Abstract
AIM: To investigate the efficacy of neoadjuvant chemoradiotherapy (NACRT) for resectability of locally advanced gastric cancer (LAGC).
METHODS: Between November 2007 and January 2014, 29 patients with LAGC (clinically T3 with distal esophagus invasion/T4 or bulky regional node metastasis) that were treated with NACRT followed by D2 gastrectomy were included in this study. Resectability was evaluated with radiologic and endoscopic exams before and after NACRT. Using three-dimensional conformal radiotherapy, patients received 45 Gy, with a daily dose of 1.8 Gy. The entire tumor extent and the regional metastatic lymph nodes were included in the gross tumor volume. Patients presenting with a resectable tumor after NACRT received a total or subtotal gastrectomy with D2 dissection. The pathologic tumor response was evaluated using Japanese Gastric Cancer Association histologic evaluation criteria. Postoperative morbidity was evaluated using the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.0. Overall survival (OS) and progression-free survival (PFS) rates were estimated using a Kaplan-Meier analysis and compared using the log-rank test.
RESULTS: All patients were assessed as unresectable cases. Twenty-four patients (24/29; 82.8%) showed LAGC on positron emission tomography-computed tomography (CT) and contrast-enhanced CT, whereas four patients (4/29; 13.8%) with vague invasion or abutment to an adjacent organ underwent diagnostic laparoscopy. One patient (1/29; 3.4%), initially assessed as a resectable case, underwent an “open and closure” after the tumor was found to be unresectable. Abutment to an adjacent organ (34.5%) was the most common reason for NACRT. The clinical response rate one month after NACRT was 44.8%. After NACRT, 69% (20/29) of patients had a resectable tumor. Of the 20 patients with a resectable tumor, 18 patients (62.1%) underwent a D2 gastrectomy. The R0 resection rate was 94.4% and two patients (2/18; 11.1%) showed a complete response. The median follow-up duration was 13.5 mo. The one-year OS and PFS rates were 72.4 and 48.9%, respectively. The one-year OS, PFS, local failure-free survival, and distant metastasis-free survival were higher in patients with a resectable tumor after NACRT (P < 0.001, P < 0.001, P < 0.001, and P = 0.078, respectively). No grade 3-4 late treatment-related toxicities or postoperative mortalities were observed.
CONCLUSION: NACRT with D2 gastrectomy showed a high rate of R0 resection and promising local control, which may increase the R0 resection opportunity resulting in survival benefit.
Keywords: Advanced gastric cancer, D2 gastrectomy, Neoadjuvant chemoradiotherapy, Combined modality therapy, Treatment outcome
Core tip: In locally advanced gastric cancer (LAGC), R0 resection is a well-established predictive factor. To achieve R0 resection, neoadjuvant approaches have been attempted. Studies of neoadjuvant chemotherapy followed by radical gastrectomy and D2 dissection have resulted in favorable outcomes and large studies of neoadjuvant chemotherapy are underway. Data on neoadjuvant chemoradiotherapy (NACRT) in LAGC are limited. This retrospective study was performed to investigate the efficacy of NACRT in LAGC. We found that NACRT increased the chance for an R0 resection and potentially enhanced survival in LAGC.
INTRODUCTION
Although the incidence of gastric cancer has decreased worldwide, it is still the second most common cancer and the third most common cause of cancer-related death in South Korea[1]. Even with the use of aggressive treatment, the five-year overall survival (OS) for advanced gastric cancer with adjacent organ invasion is < 15%[2]. To prevent recurrence and improve survival in gastric cancer patients, adjuvant treatments with radical surgery have been implemented. In European countries, perioperative chemotherapy is considered standard treatment based on the results from the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. In the United States, postoperative chemoradiotherapy (CRT) is considered standard treatment based on the Southwest Oncology Group 9008/Intergroup 0116 (SWOG 9008/INT 0116) trial[3,4].
Despite adjuvant treatment, the outcome of locally advanced gastric cancer (LAGC) remains poor[5]. To overcome the limitations of adjuvant treatment, a neoadjuvant approach has been investigated. In phase II clinical trials involving neoadjuvant chemotherapy (NAC), however, the outcomes were not satisfactory[6,7]. Therefore, the addition of radiotherapy to NAC (NACRT) was used to improve local control. In the Preoperative Chemotherapy or Radiochemotherapy in Esophagogastric Adenocarcinoma Trial (POET) where NACRT was compared with NAC, NACRT displayed a higher rate of pathologic response[8]. In the CRT for Oesophageal Cancer Followed by Surgery Study (CROSS) trial, which compared NACRT followed by surgery versus surgery alone, NACRT followed by surgery resulted in improved survival[9]. The previous trials, however, did not include advanced gastric disease, and the efficacy of NACRT in LAGC thus remains unclear.
In East Asia, radical gastrectomy and D2 dissection (D2 gastrectomy) are considered standard surgical procedures. The Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) trial failed to demonstrate a survival benefit of adjuvant CRT in patients treated with a D2 gastrectomy; therefore, postoperative chemotherapy is currently considered the standard treatment[10]. Clinical trials evaluating NAC followed by D2 gastrectomy are underway. Nevertheless, conclusive data on NACRT in LAGC have not been established so far. The aim of the present study was to investigate the role of NACRT in the treatment of LAGC in Asian patients. We also evaluated postoperative morbidity following NACRT.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the medical records of patients who were treated with NACRT between November 2007 and January 2014. Gastric cancer was histologically confirmed via endoscopic biopsy in all patients. Endoscopic and radiologic exams were used to evaluate resectability before and after NACRT. In the case of tumors with definite invasion or abutment to an adjacent organ, diagnostic laparoscopy was omitted. When the invasion or abutment was vague, diagnostic laparoscopy was implemented. The institutional review board of the Yonsei University College of Medicine approved this study.
Treatment
The NAC regimen for each patient was determined by a medical oncologist. All patients underwent three-dimensional conformal radiotherapy (RT). Gross tumor volume (GTV) was delineated using enhanced CT, positron emission tomography (PET)-CT, and endoscopic images. If the tumor abutted adjacent organs or extended to the esophagogastric junction or adjacent organs, the entire tumor extent was included in the GTV. Regional metastatic lymph nodes were included in the GTV. Clinical target volume was GTV plus a 5-10 mm margin, and the planning target volume was the clinical target volume plus a 7 mm margin. The radiation dose was 45 Gy with a daily dose of 1.8 Gy. The liver, kidneys, spinal cord, and small bowel were delineated as organs at risk.
The standard surgical procedure was total or subtotal gastrectomy with D2 dissection. When resection of an adjacent organ was inevitable, a combined resection was performed. Adjuvant chemotherapy was used. If the tumor was unresectable after NACRT, sequential chemotherapy was used.
Evaluation of response and treatment-related toxicity
After NACRT, all patients received a radiologic exam to assess tumor resectability and response. When resectability was difficult to assess using a radiologic approach, an endoscopic exam and diagnostic laparoscopy were used. The pathologic tumor response was evaluated using the Japanese Gastric Cancer Association (JGCA) histologic evaluation criteria, which are as follows: Grade 1a, viable tumor cells more than 2/3 of the tumorous area; Grade 1b, viable tumor cells more than 1/3, but less than 2/3, of the tumorous area; Grade 2, viable tumor cells less than 1/3 of the tumorous area; Grade 3, no viable tumor cells remain. We evaluated treatment-related toxicity with the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
The statistical methods used on this study were reviewed by Ha Yan Kim, a biostatistician, in Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, South Korea. χ2 tests and Fisher’s exact tests were used for comparing D2 gastrectomy group with no D2 gastrectomy group in patient and tumor characteristics. OS and progression-free survival (PFS) were calculated using the Kaplan-Meier method and compared by using a log-rank test. A P < 0.05 was considered statistically significant. All statistical analyses were performed by using SPSS version 20.0 (IBM Corp., Armonk, NY, United States).
RESULTS
Clinical characteristics
A total of 29 patients were analyzed with a median age of 53 year (range: 40-74 year). All patients were assessed as unresectable cases. Twenty-four patients (24/29; 82.8%) showed LAGC on a PET-CT and contrast-enhanced CT prior to NACRT administration. Four patients (4/29; 13.8%) with vague invasion or abutment to an adjacent organ underwent a diagnostic laparoscopy. One patient (1/29; 3.4%) underwent an “open and closure” due to tumor infiltration into the perigastric area and distal esophagus. Patient data and tumor characteristics are summarized in Table 1. The lower portion of the stomach was the most common tumor location (11/29; 37.9%). All patients had locally advanced disease characterized as clinically T3 with distal esophagus invasion/T4 or bulky regional node metastasis (Figure 1). The clinical T stage was from T3 to T4b. Six patients (6/29; 15.8%) had clinically N0 disease and 23 patients (23/29; 79.3%) had clinically N1-3 disease.
Table 1.
Patient and tumor characteristics n (%)
| Characteristics | Total | D2 gastrectomy | No D2 gastrectomy | P value |
| Age (in years) | 53 (40-74) | 54 (42-74) | 53 (40-69) | 0.929 |
| Sex | 0.362 | |||
| Male | 23 (79.3) | 13 (72.2) | 10 (90.9) | |
| Female | 6 (20.7) | 5 (27.8) | 1 (9.1) | |
| Performance status | > 0.999 | |||
| ECOG 0 | 2 (6.9) | 1 (5.6) | 1 (9.1) | |
| ECOG 1 | 27 (93.1) | 17 (94.4) | 10 (90.9) | |
| Tumor location | 0.172 | |||
| Upper | 7 (24.1) | 2 (11.1) | 5 (45.4) | |
| Mid | 0 (0) | 0 (0) | 0 (0) | |
| Lower | 11 (37.9) | 9 (50) | 2 (18.2) | |
| Upper-Mid | 3 (10.3) | 3 (16.7) | 0 (0) | |
| Mid-Lower | 0 (0) | 0 (0) | 0 (0) | |
| Upper-Lower | 8 (27.6) | 4 (22.2) | 4 (36.4) | |
| Pathology | 0.257 | |||
| Adenocarcinoma | 19 (65.5) | 13 (72.2) | 6 (54.5) | |
| Signet ring cell carcinoma | 6 (20.7) | 2 (11.1) | 4 (36.4) | |
| Adeno-signet-ring cell carcinoma | 4 (13.8) | 3 (16.7) | 1 (9.1) | |
| Differentiation | 0.619 | |||
| Well differentiated | 3 (13) | 2 (12.5) | 1 (14.3) | |
| Moderately differentiated | 10 (43.5) | 6 (37.5) | 4 (57.1) | |
| Poorly differentiated | 10 (43.5) | 8 (50) | 2 (28.6) | |
| Bormann type | 0.311 | |||
| 1 | 1 (3.4) | 0 (0) | 1 (9.1) | |
| 2 | 3 (10.3) | 3 (16.7) | 0 (0) | |
| 3 | 22 (75.9) | 13 (72.2) | 9 (81.8) | |
| 4 | 3 (10.3) | 2 (11.1) | 1 (9.1) | |
| AJCC Clinical T stage | 0.385 | |||
| T3 | 2 (6.9) | 2 (11.1) | 0 (0) | |
| T4a | 20 (69) | 11 (61.1) | 9 (81.8) | |
| T4b | 7 (24.1) | 5 (27.8) | 2 (18.2) | |
| AJCC Clinical N stage | 0.177 | |||
| N0 | 6 (20.7) | 2 (11.1) | 4 (36.4) | |
| N1 | 6 (20.7) | 4 (22.2) | 2 (18.2) | |
| N2 | 16 (55.2) | 12 (66.7) | 4 (36.4) | |
| N3a | 1 (3.4) | 0 (0) | 1 (9.1) | |
| AJCC Clinical Stage | 0.741 | |||
| 2b | 5 (17.2) | 2 (11.1) | 3 (27.3) | |
| 3a | 3 (10.3) | 2 (11.1) | 1 (9.1) | |
| 3b | 18 (62.1) | 12 (66.7) | 6 (54.5) | |
| 3c | 3 (10.3) | 2 (11.1) | 1 (9.1) |
AJCC: American joint committee on cancer; ECOG: Eastern cooperative oncology group. Results are presented as median with range in parentheses and number with column percentages in parentheses.
Figure 1.
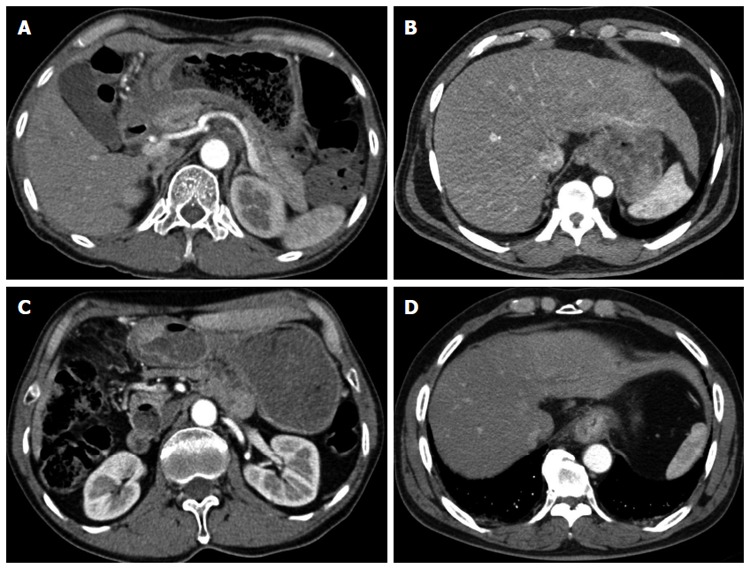
Computed tomography images of locally advanced gastric cancer. A: Direct tumor invasion of an adjacent organ; B: Tumor abutment with an adjacent organ; C: Bulky regional node metastasis; D: Invasion of the distal esophagus.
NACRT was most commonly administered due to abutment with an adjacent organ (10/29; 34.5%), while invasion of an adjacent organ (8/29; 27.6%) was the second most common reason (Table 2).
Table 2.
Cause for neoadjuvant chemoradiotherapy administration n (%)
| Cause | Value |
| Invasion of distal esophagus | 6 (20.7) |
| Abutment to adjacent organ | 10 (34.5) |
| Invasion of adjacent organ | 8 (27.6) |
| Extensive node metastasis | 4 (13.8) |
| Other | 1 (3.4) |
Treatment response and resectability after NACRT
Of the 29 patients, six (20.7%) received induction chemotherapy (ICT) prior to NACRT, and 23 (79.3%) received NACRT as their first treatment. The ICT regimens were TS-1/cisplatin (33.3%) or docetaxel/cisplatin/5-FU (66.7%). The NACRT regimen was heterogeneous. Approximately 76% of patients received cisplatin-based NACRT: TS-1/cisplatin (13/29; 44.8%), 5-FU/cisplatin (9/29; 31%).
Following ICT, all patients showed stable disease on radiologic or endoscopic exams. The first radiologic or endoscopic evaluation was performed within the first month after NACRT. The clinical response was as follows: 44.8% (13/29) of patients exhibited a partial response, 41.4% (12/29) of patients had stable disease, and 13.8% (4/29) of patients presented with progressive disease (PD). Twenty patients (20/29; 69.0%) presented with a resectable tumor after NACRT (Figure 2). Of the 20 patients, four received ICT and NACRT and 16 received NACRT as their first treatment. The patients presenting with a resectable tumor after NACRT had similar clinical characteristics to those with an unresectable tumor after NACRT.
Figure 2.
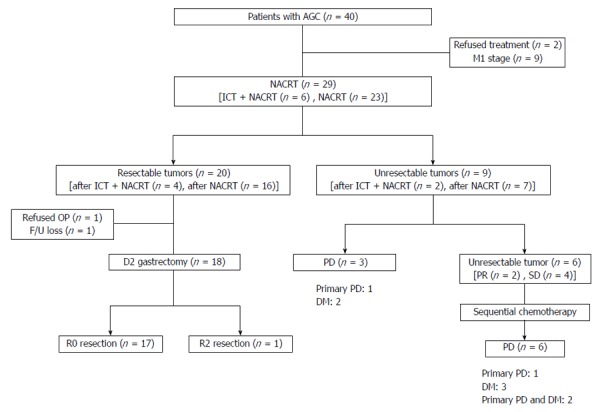
Treatment flow chart. AGC: Advanced gastric cancer; DM: Distant metastasis; F/U: Follow-up; ICT: Induction chemotherapy; NACRT: Neoadjuvant chemoradiotherapy; OP: Operation; PD: Progressive disease; PR: Partial response; SD: Stable disease.
In total, 18/29 (62%) patients underwent a D2 gastrectomy. The median interval between NACRT and surgery was 2.9 mo (range: 2.1-8.0 mo). Seven patients (7/18; 38.9%) received a subtotal gastrectomy and 11 patients (11/18; 61.1%) received a total gastrectomy. Of the 18 patients who underwent surgery, 17 (94.4%) patients underwent an R0 resection. One patient underwent an R2 resection due to peritoneal carcinomatosis.
A radiologic exam revealed a single metastatic lesion on the liver in one patient who exhibited clinical PD after NACRT. The lesion was completely dissected by wedge resection during the gastrectomy. There were no viable tumor cells in the liver specimen upon a pathologic evaluation.
Tumor pathologic response according to the JGCA histologic evaluation criteria was evaluable in 14 patients; pathologic responses of Grade 1a, 1b, 2, and 3 were observed in 4 (28.6%), 3 (21.4%), 5 (35.7%), and 2 (14.3%) patients, respectively.
After completion of NACRT, nine patients still had an unresectable tumor. Three patients presented with PD: one patient with a primary PD and two patients with distant metastases (DM). Although two patients exhibited partial response and four patients had stable disease, they were classified as unresectable and received sequential chemotherapy. Upon evaluation after sequential chemotherapy, one patient exhibited PD in the primary lesion, three patients had DM, and two patients had PD in the primary lesion and synchronous DM (Figure 2).
Survival and patterns of failure
The median follow-up duration was 13.5 mo (range: 2.1-79.8 mo). The one-year OS of all patients was 72.4% and the median OS was 21.2 mo. Of the 18 patients who underwent a radical surgery, the one-year OS was 87.7%, and the median OS was 34.3 mo. The one-year OS of the 11 patients who did not receive a radical surgery was 45%, and the median OS was 11.5 mo (P < 0.001) (Figure 3A).
Figure 3.
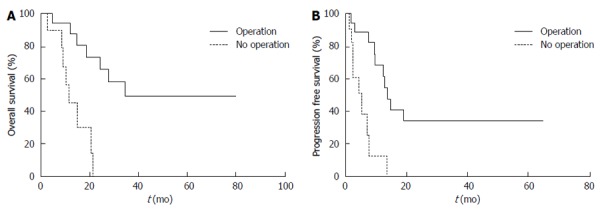
Patient survival following neoadjuvant chemoradiotherapy with or without surgery. A: Overall survival; B: Progression-free survival.
The one-year PFS of all patients was 48.9% and the median time to progression was 9.9 mo. The one-year PFS of the 18 patients who received a radical surgery was 68.4%, and the median PFS was 13.8 mo. The one-year PFS of the 11 patients who did not receive a radical surgery was 12.8%, and the median PFS was 5.2 mo (P < 0.001) (Figure 3B).
Recurrence after surgery or disease progression without surgery was observed in 19/29 patients (65.5%): two patients (6.9%) had local failure (LF) only, four patients (13.8%) had LF and synchronous DM, and 13 patients (44.8%) had DM only. Peritoneal carcinomatosis was observed in seven patients (7/29; 24.1%). LF did not occur in patients who received an R0 resection, and the nine documented recurrences in those patients were DM only: lung (n = 1), liver (n = 3), liver and pancreas (n = 1), peritoneal carcinomatosis (n = 3), and neck node (n = 1). Of the 12 patients who did not receive an R0 resection, six patients (50.0%) had LF.
The one-year LF-free survival (LFFS) was 75.9% and the median time to LF has not yet been reached. The one-year DM-free survival (DMFS) was 57.9% and the median time to DM was 12.9 mo. The one-year LFFS and DMFS of patients who underwent radical surgery were higher than those patients who did not receive radical surgery (one-year LFFS: 100% vs 27.3%; P < 0.001; and one-year DMFS: 68.4% vs 33.8%; P = 0.078). The difference in DMFS, however, was not significant.
Treatment-related toxicity
All patients completed NACRT. Six patients (6/29; 20.7%) presented with acute NACRT-related toxicities greater than grade 3, including leukopenia, nausea, anorexia, epigastric pain, and dysphagia. The grade 4 toxicity was dizziness. The most common toxicity was gastrointestinal toxicity (grade 1-2: nausea, anorexia, epigastric pain, dysphagia). Late NACRT-related toxicity greater than grade 3 did not occur.
The median postoperative hospital stay was 8 d (range: 6-27 d). Five (27.8%) of the 18 patients who received radical surgery had acute postoperative morbidity. Most of the morbidity was grade 1. Three patients had grade 1 serous drainage, one patient had a grade 1 wound seroma, and one patient had a grade 1 fever. One patient developed hypovolemic shock and pleural effusion, and recovered after hydration and antibiotic therapy. Seven days after surgery, the patient developed an abdominal abscess induced by a pancreatic fistula. Ten days after surgery, the patient presented with a wound dehiscence and underwent re-suturing. There was no late postoperative morbidity or treatment-related mortality. Any treatment related toxicity did not cause drop out from planned surgery.
DISCUSSION
This study showed a high rate of R0 resection and favorable pathologic response in Asian patients with LAGC using NACRT followed by D2 gastrectomy. Patients who underwent curative surgery showed superior outcomes when compared to those who did not undergo surgery[11]. However, radical surgery in LAGC patients is limited due to the high risk for postoperative morbidity and mortality and a low rate of R0 resection[2]. In previous prospective studies of NACRT, NACRT-treated patients showed a higher rate of pathologic complete response and pathologic response when compared with NAC. Moreover, the R0 resection rate was also higher in patients treated with NACRT[8,12]. R0 resection has been reported to be a predictive factor for survival. Klautke et al[13] reported a two-year survival rate of 42% in R0 resected patients, whereas R1 resected patients and those that were not operated on had a survival rate of 0%. In this study, 20 patients (69%) were found to have a resectable tumor after NACRT and the R0 resection rate was 94.4%. Although there is no definite standard for resectability at diagnosis of LAGC, all patients in this study had locally far advanced disease (clinically T3/4 and/or bulky metastatic lymph nodes), and initial resectability was lower than that published in previous studies; the R0 resection rate was comparable with previous studies[11,14-16].
The major pathologic response rate after NACRT was higher than that of NAC. The major pathologic response rate ranged from 15% to 39% in previous NAC studies[5-7]. In the present study, the pathologic response of JGCA Grade 1b and above was 71.4% and two patients had pathologic complete response.
One of the main reasons for the addition of RT to NAC is local control. In this study, we also observed promising local control. A phase III clinical trial recently reported on the pattern of recurrence after NACRT[12]. NACRT significantly reduced loco-regional recurrence from 34% to 14%, with only a 1% in-field recurrence. In a Japanese pilot study, no LF was observed after NACRT[17]. Other studies have also reported low LF and high local control rates after NACRT[11,13]. In this study, the one-year LFFS was 100% and no LF was observed during the follow-up period in the patients who received NACRT followed by radical surgery.
Peritoneal carcinomatosis occurred in 24.1% of the patients, which is lower than previously published[18-20]. Inoue et al[17] explained the reason for this low rate of peritoneal carcinomatosis. After the tumor cells exposed to the peritoneum or near lymphatics were treated with NACRT, they underwent necrosis and were covered with fibrous tissue. The fibrous tissue may prevent the spread of tumor cells in the peritoneum. Therefore, patients treated with NACRT developed peritoneal carcinomatosis less frequently than those without NACRT.
DM represented the major pattern of recurrence in the present study. Seventeen patients (58.6%) had DM. To the best of our knowledge, there has not yet been a comparative study to determine which chemotherapy sequence is more effective, ICT or adjuvant chemotherapy. We can only suggest that more intensive systemic chemotherapy is needed to reduce DM. The results of ongoing phase III studies of NAC will provide further insight (JCOG0501; NCT00252161, PRODIGY; NCT01515748).
In the current study, all patients completed NACRT and did not suffer from treatment-related death. Acute postoperative complications were manageable and no late postoperative morbidity or mortality was observed. Although surgeons are often concerned about postoperative morbidity and mortality following NACRT, a phase III NACRT trial showed no difference in the rate of postoperative morbidity between the treatment arms, and recent NACRT studies have reported tolerable toxicity and safety[5,9,11,17,21].
In previous studies, grade 3-4 postoperative morbidity ranged from 0 to 71.4%[13,17,21-25]. Only one patient, who developed an abdominal abscess, presented with grade 3 postoperative morbidities (hypovolemic shock, pneumonia, wound dehiscence) in this study.
Grade 3-4 postoperative mortality ranging from 0 to 12% has been documented in previous studies[13,17,21-26]. In the Japan Clinical Oncology Group 9501 trial, which compared D2 and D3 gastrectomy, the mortality rate was 0.8%. In our study, all patients who received radical surgery underwent radical node dissection, but treatment-related mortality did not occur.
We compared clinical response after NACRT with the pathologic response. Out of 18 patients, 9 (50%) showed discordance between the clinical and pathologic response. Six patients had a better pathologic response, whereas three patients had a worse pathologic response when compared with the clinical response. One patient with clinical stable disease had pathologic PD (peritoneal carcinomatosis) that was not found on the radiologic exam prior to surgery. Despite improvement of radiologic imaging techniques, evaluations with radiologic exams have a limited accuracy. Leake et al[27] highlighted the substantial value of diagnostic laparoscopy in staging patients with gastric cancer, even in the era of advanced radiologic techniques. Trip et al[21] emphasized the need for accurate staging of peritoneal carcinomatosis before radical surgery. Both studies recommended diagnostic laparoscopy in LAGC patients.
Our study had a few limitations. This was a small retrospective study that included heterogeneous chemotherapy regimens. The follow-up duration was relatively short compared to previous studies. In some patients, the resectability at diagnosis was determined using radiologic imaging only and a defined standard for resectability has not been established. Therefore, the initial resectability had limited accuracy.
Despite the limitations mentioned above, NACRT followed by D2 gastrectomy in LAGC patients showed a favorable pathologic response and promising local control. NACRT in LAGC may increase the opportunity for an R0 resection and, thus, for survival. A large prospective study of NACRT followed by D2 gastrectomy is needed to validate the efficacy of this approach.
COMMENTS
Background
In locally advanced gastric cancer (LAGC), R0 resection is a well-established predictive factor for survival. To achieve R0 resection, neoadjuvant approaches have been attempted. Studies of neoadjuvant chemotherapy followed by radical gastrectomy and D2 dissection (D2 gastrectomy) showed favorable outcomes, and large studies of NAC are currently in progress.
Research frontiers
Studies on the efficacy of neoadjuvant chemoradiotherapy (NACRT) in LAGC patients are limited. In this study, the authors document the efficacy of NACRT in an Asian cohort with LAGC.
Innovations and breakthroughs
Tumor resectability was evaluated using radiologic, endoscopic and laparoscopic exams before and after NACRT administration. Patients diagnosed with unresectable LAGC were treated with NACRT followed by D2 gastrectomy. Patients received 45 Gy radiation with a daily dose 1.8 Gy. After NACRT, resectable tumors were observed in 69% of patients. The one-year overall and progression-free survival rates were higher in patients with a resectable tumor after NACRT. NACRT followed by D2 gastrectomy in LAGC yielded a higher pathologic response than that of previous NAC studies. NACRT also showed a high rate of R0 resection and local control with tolerable toxicities and no treatment-related mortality. NACRT in LAGC may increase the opportunity for R0 resection resulting in a survival benefit.
Applications
This study showed that NACRT in LAGC patients may increase the likelihood for R0 resection and potentially improve survival. The results presented in this study may serve as a foundation for a prospective study on NACRT.
Terminology
D2 gastrectomy refers to total or subtotal gastrectomy and a D2 dissection. In East Asia, a D2 gastrectomy is the standard surgical procedure for advanced gastric cancer.
Peer-review
In this study, the authors evaluated the efficacy of NACRT on the resectability of LAGC in 29 patients that were assessed (via radiologic and endoscopic examinations) as unresectable cases. NACRT in LAGC may increase the opportunity for R0 resection resulting in a survival benefit. Although this is a retrospective study, treatment of LAGC is a critical concern and the results are clearly demonstrated. The results from this study are of clinical relevance and may serve as the foundation for future prospective studies.
Footnotes
Ethics approval: The study was reviewed and approved by the Yonsei University Health System, Severance Hospital Institutional Review Board.
Informed consent: The study demonstrated minimal risk for included patients and received a waiver of informed consent from the Institutional Review Board.
Conflict-of-interest: All authors certify that no actual or potential conflict-of-interest in relation to this article exists.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 12, 2014
First decision: October 29, 2014
Article in press: December 16, 2014
P- Reviewer: Luzza F, Tiberio GAM S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng CT, Tsai CY, Hsu JT, Vinayak R, Liu KH, Yeh CN, Yeh TS, Hwang TL, Jan YY. Aggressive surgical approach for patients with T4 gastric carcinoma: promise or myth? Ann Surg Oncol. 2011;18:1606–1614. doi: 10.1245/s10434-010-1534-x. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa T, Omura K, Kobayashi O, Nashimoto A, Takabayashi A, Yamada T, Yamaue H, Fujii M, Yamaguchi T, Nakajima T. A phase II study of preoperative chemotherapy with S-1 plus cisplatin followed by D2/D3 gastrectomy for clinically serosa-positive gastric cancer (JACCRO GC-01 study) Eur J Surg Oncol. 2010;36:546–551. doi: 10.1016/j.ejso.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita T, Sasako M, Sano T, Katai H, Furukawa H, Tsuburaya A, Miyashiro I, Kaji M, Ninomiya M. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002) Gastric Cancer. 2009;12:37–42. doi: 10.1007/s10120-008-0496-1. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–1022. doi: 10.1002/bjs.6665. [DOI] [PubMed] [Google Scholar]
- 8.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 9.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 11.Hu JB, Sun XN, Gu BX, Wang Q, Hu WX. Effect of intensity modulated radiotherapy combined with s-1-based chemotherapy in locally advanced gastric cancer patients. Oncol Res Treat. 2014;37:11–16. doi: 10.1159/000358164. [DOI] [PubMed] [Google Scholar]
- 12.Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, van der Sangen MJ, Beukema JC, Rütten H, Spruit PH, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385–391. doi: 10.1200/JCO.2013.51.2186. [DOI] [PubMed] [Google Scholar]
- 13.Klautke G, Foitzik T, Ludwig K, Ketterer P, Klar E, Fietkau R. Neoadjuvant radiochemotherapy in locally advanced gastric carcinoma. Strahlenther Onkol. 2004;180:695–700. doi: 10.1007/s00066-004-9194-z. [DOI] [PubMed] [Google Scholar]
- 14.Wydmański J, Suwinski R, Poltorak S, Maka B, Miszczyk L, Wolny E, Bielaczyc G, Zajusz A. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol. 2007;82:132–136. doi: 10.1016/j.radonc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 16.Valenti V, Hernandez-Lizoaín JL, Beorlegui MC, Diaz-Gozalez JA, Regueira FM, Rodriguez JJ, Viudez A, Sola I, Cienfuegos JA. Morbidity, mortality, and pathological response in patients with gastric cancer preoperatively treated with chemotherapy or chemoradiotherapy. J Surg Oncol. 2011;104:124–129. doi: 10.1002/jso.21947. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Yachida S, Usuki H, Kimura T, Hagiike M, Okano K, Suzuki Y. Pilot feasibility study of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced gastric cancer featuring adjacent tissue invasion or JGCA bulky N2 lymph node metastases. Ann Surg Oncol. 2012;19:2937–2945. doi: 10.1245/s10434-012-2332-4. [DOI] [PubMed] [Google Scholar]
- 18.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 19.Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono H, Nagahori Y, Hosoi H, Takahashi M, Kito F, et al. Comparison of surgical results of D2 versus D3 gastrectomy (para-aortic lymph node dissection) for advanced gastric carcinoma: a multi-institutional study. Ann Surg Oncol. 2006;13:659–667. doi: 10.1245/ASO.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 21.Trip AK, Poppema BJ, van Berge Henegouwen MI, Siemerink E, Beukema JC, Verheij M, Plukker JT, Richel DJ, Hulshof MC, van Sandick JW, et al. Preoperative chemoradiotherapy in locally advanced gastric cancer, a phase I/II feasibility and efficacy study. Radiother Oncol. 2014;112:284–288. doi: 10.1016/j.radonc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Díaz-González JA, Rodríguez J, Hernández-Lizoain JL, Ciérvide R, Gaztañaga M, San Miguel I, Arbea L, Aristu JJ, Chopitea A, Martínez-Regueira F, et al. Patterns of response after preoperative treatment in gastric cancer. Int J Radiat Oncol Biol Phys. 2011;80:698–704. doi: 10.1016/j.ijrobp.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Allal AS, Zwahlen D, Bründler MA, de Peyer R, Morel P, Huber O, Roth AD. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2005;63:1286–1289. doi: 10.1016/j.ijrobp.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 25.Fujitani K, Ajani JA, Crane CH, Feig BW, Pisters PW, Janjan N, Walsh GL, Swisher SG, Vaporciyan AA, Rice D, et al. Impact of induction chemotherapy and preoperative chemoradiotherapy on operative morbidity and mortality in patients with locoregional adenocarcinoma of the stomach or gastroesophageal junction. Ann Surg Oncol. 2007;14:2010–2017. doi: 10.1245/s10434-006-9198-2. [DOI] [PubMed] [Google Scholar]
- 26.Balandraud P, Moutardier V, Giovannini M, Giovannini MH, Lelong B, Guiramand J, Magnin V, Houvenaeghel G, Delpero JR. Locally advanced adenocarcinomas of the gastric cardia: results of pre-operative chemoradiotherapy. Gastroenterol Clin Biol. 2004;28:651–657. doi: 10.1016/s0399-8320(04)95043-9. [DOI] [PubMed] [Google Scholar]
- 27.Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, Law C, Coburn NG. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S38–S47. doi: 10.1007/s10120-011-0047-z. [DOI] [PubMed] [Google Scholar]


