Abstract
AIM: To determine the optimal type of surgery for late-stage gastric cancer with hepatic metastases.
METHODS: We retrospectively analyzed 49 gastrectomies for late-stage gastric cancer conducted in the First Hospital Affiliated to Henan University of Science and Technology between September 2003 and September 2010. All gastrectomy operations were divided into two groups: radical resection (gastrectomy and simultaneous resection of hepatic metastases, n = 31), and palliative resection (gastrectomy without hepatic resection, n = 18). All 49 patients had chemotherapy catheter implantation in the hepatic artery via the gastroduodenal artery. Postoperative complications and cumulative survival rates of the two groups were compared and analyzed.
RESULTS: There was no significant difference in the number of perioperative complications between the radical and palliative resection groups (6 and 3 cases, respectively, P > 0.05). The incidence of long-term complications including ileus (3 in the radical resection and 2 in the palliative resection groups) and anastomosis (2 cases in each group) was not significantly different (P > 0.05). The cumulative survival rate was significantly lower in the palliative resection group (P < 0.05).
CONCLUSION: Radical gastrectomy with resection of hepatic metastases and hepatoarterial catheter implantation is the recommended surgery for late-stage gastric cancer patients with hepatic metastases.
Keywords: Gastric cancer, Hepatic metastases, Cumulative survival curve, Radical gastrectomy, Palliative gastrectomy
Core tip: Late-stage gastric cancer with liver metastases is difficult to treat surgically. We developed a new surgical procedure that included radical resection of gastric cancer and liver metastases, followed by implantation of a hepatoarterial catheter for postoperative infusion chemotherapy. Hepatoarterial infusion chemotherapy is a common procedure for liver cancer. Systemic and infusion chemotherapy resulted in a better cumulative survival rate in our study. We suggest that radical resection of gastric cancer and liver metastases with hepatoarterial catheter implantation is a better choice for late-stage gastric cancer.
INTRODUCTION
Gastric cancer is a common cancer worldwide and accounts for 5.2% of all cancer deaths. In China, more than half the cases of gastric cancer are advanced when patients first present with abdominal symptoms. Hepatic metastasis is common in advanced gastric cancer and often results in death. The surgical approach to advanced gastric cancer with hepatic metastases remains debatable. Consequently, the aim of the current study was to determine the optimal type of surgery for patients with advanced gastric cancer and liver metastases.
MATERIALS AND METHODS
We conducted a retrospective study of all the operations performed in the First Hospital Affiliated to Henan University of Science and Technology, China for advanced gastric cancer with hepatic metastases from September 2003 to September 2010. All patients included in the study were diagnosed by pathological analysis and computed tomography. The exclusion criteria included patients suffering from gastric cancer without hepatic metastases, and those with gastrointestinal anastomosis or laparotomy only, without gastrectomy. Patients with gastric cancer with multiple metastases in both lobes of the liver were also excluded. The surgical approaches included radical gastrectomy with dissection of hepatic metastases or palliative gastrectomy only, without hepatectomy.
Forty-nine patients, diagnosed histologically, met the inclusion criteria. All patients were Child-Pugh Class A. Patients were divided into two surgical groups: radical resection (gastrectomy with resection of the hepatic metastases) and palliative resection (gastrectomy without hepatotomy). In the radical resection group, 13 patients had total gastrectomy, three had proximal gastrectomy, and 15 had distal gastrectomy. In the palliative resection group, six patients had total gastrectomy, five had proximal gastrectomy, and seven had distal gastrectomy. The liver metastases were classified according to The Japanese Gastric Cancer Association (Table 1)[1]. According to the classification, H-0 and H-3 did not meet our criteria. All 49 patients were H-1 and H-2 cases. In the radical resection group, there were 10 left lateral sectionectomies, five left hepatectomies, four right hepatectomies, and 12 irregular hepatectomies. All 49 patients had chemotherapy catheter implantation in the hepatic artery via the gastroduodenal artery. All patients had hepatic arterial infusion (HAI) chemotherapy with 5-fluorouracil (5-FU) and systemic chemotherapy with CF or XELOX following surgery. Surgery in both groups of patients was performed by two experienced surgeons.
Table 1.
Classification of hepatic metastases from gastric cancer proposed by the Japanese Gastric Cancer Association
| H-0 | No liver metastases |
| H-1 | Liver metastases limited to one lobe of the liver |
| H-2 | Isolated diverse metastases in both lobes of the liver |
| H-3 | Multiple distributed metastases in both lobe of the liver |
The characteristics of the two groups are listed in Table 2. The baseline characteristics were analyzed using the χ2 test and Student’s t test where appropriate. The between-group difference in sex was compared by Student’s t test, and the other factors were analyzed using Fisher’s exact test. The Kaplan-Meier test was used to analyze survival curves. All statistical analyses were conducted using SPSS version 16.0 statistical software and P ≤ 0.05 was considered statistically significant.
Table 2.
Baseline characteristics
| Radical resection group | Palliative resection group | P value | |
| Number of patients | 31 | 18 | N/A |
| Sex (M/F) | 19/12 | 13/5 | 0.541 |
| Age (yr) | 56.2 ± 14.3 (22-83) | 59.1 ± 12.7 (34-79) | 0.632 |
| Liver metastases | 0.124 | ||
| H1 | 24 | 10 | |
| H2 | 7 | 8 | |
| Location of tumor | 0.892 | ||
| Gastric antrum | 15 | 7 | |
| Body of stomach | 8 | 5 | |
| Gastric fundus and cardia | 6 | 5 | |
| Whole stomach | 2 | 1 | |
| Pattern of gastrectomy | 0.255 | ||
| Proximal gastrectomy | 3 | 5 | |
| Distal gastrectomy | 15 | 7 | |
| Total gastrectomy | 13 | 6 | |
| Pattern of reconstruction | 0.251 | ||
| Billroth II | 12 | 5 | |
| Roux-en-Y | 16 | 8 | |
| Gastric remnant esophageal anastomose | 3 | 5 |
RESULTS
Of the 49 patients included in the present study, 46 had satisfactory recovery and good follow-up of 3-5 years, and three were lost. The follow-up of all patients was terminated in September 2013. The endpoint of the follow-up was death. There were no serious complications, perioperative hemorrhage, anastomotic leakage, perioperative deaths or postoperative hepatic dysfunction in either group. In the radical and palliative resection groups there were four and three cases of pneumonia, respectively; all of these patients suffered from fever and expectoration postoperatively, and all recovered well following administration of anti-inflammatory agents. In the radical resection group there were two cases of biliary leakage, however, as the leakages were not serious, they were discharged 10 and 14 d postoperatively, respectively, once leakage ended.
One serious long-term complication was postoperative ileus, with three cases in the radical resection group and two in the palliative resection group; however, all recovered well. There were two cases of anastomotic inflammation in each group, with complaints of repeated acid regurgitation, eructation, or bloating immediately after the operation. These complaints subsided following 2-4 wk treatment with Motilium and omeprazole. There was no significant difference between the two groups in the incidence of the above-mentioned postoperative complications (P = 0.503). The complications of the two groups are listed in Table 3.
Table 3.
Postoperative complications
| Radical resection group | Palliative resection group | P value | |
| Perioperative complications | 6 | 3 | 0.567 |
| Perioperative death | 0 | 0 | |
| Anastomotic leakage | 0 | 0 | |
| Liver failure | 0 | 0 | |
| Pneumonia | 4 | 3 | |
| Biliary leakage1 | 2 | 0 | |
| Long-term complication | 6 | 5 | 0.503 |
| Ileus | 3 | 2 | |
| Anastomosis2 | 2 | 2 | |
| Chemotherapy Catheter blockage | 1 | 1 |
Two cases in the radical resection group had biliary leakage. Both recovered and were discharged at 10 and 14 d postoperatively, once leakage stopped;
Two cases in each group suffered from anastomosis with moderate to severe discomfort. Both complained of acid regurgitation, eructation or bloating; both of which were alleviated after 2-4 wk domperidone and omeprazole.
The median survival rate was 2 and 1 year for the radical resection and palliative resection groups, respectively. The 1-, 3- and 5-year cumulative survival rate was 66.7%, 23.3% and 16.7%, respectively, for the radical resection group. Five patients in the radical resection group were still alive after 5 years follow-up and two of these patients were tumor free. The 1-, 2- and 3-year cumulative survival rate was 31.2%, 6.3% and 0%, respectively, for the palliative resection group. The cumulative survival rate between the two groups was significantly different (Figure 1, P = 0.002). One patient in the radical resection group presented with tumor recurrence 1 year postoperatively and consequently required a second operation, including total gastrectomy with six cycles of postoperative chemotherapy with docetaxel, cisplatin and 5-FU. This patient unfortunately suffered tumor recurrence again, with multiple liver metastases, 6 mo after the second operation and died 10 mo later.
Figure 1.
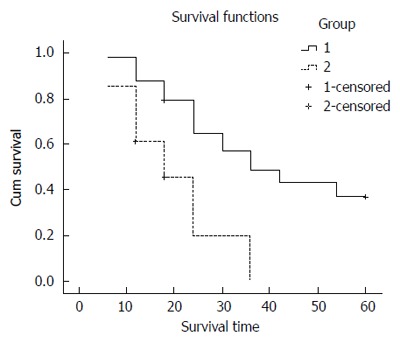
Cumulative survival rate for radical resection group (Group 1) and palliative resection group (Group 2). Significantly longer survival was observed for the radical resection group.
In the radical resection group, two patients died due to brain infarction 1 and 2 years postoperatively, and another patient died due to severe pneumonia and respiratory failure 4 years after the operation. All other deaths in both groups were due to tumor recurrence or metastases.
DISCUSSION
Advanced gastric cancer with liver metastases is associated with high mortality rates with 4%-14% of patients having liver metastases at their first diagnosis of gastric cancer following poor prognosis[2]. Controversy remains in almost every aspect of this field. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial[3] trial recommended perioperative chemotherapy because it could improve 5-year survival rate. However, the following study of EORTC[4] demonstrated no advantage in 5-year survival, with only an improvement of R0 resection rate. Controversy also existed in terms of the optimal surgical choice for advanced gastric cancer. Reports by Imamura et al[5] and Ambiru et al[6] of a 5-year survival rate of 0 and 5%, respectively, following extensive gastrectomy with hepatectomy due to advanced gastric cancer with liver metastases rendered this particular operation controversial. One of the reasons for such poor outcomes include the fact that some of the patients suffered from multiple or simultaneous extrahepatic metastases at the time of surgery[7], possibly increasing the difficulty of the operation[8]. Additional studies have focused on this dilemma with exciting results. Koga et al[9] reported a 5-year survival rate of 42% in patients that underwent extensive gastrectomy with hepatectomy. Lim et al[10] reported two patients with advanced gastric cancer with liver metastases who were alive without tumor recurrence 8 years after surgery. In addition, Munekage et al[11] reported an even longer survival time without tumor recurrence of 10 years following extensive gastrectomy with liver metastases resection. The present study evaluated whether extensive resection is a viable option for patients suffering from advanced gastric cancer with liver metastases.
Of the 49 operations for advanced gastric cancer analyzed in the present study, 31 patients underwent radical resection with hepatectomy and 18 patients underwent palliative resection without resection of liver metastases. The perioperative and long-term complications in both groups were similar. Radical resection did not result in increased incidence of severe complications such as perioperative death due to serious hemorrhage, serious biliary leakage, or severe inflammation. Long-term complications including intestinal obstruction and anastomotic inflammation were easily treatable and did not affect quality of life. There was however a significantly higher cumulative survival rate in the radical resection with hepatectomy group compared with the palliative resection group. Although 16.7% of the radical resection group, including two patients that did not experience tumor recurrence, survived > 5 years postoperatively, all patients in the palliative resection group without hepatectomy died within 3 years postoperatively. Such results are in accordance with previous studies[12].
HAI chemotherapy is an optimal choice for liver metastases because it derives its blood from the liver arterial system. 5-FU is a rational drug for HAI due to its short half-life, steep dose-response curve, high total body clearance, and high hepatic extraction. So, we routinely implanted a pump for HAI during and after the operation. HAI for liver metastases is also widely accepted, with a good outcome. The CALBG study shown a significant increase in survival time, with a longer time to hepatic progression for unresectable colorectal cancer[13]. For the radical resection patients, HAI with systemic chemotherapy also showed a significantly better outcome compared to systemic chemotherapy alone[14]. Power and Kemeny[15] also recommended combination of HAI and systemic chemotherapy in order to increase disease-free survival and hepatic disease-free survival.
The current study had several limitations including a systemic bias. Two different surgeons, with potentially varying opinions and criteria, conducted the operations in the two groups analyzed. This may have resulted in bias in the choice of surgery and thereby significantly skewed the results. It remains clear, regardless, that radical resection is a viable option for patients with liver metastases located in one lobe of the liver or in the same segment of the liver. It is important to note, however, that patients with > 4 metastases[16] or with metastases > 4 cm[17] remain at risk of poor prognosis.
In conclusion, the current study indicates that, if all liver metastases can be removed immediately, radical gastrectomy and hepatectomy with hepatoarterial catheter implantation is a viable option for late-stage gastric cancer with liver metastases. In addition, postoperative delivery of HAI chemotherapy is efficient and safe. Systemic chemotherapy with HAI chemotherapy after radical resection of gastric cancer may lead to better results.
COMMENTS
Background
Surgery for late-stage gastric cancer is controversial, especially when liver metastases are involved. Radical resection of gastric cancer and liver metastases with postoperative systemic chemotherapy cannot provide satisfactory results. Liver recurrence is always found soon after the operation and may lead to death later. Hepatoarterial infusion (HAI) chemotherapy is considered to be a better choice for liver cancer. So, the authors developed a new surgical approach for late-stage gastric cancer that included radical resection of gastric cancer and all metastases, with additional implantation of a hepatoarterial catheter for postoperative infusion chemotherapy.
Research frontiers
Many studies have focused on surgery and chemotherapy for gastric cancer. Which is the best surgical approach for late-stage gastric cancer has not reached a consensus yet. Chemotherapy regimen is also controversial. The hotspots in this field are to find a better surgical approach that leads to less recurrence and prolonged survival, especially, when liver metastases are present.
Innovations and breakthroughs
The authors developed a novel surgical approach to radically resect gastric cancer and liver metastases and associated lymph nodes, followed by implantation of a hepatoarterial catheter. Using the catheter, hepatoartery infusion chemotherapy was administered, which may be the main improvement in the procedure. HAI chemotherapy is common for liver cancer. This study showed good results after this surgery with systemic and infusion chemotherapy.
Applications
The simultaneous systemic and infusion chemotherapy can provide better results. The key point is the implantation of the hepatoarterial catheter during the operation.
Terminology
The authors defined late-stage gastric cancer as gastric cancer with liver metastases. Late-stage gastric cancer is difficult to treat and has a high rate of recurrence. Additional implantation of a hepato-arterial catheter makes infusion chemotherapy easy. This is a common method for liver cancer, which provides better results.
Peer-review
Treatment outcome of solid cancer might depend on the volume of tumor burden. Better survival in the group of radical surgery and intra-arterial chemotherapy than that in the group of palliative surgery and chemotherapy could be explained by the difference in tumor burden between both groups. Results of this study may provide useful information to oncologists who always prefer systemic chemotherapy for gastric cancer patients with liver metastasis.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 5, 2014
First decision: October 14, 2014
Article in press: December 8, 2014
P- Reviewer: Coccolini F, Nakajima T S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Wang CH
References
- 1.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 2.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, Kitamura H, Ikegami T, Miyagawa SI, Kawasaki S. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001;96:3178–3184. doi: 10.1111/j.1572-0241.2001.05278.x. [DOI] [PubMed] [Google Scholar]
- 6.Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, Shimizu Y, Nakajima N. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–283. doi: 10.1016/s0002-9610(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 7.Marrelli D, Roviello F, De Stefano A, Fotia G, Giliberto C, Garosi L, Pinto E. Risk factors for liver metastases after curative surgical procedures for gastric cancer: a prospective study of 208 patients treated with surgical resection. J Am Coll Surg. 2004;198:51–58. doi: 10.1016/j.jamcollsurg.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Tiberio GA, Coniglio A, Marchet A, Marrelli D, Giacopuzzi S, Baiocchi L, Roviello F, de Manzoni G, Nitti D, Giulini SM. Metachronous hepatic metastases from gastric carcinoma: a multicentric survey. Eur J Surg Oncol. 2009;35:486–491. doi: 10.1016/j.ejso.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, Yamaguchi T. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol. 2007;37:836–842. doi: 10.1093/jjco/hym113. [DOI] [PubMed] [Google Scholar]
- 10.Lim JK, Ahn JB, Cheon SH, Chang H, Jung JY, Rha SY, Roh JK, Noh SH, Kim HG, Chung HC, et al. Long-term survival after surgical resection for liver metastasis from gastric cancer: two case reports. Cancer Res Treat. 2006;38:184–188. doi: 10.4143/crt.2006.38.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munekage M, Okabayashi T, Hokimoto N, Sugimoto T, Maeda H, Namikawa T, Dabanaka K, Kobayashi M, Araki K, Hanazaki K. A case with synchronous multiple liver metastases from gastric carcinoma: postoperative long-term disease-free survival. Langenbecks Arch Surg. 2009;394:749–753. doi: 10.1007/s00423-008-0434-z. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Song MQ, Lin HZ, Hao LH, Jiang XJ, Li ZY, Chen YX. Chemotherapy and resection for gastric cancer with synchronous liver metastases. World J Gastroenterol. 2013;19:2097–2103. doi: 10.3748/wjg.v19.i13.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481) J Clin Oncol. 2006;24:1395–1403. doi: 10.1200/JCO.2005.03.8166. [DOI] [PubMed] [Google Scholar]
- 14.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 15.Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. Mol Cancer Ther. 2009;8:1015–1025. doi: 10.1158/1535-7163.MCT-08-0709. [DOI] [PubMed] [Google Scholar]
- 16.Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, Ikeda Y, Ezaki T, Fukuzawa S, Takenaka K, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepatogastroenterology. 2003;50:1560–1563. [PubMed] [Google Scholar]
- 17.Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, Katai H, Kosuge T, Sasako M. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95:534–539. doi: 10.1002/jso.20739. [DOI] [PubMed] [Google Scholar]


