| Pdk2 | pyruvate dehydrogenase kinase type 2 | TCACTCTCCCTCCCATCAA | CGCCTCGGTCACTCATTT | 75 |
| Nos3 | nitric oxide synthase (endothelial type) | CAAGTCCTCACCGCCTTTT | GACATCACCGCAGACAAACA | 138 |
| Acc1 | acetyl-CoA carboxylase type 1 | AGGAAGATGGTGTCCGCTCTG | GGGGAGATGTGCTGGGTCAT | 145 |
| Fas | fatty acid synthase | CTTGGGTGCCGATTACAACC | GCCCTCCCGTACACTCACTC | 163 |
| Acly | ATP: citrate lyase | GACCAGAAGGGCGTGACCAT | GTTGTCCAGCATCCCACCAGT | 96 |
| Hsl | hormone-sensitive lipase | CCCATAAGACCCCATTGCCTG | CTGCCTCAGACACACTCCTG | 94 |
| Lpl | lipoprotein lipase | GAAGGGGCTTGGAGATGTGG | TGCCTTGCTGGGGTTTTCTT | 103 |
| Atgl | triacylglycerol lipase (adipose tissue) | CGGTGGATGAAGGAGCAGACA | TGGCACAGACGGCAGAGACT | 138 |
| Cpt1 | carnitine palmitoleoyl transferase (liver) | CCGCTCATGGTCAACAGCA | CAGCAGTATGGCGTGGATGG | 105 |
| Cpt2 | carnitine palmitoleoyl transferase (muscle) | TGCTTGACGGATGTGGTTCC | GTGCTGGAGGTGGCTTTGGT | 152 |
| Lcad | long-chain acyl-CoA dehydrogenase | ATGCCAAAAGGTCTGGGAGT | TCGACCAAAAAGAGGCTAATG | 148 |
| Rplp0 | 60S acidic ribosomal protein 0 | GAGCCAGCGAAGCCACACT | GATCAGCCCGAAGGAGAAGG | 62 |
Measurement of tissue blood flows
On day 27, the rats were implanted with two cannulas through the left carotid artery using Intramedic PE-10 polyethylene tubing (Becton Dickinson, Parsipanny, NJ USA), under isoflurane anaesthesia. The first carotid cannula was used to draw blood from descending aorta and the other to inject microspheres directly into the heart outflow following the instructions provided by the supplier. At 12 h intervals, the viability of the cannulas was checked (without disturbing the animals) by drawing blood up a few mm, followed by refilling with heparinized saline. On day 30 the rats were transferred to smaller individual cages, shielded from the operators. Two hours later, they were injected through the left ventricle cannula with 105 red latex beads (Molecular Probes, Carlsbad, CA USA) suspended in 0.1 ml of 9 g/L NaCl. At the same time, blood (about 0.2 mL) was slowly drawn (exactly during 60 s) through the other cannula. The rats were, then, anesthetized with isoflurane, and larger blood samples were obtained from the exposed aorta. Blood and tissue samples were frozen and kept at -80°C. After sacrifice the position of the cannulas was checked; no placement errors, nor cannula clotting were found. The weights of all organs analysed were measured.
Blood and tissue samples of known volume/weight were digested with 4 M KOH for 24 hours at 25°C with occasional stirring. The samples were filtered through glass-fibre filters (GF/D, 2.5 μm, Whatman, Maidstone, Kent UK) and rinsed with Tween-20 (20 g/L) followed by distilled water. Then, the fluorospheres were extracted from the filter with 2.5 ml of etoxyethyl acetate. The fluorescence at red (565 nm) excitation wavelength was measured at 598 nm emission wavelength in a spectrofluorimeter. Samples were adequately diluted and compared against tissue blanks, made with pieces of the same tissues from rats of the first experiment, which had not received fluorescent beads. At least two samples and/or extractions for each tissue were used / carried out. Samples were processed according to the bead supplier specifications. The number of beads in tissue samples was estimated from the organic extract fluorescence. The distribution of bead fluorescence equivalents vs. bead controls was used to obtain a percent distribution of blood flow between organs, since the amount of beads injected was known: a sample of the injected material was also analysed to correct for possible errors in the evaluation of injected bead numbers.
Calculation of cardiac output was done by measuring the amount of beads in the blood drawn for one minute from the artery. Bead concentration and the known amount of beads injected allowed the calculation of cardiac output. Absolute blood flows were calculated from the number of beads leaving the heart per unit of time and blood volume and the percentage of beads retained in the organs of the rats.
Statistics
Comparisons between groups were done with two- or three-way ANOVA analyses using the Statgraphics Centurion XVI software (Warrenton, VA USA). Analyses of correlations and curve fitting (i.e. for V0 estimation) were carried out using Prism 5 program (GraphPad Software, San Diego CA USA).
Results
The initial and final weights, energy intake, and the basic plasma parameters are shown in Table 2. Body weight changes and energy data just repeat what we have previously published [22]. Plasma glucose, triacylglycerols, total cholesterol and urea were within the normal range, showing no significant differences between groups, except for higher glucose and lower urea in cafeteria diet-fed rats.
Table 2. Body weight, energy intake and plasma metabolites of control and cafeteria diet-fed rats.
| tissue | units | male control | male cafeteria | female control | female cafeteria | P sex | i | P diet |
|---|---|---|---|---|---|---|---|---|
| Initial body weight | g | 241±6 | 256±6 | 161±6 | 173±7 | <0.0001 | NS | |
| Final body weight | g | 372±6 | 420±20 | 232±8 | 277±15 | <0.0001 | <0.0001 | |
| Energy intake | MJ/30d | 8.71±0.45 | 19.7±0.99 | 6.33±0.39 | 17.9±0.98 | <0.0001 | 0.0121 | |
| Plasma glucose | mM | 7.80±0.32 | 8.25±0.33 | 6.60±0.26 | 8.78±0.24 | NS | i | 0.0002 |
| Plasma triacylglycerols | mM | 1.50±0.06 | 1.50±0.01 | 1.69±0.06 | 1.51±0.03 | 0.0390 | NS | |
| Plasma cholesterol | mM | 1.97±0.07 | 2.28±0.21 | 1.98±0.16 | 2.07±0.19 | NS | NS | |
| Plasma urea | mM | 3.90±0.17 | 3.82±0.20 | 5.13±0.25 | 3.78±0.20 | 0.0094 | i | 0.0025 |
The data are the mean ± sem of 6 different animals. Up to three significant digits are shown for each mean value. Statistical significance of the differences between groups (2-way anova): the columns show the P values for sex, diet and their interaction; a i in the interaction column indicates a significant interaction between the two factors analysed.
Table 3 shows the WAT site mass cellularity and protein content for female and male rats subjected to control and cafeteria diets. There were significant differences between sites for total mass, mean cell size, number of cells per unit of tissue weight and in the whole site. Sex effects were limited to SC and RP sites' mass and cell content; PG WAT also showed significant changes linked to sex for site cell and protein content. No effects attributable to sex were observed on ME WAT. With respect to the effects of diet, all sites showed significant differences for all the parameters included in Table 3, except for RP WAT protein content.
Table 3. White adipose tissue mass and cell size in adipose tissue sites of control and cafeteria diet-fed rats.
| parameter | units | site | male control | male cafeteria | female control | female cafeteria | P-site | P sex | i | P diet |
|---|---|---|---|---|---|---|---|---|---|---|
| WAT site mass | g | SC | 12.3±0.2 | 19.9±1.0 | 7.02±0.25 | 12.3±0.6 | <0.0001 S>P>M≈R | <0.0001 | <0.0001 | |
| ME | 4.94±0.64 | 8.38±0.95 | 3.92±0.33 | 9.02±1.25 | NS | <0.0001 | ||||
| PG | 7.34±0.50 | 12.8±1.6 | 4.83±0.39 | 11.8±1.70 | NS | <0.0001 | ||||
| RP | 6.29±0.80 | 9.98±1.38 | 2.79±0.35 | 7.81±0.77 | 0.0051 | 0.0001 | ||||
| mean cell size | nL | SC | 7.72±0.88 | 8.34±0.49 | 7.15±0.39 | 9.89±0.25 | <0.0001 R>P≈S>M | NS | 0.0088 | |
| ME | 6.76±0.21 | 7.12±1.28 | 4.19±0.84 | 9.33±0.61 | NS | i | 0.0065 | |||
| PG | 7.72±0.66 | 9.16±0.84 | 8.35±0.58 | 11.1±0.6 | NS | 0.0061 | ||||
| RP | 9.74±0.25 | 9.48±0.56 | 9.43±0.78 | 12.1±0.4 | NS | i | 0.0439 | |||
| number of cells in the whole site | 106·cells | SC | 1474±121 | 2556±69 | 1000±73 | 1473±213 | <0.0001 S>P≈M>R | <0.0001 | i | <0.0001 |
| ME | 658±69 | 1392±265 | 906±129 | 959±116 | NS | 0.0411 | ||||
| PG | 883±25 | 1382±85 | 579±31 | 1069±145 | 0.0019 | <0.0001 | ||||
| RP | 681±65 | 1072±165 | 298±37 | 639±46 | 0.0003 | 0.0009 | ||||
| number of cells /g tissue | 106·cells/g | SC | 122±9 | 122±8 | 142±7 | 101±3 | 0.0005 M>S≈P≈R | NS | i | 0.0122 |
| ME | 149±5 | 134±21 | 230±35 | 109±7 | NS | i | 0.0051 | |||
| PG | 134±10 | 104±7 | 123±8 | 87±4 | NS | 0.0007 | ||||
| RP | 103±3 | 102±5 | 110±9 | 83.0±2.6 | NS | i | 0.0262 | |||
| protein content/g tissue | mg/g | SC | 63.1±11.6 | 35.0±3.9 | 51.2±3.8 | 41.2±4.4 | <0.0001 M>R>S≈P | NS | 0.0105 | |
| ME | 74.2±7.4 | 60.9±4.7 | 86.2±4.2 | 57.5±2.9 | NS | 0.0010 | ||||
| PG | 44.3±1.6 | 35.0±2.0 | 54.4±2.4 | 47.6±2.6 | <0.0001 | 0.0015 | ||||
| RP | 65.1±6.3 | 63.7±5.2 | 62.9±4.7 | 50.7±1.3 | NS | NS |
The data are the mean ± sem of 6 different animals. Up to three significant digits are shown for each mean value. SC/S = subcutaneous inguinal: ME/M = mesenteric; PG/P = perigonadal (periovaric, epididymal), RP/R = retroperitoneal. Statistical significance of the differences between groups (2-way anova): the columns show the P values for sex, diet and their interaction. A i in the interaction column indicates a significant interaction between the two factors analysed. The statistical significance of differences between sites was estimated by a 3-way-ANOVA. P values >0.05 are presented as NS; the sequences indicate overall significant differences between sites; post-hoc Duncan test: the sign > indicates significantly higher values at the left; commas are equivalent to NS.
Rats fed the cafeteria diet had larger WAT depots, but the relative size order was unchanged. Mean cell size followed the same pattern in all four groups, irrespective of global higher sizes in cafeteria diet-fed rats: RP>PG≈SC>ME.
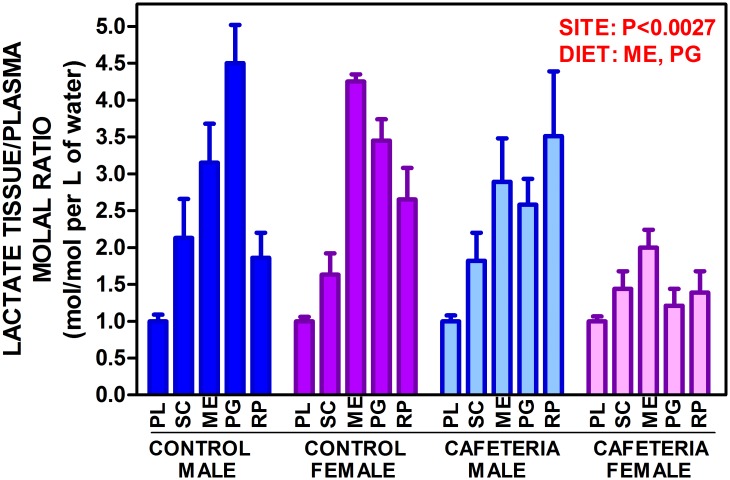
Tissue lactate levels (Table 4) were lower in tissue (μmol/g) than in plasma (mM), and were not affected by sex, except for RP. Cafeteria diet decreased tissue (and plasma) lactate (significant for plasma, ME and PG). When the data were expressed in molal units, the picture was quite different (Fig. 1). The molal concentration ratios for WAT versus plasma were in all cases higher than 1, i.e. tissue lactate concentration in the water available (i.e. discounting protein and fat) was up to four-fold higher than in arterial plasma. There was a significant effect of site and of diet for PG and ME, with no effects of sex. The patterns of molal ratios between tissue and plasma lactate, however, were different in males PG>ME>SC>RP and females ME>PG>RP>SC. Cafeteria diet changed the patterns to RP>ME>PG>SC in males and ME>SC>RP>PG in females.
Table 4. Lactate levels in plasma and adipose tissue sites of control and cafeteria diet-fed rats.
| tissue | units | male control | male cafeteria | female control | female cafeteria | P sex | i | P diet |
|---|---|---|---|---|---|---|---|---|
| plasma | mM | 3.10±0.29 | 2.64±0.21 | 3.78±0.24 | 2.57±0.21 | NS | 0.0028 | |
| subcutaneous WAT | μmol/g | 2.16±0.71 | 1.57±0.35 | 2.37±0.37 | 1.43±0.22 | NS | NS | |
| mesenteric WAT | μmol/g | 1.98±0.33 | 1.80±0.37 | 2.87±0.17 | 1.04±0.13 | NS | i | 0.0019 |
| perigonadal WAT | μmol/g | 2.00±0.22 | 1.16±0.26 | 2.12±0.12 | 0.45±0.10 | NS | <0.0001 | |
| retroperitoneal WAT | μmol/g | 0.803±0.152 | 0.781±0.134 | 1.58±0.21 | 0.522±0.116 | 0.0026 | i | NS |
The data are the mean ± sem of 6 different animals. Up to three significant digits are shown for each mean value. Statistical significance of the differences between groups (2-way anova): the columns show the P values for sex, diet and their interaction; a i in the interaction column indicates a significant interaction between the two factors analysed. The statistical significance of differences between sites was estimated by a 3-way-ANOVA; the P value was <0.0001; post-hoc Duncan test (same conventions as in Table 3): plasma > mesenteric, subcutaneous > perigonadal > retroperitoneal).
Fig 1. Lactate adipose tissue/plasma molal ratio of control- and cafeteria diet-fed male and female rats.
The data are the mean ± sem of 6 animals per group, and are expressed in moles/L of water in the tissue divided by moles/L of water in plasma. PL = plasma; SC = subcutaneous WAT; ME = mesenteric WAT; PG = perigonadal WAT; RP = retroperitoneal WAT. Statistical differences between groups: two-way anova for sex and diet and three-way anova for "site" are marked in red.
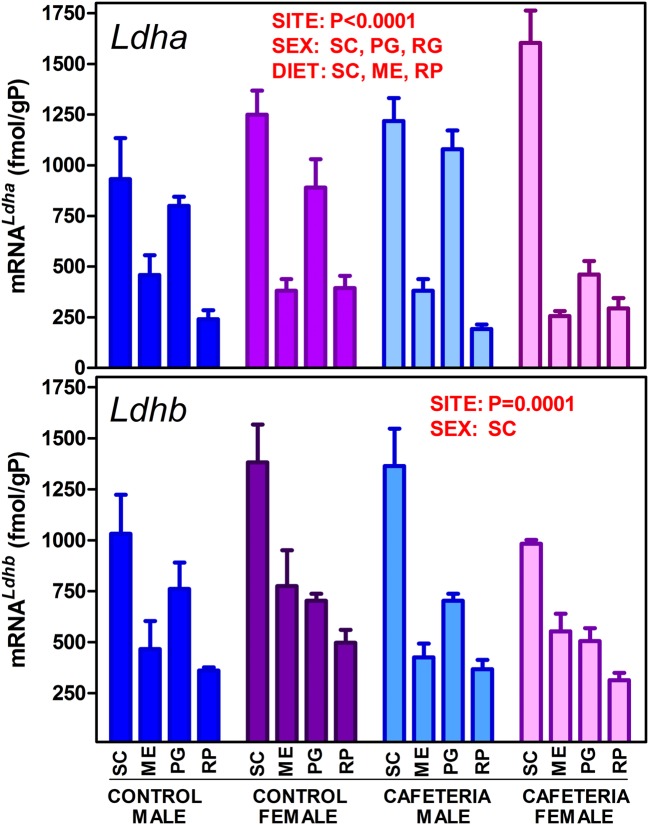
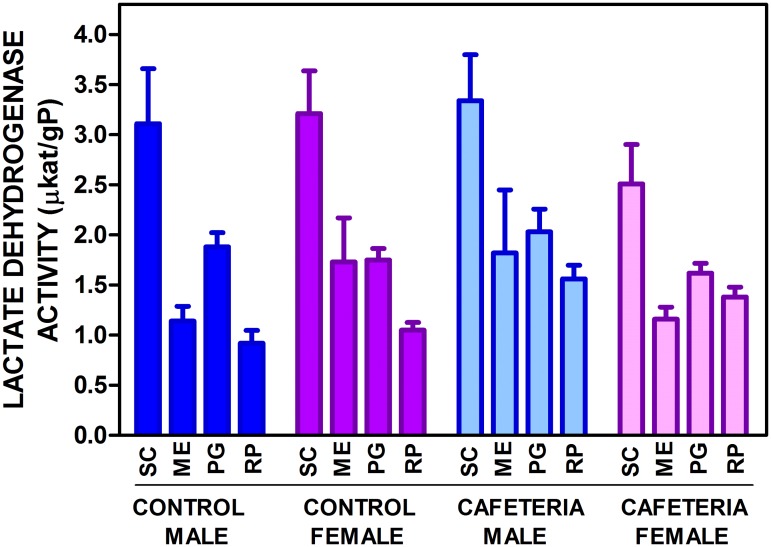
The levels of expression of the genes for the main lactate dehydrogenase isoenzymes in WAT (Ldha and Ldhb) are presented in Fig. 2. There was a marked difference in expression of both genes depending on WAT site. The patterns of distribution were fairly similar for both genes, with maximal expression in SC, and minimal in RP. However, there were significant differences induced by sex and diet. The effect of sex was less marked on Ldhb, which did not show significant effects of diet either, in contrast with Ldha. The actual tissue enzyme activities are shown in Fig. 3. The patterns were comparable to those of Ldha and Ldhb expressions, but we must assume that the lactate dehydrogenase activity measured in tissue (in fact Vmax under the conditions of measurement) was the result of the sum of both isoenzyme activities. No differences were observed for sex and only RP showed a significant effect of diet; however, the variable "site" was highly significant as in the gene expression analysis. In all groups, the patterns were comparable, but not identical, with maximal enzyme activity in SC.
Fig 2. Lactate dehydrogenase genes (Ldha and Ldhb) expression in WAT sites of female and male rats fed control or cafeteria diet.
The data are the mean ± sem of 6 animals per group, and are expressed in fmol/gP (g of tissue protein) to render the figures comparable. The conventions used are the same as in Fig. 1.
Fig 3. Total tissue lactate dehydrogenase activity in WAT sites of female and male rats fed control or cafeteria diet.
The data are the mean ± sem of 6 animals per group, and are expressed in μKat/gP (g of tissue protein) to render the figures comparable. The conventions used are the same as in Fig. 1.
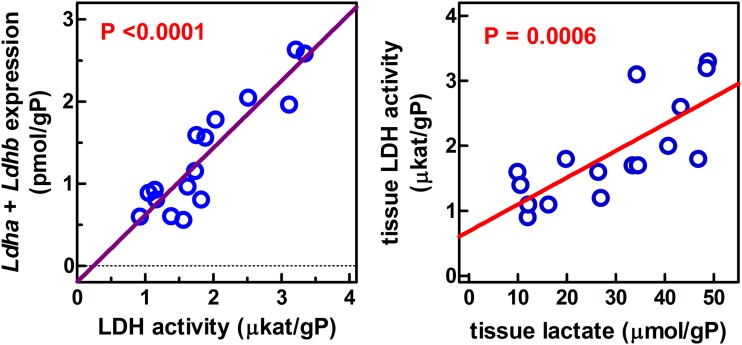
The comparison of enzyme activity vs. the expression of the genes for the two isoenzymes implicated in that activity could not be done in a direct way, since gene expression of an enzyme seldom can be directly correlated with its activity in a tissue. However, we plotted (Fig. 4) the mean values for lactate dehydrogenase enzyme activity per g of tissue protein of all groups (i.e. different sex and diet) vs. the sum of the corresponding Ldha and Ldhb expressions, also referred to g of tissue protein. We obtained a significant correlation between expression and enzyme activity (P<0.0001 vs. zero). When the individual rat data were plotted, we obtained a little more dispersion, but the slope of the regression line and the P values vs. zero were practically unchanged. The separate analysis of Ldha and Ldhb gave similar results (the slopes were practically halved with respect to the sum of expressions, but were superimposable for both genes). In both cases, the correlation was also significant. These data point at a direct relationship between the expression of both genes and the enzyme activity, but also that their contribution to final lactate dehydrogenase activity was similar for both isoenzymes (heart type and muscle type); thus the isozyme pattern of rat WAT is HHMM. When lactate dehydrogenase activity was plotted versus tissue lactate expressing both entities per g of protein, a significant correlation was observed between both parameters.
Fig 4. Linear correlation analysis of the relationship between lactate dehydrogenase activity and expression or tissue lactate content in WAT sites of male and female rats fed a control or cafeteria diet.
LDH = lactate dehydrogenase; gP = g of protein. Each circle represents the mean value for one group of rats (i.e. female/male, control/cafeteria). Enzyme activities are those presented in Fig. 3; expression data are the sum of Ldha and Ldhb (Fig. 2) for the corresponding groups; tissue lactate were those shown in Table 4. Correlation between lactate dehydrogenase activity and gene expression: r2 = 0.828, significantly different from zero (P<0.0001). When all the individual data (i.e. all sites and groups) were plotted, the corresponding values were r2 = 0.380, and P<0.0001. The separate analyses of Ldha and Ldhb (using the mean values) gave similar results Ldha r2 = 0.642, P = 0.0002, and Ldhb r2 = 0.840, P<0.0001. Correlation between tissue lactate dehydrogenase activity and lactate content: r2 = 0.583, significantly different from zero (P = 0.0006). When all the individual data (i.e. all sites and groups) were plotted instead of only the means, the corresponding values were r2 = 0.307, and P<0.0001.
Table 5 presents the expression data for enzymes related with glucose oxidation. As observed for the lactate dehydrogenase genes, there was a marked significant effect of site in all cases. There were no effects of sex on Glut4, but females had higher hexokinase 2 expression, significant in ME and RP. The control of glucose entry in the cell was more affected by diet: Glut4 and Hk2 expressions were significantly lower for all cafeteria diet groups with the exception of SC. Glucose-6P dehydrogenase gene (G6pd) expression was also lowered by cafeteria diet, significantly for ME and RP, the latter with higher values in females.
Table 5. Gene expression in WAT of control and cafeteria diet-fed rats in fmol/g protein. I Glucose oxidation and nitric oxide synthase.
| protein | gene | WAT | male control | male cafeteria | female control | female cafeteria | P site | P sex | i | P diet |
|---|---|---|---|---|---|---|---|---|---|---|
| glucose transporter 4 | Glut4 | SC | 108±29 | 123±25 | 161±184 | 115±8 | <0.0001 S≈P>M≈R | NS | NS | |
| ME | 31.2±8.6 | 15.8±3.0 | 116±36 | 12.2±0.6 | NS | 0.0189↓ | ||||
| PG | 109±8 | 70.2±2.7 | 160±54 | 32.6±6.6 | NS | 0.0134↓ | ||||
| RP | 44.0±6.5 | 25.2±3.9 | 119±43 | 26.0±5.8 | NS | 0.0088↓ | ||||
| hexokinase 2 | Hk2 | SC | 11.7±2.5 | 13.0±1.9 | 18.9±3.7 | 14.7±0.9 | <0.0001 S≈P>M≈R | NS | NS | |
| ME | 7.9±1.2 | 2.7±0.5 | 16.8±3.2 | 1.5±0.2 | 0.0304f | i | <0.0001↓ | |||
| EC 2.7.1.1 | PG | 16.9±2.8 | 14.6±2.0 | 25.6±5.7 | 10.5±2.1 | NS | 0.0266↓ | |||
| RP | 4.1±0.4 | 2.9±0.4 | 13.5±4.5 | 3.3±0.6 | 0.0084f | i | 0.0030↓ | |||
| glucose-6P-dehydrogenase | G6pd | SC | 334±73 | 413±51 | 257±25 | 302±36 | 0.0001 S>P>M≈R | NS | NS | |
| ME | 129±18 | 75.6±9.3 | 202±32 | 62.9±4.6 | NS | i | <0.0001↓ | |||
| EC 1.1.1.49 | PG | 129±12 | 188±23 | 285±51 | 145±16 | NS | i | NS | ||
| RP | 84.2±14.2 | 67.0±5.7 | 183±35 | 82.0±9.3 | 0.0048f | 0.0037↓ | ||||
| pyruvate dehydrogenase kinase 4 | Pdk4 | SC | 181±73 | 187±41 | 22.7±2.6 | 90.2±21.6 | <0.0001 S>P≈M≈R | 0.0113m | NS | |
| ME | 14.2±3.7 | 14.4±2.1 | 7.6±3.9 | 9.1±1.7 | NS | NS | ||||
| EC 2.7.11.2 | PG | 14.5±5.4 | 20.6±3.2 | 8.5±3.2 | 11.8±2.2 | NS | NS | |||
| RP | 13.5±3.5 | 12.9±1.6 | 6.1±1.0 | 17.6±3.7 | NS | NS | ||||
| pyruvate dehydrogenase kinase 2 | Pdk2 | SC | 263±87 | 167±41 | 146±13 | 302±36 | <0.0001 S>M≈P≈M>R | NS | NS | |
| ME | 120±24 | 34.2±1.7 | 193±51 | 22.9±5.3 | NS | 0.0009↓ | ||||
| EC 2.7.11.2 | PG | 91.7±12.8 | 90.1±11.4 | 86.6±16.8 | 41.8±3.1 | 0.0426m | NS | |||
| RP | 48.6±7.6 | 27.9±3.9 | 63.9±10.4 | 31.5±6.1 | NS | NS | ||||
| nitric oxide synthase (endothelial type) | Nos3 | SC | 75.4±9.5 | 88.9±6.0 | 43.2±2.8 | 65.5±7.2 | <0.0001 S>P>M≈R | 0.0009m | 0.0190↑ | |
| ME | 21.6±2.6 | 15.3±2.3 | 18.5±1.0 | 10.4±1.5 | NS | 0.0054↓ | ||||
| EC 1.14.13.39 | PG | 31.6±6.3 | 32.9±4.6 | 22.6±2.4 | 13.3±1.9 | 0.0030m | NS | |||
| RP | 14.7±1.1 | 11.6±1.7 | 16.1±2.2 | 9.4±1.2 | NS | 0.0051↓ |
The data are presented as fmol of the corresponding mRNA for g of tissue protein, and are the mean ± sem of 6 different animals. Up to three significant digits are shown for each mean value. SC/S = subcutaneous inguinal: ME/M = mesenteric; PG/P = perigonadal (periovaric, epididymal), RP/R = retroperitoneal. Statistical significance of the differences between groups (2-way anova): the columns show the P values for sex, diet and their interaction. A superscript f represents higher (overall) values for females and m for males. The arrows show the direction of significant changes for diet: ↓ indicate (overall) slower values for cafeteria diet-fed rats; a i in the interaction column indicates a significant interaction between the two factors analysed. The statistical significance of differences between sites was estimated by a 3-way-ANOVA; post-hoc Duncan test (same conventions as in Table 1)
The expressions of the enzymes controlling the critical step of conversion of 3C pyruvate to 2C acetyl-CoA, Pdk4 and Pdk2, showed little change because of sex, with only higher male values for SC in Pdk4 and PG in Pdk2, and no changes at all induced by the cafeteria diet (with only a significant decrease in ME for Pdk2).
The expressions of the main genes controlling enzymes of lipid metabolism are presented in Table 6. The effect of "site" was significant for all genes studied with the exception of Acly, which was not. The differences related to sex were not evenly distributed, with higher expression values for males, the exceptions being Acc1 (ME and RP) and Acly (ME) which showed higher specific mRNA concentrations in females. In all other cases, males showed higher mean values: Fas (SC and RP), Lpl (SC), Atgl (SC and PG), Cpt1 (all sites, except ME), Cpt2 (PG) and Lcad (SC).
Table 6. Gene expression in WAT of control and cafeteria diet-fed rats in fmol/g protein.
II Lipid metabolism.
| protein | gene | WAT | male control | male cafeteria | female control | female cafeteria | P site | P sex | i | P diet |
|---|---|---|---|---|---|---|---|---|---|---|
| acetyl-CoA carboxylase 1 EC 6.4.1.2 | Acc1 | SC | 74.0±16.3 | 59±6 | 149±37 | 40.3±5.0 | 0.0020R>S≈M≈P | NS | 0.0024↓ | |
| ME | 30.6±4.0 | 14.6±1.6 | 165±49 | 9.2±2.1 | 0.0134f | i | 0.0019↓ | |||
| PG | 126±12 | 53.4±9.5 | 156±66 | 28.0±4.6 | NS | 0.0064↓ | ||||
| RP | 81.0±7.2 | 33.5±4.8 | 542±206 | 27.6±2.7 | 0.0087f | i | 0.0019↓ | |||
| fatty acid synthase EC 2.3.1.85 | Fas | SC | 7620±4110 | 8762±1955 | 2447±403 | 540±47 | <0.0001 S>M≈P≈R | 0.0202m | NS | |
| ME | 192±74 | 110±22 | 1660±536 | 67.3±13.9 | NS | i | 0.0268↓ | |||
| PG | 1269±250 | 701±166 | 2106±795 | 319±45 | NS | 0.0056↓ | ||||
| RP | 572±85 | 204±28 | 3318±1130 | 190±32 | 0.0050m | i | 0.0007↓ | |||
| ATP: citrate lyase EC 4.1.3.8 | Acly | SC | 167±81 | 174±43 | 186±54 | 71.5±7.7 | NSS>M | NS | NS | |
| ME | 22.2±2.9 | 9.9±2.3 | 160±34 | 6.4±1.5 | 0.0020f | i | 0.0003↓ | |||
| PG | 109±10 | 88.6±13.9 | 228±102 | 39.9±6.3 | NS | 0.0391↓ | ||||
| RP | 25.1±4.7 | 14.6±2.4 | 295±132 | 13.3±2.6 | NS | NS | ||||
| hormone-sensitive lipase EC 3.1.1.79 | Hsl | SC | 631±131 | 648±138 | 486±37 | 660±63 | <0.0001 S≈P>R>M | NS | NS | |
| ME | 166±38 | 130±23 | 218±61 | 110±12 | NS | NS | ||||
| PG | 674±59 | 670±70 | 664±64 | 461±45 | NS | NS | ||||
| RP | 458±39 | 344±49 | 645±84 | 295±34 | NS | i | 0.0002↓ | |||
| lipoprotein lipase EC 3.1.1.34 | Lpl | SC | 6300±1305 | 6700±1110 | 3790±440 | 4520±670 | <0.0001 S>P>M≈R | 0.0365m | NS | |
| ME | 1190±38 | 1630±470 | 1100±80 | 1430±260 | NS | NS | ||||
| PG | 3970±720 | 3790±440 | 4320±290 | 2080±250 | NS | i | 0.0209↓ | |||
| RP | 2610±350 | 1750±110 | 3440±820 | 1470±150 | NS | 0.0052↓ | ||||
| triacylglycerol lipase (adipose type) EC 3.1.1.3 | Atgl | SC | 1320±300 | 1680±270 | 701±26 | 1470±150 | <0.0001 S,P>R>M | 0.0426m | NS | |
| ME | 429±95 | 412±92 | 338±95 | 403±79 | NS | NS | ||||
| PG | 1450±300 | 1790±290 | 1040±150 | 1180±90 | 0.0332m | NS | ||||
| RP | 700±48 | 608±74 | 732±51 | 536±68 | NS | 0.0400↓ | ||||
| carnitine palmitoyl-transferase (liver type) EC 2.3.1.21 | Cpt1 | SC | 39.6±14.8 | 58.9±9.0 | 19.4±3.9 | 37.9±7.3 | <0.0001 P>S>M>R | 0.0426m | NS | |
| ME | 14.0±2.7 | 3.8±0.9 | 13.7±3.6 | 0.7±0.1 | NS | 0.0001↓ | ||||
| PG | 16.3±1.7 | 18.9±1.1 | 10.8±1.7 | 7.7±1.2 | <0.0001m | NS | ||||
| RP | 3.3±0.5 | 3.4±0.5 | 2.0±0.4 | 2.6±0.2 | 0.0200m | NS | ||||
| carnitine palmitoyl-transferase (muscle type) EC 2.3.1.21 | Cpt2 | SC | 10.6±3.0 | 17.9±2.8 | 17.0±1.8 | 13.4±12.7 | <0.0001 P>S>M>R | NS | i | NS |
| ME | 10.7±1.5 | 7.8±1.0 | 14.9±3.6 | 7.5±1.3 | NS | 0.0214↓ | ||||
| PG | 25.1±4.3 | 20.5±1.7 | 19.2±3.4 | 11.7±1.7 | 0.0332m | NS | ||||
| RP | 13.4±1.7 | 11.3±1.7 | 20.9±3.4 | 10.5±1.9 | NS | 0.0130↓ | ||||
| long-chain acyl-CoA dehydrogenase EC 1.3.8.8 | Lcad | SC | 1076±347 | 1370±155 | 365±23 | 733±117 | <0.0001 S>P>M≈R | 0.0037m | NS | |
| ME | 166±9 | 196±14 | 298±60 | 179±32 | NS | NS | ||||
| PG | 353±72 | 653±99 | 412±91 | 359±37 | NS | NS | ||||
| RP | 204±22 | 379±29 | 302±49 | 207±29 | NS | NS |
The data are presented as fmol of the corresponding mRNA for g of tissue protein, and are the mean ± sem of 6 different animals. Up to three significant digits are shown for each mean value. SC/S = subcutaneous inguinal: ME/M = mesenteric; PG/P = perigonadal (periovaric, epididymal), RP/R = retroperitoneal. Statistical significance of the differences between groups (2-way anova): the columns show the P values for sex, diet and their interaction. A superscript f represents higher (overall) values for females and m for males. The arrows show the direction of significant changes for diet: ↓ indicate (overall) slower values for cafeteria diet-fed rats; a i in the interaction column indicates a significant interaction between the two factors analysed. The statistical significance of differences between sites was estimated by a 3-way-ANOVA; post-hoc Duncan test (same conventions as in Table 3). P values >0.05 are presented as NS.
The effects of diet resulted in all cases in either no change or decreased expression; no increased expressions were observed when comparing cafeteria diet and controls. In the case of Acc1, all sites showed significant effects of diet, and in Fas, all showed significant changes except SC. Acly decreased its expression with diet in ME and PG; Hsl showed changes only in RP, Lpl in PG and RP. Atgl also decreased its expression in RP; Cpt1 in ME and Cpt2 in RP and ME; there were no changes at all in Lcad.
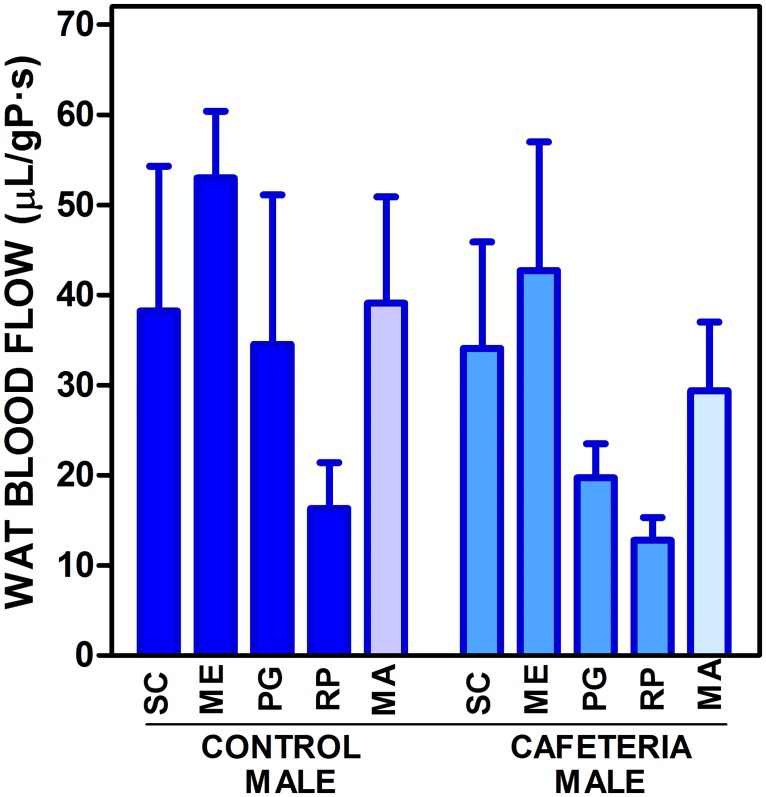
The estimation of WAT blood flow in male rats fed the control or cafeteria diets is shown in Fig. 5. The data are referred to g of tissue protein for direct comparisons with the other Figures and Tables. There was a trend to show lower blood flows for the WAT sites, including the composite value of all four, MA, in cafeteria diet-fed rats but there were no statistically significant differences either for the four studied sites or their composite; there was no significant difference for "site" either.
Fig 5. WAT blood flow in male rats fed control or cafeteria diets.
The data are the mean ± sem of 6 animals per group, and are expressed in μL/s·gP (g of tissue protein). The conventions used are the same as in Fig. 1; MA = mean value for SC, MS, PG and RP taking into account their combined masses.
Table 5 also presented the expression of the most abundant gene for an isoenzyme of nitric oxide synthase (endothelial type) Nos3. Sex showed significantly higher values for males in SC and PG. Feeding a cafeteria diet increased the expression of Nos3 in SC, and decreased it in ME and RP.
Discussion
The production of lactate from glucose by WAT is well known [26–28], and is related to glucose availability [46] in a way similar to that of the Cori cycle [5] between muscle and the splanchnic bed. However, there are reports on the active use of lactate as lipogenic substrate by WAT [6,7]. The release of lactate from human and rat subcutaneous WAT has been proven in vivo [26,47], and we have observed, in cultured 3T3L1 adipocytes [25], that these cells produced an inordinately large amount of lactate when medium glucose concentration was high. A number of elegant studies, have found that the oxygen consumption by WAT under basal conditions is low, justifying a normal operation of the tissue even at low concentrations of oxygen [29,30,48].
The 3T3L1 adipocytes converted almost quantitatively glucose to lactate, which represents a negligible consumption of oxygen under conditions of normoxia [25]. Consequently, the hypothesis that limited blood (and thence oxygen) supply to WAT, a mechanism of defence against excess energy substrate availability [49], may elicit inflammation because of hypoxia [50,51] should be revised, at least for adipocytes, the main component of WAT.
In this study, we found a considerable difference in the potential for lactate production between different WAT sites as shown by different levels of activity and gene expression for lactate dehydrogenase. A high enzyme activity is no proof in itself that lactate is being produced in a given tissue without using dynamic (albeit invasive) tracer studies. However, the data presented in Fig. 4 show that there is a close correlation between WAT lactate content and lactate dehydrogenase activity, regardless of site, sex and dietary treatment. These data strongly suggest that the actual lactate tissue levels are a consequence of the lactate dehydrogenase activity.
Furthermore, the plasma/tissue molal lactate ratios proved that, in all samples, lactate concentration was higher in water tissue than in plasma, i.e. the lactate in WAT could not come from plasma (uphill gradient) but was produced in WAT and released to plasma. Evidently, the main ultimate destination of this lactate is the liver, where it may be used for gluconeogenesis, lipogenesis or oxidized to supply energy. Gluconeogenesis is highly improbable under conditions of excess glucose [52]. Oxidation to CO2 for energy is improbable too in liver and other tissues because of the availability of glucose and the induction of insulin resistance by lactate itself [53]. The large presence of fatty acids in cafeteria rats further increased insulin resistance [54]. In consequence, the most probable fate for most of the lactate produced by adipocytes will be its incorporation to the hepatic lipogenic pathway, and their final release as lipoprotein triacylglycerols.
Adipose tissue is one of the main body producers of lactate [13]. In postabsorptive state humans, whole adipose tissue releases 60–150 μmol/min; other important lactate producing tissues are the brain (with a contribution of around 50 μmol/min) and skeletal muscle. However, although skeletal muscle is a main site of lactate production during exercise [5], it also plays an important role in lactate clearance under basal conditions [13].
It is unclear why WAT contains both muscle and heart type lactate dehydrogenase isoenzymes, (HHMM) with different affinities for lactate and markedly distinct functions in different organs [55]. In the present conditions, both Ldha and Ldhb genes behaved in a similar way and both (and especially their combined expression) was closely adjusted to the total enzyme activity measured, which points to a similar (or shared) regulation in vivo, and also to a gene expression-linked mechanism of regulation of the enzyme activity. The similar turnover rate of the isoenzymes [55] agrees with this interpretation.
The limited changes in circulating lactate suggest that its turnover in blood was fast [56] in agreement with the high capacity of liver to take up and metabolize lactate [57] as indicated above. However, given the relatively large mass of adipose tissue, at least in the overweight cafeteria diet-fed rats, the effect of a significant breakup of glucose to produce lactate is far from being negligible and undoubtedly may help downregulate glycaemia in a significant proportion, at least under basal, i.e. non-exercise, conditions.
Intake of a hyperlipidic cafeteria diet results in a proinflammatory state [58], which is often associated with hypoxia [59,60]. However, contrary to what was expected, no increases in lactate dehydrogenase activity, neither of tissue lactate concentrations were found in the WAT of cafeteria diet-fed rats. The close correlation observed between lactate dehydrogenase activity and tissue lactate content, irrespective of site, sex and diet, suggests that hypoxia could not be a critical factor in the regulation of lactate production. This is again in agreement with the need to revise the purported relationship between hypoxia and inflammation.
There were sex-related differences in the expression of a number of key enzymes related to lipid synthesis, but the most marked effects were attributable to the exposure to cafeteria diet, providing an excess of energy. The consequence was an increased WAT mass [61], via increases in both cell numbers and cell size, as observed here and in agreement with previous studies [62]. However, the factor "site" was markedly relevant for practically all parameters studied. The data on the expression of genes involved in lipogenesis from glucose show clearly that cafeteria diet had a marked overall influence; the entry of glucose into WAT cells was probably limited, as shown by lower Glut4 and Hk2 expressions. This decrease may be part of the tissue mechanism of defence against a sustained excess of energy supply and the generalized insulin resistance [63] that makes WAT the ultimate destination for excess circulating glucose [49,64]. The relative lack of changes in the expression of pyruvate dehydrogenase kinases suggest that the oxidation of pyruvate to acetyl-CoA was practically unaffected by diet, i.e. this was not, probably, an outlet for excess glucose carbon towards lipogenesis, at least in WAT. Notwithstanding, the maintenance of these expressions unchanged also hinted to a limited success of glucose transporter 4 and hexokinase decreases in gene expression to stem the flow of glucose into the cells. All the genes controlling lipogenesis, from G6pd role in the production of NADPH, to the cytoplasm supplier of acetate Acly, and to the proper lipogenic enzyme genes Acc1, and Fas, showed steady decreases in expression under the hyperlipidic cafeteria diet, as previously observed [22,65]. Lipoprotein lipase expression was also decreased, but only in RP and PG. The limitations induced in lipid metabolism by cafeteria feeding were less marked on the lipase genes Hsl and Atgl, which showed decreases only in RP.
WAT lipid utilization, thus, was also limited under cafeteria feeding, an obvious conclusion because of increases in the rat global fat mass and in all WAT sites. The expression of genes related to the transfer of acyl-CoA to the mitochondria was also limited (Cpt1 and Cpt2), and those controlling the oxidation of fatty acids was unchanged (albeit maintained at low levels) as shown by the lack of effect of diet on Lcad expression. These data agree with those of cultured adipocytes, in which the use of glucose as substrate for lipogenesis was largely shunted to the production of 3C (lactate) fragments [25] for eventual exportation to the liver. However, the increase in WAT mass and triacylglycerol accumulation of cafeteria rats is difficult to explain with lowered WAT glucose uptake, limited lipogenesis and maintained lactate production capability, unless, incorporation of blood-borne lipids is factored in.
At first, we assumed that the contrast between lactate levels and lactate dehydrogenase activity in a given WAT site could be due to differences in blood flow. The control of blood flow is probably a key mechanism of control of substrate access to WAT [49,66,67], and its limitation under conditions of excess available energy is counterbalanced by the eventual hypoxic effects induced by this limitation [66,68]. The higher production of NOx in the obese [69] and its effects countering the vasoconstriction elicited by other factors [70,71] agrees with that interpretation. We have observed decreases in WAT expression of Nos3, the endothelial nitric oxide synthase gene [72], a key controller of blood flow [73], in most sites of rats fed the cafeteria diet; but it increased in subcutaneous WAT. However, the blood flow study (done only in males to limit animal lives and costs), showed a limited variability (not significant) from site to site in this parameter, which hints to a general uniform control of WAT blood flow comparable to the ability to uniformly depose fat in all WAT sites and other organs [22] under comparable energy availability conditions.
In any case, the data on blood flow did not explain the differences in lactate and lactate dehydrogenase activity. In spite of the efforts invested, no proof has been found linking blood flow (or its control) to WAT lactate production or release. Thus, lactate production by WAT seems to be essentially unrelated to hypoxia and inflammation.
A main conclusion of this study is the production of lactate by WAT irrespective of site, diet or sex, and that this production is a direct consequence of lactate dehydrogenase activity in the tissue. Furthermore, this activity is a direct correlate of the main lactate dehydrogenase controlling genes' expression. In sum, the ability to produce lactate by WAT is not directly dependent of WAT metabolic condition. We postulate that a main function of the lactate dehydrogenase path of WAT may be that of converting excess circulating glucose to 3C fragments as a way to control glycaemia and/or providing shorter chain substrates for use as energy source elsewhere. The higher circulating lactate levels of obese humans [27] supports this interpretation, and suggests a potential role of WAT in the control of glycaemia, at least in obese individuals.
More information must be gathered before a conclusive role of WAT in the control of glycaemia and the full existence of a renewed glucose-lactate-fatty acid cycle [74] is definitely established.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was done with the partial support of grants of the Plan Nacional de Investigación en Biomedicina (SAF2012-34895) and the Plan Nacional de Ciencia y Tecnología de los Alimentos (AGL-2011-23635) of the Government of Spain, as well as of CIBER-OBN (Institute of Health Carlos III). S. Agnelli was the recipient of a Leonardo da Vinci fellowship, and S. Arriarán a predoctoral fellowship of the Catalan Government, in both cases covering part of the time invested in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sahlin K. Lactate formation and tissue hypoxia. J Appl Physiol. 1989;67: 2640 [DOI] [PubMed] [Google Scholar]
- 2. Rapoport TA, Heinrich R, Rapoport SM. The regulatory principles of glycolysis in erythrocytes in vivo and in vitro. A minimal comprehensive model describing steady states, quasi-steady states and time-dependent processes. Biochem J. 1976;154: 449–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohr C, Hasselbalch K, Krogh A. Über einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Scand Arch Physiol. 1904;16: 402–412. [Google Scholar]
- 4. Ross BD, Hems R, Krebs HA. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967;102: 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cori CF. The glucose-lactic acid cycle and gluconeogenesis. Curr Top Cell Regul. 1981;18: 377–387. [PubMed] [Google Scholar]
- 6. O'Hea EK, Leveille GA. Significance of adipose tissue and liver as sites of fatty acid synthesis in the pig and the efficiency of utilization of various substrates for lipogenesis. J Nutr. 1969;99: 338–344. [DOI] [PubMed] [Google Scholar]
- 7. Granata AL, Midrio M, Corsi A. Lactate oxidation by skeletal muscle during sustained contraction in vivo. Pflügers Archiv-Eur J Physiol. 1976;366: 247–250. [DOI] [PubMed] [Google Scholar]
- 8. Lopaschuk GD, Collinsnakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 9. López-Lázaro M. The Warburg effect: Why and how do cancer cells activate glycolysis in the presence of oxygen? AntiCancer Agents Med Chem. 2008;8: 305–312. [DOI] [PubMed] [Google Scholar]
- 10. Warburg O. On the origin of cancer cells. Science. 1956;132: 309–314. [DOI] [PubMed] [Google Scholar]
- 11. Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat-heart. Biochem J. 1980;186: 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saggerson ED, Mcallister TWJ, Baht HS. Lipogenesis in rat brown adipocytes—effects of insulin and noradrenaline, contributions from glucose and lactate as precursors and comparisons with white adipocytes. Biochem J. 1998;251: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010;199: 499–508. 10.1111/j.1748-1716.2010.02122.x [DOI] [PubMed] [Google Scholar]
- 14. Dringen R, Schmoll D, Cesar M, Hamprecht B. Incorporation of radioactivity form [14C] lactate into the glycogen of cultured mouse astroglial cells- Evidence for gluconeogenesis in brain cells. Biol Chem Hoppe-Seyler. 1993;374: 343–347. [DOI] [PubMed] [Google Scholar]
- 15. Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, et al. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol. 2005;99: 201–212. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Matsuura Y, Kumagai S, Matsuzaka A, Hirakoba K, Asano K. Relationships of anaerobic threshold and onset of blood lactate accumulation with endurance performance. Eur J Appl Physiol. 1983;52: 51–56. [DOI] [PubMed] [Google Scholar]
- 17. Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90: 3191–3196. [DOI] [PubMed] [Google Scholar]
- 18. Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Cli Nutr. 2005;81: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herschap B, Zakhia D, Nicolas M. Intraprostatic adipose tissue. International J Surg Pathol. 2011;19: 190–190. 10.1177/1066896910397611 [DOI] [PubMed] [Google Scholar]
- 20. Nadra K, Médard JJ, Quignodon L, Verheijen MHG, Desvergne B, Chrast R. Epineural adipocytes are dispensable for Schwann cell myelination. J Neurochem. 2012;123: 662–667. 10.1111/j.1471-4159.2012.07896.x [DOI] [PubMed] [Google Scholar]
- 21. Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complicat. 2012;26: 1–9. 10.1016/j.jdiacomp.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 22. Romero MM, Roy S, Pouillot K, Feito M, Esteve M, Grasa MM, et al. Treatment of rats with a self-selected hyperlipidic diet, increases the lipid content of the main adipose tissue sites in a proportion similar to that of the lipids in the rest of organs and tissues. PLOS One. 2014;9: e90995 10.1371/journal.pone.0090995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alemany M, Fernández-López JA. Adipose tissue: something more than just adipocytes. Curr Nutr Food Sci. 2006;2: 141–150. [Google Scholar]
- 24. Cinti S. The adipose organ. Prostag Leukotr Essent Fatty Acids. 2005;73: 9–15. [DOI] [PubMed] [Google Scholar]
- 25. Sabater D, Arriarán S, Romero MM, Agnelli S, Remesar X, Fernández-López JA, et al. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci Rep. 2014;4: 3663 10.1038/srep03663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagström E, Arner P, Ungerstedt U, Bolinder J. Subcutaneous adipose tissue: a source of lactate production after glucose ingestion in humans. Am J Physiol. 1990;258: E888–E893. [DOI] [PubMed] [Google Scholar]
- 27. DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 1992;6: 2405–2412. [DOI] [PubMed] [Google Scholar]
- 28. van der Merwe MT, Crowther NJ, Schlaphoff GP, Boyd IH, Gray IP, Joffe BI, et al. Lactate and glycerol release from the subcutaneous adipose tissue of obese urban women from South Africa; Important metabolic implications. J Clin Endocrinol Metab. 1998;83: 4084–4091. [DOI] [PubMed] [Google Scholar]
- 29. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56: 901–911. [DOI] [PubMed] [Google Scholar]
- 30. Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes. 2013;62: 1417–1425. 10.2337/db12-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slawik M, Vidal-Puig AJ. Adipose tissue expandability and the metabolic syndrome. Genes Nutr. 2007;2: 41–45. 10.1007/s12263-007-0014-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Borst B, Schols AMWJ, de Theije C, Boots AW, Köhler SE, Goossens GH, et al. Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J Appl Physiol. 2013;114: 1619–1628. 10.1152/japplphysiol.00460.2012 [DOI] [PubMed] [Google Scholar]
- 33. Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflügers Archiv-Eur J Physiol. 2007;455: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alemany M. The defense of adipose tissue against excess substrate-induced hyperthrophia: Immune system cell infiltration and arrested metabolic activity. J Clin Endocrinol Metab, 2011;96: 66–68. 10.1210/jc.2010-2541 [DOI] [PubMed] [Google Scholar]
- 35. Alemany M. Steroid hormones interrelationships in the metabolic syndrome: An introduction to the ponderostat hypothesis. Hormones. 2012;11: 272–289. [DOI] [PubMed] [Google Scholar]
- 36. Ferrer-Lorente R, Cabot C, Fernández-López JA, Alemany M. Combined effects of oleoyl-estrone and a β3-adrenergic agonist (CL316,243) on lipid stores of diet-induced overweight male Wistar rats. Life Sci. 2005;77: 2051–2058. [DOI] [PubMed] [Google Scholar]
- 37. Vytašek R. A sensitive fluorometric assay for the determination of DNA. Anal Biochem. 1982;120: 243–248. [DOI] [PubMed] [Google Scholar]
- 38. Consortium GSP. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428: 493–521. [DOI] [PubMed] [Google Scholar]
- 39. Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obesity. 1994;18: 79–83. [PubMed] [Google Scholar]
- 40. Lowry OH, Rosebrough RW, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193: 265–275. [PubMed] [Google Scholar]
- 41. Soley M, Alemany M. A rapid method for the estimation of amino acid concentration in liver tissue. J Biochem Biophys Meth. 1980;2: 207–211. [DOI] [PubMed] [Google Scholar]
- 42. Vassault A. Lactate dehydrogenase. UV method with pyruvate and NADH+ . Meth Enzym Anal. 1983;3: 118–126. [Google Scholar]
- 43. Romero MM, Grasa MM, Esteve M, Fernández-López JA, Alemany M. Semiquantitative RT-PCR measurement of gene expression in rat tissues including a correction for varying cell size and number. Nutr Metab. 2007;4: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stern-Straeter J, Bonaterra GA, Hörmann K, Kinscherf R, Goessler UR. Identification of valid reference genes during the differentiation of human myoblasts. BMC Mol Biol. 2009;10: 66 10.1186/1471-2199-10-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bamias G, Goukos D, Laoudi E, Balla IG, Siakavellas SI, Daikos GL, et al. Comparative study of candidate housekeeping genes for quantification of target gene messenger RNA expression by real-time PCR in patiens with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19: 2840–2847. 10.1097/01.MIB.0000435440.22484.e8 [DOI] [PubMed] [Google Scholar]
- 46. Muñoz S, Franckhauser S, Elias I, Ferré T, Hidalgo A, Monteys AM, et al. Chronically increased glucose uptake by adipose tissue leads to lactate production and improved insulin sensitivity rather than obesity in the mouse. Diabetologia. 2010;53: 2417–2430. 10.1007/s00125-010-1840-7 [DOI] [PubMed] [Google Scholar]
- 47. Crandall DL, Fried SK, Francendese AA, Nickel M, DiGirolamo M. Lactate release from isolated rat adipocytes: Influence of cell size, glucose concentration; insulin and epinephrine. Horm Metab Res. 1983;15: 326–329. [DOI] [PubMed] [Google Scholar]
- 48. Ellmerer M, Schaupp L, Sendlhofer G, Wutte A, Brunner GA, Trajanoski Z, et al. Lactate metabolism of subcutaneous adipose tissue studied by open flow microperfusion. J Clin Endocrinol Metab. 1998;83: 4394–4401. [DOI] [PubMed] [Google Scholar]
- 49. Alemany M. Regulation of adipose tissue energy availability through blood flow control in the metabolic syndrome. Free Radical Biol Med. 2012;52: 2108–2119. 10.1016/j.freeradbiomed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 50. Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia. 2013;56: 1403–1412. 10.1007/s00125-013-2885-1 [DOI] [PubMed] [Google Scholar]
- 51. Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447: 660–665. 10.1016/j.bbrc.2014.04.062 [DOI] [PubMed] [Google Scholar]
- 52. Rognstad R. Control of lactate gluconeogenesis by glucose in rat hepatocytes. Arch Biochem Biophys. 1982;217: 498–502. [DOI] [PubMed] [Google Scholar]
- 53. Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol. 2002;283: E233–E240. [DOI] [PubMed] [Google Scholar]
- 54. Zhang LY, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid β-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta. 2010;1801: 1–22. 10.1016/j.bbalip.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 55. Nadal-Ginard B. Regulation of lactate dehydrogenase levels in the mouse. J Biol Chem. 1978;253: 170–177. [PubMed] [Google Scholar]
- 56. Freminet A, Leclerc L, Gentil M, Poyart C. Effect of fasting on the rates of lactate turnover and oxidation in rats. FEBS Lett. 1975;60: 431–434. [DOI] [PubMed] [Google Scholar]
- 57. Felipe A, Remesar X, Pastor-Anglada M. L-Lactate uptake by rat liver. Effect of food deprivation and substrate availability. Biochem J. 1991;273: 195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146: 4192–4199. [DOI] [PubMed] [Google Scholar]
- 59. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92: 347–355. [DOI] [PubMed] [Google Scholar]
- 60. O'Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia.2011;54: 1480–1490. 10.1007/s00125-011-2103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sclafani A, Springer D. Dietary obesity in adult rats: Similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17: 461–471. [DOI] [PubMed] [Google Scholar]
- 62. Rafecas I, Esteve M, Fernández-López JA, Remesar X, Alemany M. Deposition of dietary fatty acids in young Zucker rats fed a cafeteria diet. Int J Obesity. 1992;16: 775–787. [PubMed] [Google Scholar]
- 63. Davidson MB, Garvey D. Studies on mechanisms of hepatic insulin resistance in cafeteria- fed rats. Am J Physiol. 1993;264: E18–E23. [DOI] [PubMed] [Google Scholar]
- 64. Alemany M. Adipose tissue hypoxia, a conceptual alometric view. J Endocrinol Metab. 2011;1: 155–158. [Google Scholar]
- 65. Priego T, Sánchez J, Picó C, Palou A. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity. 2008;16: 819–826. 10.1038/oby.2007.117 [DOI] [PubMed] [Google Scholar]
- 66. Heinonen I, Kemppainen J, Kaskinoro K, Knuuti J, Boushel R, Kalliokoski KK. Capacity and hypoxic response of subcutaneous adipose tissue blood flow in humans. Circ J. 2014;78: 1501–1506. [DOI] [PubMed] [Google Scholar]
- 67. Andersson J, Karpe F, Sjöström LG, Riklund K, Söderberg S, Olsson T. Association of adipose tissue blood flow with fat depot sizes and adipokines in women. Int J Obesity. 2012;36: 783–789. 10.1038/ijo.2011.152 [DOI] [PubMed] [Google Scholar]
- 68. Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric oxide and β-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation. 2004;109: 47–52. [DOI] [PubMed] [Google Scholar]
- 69. Codoñer-Franch P, Tavárez-Alonso S, Murria-Estal R, Megías-Vericat J, Tortajada-Girbés M, Alonso-Iglesias E. Nitric oxide production is increased in severely obese children and related to markers of oxidative stress and inflammation. Atherosclerosis. 2001;215: 475–480. [DOI] [PubMed] [Google Scholar]
- 70. Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53: 2060–2066. [DOI] [PubMed] [Google Scholar]
- 71. Toda N, Okamura T. Obesity impairs vasodilatation and blood flow increase mediated by endothelial nitric oxide: An overview. J Clin Pharmacol. 2013; 53:1228–1239. 10.1002/jcph.179 [DOI] [PubMed] [Google Scholar]
- 72. Ribiere C, Jaubert AM, Gaudiot N, Sabourault D, Marcus ML, Boucher JL, et al. White adipose tissue nitric oxide synthase: A potential source for NO production. Biochem Biophys Res Commun. 1996;222: 706–712. [DOI] [PubMed] [Google Scholar]
- 73. Gardiner SM, Compton AM, Bennett T, Palmer RM, Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990;15: 486–492. [DOI] [PubMed] [Google Scholar]
- 74. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolc disturbances of diabetes mellitus. Lancet. 1963;281: 785–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.