Abstract
AIM: To clarify the correlation with phenotypic expression, clinicopathological features, genetic alteration and microsatellite-instability status in small intestinal adenocarcinoma (SIA).
METHODS: The cases of 47 patients diagnosed with primary SIAs that were surgically resected at our institution in 1975-2005 were studied. We reviewed clinicopathological findings (age, gender, tumor size, gross appearance, histological morphologic type, invasion depth, lymphatic permeation, venous invasion, and lymph node metastasis), and the immunohistochemical expression of MUC5AC, MUC6, MUC2, CD10, and mismatch-repair (MMR) proteins (MLH1 and MSH2). We analyzed KRAS and BRAF gene mutations, and the microsatellite instability (MSI) status. The immunohistochemical staining of CD10, MUC2, MUC5AC and MUC6 was considered positive when distinct staining in > 5% of the adenocarcinoma cells was recorded. To evaluate of MMR protein expression, we used adjacent normal tissue including lymphoid follicles, inflammatory cells, and stromal cells as an internal positive control. Sections without nuclear staining in the tumor cells were considered to have lost the expression of the respective MMR protein.
RESULTS: There were 29 males and 18 females patients (mean age 59.9 years, range: 23-87 years). Tumors were located in the duodenum in 14 cases (30%), the jejunum in 21 cases (45%), and the ileum in 12 cases (25%). A phenotypic expression analysis revealed 20 MUC2-positive tumors (42.6%), 11 MUC5AC-positive (23.4%), 4 MUC6-positive (8.5%), and 7 CD10-positive (14.9%). The tumor sizes of the MUC2(+) tumors were significantly larger than those of the MUC2(-) tumors (mean, 5.7 ± 1.4 cm vs 4.7 ± 2.1 cm, P < 0.05). All three tumors with adenomatous component were positive for MUC2 (P < 0.05). Polypoid appearance was seen significantly more frequently in the CD10(+) group than in the CD10(-) group (P < 0.05). The tumor size was significantly larger in the CD10 (+) group than in the CD10(-) group (mean, 5.9 ± 1.4 cm vs 5.0 ± 2.1 cm, P < 0.05). Of 34 SIAs with successfully obtained MSI data, 4 were MSI-high. Of the 4 SIAs positive for both MUC5AC and MUC2, 3 showed MSI-H (75%) and 3 were mucinous adenocarcinoma (75%). KRAS mutations were detected in 4 SIAs. SIAs had KRAS mutation expressed only MUC2, but were negative for MUC5AC, MUC6 and CD10.
CONCLUSION: These findings suggest that the phenotypic expression of SIAs is correlated with their biological behavior, genetic alteration, and MSI status.
Keywords: Small intestine, Adenocarcinoma, Mucin, CD10, Microsatellite instability
Core tip: This study analyzed the immunohistochemical expression of mucin core proteins (MUC5AC, MUC6 and MUC2), CD10 and mismatch-repair proteins (MLH1, MSH2), microsatellite instability (MSI), and the mutational status of KRAS and BRAF in 47 primary small intestinal adenocarcinoma. We suggest that the mucin phenotype and CD10 expression of small intestinal adenocarcinoma correlates with biological behavior, genetic alteration, and MSI status. Notably, the preservation of CD10 expression may be correlated with favorable biological behavior. The SIA with co-expression of MUC5AC and MUC2 was correlated with MSI-H status and mucinous adenocarcinoma, similar to colorectal carcinoma.
INTRODUCTION
Primary small intestinal adenocarcinomas (SIAs) are rare, accounting for only about 0.8% of all malignant gastrointestinal tumors, although the small intestine represents 75% of the length and 90% of the mucosal surface of the gastrointestinal tract[1,2]. Despite major advances in imaging and therapeutic techniques, the prognosis of SIAs remains dismal, with an overall 5-year survival rate of 25%-30%[3,4].
Mucins are a family of high-molecular-weight, heavily glycosylated proteins that are widely produced by epithelial tissue. In the alimentary canal, MUC2 is produced by goblet cells of the small and large intestinal mucosa, and MUC5AC is produced by gastric foveolar epithelium. MUC6 is produced by the gastric pyloric gland and duodenal Brunner’s glands. Compared to normal mucosa, adenocarcinomas occasionally produce different types of mucin. Some researchers have reported that in gastric carcinoma (GC) or colorectal carcinoma (CRC), the mucin phenotype was related to the tumor growth pattern, appearance, aggressiveness, prognosis, genetic alternation and microsatellite instability (MSI) status. For example, it was reported that MUC2-positive CRCs have a relatively favorable prognosis[5], whereas loss of MUC2 was an adverse prognostic factor in CRCs[6]. MUC5AC was often found to be expressed in CRCs with MSI-high (MSI-H) and/or mucinous adenocarcinomas, and intriguingly, this type of CRCs often express MUC2 as well[7,8].
The “serrated pathway” is an evolving pathway in the carcinogenesis of CRC, characterized by BRAF gene mutations and MSI-H[9]. Sessile serrated adenomas, considered precursors of MSI-H CRCs, often express both MUC2 and MUC5AC, the same as MSI-H CRCs do[10].
It has been suggested that not only mucin expression but also CD10 expression, which is usually seen in the brush border of the small intestinal villi, are correlated with aggressive biological behavior of CRCs. CD10-positive CRCs in particular were suggested to be prone to the development of metastasis to the liver[11,12]. Analyses of the mucin core proteins and CD10 expression in adenocarcinomas of the alimentary canal are thus important to determine these tumors’ biological behavior and carcinogenesis.
In SIAs, however, the phenotypic expressions of mucin core proteins and CD10 have not been established, and the clinicopathological features and pathogenesis of SIAs are less known than those of CRCs.
The purposes of the present study were to evaluate the mucin core proteins and CD10 expression of primary SIAs and to clarify their correlation with the phenotypic expression, clinicopathological features, biological behavior, genetic alterations and MSI status.
MATERIALS AND METHODS
Patients
The cases of 47 patients diagnosed with primary SIAs that were surgically resected and diagnosed at the Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University (Fukuoka, Japan) between January 1975 and December 2005 were studied.
To prevent confusion with the findings of carcinomas in neighboring organs, all carcinomas involving the pylorus, ileocecal valve, and ampulla of Vater were excluded from the analysis. The cases of patients diagnosed with hereditary nonpolyposis colorectal cancer (HNPCC), familial adenomatous polyposis (FAP), celiac disease or inflammatory bowel disease (IBD) were also excluded. The study was approved by the Institutional Review Board of Kyushu University (IRB#25-191).
Histological evaluation
The surgically resected small intestine specimens were each fixed in 10% formalin and examined macroscopically. The entire tumor tissue was sliced in a serial fashion at approx. 3 to 5 mm thickness, and the tissue slices were routinely processed to paraffin blocks and stained with hematoxylin and eosin (HE). Clinicopathologic findings (patient age, gender, tumor size, gross appearance, histological morphologic type, invasion depth, lymphatic permeation, venous invasion, and lymph node metastasis) were reviewed. Representative sections were also used for the subsequent immunohistochemical stains and molecular analysis.
Immunohistochemistry
Immunohistochemical staining was performed using 4-μm-thick formalin-fixed, paraffin-embedded tissue sections and the primary antibodies for CD10 (56C6, dilution 1:100, Leica, Newcastle, United Kingdom), MUC2 (Ccp58, dilution 1:200, Leica), MUC5AC (CLH1, dilution 1:200, Leica), MUC6 (CLH5, dilution 1:200, Leica,), MLH1 (G168-728, dilution 1:50, BD Bioscience, San Diego, CA) and MSH2 (FE11, dilution 1:200, Oncogene Research Products, La Jolla, CA). A biotin-free, horseradish peroxidase (HRP) enzyme-labeled polymer method (Envision+ system; Dako, Carpinteria, CA) was used, with hematoxylin counterstaining.
The immunohistochemical staining of CD10, MUC2, MUC5AC and MUC6 was considered positive when distinct staining in more than 5% of the adenocarcinoma cells was recorded, in reference to the phenotypic expressions of previous studies[13,14]. The adenomatous area of the SIAs was excluded in the evaluation of immunohistochemical staining.
For the evaluation of mismatch-repair (MMR) protein expression (i.e., MLH1 and MSH2), adjacent normal tissue including lymphoid follicles, inflammatory cells, and stromal cells was used as an internal positive control. Sections without nuclear staining in the tumor cells were considered to have lost the expression of the respective MMR protein.
Sections were viewed by three independent observers (R.K., K.K. and M.H.) with conflicts resolved using a conference microscope.
Mutational analysis for KRAS and BRAF
Genomic DNA was extracted from paraffin sections using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. To detect KRAS mutation in exon 2, and BRAF mutation in exon 15, we performed a polymerase chain reaction (PCR) followed by sequencing, as described[15]. The primer sets used are listed in Table 1.
Table 1.
Primers used in this study
| Primer | ||
| KRAS | Forward | 5’-GGCCTGCTGAAAATGACTGA-3’ |
| Reverse | 5’-GTTGGATCATATTCGTCCAC-3’ | |
| BRAF | Forward | 5’-CCTTTACTTACTACACCTCAG-3’ |
| Reverse | 5’-CATCCACAAAATGGATCCAG-3’ | |
| BAT25 | Forward1 | 5’-TCGCCTCCAAGAATGTAAGT-3’ |
| Reverse | 5’-TCTGCATTTTAACTATGGCTC-3’ | |
| BAT26 | Forward1 | 5’-TGACTACTTTTGACTTCAGCC-3’ |
| Reverse | 5’-AACCATTCAACATTTTTAACCC-3’ | |
| D2S123 | Forward1 | 5’-GAAACAGGATCCTGCCTTTA-3’ |
| Reverse | 5’-GGACTTTCCACCTATGGGAC-3’ | |
| D5S346 | Forward1 | 5’-GACTCACTCTAGTATAAATCGGG-3’ |
| Reverse | 5’-GAGCAGATAAGACAGTATTACTAGTT-3’ | |
| D17S250 | Forward1 | 5’-GGGAAGAATCAAATAGACAAT-3’ |
| Reverse | 5’-GCTGGCCATATATATATTTAAACC-3’ |
Forward primers for microsatellite instability (MSI) analysis were fluorescently-labeled with 6-carboxyfluorescein (6-FAM).
MSI analysis
DNA isolated from the adenocarcinomas and non-neoplastic normal mucosa was amplified. The MSI status was determined using the Bethesda panel established by the United States National Cancer Institute, including mononucleotide (BAT25 and BAT26) and dinucleotide (D2S123, D5S346, D17S250) repeats[16]. The primer sets used are listed in Table 1. PCR products were separated on an ABI 310 automated sequencer (Applied Biosystems, Warrington, United Kingdom). The data analysis was done by GeneScan software (Applied Biosystems). Tumors with two or more of the markers exhibiting instability were classified as MSI-H. Tumors with only one marker exhibiting instability were classified as MSI-low (MSI-L), and those showing no markers with instability were classified as microsatellite stable (MSS).
Statistical analysis
All calculations were made using JMP Statistical Discovery Software (version 8.0; SAS, Cary, NC). We used Fisher’s exact test, the χ2 test or Wilcoxon signed-rank test to evaluate the association between the phenotypic expression and clinicopathological features or genetic alteration. The results were considered significant when the P value was < 0.05. The statistical methods of this study were reviewed by Mototsugu Shimokawa from Clinical Research Institute, National Hospital Organization Kyushu Cancer Center.
RESULTS
Clinicopathological findings
The clinicopathological findings of the 47 SIAs are shown in Table 2. There were 29 male and 18 female patients with a mean age of 59.9 years (range 23-87 years). Tumors were located in the duodenum in 14 cases (30%), the jejunum in 21 cases (45%), and the ileum in 12 cases (25%). The tumor showed polypoid growth in 12 cases, and ulcerative growth in 35 cases. The tumor size ranged from 1.5 to 10.0 cm (mean, 5.1 cm). Two tumor invaded the lamina propria mucosae or the submucosa (pT1, classified invasion depth according to the UICC TNM classification of malignant tumors[17]), 4 tumors invaded the muscularis propria (pT2), 31 tumors invaded into the subserosa (pT3), and 10 tumors had perforated the visceral serosa or directly invaded other organs (pT4). Of the 25 tumors resected with lymph nodes, 16 had lymph node metastases (data not shown). Thirty-three tumors were well or moderately differentiated (tubular), 9 were poorly differentiated, and 5 were mucinous adenocarcinomas. Lymphatic permeation was detected in 33 tumors (70%), and venous invasion was detected in 12 tumors (26%). Three tumors had an adjacent adenomatous component, which showed a tubulo-villous structure.
Table 2.
Clinicopathological findings of all 47 cases of small intestinal adenocarcinomas n (%)
| All cases (n = 47) | |
| Age (yr) | |
| Mean (range) | 59.9 (23-87)1 |
| Gender | |
| Male | 29 |
| Female | 18 |
| Gross appearance | |
| Polypoid | 12 |
| Ulcerative | 35 |
| Size (cm) (mean) | 5.13 |
| Location | |
| Duodenum | 14 |
| Jejunum | 21 |
| Ileum | 12 |
| Histological subtype | |
| Tubular2 | 33 |
| Poorly | 9 |
| Mucinous | 5 |
| Invasion depth3 | |
| T1 | 2 |
| T2 | 4 |
| T3 | 18 |
| T4 | 23 |
| Lymphatic permeation | 33 (70) |
| Venous invasion | 12 (26) |
| Adenomatous component | 3 (6) |
Age of one patient was unknown;
"Tubular" contains well or moderately differentiated Adenocarcinoma;
UICC TNM classification is used.
Expressions of mucin core proteins and CD10
The phenotypic expression analysis of the 47 SIAs revealed 20 tumors (42.6%) positive for MUC2, 11 tumors (23.4%) positive for MUC5AC, 4 tumors (8.5%) positive for MUC6, and 7 tumors (14.9%) positive for CD10. The correlations between clinicopathological features and the expressions of mucin core proteins and CD10 are summarized in Table 3.
Table 3.
Clinicopathological features and expression of mucin core proteins and CD10
|
MUC5AC |
MUC6 |
MUC2 |
CD10 |
|||||
| Positive (n = 12) | Negative (n = 35) | Positive (n = 4) | Negative (n = 43) | Positive (n = 20) | Negative (n = 27) | Positive (n = 7) | Negative (n = 40) | |
| Age (yr) | ||||||||
| Mean ± SD | 63.4 ± 15.11 | 58.8 ± 16.5 | 76.3 ± 10.01 | 58.7 ± 15.9 | 62.3 ± 15.3 | 58.0 ± 16.71 | 60.1 ± 15.1 | 59.8 ± 16.51 |
| (range) | (35-85) | (23-87) | (67-87) | (23-85) | (38-87) | (23-81) | (38-87) | (23-85) |
| Gender (male/female) | 10/2 | 19/16 | 3/1 | 26/17 | 9/11 | 18/9 | 3/4 | 27/14 |
| Gross appearance | ||||||||
| (polypoid/ulcerated) | 3/9 | 9/26 | 2/2 | 10/33 | 6/14 | 6/21 | 4/3a | 8/32a |
| Size (cm) | 5.3 ± 2.2 | 5.1 ± 1.9 | 5.0 ± 1.8 | 5.1 ± 2.0 | 5.7 ± 1.6a | 4.7 ± 2.2a | 5.9 ± 1.4a | 5.0 ± 2.1a |
| Location | ||||||||
| (duodenum/jejunum/ileum) | 2/5/5 | 12/16/7 | 2/1/1 | 12/20/11 | 6/11/3 | 8/10/9 | 4/2/1 | 10/19/11 |
| Histological subtype | ||||||||
| (tubular2/poorly/mucinous) | 6/3/3 | 27/6/2 | 3/0/1 | 30/9/4 | 13/2/5 | 10/7/0 | 7/0/0 | 29/9/5 |
| Invasion depth3 | ||||||||
| T1 | 0 | 2 | 0 | 2 | 2 | 0 | 2a | 0a |
| T2 | 2 | 2 | 2 | 2 | 2 | 2 | 2a | 2a |
| T3 | 2 | 16 | 0 | 18 | 6 | 12 | 1a | 17a |
| T4 | 8 | 15 | 2 | 21 | 10 | 13 | 2a | 21a |
| Lymphatic permeation n (%) | 10 (83) | 23 (66) | 2 (50) | 31 (72) | 12 (60) | 21 (78) | 3 (43) | 30 (75) |
| Venous invasion n (%) | 3 (25) | 9 (26) | 1 (25) | 11 (26) | 5 (25) | 7 (26) | 2 (29) | 10 (25) |
| Adenomatous component n (%) | 0 (0) | 3 (9) | 1 (25) | 2 (5) | 3 (15)a | 0 (0)a | 2 (29)a | 1 (3)a |
Age of one patient was unknown;
"tubular" contains well or moderately differentiated adenocarcinoma;
UICC TNM classification is used.
P < 0.05 vs positive group and negative group in the same mucin core protein or CD10. In comparison invasion depth, T1 vs T2-T4).
All mucin core proteins were expressed in the cytoplasm of the tumor cells. The tumor sizes of the MUC2(+) tumors were significantly larger than those of the MUC2(-) tumors (mean, 5.7 ± 1.6 cm vs 4.7 ± 2.2 cm, P < 0.05). All 3 tumors with adenomatous component were positive for MUC2 (P < 0.05). The MUC5AC(+) SIAs were more prevalent in the males than the females (9 males vs 2 females). The ages of the patients in the MUC6(+) group tended to be higher than those of the MUC6(-) group (mean, 76.3 ± 10.0 years vs 58.7±15.9 years).
CD10 expression was observed along the luminal surface of the tumor glands. In the CD10(+) group, CD10 was more frequently expressed in the superficial areas of the adenocarcinomas than in their deep area, and the deep area of the CD10-negative adenocarcinomas showed the same tubular shape but more high-grade atypia compared to the surperficial area of the CD10-positive adenocarcinomas (Figure 1). Polypoid appearance was seen significantly more frequently in the CD10(+) group compared to the CD10(-) group (P < 0.05). The tumor size was significantly larger in the CD10(+) group than in the CD10(-) group (mean, 5.9 ± 1.4 cm vs 5.0 ± 2.1 cm, P < 0.05). The invasion depth was significantly deeper in the CD10(-) group than in the CD10(+) group (T1 vs T2-T4, P < 0.05). The incidence of lymphatic permeation tended to be less in the CD10(+) group than in the CD10(-) group (Figure 2).
Figure 1.
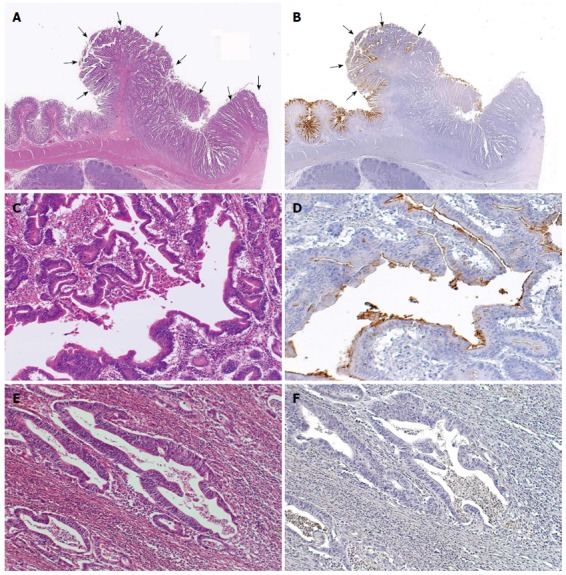
Representative CD10-positive small intestinal adenocarcinoma case. A: Well differentiated adenocarcinoma (pointed by arrows) (HE stain, × 12.5); B: CD10-positive glands were seen in the superficial area and the edge of the adenocarcinoma (left, pointed by arrows), while CD10-negative glands were seen in the center or deep area of the tumor (right) (CD10, × 12.5); C, D: These glands in the surface area were positive for CD10 (HE stain and CD10, × 200); E, F: Other glands in the deeper invasive area were negative for CD10 and showed more evident loss of nuclear polarity compared to the CD10-positive glands (HE stain and CD10, × 200).
Figure 2.
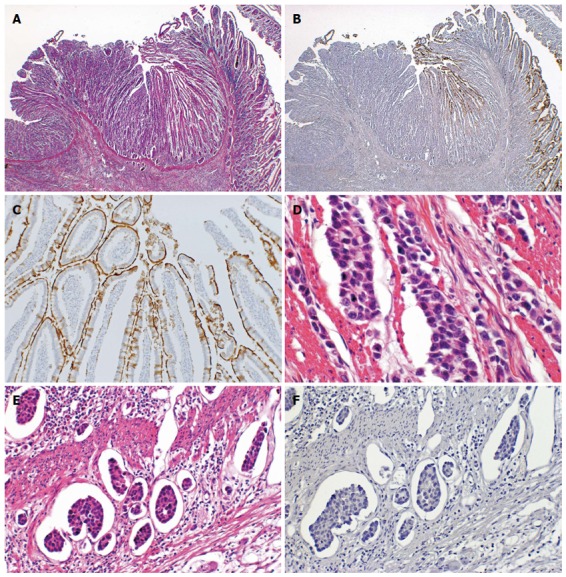
Representative CD10-negative small intestinal adenocarcinoma case. A: Normal small intestinal mucosa was observed on the right side, whereas the adenocarcinoma was observed on the left side (HE stain, × 12.5); B: Normal mucosa was positive for CD10, but adenocarcinoma was negative for CD10 (CD10, × 12.5); C: The brush border of the normal mucosa was positive for CD10 (CD10, × 100); D: This SIA case showed a poorly differentiated adenocarcinoma (HE satin, × 200); E: Lymphatic permeation was frequently seen (HE stain, × 200); F: The carcinoma cells were negative for CD10 (CD10, × 200).
We found 16 SIAs that expressed only intestinal markers (MUC2 and/or CD10), 7 SIAs that expressed only gastric markers (MUC5AC and/or MUC6), and 6 SIAs that expressed both gastric and intestinal markers, including 4 SIAs that were positive for both MUC5AC and MUC2.
Expression of MLH1 and MSH2
Four of the 47 cases could not be evaluated for MLH1 due to poor staining quality. One of the remaining 43 tumors showed loss of MLH1, and 2 of the 47 tumors showed loss of MSH2.
MSI analysis
MSI data were obtained from 34 tumors. The phenotypes were MSI-H in 4 tumors (12%), MSI-L in 8 tumors (24%), and MSS in 22 tumors (64%). Immunohistochemical loss of MMR protein was detected in 2 of 4 MSI-H tumors (1 tumor showed loss of MLH1, and the other showed loss of MSH2), 1 of 8 MSI-L tumors (loss of MSH2), and no MMS tumors (Table 4).
Table 4.
Summarized data of microsatellite instability status, expression of mismatch repair proteins and KRAS mutations
| MSI |
Expression of MMR protein |
KRAS mutation base exchange (codon) | Age | Gender | ||
| MLH1 | MSH2 | |||||
| Case 6 | MSI-H | + | + | - | 55 | M |
| Case 36 | MSI-H | + | - | - | 85 | M |
| Case 31 | MSI-H | - | + | - | 67 | M |
| Case 34 | MSI-H | + | + | - | 71 | M |
| Case 12 | MSI-L | + | + | GGT>GAT(12) | 69 | M |
| Case 28 | MSI-L | + | - | - | 41 | M |
| Case 24 | MSI-L | + | + | - | 60 | M |
| Case 40 | MSI-L | + | + | - | 35 | M |
| Case 25 | MSI-L | + | + | - | 53 | F |
| Case 5 | MSI-L | + | + | - | 23 | F |
| Case 44 | MSI-L | + | + | - | 87 | F |
| Case 38 | MSI-L | + | + | - | 67 | F |
| Case 41 | MSS | + | + | GGC>GAC(13) | 45 | F |
| Case 20 | MSS | + | + | GGT>GAT(12) | 59 | F |
| Case 46 | MSS | + | + | GGT>GTT(12) | 40 | F |
MSI: Microsatellite instability; MSI-H: MSI-high; MSI-L: MSI-low; MMR: Mismatch repair; M: Male; F: Female.
The data of MSI status and clinicopathological features is shown in Table 5. The MSI-H SIAs were composed of 2 well differentiated adenocarcinomas and 2 mucinous adenocarcinomas (Figure 3). The tumor size was significantly larger in MSI-H SIAs than in MSI-L and MSS SIAs (mean 7.7 ± 1.1 cm vs 5.1 ± 2.0 cm, P < 0.05).
Table 5.
Clinicopathological features, microsatellite instability status and KRAS mutation
|
MSI |
KRAS |
|||
| MSI-H (n = 4) | MSI-L/MSS (n = 30) | Mutational type (n = 4) | Wild type (n = 33) | |
| Age (yr) | 69.5 ± 12.3 | 59.6 ± 15.91 | 53.3 ± 13.2 | 60.8 ± 16.21 |
| Gender (male/female) | 4/0 | 16/14 | 1/3 | 22/11 |
| Gross appearance (polypoid/ulcerated) | 1/3 | 6/24 | 2/2 | 9/24 |
| Size (cm) | 7.7 ± 1.1a | 5.1 ± 2.0a | 4.4 ± 1.0 | 5.5 ± 2.0 |
| Location (duodenum/jejunum/ileum) | 0/2/2 | 7/15/8 | 1/2/1 | 9/15/9 |
| Histological subtype (tubular2/poorly/mucinous) | 2/0/2 | 22/6/2 | 4/0/0 | 22/8/3 |
| Invasion depth3 | ||||
| T1 | 0 | 1 | 0 | 1 |
| T2 | 0 | 2 | 0 | 3 |
| T3 | 2 | 13 | 1 | 13 |
| T4 | 2 | 14 | 3 | 15 |
| Lymphatic permeation n (%) | 3 (75) | 23 (77) | 4 (100) | 22 (67) |
| Venous invasion n (%) | 1 (25) | 9 (30) | 1 (25) | 10 (30) |
| Adenomatous component n (%) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
Age of one patient was unknown;
"Tubular" contains well or moderately differentiated adenocarcinoma;
UICC TNM classification is used.
P < 0.05 vs control.
Figure 3.
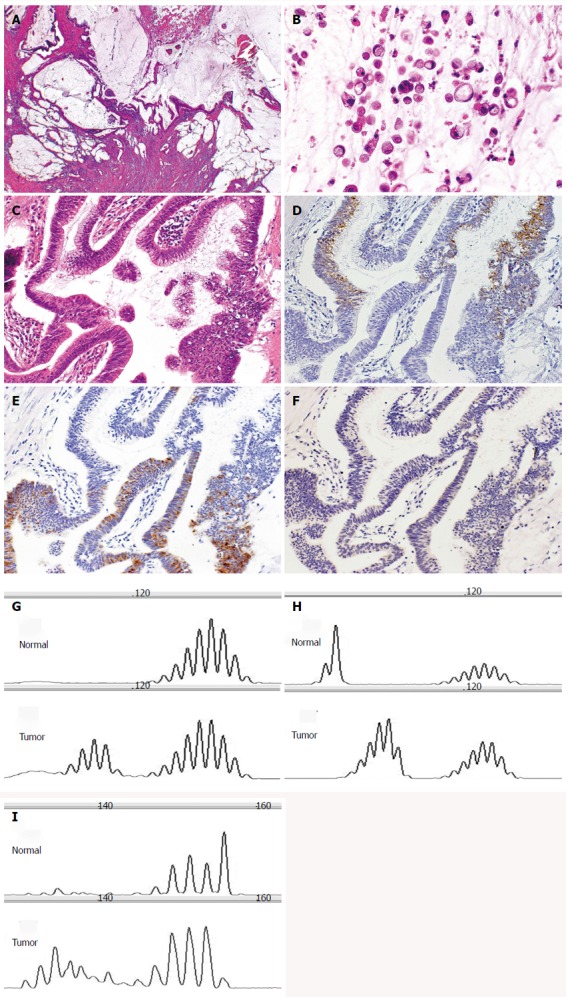
Small intestinal adenocarcinoma with microsatellite instability-high (case 36). A: The tumor produced abundant mucin (HE stain, × 20); B: The tumor focally showed signet ring-like carcinoma cells (HE stain, × 200); C: The carcinomatous gland was composed of columnar cells with intracytoplasmic mucin (HE stain, × 200); D: These glands were positive for MUC2 (MUC2, × 200); E: positive for MUC5AC (MUC5AC, × 200); F: But negative for MUC6 (MUC6, × 200). In these microsatellites, the tumor was unstable. Microsatellite analysis of paired normal and tumor DNA using G: BAT25; H: BAT26; I: D17S250.
Frequency and associations of tumor gene mutations
The mutation analysis was successfully conducted in 37 of the 47 cases. The genetic mutations and MSI status findings are summarized in Table 4. KRAS mutations were prevalent in 11% (4 of 37) of the tumors. The most prevalent KRAS mutation was GGT > GAT(G12D) within codon 12, which was detected in 2 cases. One case showed GGT>GTT(G12V) within codon 12, and one case showed GGC>GAC(G13D) within codon 13. The data of KRAS mutation and clinicopathological features is shown in Table 5. No BRAF V600E mutation was found in any cases.
Correlations among the mucin phenotype, CD10 expression, genetic alteration and MSI status
Three of the 4 SIAs with KRAS mutation were positive for only MUC2, but negative for MUC5AC, MUC6 and CD10 (75%). Three of the 4 SIAs positive for both MUC5AC and MUC2 showed MSI-H (75%) (Table 6). One of the 4 MSI-H SIA was immunohistochemically negative for all antibodies (MUC5AC, MUC6, MUC2 and CD10).
Table 6.
Summarized data of 4 small intestinal adenocarcinomas with co-expression of MUC5AC and MUC2
| Age | Gender | Location | Histological subtype | Invasion depth | Lymphatic permeation | Venous invasion | Lymph node metastasis | MSI | |
| case 6 | 55 | m | Jejunum | Tubular | T3 | - | - | - | MSI-H |
| case 29 | 61 | m | Jejunum | Mucinous | T4 | + | - | No data | MSS |
| case 31 | 67 | m | Ileum | Mucinous | T4 | + | - | No data | MSI-H |
| case 36 | 85 | m | Ileum | Mucinous | T4 | + | - | - | MSI-H |
MSI: Microsatellite instability; MSI-H: MSI-high; MSS: Microsatellite stable; M: Male.
DISCUSSION
CD10 is a membrane-bound, zinc-containing metalloendopeptidase, that is expressed in a wide variety of normal and neoplastic cells including pre-cursor B and T cells, follicle center B cells in the hematopoietic system, myoepithelial cells of the breast and salivary glands, basaloid cells of the prostatic gland, glomerular cells of the kidney, and stromal cells in the endometrium[18,19]. In the alimentary canal, normal small intestine shows immunoreactivity for CD10 in the brush border. On the other hand, except for intestinal metaplasia of the stomach, the normal gastric foveolae and the colorectal epithelium show no CD10 expression.
In our evaluation of the distribution of CD10, we observed CD10 expression in the superficial area of the SIAs. The expression diminished with greater depth and was not detected in the deepest area of almost all of the CD10(+) SIAs. Yao et al[13] reported that the CD10 expression of CRCs was more frequently observed in the center or deep area of the tumors than their periphery. The distribution of CD10-positive areas seemed to be opposite in SIAs and CRCs.
Regarding the CD10 expression of advanced CRCs, the significant correlations have been reported between CD10 expression and a high incidence of liver metastasis[11,12,20], and between CD10 expression and venous invasion[13]. Carl-McGrath et al[21] reported that CD10 was significantly up-regulated in GCs and lymph node metastases. Although the concrete function of CD10 in carcinogenesis remains unclear, the acquisition of CD10 expression correlates with the malignant potential in CRCs and GCs. In the present study, the incidence of lymphatic permeation tended to be lower in the CD10(+) SIAs than in the CD10(-) SIAs, regardless of the tendency for larger tumor sizes in the CD10(+) SIAs. The CD10-positive adenocarcinoma glands showed lower atypia than the CD10-negative adenocarcinoma glands did in the same SIAs. These results suggest that CD10(+) SIAs are less aggressive than CD10(-) SIAs, and that the loss of CD10 expression in SIAs may be correlated with the phenotypic dedifferentiation and malignant phenotype.
The relationship between mucin phenotype and clinicopathological or biological features has been investigated in various types of GC and CRC[7,8,11-14,22-25]. It is well established that MUC2 expression is related to the progression or prognosis of CRC[5,6,25], an association that suggests that the expression of MUC2 is also related to the biological behavior of SIA. Although in the present study the tumor sizes of the MUC2(+) SIAs were significantly larger than those of the MUC2(-) SIAs, the frequency of lymphatic permeation tended to be lower in the MUC2(+) SIAs than in the MUC2(-) SIAs. Poorly differentiated adenocarcinomas were prevalent in the MUC2(-) SIA group. From these findings, we infer that MUC2(-) SIAs are more aggressive than MUC2(+) SIAs. Loss of MUC2 expression in SIAs may also be correlated with malignant potential.
The serrated neoplastic pathway of CRCs is characterized by BRAF mutation, abnormal DNA methylation, and MSI-H status, and some authors suggested that serrated polyps were the precursor of MSI-H CRCs[9,26]. The MUC5AC+/ MUC2+ mucinous phenotype is common in serrated polyps (including hyperplastic polyps and serrated adenomas) and MSI-H CRCs[10,27,28]. Arai et al[28] also demonstrated a higher proportion of MSI-H in mucinous adenocarcinoma. In the present study, we observed the 4 SIAs that expressed both MUC5AC and MUC2, and these 4 SIAs contained 3 MSI-H tumors and 3 mucinous adenocarcinomas. The data suggested that the co-expression of MUC5AC and MUC2 in SIAs was also correlated with MSI-H tumors and mucinous adenocarcinomas as in CRCs.
We did not detect any serrated polyps or any mutations in codon 600 of BRAF gene. Fu et al[29] investigated 99 duodenal adenocarcinomas and detected no BRAF mutation among them, and therefore they suggested that BRAF mutations are not involved in MSI-H duodenal adenocarcinomas. In other investigations, the BRAF mutations were rare in SIAs[30,31]. In the carcinogenesis of SIAs, it is still unclear whether there is a serrated neoplastic pathway as same as in CRCs.
The present study revealed that 4 of 34 (12%). SIAs had microsatellite instability. This result is in line with reports by other investigators, who described the frequency of MSI-H of SIAs of 5% to 30%[32-35]. We also found that 2 of 4 MSI-H tumors showed the immunohistochemical loss of MMR protein expression, which affected MLH1 in 1 case and MSH2 in 1 case. Mangold et al[36] reported that 32% of CRCs from MLH1 mutation-carrier patients showed partly positive or weak staining of MLH1. In the present study, we considered only the completely negative staining of MLH1 as the loss of MLH1 protein, and thus some of the SIAs we assessed as positive for MLH1 protein could be false-positive. Planck et al[35] investigated a large population-based series of 89 SIAs, and eight of 15 MSI-H tumors expressed both MLH1 and MSH2. We suggest that the immunohistochemical staining of MLH1 and MSH2 proteins is generally useful to detect MSI-H SIAs, but a molecular analysis is necessary to detect some MSI-H SIAs.
Previous studies detected KRAS mutations in 14%-83% of SIA cases[32,34,37-39]. We detected mutations in KRAS codon 12 and codon 13 in 4 of 37 SIAs. The frequency and the location were in line with those of the previous studies. Three of the 4 present SIAs with KRAS mutations were positive for only MUC2 (75%). We found a tendency for the SIAs with KRAS mutations to have the colonic phenotype.
In conclusion, we suggest that the mucinous phenotype and CD10 expression of SIAs also correlates with the tumors’ biological behavior, genetic alteration, and MSI status. In particular, the preservation of CD10 expression, correlated with larger tumor size, polypoid appearance and less lymphatic permeation, features that are suggestive of less aggressive biological behavior. The co-expression of MUC5AC and MUC2 of the SIAs was correlated with the MSI-H status and mucinous adenocarcinoma, as in CRCs.
ACKNOWLEDGMENTS
We are grateful to Dr. Shimokawa M, National Hospital Organization Kyushu Cancer Center, for reviewing the statistical method of this study.
COMMENTS
Background
Mucins are a family of high-molecular-weight, heavily glycosylated proteins that are widely produced by epithelial tissue. In the alimentary canal, MUC2 is produced by goblet cells of the small and large intestinal mucosa, and MUC5AC is produced by gastric foveolar epithelium.
Research frontiers
The purposes of the present study were to evaluate the mucin core proteins and CD10 expression of primary small intestinal adenocarcinomas (SIAs) and to clarify their correlation with the phenotypic expression, clinicopathological features, biological behavior, genetic alterations and microsatellite instability status.
Innovations and breakthroughs
The authors considered only the completely negative staining of MLH1 as the loss of MLH1 protein, and thus some of the SIAs we assessed as positive for MLH1 protein could be false-positive.
Peer-review
The paper by Kumagai et al entitled “Mucinous phenotype and CD10 expression of primary adenocarcinoma of the small intestine: Possible association with biological behavior, genetic alteration and microsatellite instability status” investigates the expression of CD10, MUC5A, MUC2, MUC6 and proteins related to microsatellite instability/mismatch repair proteins (MLH1 and MSH2) in 47 cases of small bowel adenocarcinoma, evaluated by immunohistochemistry. Then, Authors correlated the immunohistochemical pattern of expression of these molecules to size, grade and stage of the tumor.
Footnotes
Ethics approval: The study was reviewed and approved by the Institutional Review Board of Kyushu University (IRB#25-191).
Informed consent: Informed consent was not obtained, but this study is not an interventional study and we completely anonymized or omitted all the information that might disclose the identity of the subjects.
Conflict-of-interest: The authors declare that there are no conflicts of interest to disclose.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 13, 2014
First decision: October 29, 2014
Article in press: December 22, 2014
P- Reviewer: Boscá L, Ierardi E S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Delaunoit T, Neczyporenko F, Limburg PJ, Erlichman C. Pathogenesis and risk factors of small bowel adenocarcinoma: a colorectal cancer sibling? Am J Gastroenterol. 2005;100:703–710. doi: 10.1111/j.1572-0241.2005.40605.x. [DOI] [PubMed] [Google Scholar]
- 2.Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19:58–69. doi: 10.1016/j.annepidem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–526. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 4.Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer. 1999;86:2693–2706. doi: 10.1002/(sici)1097-0142(19991215)86:12<2693::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Elzagheid A, Emaetig F, Buhmeida A, Laato M, El-Faitori O, Syrjänen K, Collan Y, Pyrhönen S. Loss of MUC2 expression predicts disease recurrence and poor outcome in colorectal carcinoma. Tumour Biol. 2013;34:621–628. doi: 10.1007/s13277-012-0588-8. [DOI] [PubMed] [Google Scholar]
- 6.Lugli A, Zlobec I, Baker K, Minoo P, Tornillo L, Terracciano L, Jass JR. Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol. 2007;60:534–539. doi: 10.1136/jcp.2006.039552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res. 2000;6:1909–1916. [PubMed] [Google Scholar]
- 8.Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–477. doi: 10.1046/j.1440-1827.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 9.Patai AV, Molnár B, Tulassay Z, Sipos F. Serrated pathway: alternative route to colorectal cancer. World J Gastroenterol. 2013;19:607–615. doi: 10.3748/wjg.v19.i5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita K, Hirahashi M, Yamamoto H, Matsumoto T, Gushima M, Oda Y, Kishimoto J, Yao T, Iida M, Tsuneyoshi M. Mucin core protein expression in serrated polyps of the large intestine. Virchows Arch. 2010;457:443–449. doi: 10.1007/s00428-010-0959-8. [DOI] [PubMed] [Google Scholar]
- 11.Yao T, Takata M, Tustsumi S, Nishiyama K, Taguchi K, Nagai E, Tsuneyoshi M. Phenotypic expression of gastrointestinal differentiation markers in colorectal adenocarcinomas with liver metastasis. Pathology. 2002;34:556–560. doi: 10.1080/0031302021000035965-4. [DOI] [PubMed] [Google Scholar]
- 12.Ohji Y, Yao T, Eguchi T, Yamada T, Hirahashi M, Iida M, Tsuneyoshi M. Evaluation of risk of liver metastasis in colorectal adenocarcinoma based on the combination of risk factors including CD10 expression: multivariate analysis of clinicopathological and immunohistochemical factors. Oncol Rep. 2007;17:525–530. [PubMed] [Google Scholar]
- 13.Yao T, Tsutsumi S, Akaiwa Y, Takata M, Nishiyama K, Kabashima A, Tsuneyoshi M. Phenotypic expression of colorectal adenocarcinomas with reference to tumor development and biological behavior. Jpn J Cancer Res. 2001;92:755–761. doi: 10.1111/j.1349-7006.2001.tb01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenstein IL, Amid PK, Shulman AG. The iliopubic tract. The key to inguinal herniorrhaphy? Int Surg. 1990;75:244–246. [PubMed] [Google Scholar]
- 15.Fujita K, Yamamoto H, Matsumoto T, Hirahashi M, Gushima M, Kishimoto J, Nishiyama K, Taguchi T, Yao T, Oda Y. Sessile serrated adenoma with early neoplastic progression: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:295–304. doi: 10.1097/PAS.0b013e318205df36. [DOI] [PubMed] [Google Scholar]
- 16.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 17.Sobin LH, Gospodarowicz MK, Wittekind C. UICC TNM classification of Malignant tumors. 7th ed. New York: John Wiley & Sons; 2009. [Google Scholar]
- 18.Iwase T, Kushima R, Mukaisho K, Mitsufuji S, Okanoue T, Hattori T. Overexpression of CD10 and reduced MUC2 expression correlate with the development and progression of colorectal neoplasms. Pathol Res Pract. 2005;201:83–91. doi: 10.1016/j.prp.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda M, Johbu I, Yoshihiro S, Nobue K, Keiichi T, Naoki O, Yoshiyuki O. Availability of CD10 as a Histopathological Diagnostic Marker. Acta Histochem Cytochem. 2005;38:17–24. [Google Scholar]
- 20.Fujimoto Y, Nakanishi Y, Sekine S, Yoshimura K, Akasu T, Moriya Y, Shimoda T. CD10 expression in colorectal carcinoma correlates with liver metastasis. Dis Colon Rectum. 2005;48:1883–1889. doi: 10.1007/s10350-005-0141-6. [DOI] [PubMed] [Google Scholar]
- 21.Carl-McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Röcken C. The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol. 2004;25:1223–1232. [PubMed] [Google Scholar]
- 22.Yasugi A, Yashima K, Hara A, Koda M, Kawaguchi K, Harada K, Andachi H, Murawaki Y. Fhit, Mlh1, P53 and phenotypic expression in the early stage of colorectal neoplasms. Oncol Rep. 2008;19:41–47. [PubMed] [Google Scholar]
- 23.Koseki K. Subclassification of Well Differentiated Gastric Cancer with Reference to Biological Behavior and Malignancy, Gastric Type vs. Intestinal Type, and Papillary Carcinoma vs. Tublar Carcinoma. Stomach and Intestine. 1999;34:507–512. [Google Scholar]
- 24.Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Gastric or intestinal phenotypic expression in the carcinomas and background mucosa of multiple early gastric carcinomas. Histopathology. 2000;37:513–522. doi: 10.1046/j.1365-2559.2000.01008.x. [DOI] [PubMed] [Google Scholar]
- 25.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 26.Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 27.Jass JR. Mucin core proteins as differentiation markers in the gastrointestinal tract. Histopathology. 2000;37:561–564. doi: 10.1046/j.1365-2559.2000.01032.x. [DOI] [PubMed] [Google Scholar]
- 28.Arai T, Kasahara I, Sawabe M, Kanazawa N, Kuroiwa K, Honma N, Aida J, Takubo K. Microsatellite-unstable mucinous colorectal carcinoma occurring in the elderly: comparison with medullary type poorly differentiated adenocarcinoma. Pathol Int. 2007;57:205–212. doi: 10.1111/j.1440-1827.2007.02082.x. [DOI] [PubMed] [Google Scholar]
- 29.Fu T, Pappou EP, Guzzetta AA, Jeschke J, Kwak R, Dave P, Hooker CM, Morgan R, Baylin SB, Iacobuzio-Donahue CA, et al. CpG island methylator phenotype-positive tumors in the absence of MLH1 methylation constitute a distinct subset of duodenal adenocarcinomas and are associated with poor prognosis. Clin Cancer Res. 2012;18:4743–4752. doi: 10.1158/1078-0432.CCR-12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bläker H, Helmchen B, Bönisch A, Aulmann S, Penzel R, Otto HF, Rieker RJ. Mutational activation of the RAS-RAF-MAPK and the Wnt pathway in small intestinal adenocarcinomas. Scand J Gastroenterol. 2004;39:748–753. doi: 10.1080/00365520410005847. [DOI] [PubMed] [Google Scholar]
- 31.Schönleben F, Qiu W, Allendorf JD, Chabot JA, Remotti HE, Su GH. Molecular analysis of PIK3CA, BRAF, and RAS oncogenes in periampullary and ampullary adenomas and carcinomas. J Gastrointest Surg. 2009;13:1510–1516. doi: 10.1007/s11605-009-0917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arai M, Shimizu S, Imai Y, Nakatsuru Y, Oda H, Oohara T, Ishikawa T. Mutations of the Ki-ras, p53 and APC genes in adenocarcinomas of the human small intestine. Int J Cancer. 1997;70:390–395. doi: 10.1002/(sici)1097-0215(19970207)70:4<390::aid-ijc3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Michel S, Kloor M, Singh S, Gdynia G, Roth W, von Knebel Doeberitz M, Schirmacher P, Bläker H. Coding microsatellite instability analysis in microsatellite unstable small intestinal adenocarcinomas identifies MARCKS as a common target of inactivation. Mol Carcinog. 2010;49:175–182. doi: 10.1002/mc.20587. [DOI] [PubMed] [Google Scholar]
- 34.Warth A, Kloor M, Schirmacher P, Bläker H. Genetics and epigenetics of small bowel adenocarcinoma: the interactions of CIN, MSI, and CIMP. Mod Pathol. 2011;24:564–570. doi: 10.1038/modpathol.2010.223. [DOI] [PubMed] [Google Scholar]
- 35.Planck M, Ericson K, Piotrowska Z, Halvarsson B, Rambech E, Nilbert M. Microsatellite instability and expression of MLH1 and MSH2 in carcinomas of the small intestine. Cancer. 2003;97:1551–1557. doi: 10.1002/cncr.11197. [DOI] [PubMed] [Google Scholar]
- 36.Mangold E, Pagenstecher C, Friedl W, Fischer HP, Merkelbach-Bruse S, Ohlendorf M, Friedrichs N, Aretz S, Buettner R, Propping P, et al. Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol. 2005;207:385–395. doi: 10.1002/path.1858. [DOI] [PubMed] [Google Scholar]
- 37.Rashid A, Hamilton SR. Genetic alterations in sporadic and Crohn’s-associated adenocarcinomas of the small intestine. Gastroenterology. 1997;113:127–135. doi: 10.1016/s0016-5085(97)70087-8. [DOI] [PubMed] [Google Scholar]
- 38.Sutter T, Arber N, Moss SF, Findling RI, Neugut AI, Weinstein IB, Holt PR. Frequent K-ras mutations in small bowel adenocarcinomas. Dig Dis Sci. 1996;41:115–118. doi: 10.1007/BF02208591. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama K, Yao T, Yonemasu H, Yamaguchi K, Tanaka M, Tsuneyoshi M. Overexpression of p53 protein and point mutation of K-ras genes in primary carcinoma of the small intestine. Oncol Rep. 2002;9:293–300. [PubMed] [Google Scholar]


