Abstract
Disruption of the reconsolidation of conditioned fear memories has been suggested as a non-pharmacological means of preventing the return of learned fear in human populations. A reconsolidation update paradigm was developed in which a reconsolidation window is opened by a single isolated retrieval trial of a previously reinforced CS+ which is then followed by Extinction Training within that window. However, follow-up studies in humans using multi-methods fear conditioning indices (e.g., fear-potentiated startle, skin conductance, US-expectancy) have failed to replicate the retrieval + extinction effects. In the present study, we further investigated the retrieval + extinction reconsolidation update paradigm by directly comparing the acquisition, extinction, and return of fear-potentiated startle in the absence or presence of US-expectancy measures (using a trial-by-trial response keypad) with and without retrieval of a previously acquired CS-US association. Participants were fear conditioned to two visual cue CS+'s, one of which was presented as a single, isolated retrieval trial before Extinction Training and one that was extinguished as usual. The results show that the inclusion of US-expectancy measures strengthens the CS–US association to provide enhanced fear conditioning and maintenance of fear memories over the experimental sessions. In addition, in the groups that used on-line US-expectancy measures, the retrieval + extinction procedure reduced reinstatement of fear-potentiated startle to both previously reinforced CS+'s, as compared to the extinction as usual group.
Keywords: Fear extinction, Reconsolidation, Spontaneous recovery, Reinstatement, Fear-potentiated startle, US expectancy
1. Introduction
The fear-related symptoms of anxiety disorders such as panic disorder, specific phobia, and posttraumatic stress disorder (PTSD) have been conceptualized within the framework of fear conditioning (Bouton, Mineka, & Barlow, 2001; Friedman, 2000; Mineka & Zinbarg, 2006; Norrholm & Jovanovic, 2010; Wolpe & Rowan, 1988). Empirical evidence suggests that these symptoms can arise as a result of impaired fear inhibition (Jovanovic, Kazama, Bachevalier, & Davis, 2011; Jovanovic & Norrholm, 2011; Jovanovic et al., 2009, 2010), over-generalization of fear responses (Lissek, 2012), and/or dysregulation of fear extinction learning (Norrholm & Jovanovic, 2011; Norrholm, Anderson, et al., 2011; Norrholm, Jovanovic, et al., 2011) and extinction recall (Milad et al., 2008, 2009). According to the principles of fear conditioning, the traumatic event serves as an unconditioned stimulus (e.g., improvised explosive device detonation; termed the US) and previously neutral ambient cues (e.g., smoke, palm trees) in the trauma environment become associated with this “US” as a result of trauma exposure. As a result, intense fear reactions can develop to these cues such that the original fear memory is maintained over time even upon return to safety. From a clinical perspective, the most effective interventions for treating anxiety disorders are extinction-based exposure therapies in which the anxiety disorder patient is exposed to traumatic stimuli in the absence of noxious consequences (Rothbaum & Schwartz, 2002). Despite the success of these interventions, anxiety disorder symptoms can relapse following the completion of treatment (e.g., the clinical homolog of the laboratory observation of the return of fear; (Craske, 1999).
The return of conditioned fear can occur as a result of a change in context (termed renewal; (Bouton, 2004), through the passage of time (termed spontaneous recovery; (Bouton, 1993; Pavlov, 1927), or through re-exposure to the US (termed reinstatement; (Bouton & Bolles, 1979; Rescorla & Heth, 1975). Each of the latter phenomena can be observed by accessing the original fear memory that remains intact after new extinction learning has taken place. Current data in the literature strongly suggest that extinction is a form of new learning in which the organism learns that the CS is no longer associated with the US and that this new memory trace competes with the original fear memory trace (Bouton, 1993; Myers & Davis, 2002).
While a large number of studies suggest that the original fear memory (a consolidated CS–US association) remains intact following extinction learning, it is possible to return this memory into a labile state through the process of reconsolidation. Retrieval of the original fear memory during reconsolidation renders the memory vulnerable to disruption and its return to stability requires de novo protein and RNA synthesis (Dudai, 2004; Lee, 2009; Nader, Schafe, & LeDoux, 2000; Sara, 2000; Tronson & Taylor, 2007); this period of lability has been termed the reconsolidation window and has been suggested to last for several hours (Duvarci & Nader, 2004). Previous studies have demonstrated disruption of reconsolidation of the fear memory through pharmacological manipulation in both animals (e.g., inhibition of protein synthesis; as in (Nader, Schafe, & LeDoux, 2000) and, more recently, humans (e.g., b-adrenergic receptor antagonism; as in (Soeter & Kindt, 2011a). It is of great clinical interest to develop potential non-pharmacological mechanisms for disrupting reconsolidation of fear and several recent investigations have explored this possibility.
One potential non-pharmacological avenue for disrupting the reconsolidation of fear memories was initially investigated in rodents by Monfils, Cowansage, Klann, and LeDoux (2009) and involved the presentation of an isolated retrieval trial (to render the fear memory labile) followed by Extinction Training (to foster new learning of the now nonthreatening nature of the previously reinforced CS; (Monfils et al., 2009) during the reconsolidation time window. The rationale behind this model was that the isolated retrieval trial (reactivation of a previously reinforced CS) would result in a persistent revaluation of the CS as safe and, as such, weaken the previously acquired CS–US association. This weakening of the fear memory would then in turn prevent the return of fear through renewal, reinstatement, or spontaneous recovery. In their study, the Monfils group, in fact, showed that the reactivation of an isolated CS followed by Extinction Training during the reconsolidation window prevented the return of fear.
Due to the potential impact on the dogma of fear learning and the translation to clinical practice, the retrieval + extinction paradigm introduced by Monfils et al. (2009) has been further examined by other groups in several preclinical human studies using validated psychophysiological measures of fear, namely skin conductance responding and fear-potentiated startle. For example, using skin conductance, Schiller et al. (2010) showed that Extinction Training administered 10 min after a reactivated cue (during the reconsolidation window) prevented spontaneous recovery as compared to groups that underwent extinction 6 h after reactivation (outside the reconsolidation window) and to groups that did not receive a reactivated CS (Schiller et al., 2010). In addition, the Schiller et al. (2010) group demonstrated that the blockade of fear return was cue specific, extended to reinstatement as well as recovery, and persisted for up to a year. Oyarzun et al. (2012) replicated this finding by demonstrating that reinstatement of fear, as measured by skin conductance, to visual stimuli previously paired to an aversive auditory US could be attenuated by presenting an isolated retrieval trial 10 min prior to Extinction Training (Oyarzun et al., 2012). Using fear-potentiated startle measures coupled with skin conductance and on-line ratings of distress (2011) or US-expectancy (2013), Kindt and Soeter (Kindt & Soeter, 2013; Soeter & Kindt, 2011a) did not replicate the finding that return of fear is prevented when Extinction Training occurs within the reconsolidation window. Recently, Golkar, Bellander, Olsson, and Ohman (2012), using both skin conductance and fear-potentiated startle methods, presented both fear-relevant and fear-irrelevant cues in the Schiller et al. (2010) experimental design and did not replicate the disruption of reconsolidation effect either (Golkar et al., 2012).
As described by Schiller and Phelps (2011) and Auber, Tedesco, Jones, Monfils, and Chiamulera (2013), there are many contributing factors to consider with regard to the conditions under which the retrieval + extinction-induced disruption of reconsolidation can be observed (Auber et al., 2013; Schiller & Phelps, 2011). For example, differing methodological approaches have been employed to index fear conditioning (skin conductance only (Oyarzun et al., 2012; Schiller et al., 2010) vs. concurrent startle, skin conductance, and on-line ratings (Kindt & Soeter, 2013; Soeter & Kindt, 2011b) and in the selection of CS type (geometric shapes (Oyarzun et al., 2012; Schiller et al., 2010) vs. fear-relevant spider images (Soeter & Kindt, 2011b). In addition, there is evidence to suggest that individual differences among participants, such as genetic polymorphisms, may influence the effect of disruption of reconsolidation (Agren, Furmark, Eriksson, & Fredrikson, 2012). Further investigations of reconsolidation update mechanisms are certainly warranted given the inconclusive data reported to date, the potential boundary conditions that may explain reported discrepancies, and the compelling clinical potential of this type of paradigm.
The translation of the Monfils et al. (2009) study into the human preclinical arena (e.g., Kindt & Soeter, 2013) requires certain methodological adjustments in order to account for obvious species-specific differences in the expression of fear behavior as well as the means of effectively capturing these data. For example, human studies often include the use of verbal instructions and on-line ratings of participant US-expectancy, threat, or distress (Norrholm et al., 2006; Vervliet, Kindt, Vansteenwegen, & Hermans, 2010) to enhance learning and foster attention to the experimental contingencies. The inconclusive results observed to date in the study of reconsolidation update mechanisms may be explained, in part, by these procedural differences and the underlying neurobiological learning mechanisms accessed by these divergent procedures. Kindt and Soeter (2013) employed a multi-method approach that included the use of an on-line measure of US-expectancy; a behavior that recruits higher cortical brain regions involved in declarative knowledge (Weike, Schupp, & Hamm, 2007). The results reported by the Monfils et al. (2009) coupled with prior animal work on fear memory formation and reconsolidation (e.g., (Davis, 1997; Han et al., 2009; LeDoux, 2000; Nader et al., 2000)) implicate the amygdala as a potential anatomical substrate for previously observed retrieval + extinction effects. This possibility was directly investigated in humans by Agren, Furmark, et al. (2012) through the integration of brain imaging and skin conductance measures. Agren and others reported that retrieval + extinction with a 10-min, but not 6-h, interval between retrieval and extinction: (1) attenuated reinstatement of fear, (2) significantly reduced amygdala activity during fear memory retrieval, and (3) weakened coupling between the amygdala and other brain regions important for fear memory recall and return of fear (Agren, Engman, et al., 2012; Agren, Furmark, et al., 2012). Thus, it remains possible that the use of on-line ratings and subsequent recruitment of cortical areas disrupts the retrieval + extinction effect based in the amygdala.
The current study expands on recent investigations of reconsolidation update mechanisms by directly comparing the absence or presence of on-line US-expectancy measures during administration of the retrieval + extinction paradigm introduced by Monfils et al. (2009) translated to humans by Schiller et al. (2010) and subsequently investigated by several groups. The results will be discussed in terms of the effects of expectancy ratings on (a) fear acquisition and extinction and (b) extinction during reconsolidation.
2. Materials and methods
2.1. Participants
55 Subjects (20 males/35 females) with a mean age of 20.8 + 1.7 years old participated in the study after signing an informed consent form approved by the Emory University Institutional Review Board, the Atlanta VAMC Research and Development Committee, and the US Army Medical Research and Materiel Command (USAMRMC)/Office of Research Protections (ORP)/Human Research Protection Office (HRPO). The psychiatrically healthy volunteers included in this study were recruited as part of a larger investigation of fear inhibition and generalization in combat veterans at the Atlanta VAMC. Requirements for participation included no significant visual impairment (corrected 20/20 vision) and tone detection at 30 dB of frequencies ranging from 250 to 4000 Hz (assessed with a Grason-Stadler Model GS1710 pure threshold audiometer). Participants were screened for current or past psychiatric illness through self-report measures and administration of the Structured Clinical Interview for DSM-IV Axis I Disorders, SCID-1. Participants were also screened for illicit drug use via urine toxicology analysis and excluded for current drug or alcohol abuse or dependency. The participants were assigned to one of four age- and sex-matched experimental groups: No Retrieval/No Keypad (n = 10), No Retrieval/Keypad (n = 13), Retrieval/No Keypad (n = 20), or Retrieval/Keypad (n = 12).
2.2. Trial definitions
The eyeblink component of the acoustic startle response was measured according to previously published methods (Norrholm et al., 2006; Norrholm, Anderson, et al., 2011; Norrholm, Jovanovic, et al., 2011). The startle probe was a 108-dB [A], 40 ms burst of white noise with near instantaneous rise time delivered binaurally with headphones. Acoustic startle response magnitude was recorded via electromyography (EMG) readings of the right orbicularis oculi muscle. Two 5 mm Ag/AgCl electrodes filled with electrolyte gel were placed 1 cm below the pupil and 1 cm below the lateral canthus. EMG signals were amplified and digitalized with the BIOPAC MP150 monitoring system (Biopac Systems, Inc., Aero Camino, CA). Impedances through these electrodes were less than 6 kX. Startle magnitude was determined as the peak amplitude of the EMG contraction 20–250 ms following the acoustic stimulus. Similar to several of our previous studies (e.g., (Jovanovic et al., 2005; Norrholm et al., 2008), the aversive stimulus (US) was a 250 ms, 140 p.s.i. airblast directed at the larynx. The CSs were geometric shapes presented on a computer monitor approximately 1 m in front of the participant. On CS+ trials (Fear Acquisition), the shape was presented for 6s total, with the 40 ms startle probe presented 5210 ms after CS onset followed 500 ms later by the 250 ms, 140 p.s.i. airblast that co-terminated with CS presentation. On CS- trials (Fear Acquisition) and nonreinforced CS+ trials (Extinction, Test, Re-extinction, Reinstatement), the shape was presented for 6 s total, with the startle probe occurring 5960 ms after CS onset. On noise alone (NA) trials, the 40 ms startle probe was presented alone without the CS's. Startle trials were averaged across blocks (4 trials per block) in order to reduce variability as in our previous work (e.g., Norrholm et al., 2008).
2.3. Session definitions
The experimental sessions occurred over three consecutive days (see Fig. 1). The Fear Acquisition session occurred on Day 1, the Retrieval trial (or equivalent passage of time) and Extinction Training sessions were administered on Day 2, and the Extinction Test (to as sess extinction retention or spontaneous recovery), Re-extinction, and the Reinstatement Tests occurred on Day 3. All test sessions occurred in the same context. The Fear Acquisition session began with a 1-min acclimation period followed by a habituation phase consisting of three noise alone (NA) presentations. Next, a CS habituation phase was presented consisting of four presentations of each CS without the airblast US. After habituation to the CSs, the Fear Acquisition session continued with three blocks of four trials of each trial type (CS+a, CS+b, CS , NA). Shapes were counterbalanced across participants for each CS type. The Fear Acquisition session used a 100% schedule of reinforcement for the CS+'s such that each presentation of the CS+'s was reinforced with the airblast US. The inter-trial interval (ITI) was randomized between 9 and 22 s.
Fig. 1.
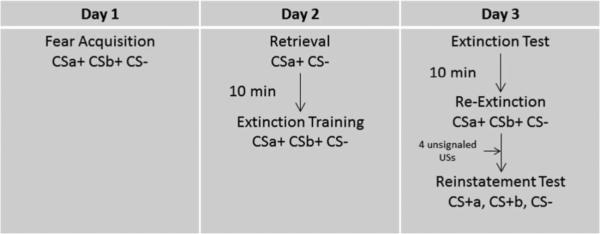
Representative schematic of the experimental design.
Twenty-four hours after Fear Acquisition, participants were either presented with a single non-reinforced presentation of CS+a and the CS– (Retrieval groups) or an equivalent period of time (No Retrieval groups). The CS+b cue was not reactivated. Ten minutes after the Retrieval trial, participants were administered the Extinction Training session. Extinction Training consisted of six blocks of four presentations each of the CS's and NA. Twenty-four hours after Extinction Training, an Extinction Test of extinction retention or spontaneous recovery was presented. The Extinction Test consisted of one block of four trials of each CS and NA. Ten minutes after the Extinction Test, Re-extinction occurred using the same protocol as that used for Extinction Training. Finally, a Reinstatement Test was presented at the conclusion of Re-extinction. The Reinstatement Test consisted of four unsignaled presentations of the airblast US followed by one block of four presentations of each CS and NA. In order to remove any sensitization effects of the US on NA trials, 6 NA startle probe trials were delivered prior to the block with the CS and NA trials.
2.4. US-expectancy
A three-button response keypad (SuperLab, Cedrus Corporation, San Pedro, CA) was used during each acoustic startle session to record the expectancy of the participants of the US on each CS presentation. Participants received verbal instructions prior to each session on how to respond with the keypad. Participants were instructed to press a button marked “+” if they expected the shape to be followed by the US, a button marked “–” if they did not expect the airblast US, or a button marked “0” if they were uncertain. Instructions were to press the button within three seconds of conditioned stimulus onset. Any responses occurring after the airblast US were discarded.
2.5. Data analysis
US-expectancy ratings (scored as –1 for the “–“ button, 0 for the “0” button, and 1 for the “+” button) during fear acquisition were analyzed using repeated measures analysis of variance (RM-ANOVA) with Trial (16 levels: 4 trials during CS Habituation and 12 trials during Acquisition) and Trial Type (3 levels: CS+a, CS+b, CS–) as within-subject factors and Retrieval group (2 levels: Yes, No) as the between-groups factor. Startle response potentiation to CS+ trials was tested with a RM-ANOVA with Block (4 levels: 1 block of CS Habituation, 3 blocks of Acquisition) and Trial Type (3 levels: NA, CS+a, CS+b) as within-subjects factors and Retrieval group (2 levels) and Keypad group (2 levels: Yes, No) as between-groups variables. The degree of potentiation to the CS's was compared by calculating a fear-potentiated startle score. Fear-potentiated startle was expressed as a Difference score using the formula: Difference score = [Mean startle magnitude to probe in presence of CS] – [Mean startle magnitude to startle probe alone (NA) for each session]. The Difference score was then used as the dependent variable in further analyses of startle data. During fear acquisition, a RM-ANOVA comparing Block (4 levels) and Trial Type (3 levels: CS+a, CS+b, CS–) as within-subjects factors and the same between-groups factors as in the previous analysis.
In order to test effects of Extinction Training on Day 2, we used RM-ANOVA with US-expectancy as the dependent variable with Trial (2 levels:1st Extinction trial, last Extinction trial) and Trial Type (2 levels: CS+a, CS+b) as within-subjects factors and Retrieval group (2 levels) and as the between-groups factors. Given that the US-expectancy ratings were derived from the keypad, these analyses were limited to the Keypad groups and included only Retrieval group as a between-group factor. Analyses of fear-potentiated startle used the Difference score as the dependent variable and Block (2 levels: 1st and last block of Extinction) and Trial Type (2 levels) as within-subject factors and Retrieval and Keypad groups as between-group factors. Analyses of extinction focused on comparing the two CS+'s: CS+a was the reactivated cue and the CS+b was the non-reactivated cue.
For Day 3, we tested spontaneous recovery of fear by comparing the last trial of Extinction Training on the previous day with the first trial of the Extinction Test session for US-expectancy. Fear-potentiated startle was analyzed by comparing the last block of Extinction with the first Test block. Trial Type, Retrieval and Keypad groups were defined the same as above. Re-extinction used RM-ANOVA to compare US-expectancy on the first and last trial of Re-extinction and fear-potentiated startle on the first and last block of Re-extinction, with all other factors the same as above. Finally, Reinstatement tested the increase in US-expectancy after the delivery of 4 unpaired airblasts by comparing the last trial of Re-extinction and the first trial of the Reinstatement Test. As in the analyses above, fear-potentiated startle compared the last block of Re-extinction to the Reinstatement Test block. Fear-potentiated startle was calculated using the Difference score of CS minus NA, as described above. Because unpaired airblasts increase baseline startle responses (i.e. NA), 6 startle probes were delivered in order to normalize the NA level. The NA used as the reference in the calculation of fear-potentiated startle during Reinstatement did not include those trials, but only the 4 in the Reinstatement Test.
Interaction effects of Retrieval and Keypad groups were followed-up by comparisons within the four groups defined above: No Retrieval/No Keypad, No Retrieval/Keypad, Retrieval/No Keypad, or Retrieval/Keypad. Given the differences in cell size, we report effect sizes for these analyses using partial Eta squared. All analyses were performed using SPSS 20.0 for Windows, with alpha level set at .05.
3. Results
3.1. Day 1: fear acquisition
We examined US-expectancy ratings on each trial during conditioning using the response keypad comparing the two CS+'s and the CS–. Participants responded with “1” if they expected the air-blast on the trial, “0” if they were uncertain, and “–1” if they did not expect an airblast. Instructions were to press the button within three seconds of CS appearance. All participants were compliant with this timeframe, in fact, most responses occurred within the first 1500 ms of CS onset. Fig. 2A shows the US-expectancy across Trials and Trial Type. A RM-ANOVA reveled a significant Trial by Trial Type interaction, F(30, 690) = 35.00, p < .001, with US-expectancy increasing to both CS+'s and decreasing to CS– across trials during Fear Acquisition. In the groups that included the Keypad, the No Retrieval and Retrieval groups did not differ on US-expectancy during Acquisition. Although the Retrieval trial occurred after the Acquisition session, we compared the two groups in order to ensure that there were no prior differences in the groups.
Fig. 2.
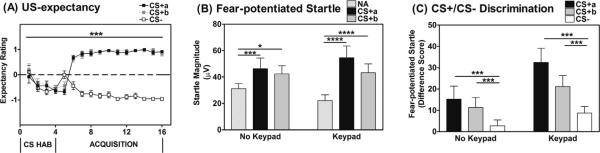
Fear Acquisition. (A) For groups with a Keypad, participants displayed significant discrimination between the reinforced CS+'s and the CS–. CS HAB = CS habituation phase. For each trial, participant responses were coded as “+1” if they expected the airblast on the trial, coded “0” if they were uncertain, and coded “–1” if they did not expect an airblast. (B) Participants with and without a Keypad showed robust fear-potentiated startle to the CS+a and CS+b with an enhanced degree of fear-potentiated startle to both the CS+a, and CS+b in those who used a Keypad. (C) Fear-potentiated startle calculated as a Difference Score between the CS+'s and NA examined during the last block of Acquisition was significantly higher for the CS+a compared to the CS–, with no interaction effects with either Retrieval group or Keypad group. **** p < 0.0001, *** p < 0.001, p < 0.05; Difference Score = [startle magnitude in the presence of a CS] – [startle magnitude in response to noise alone (NA)].
Startle magnitude was significantly potentiated during acquisition to both CS+'s compared to NA trials (see Fig. 2B), as seen by a significant Block by Trial Type interaction, F(6, 318) = 14.20, p < .001. Contrasts showed that startle magnitude to both CS+s was significantly higher than NA (both p's < .001). While there was no effect of Retrieval group during Acquisition, there was a significant interaction of Trial Type and Keypad group, F(2, 106) = 7.41, p < .001. We examined the effect of Trial Type in each group separately: while those without the Keypad still showed significant conditioning effects to CS+a (p = .02) and CS+b (p = .0003), the increase in startle magnitude was more robust in those who used a Keypad to both the CS+a (p = .00002), and CS+b (p = .000006). Fear-potentiated startle calculated as a Difference Score between the CS+'s and NA examined during the last block of Acquisition was significantly higher for the two CS+ trials compared to the CS–, F(2, 106) = 13.26, p < .001, with no interaction effects with either Retrieval group or Keypad group (Fig. 2C). Contrast analyses showed that CS+a and CS+b had significantly greater fear-potentiated startle than the CS– (F(1, 53) = 19.20, p < .001 and F(1, 53) = 11.32, p < .001, respectively).
3.2. Day 2: Extinction Training
For the Extinction Training session, we examined US-expectancy to the CS+trials during the first and last block of extinction with Retrieval group as the between-groups factor. We found a significant main effect of Trial, F(1, 23) = 58.90, p < .001, and no main or interaction effects of Retrieval group. There was also no main or interaction effect of Trial Type, indicating that US-expectancy ratings to both CS+s were equally diminished during Extinction Training (see Fig. 3).
Fig. 3.
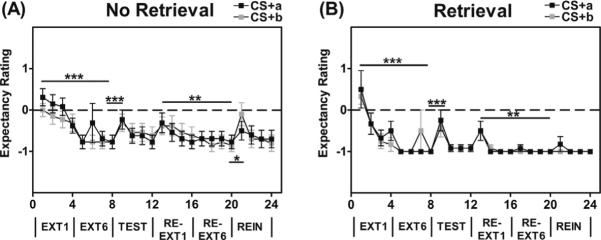
US-expectancy ratings for the (A) No Retrieval group and (B) Retrieval group, during Extinction Training (EXT), Extinction Test (TEST), Re-extinction (RE-EXT), and Reinstatement Test (REIN). During Extinction Training, US-expectancy ratings to both CS+s (CS+a = reactivated cue; CS+b = non-reactivated cue) were equally diminished in the Retrieval and No Retrieval groups. During the Extinction Test, both Trial Types showed a significant increase in US-expectancy, when comparing US-expectancy ratings on the last trial of extinction (EXT6) and the first test trial (EXT1) for both CS+s. Next, analysis of US-expectancy on the first and last trial during Re-extinction revealed a significant decrease from RE-EXT1 to RE-EXT6. After Re-extinction, we tested for reinstatement effects on US-expectancy by comparing the last trial of Re-extinction to the first trial of the Reinstatement Test after the delivery of 4 unsignaled airblast USs. This analysis revealed a significant main effect of Reinstatement, as well as an interaction effect of with Retrieval group, in that the group that received the retrieval cue showed less of an increase in US-expectancy during Reinstatement compared to the group who did not get a reactivation cue. *** p < 0.001, ** p < 0.01.
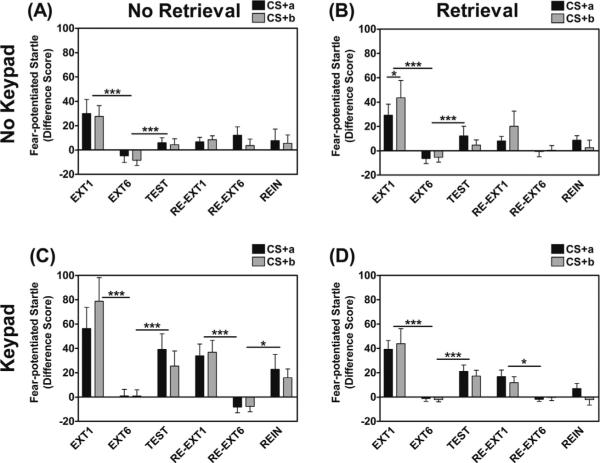
Fig. 4 shows startle data across study Days 2 and 3. Comparing fear-potentiated startle on the first and last block of Extinction Training across the above groups revealed a significant main effect of extinction Block, F(1, 51) = 31.64, p < .001 and an interaction of Block and Trial Type, F(1, 51) = 5.69, p = .02. However, analyses of each Trial Type separately showed that there was significant extinction to CS+a trials, F(1, 54) = 35.20, p < .001, and to CS+b trials, F(1, 54) = 32.36, p < .001. In addition, there was a significant 3-way interaction of Trial Type × Retrieval group × Keypad group, F(1, 51) = 3.98, p = .05. The interaction was followed up by comparing Trial Type (CS+a vs CS+b) within each of four groups: (a) No Retrieval and No Keypad; (b) Retrieval and No Keypad; (c) No Retrieval and Keypad, and (d) Retrieval and Keypad. This analysis revealed that only the Retrieval and No Keypad group showed lower fear-potentiated startle to the reactivated CS+ compared to the non-reactivated CS+ during extinction, F(1, 19) = 5.75, p = .03, η2 = 0.23 (Fig. 4B). The two CS+ trials did not differ from each other in any of the other groups (No Retrieval/No Keypad: p = 0.75, η2 = 0.01; No Retrieval/Keypad: p = 0.10, η2 = 0.21; Retrieval/Keypad: p = 0.55, η2 = 0.03). Finally, there was a significant main effect of Keypad, with higher fear-potentiated startle to both Trial Types in the groups that used the Keypad, F(1, 51) = 4.25, p = .04 (Fig. 4C and D).
Fig. 4.
Fear-potentiated startle data for the Extinction Training (EXT), Extinction Test (TEST), Re-extinction (RE-EXT), and Reinstatement Test (REIN) sessions for the four experimental groups: (A) No Retrieval/No Keypad; (B) Retrieval/No Keypad; (C) No Retrieval/Keypad, and (D) Retrieval/Keypad. All groups showed significant extinction of fear-potentiated startle from EXT1 to EXT6. However, only the Retrieval/No Keypad group showed lower fear-potentiated startle to the reactivated CS+ (CS+a) compared to the non-reactivated CS+(CS+b) during Extinction Training. The two CS+ trials did not differ from each other in any of the other groups. There was higher fear-potentiated startle in the groups that used the Keypad compared to the No Keypad groups. All four groups showed a significant increase from the last block of Extinction Training (EXT6) to the Extinction Test (TEST), however there was also a significant main effect of Keypad group. Re-extinction (RE-EXT1 vs RE-EXT6) was only significant in the two groups that used a Keypad. None of the groups showed any effects of Trial Type, i.e. both CS+ were re-extinguished in the Keypad groups, and neither in the No Keypad groups. Significant reinstatement (RE-EXT6 vs REIN) was only observed in the No Retrieval/Keypad group, with no effect of Trial Type.
3.3. Day 3: Extinction Test
Next, we administered an Extinction Test to assess the degree of extinction retention or spontaneous recovery 24 h after Extinction Training by comparing US-expectancy ratings on the last trial of extinction and the first test trial for both CS+'s. We again found that both Trial Types showed a significant increase in US-expectancy, F(1, 23) = 19.21, p < .001, but no interaction or main effects of Trial Type or Retrieval group.
With respect to fear-potentiated startle, we also found a significant increase to the Test block compared to the last block of Extinction Training, F(1, 50) = 20.23, p < .001, with no interaction effects with Retrieval or Keypad group. There was also no interaction effect with Trial Type, indicating that the CS+a and CS+b showed similar levels of spontaneous recovery. However, there was a significant main effect of Keypad group, F(1, 50) = 11.44, p = .001, with the Keypad group showing higher levels of fear-potentiated startle than the No Keypad group (Fig. 4C and D).
3.4. Day 3: Re-extinction
Analysis of US-expectancy on the first and last trial during Re-extinction revealed a significant decrease across Trials, F(1, 23) = 11.31, p = .003, but again no interaction effects of Retrieval group, Keypad group, or Trial Type. On the other hand, the startle data showed a significant 3-way interaction of Block (1st vs 6th Block of Re-extinction) × Retrieval group × Keypad group, F(1, 50) = 4.55, p = .04. We subsequently compared the Re-extinction blocks within each of the four experimental groups. These results showed significant re-extinction only in the two groups that used a Keypad: the No Retrieval/Keypad group, F(1, 12) = 31.34, p < .001, η2 = 0.72, and the Retrieval/Keypad group, F(1, 11) = 7.08, p = .02, η2 = 0.39. The groups that did not use a Keypad did not demonstrate significant re-extinction (No Retrieval/No Keypad: p = 0.97, η2 = 0.00; Retrieval/No Keypad: p = 0.21, η2 = 0.08). None of the groups showed any main or interaction effects of Trial Type, i.e. both CS+'s were re-extinguished in the Keypad groups, and neither in the No Keypad groups. There were no effects of Retrieval group.
3.5. Day 3: Reinstatement Test
After Re-extinction, we tested for reinstatement effects on US-expectancy by comparing the last trial of Re-extinction to the first trial of Reinstatement after the delivery of 4 unsignaled airblast USs. Trial Type was included as a within-subjects factor, and the Retrieval group as a between-groups factor. This analysis revealed a significant main effect of Trial, F(1,19) = 10.19, p = .005, as well as an interaction effect of Trial by Retrieval group, F(1, 19) = 4.89, p = .04. The group that received the reactivation cue showed less of an increase in US-expectancy during Reinstatement compared to the group who did not get a reactivation cue, see Fig. 3.
The same analysis of Trial Type and Block (last block of Re-extinction compared to Reinstatement Test block) as within-subjects factors and Retrieval group and Keypad group as between-groups factors with fear-potentiated startle as the dependent variable revealed a main effect of Trial Type, F(1, 44) = 7.50, p = .009, with higher startle during the Reinstatement Test to the reactivated cue (CS+a) compared to the non-reactivated cue (CS+b); this should not to be confused with reinstatement of fear which is based on the within-subject change from the end of re-extinction to the Reinstatement Test (see next set of analyses). There was also a significant 3-way interaction of Block × Retrieval group × Keypad group, F(1, 44) = 5.68, p = .02. Analyses of Block within each of the four groups revealed significant reinstatement in the No Retrieval/Keypad group, F(1, 8) = 6.56, p = .03, η2 = 0.45, and no effect of Block in the Retrieval/Keypad (p = 0.47, η2 = 0.05), Retrieval/No Keypad (p = 0.06, η2 = 0.18), or No Retrieval/No Keypad groups (p = 0.89, η2 = 0.003), see Fig. 4.
4. Discussion
The purpose of the present study was to further examine the putative reconsolidation update mechanism reported in rodents by Monfils et al. (2009) that has since been translated to human studies (e.g., Schiller et al., 2010). Previous results from the Monfils group and others (e.g., Oyarzun et al., 2012; Schiller, Raio, & Phelps, 2012; Schiller et al., 2010) demonstrated a blockade of the return of fear when the reconsolidation window was opened by an isolated single exposure to a previously reinforced CS+ followed by Extinction Training. Recent skin conductance and fear-potentiated startle data from other groups (e.g., Soeter & Kindt, 2011a, Kindt and Soeter (2013); Golkar et al., 2012) failed to replicate these findings. One of the significant discrepancies between the Schiller human study and the procedures used by other groups was the inclusion of on-line US-expectancy measures. In the current study, we performed a direct examination of the effects of retrieval + extinction in the presence or absence of a response keypad to report participant US-expectancy.
4.1. Summary of Findings
The primary findings of the present study were as follows: (1) all participants acquired robust fear-potentiated startle responses to the reinforced CS+a and CS+b as compared to NA trials and significant discrimination between the reinforced CS+'s and the CS , (2) the inclusion of the Keypad for US-expectancy enhanced the degree of fear-potentiated startle as compared to groups without the Keypad during Fear Acquisition, (3) the inclusion of the Keypad increased participant retention of fear-potentiated startle to the CS+'s at the beginning of Extinction Training as compared to those groups without the Keypad, (4) in the No Keypad/Retrieval group, there was significantly less fear-potentiated startle to the reactivated CS+a as compared to the control CS+b during Extinction Training, (5) all groups displayed some level of spontaneous recovery of fear-potentiated startle to both previously reinforced CS+'s during the Extinction Test with the Keypad groups showing a greater degree of fear recovery as compared to the No Keypad groups, (6) only the groups with a Keypad showed significant re-extinction to both previously reinforced CS+'s, (7) only the No Retrieval/Keypad group showed significant reinstatement of fear-potentiated startle to the previously reinforced CS+'s.
4.2. Relationship between on-line US-expectancy measures and psychophysiological indices
As in several of our previous studies (e.g., Norrholm et al., 2008), a three-button response keypad was used as a measure of US-expectancy on a trial-by-trial basis during each of the test sessions previously described. Traditionally, these data provide the experimenter with validation that the CS–US association was successfully learned during fear acquisition in that there is often a high correlation between physiological indices of fear (e.g., startle, skin conductance) and US-expectancy responses (e.g., Norrholm, Anderson, et al., 2011; Norrholm, Jovanovic, et al., 2011). As the study of conditioned fear is extended into fear extinction and return of fear paradigms (which often include multiple test sessions with varying temporal and contextual factors), dissociations begins to emerge between physiological measures and US-expectancy responses (see (Kindt & Soeter, 2013; Norrholm et al., 2008; Soeter & Kindt, 2010, 2011b; Weike et al., 2007). For example, in the current study, participant keypad responses increase from a level of “safety” at the end of the Extinction Test to that of “uncertainty” at the beginning of the re-extinction session (see Fig. 3). This is in contrast to the level of fear-potentiated startle observed at the beginning of re-extinction in the keypad groups (see Fig. 4). This dissociation is consistent with previous reports using on-line US-expectancy measures during extended fear extinction paradigms (Kindt & Soeter, 2013; Norrholm et al., 2008). As discussed by Boddez et al. (2013), this dissociation is not surprising and may be due, in part, to (1) the subjective nature of US-expectancy, (2) the failure of US-expectancy measures to capture arousal, and (3) the sensitivity of this measure to experimental demand, or the experimental artifact created when a participant changes his or her behavior based on their interpretation of the experiment's purpose (Grillon, 2008; Lipp, 2006).
Based on a large body of evidence accumulated over the past two decades, it is clear that fear-potentiated startle, skin conductance response, and US-expectancy measures can be used as complementary research tools in the area of human fear conditioning (Jovanovic et al., 2013; Kindt & Soeter, 2013). However, each methodology has its own inherent strengths and limitations. Fear-potentiated startle provides a non-zero baseline, is a proxy measure of amygdala activity, and shows sensitivity to the valence of experimental stimuli (for review see Davis, Falls, Campeau, & Kim, 1993; Glover et al., 2011). A potential limitation of the use of fear-potentiated startle is the potential for the acoustic startle probes to be perceived as aversive and an additional unconditioned stimulus (e.g., Golkar et al., 2012). Skin conductance response is generally viewed as a measure of arousal and a less specific measure of valence and/or fear (e.g., Glover et al., 2011; Kindt & Soeter, 2013). SCR is also preferentially used in conjunction with neuroim-aging studies (Agren, Engman, et al., 2012; Milad et al., 2008) since startle introduces movement artifact in the scanner. As previously described, US-expectancy measures tend to show greatest sensitivity at the peak of fear acquisition (i.e., at the end of fear conditioning and at the outset of Extinction Training) and much less sensitivity once the initial fear memory has been extinguished; this pattern of US-expectancy responding has been observed in response to prepared stimuli, such as fear-relevant images of spiders (Kindt & Soeter, 2013) and non-prepared stimuli, such as neutral shapes (Norrholm et al., 2008, current results). In addition, these measures have been employed concurrently and there is little evidence to suggest that either the administration of acoustic startle probes or the active recording of one's US-expectancy disrupts skin conductance responses (for discussion see Soeter & Kindt, 2011a, 2011b).
4.3. The effect of US-expectancy measures on fear acquisition and extinction
The results of the present study indicated that the presence of a keypad for on-line assessment of US-expectancy enhanced the acquisition, maintenance, spontaneous recovery, and reinstatement of fear-potentiated startle as compared to test groups without the US-expectancy measure. Critics of the use of on-line US-expectancy measures have argued that the inclusion of these procedures intentionally draws participant attention to the experimental contingencies and, as such, potentially influences pure conditioning (Boddez et al., 2013; Lipp, 2006). This criticism has its roots in early work by Razran (1955) who believed that the effect of a participant's conscious awareness of the CS–US association was an “unfortunate error” and not reflective of true conditioning (Razran, 1955). More recently, it has been argued that active awareness of experimental contingencies is essential to fear conditioning and provides greater ecological validity (De Houwer & Beckers, 2002; Grillon, 2002). While initial fear conditioning was evident in the groups that did not have on-line US-expectancy measures in the present study, it appears as though active detection of the experimental contingencies via US-expectancy responses produced a stronger CS–US association that was more readily retrieved following Extinction Training. The observed enhancement of fear conditioning associated with on-line US-expectancy ratings may also reflect recruitment of two mechanisms of learning described by Ohman and Mineka (2001): (1) contingency learning and (2) fear module-based emotional learning (Ohman & Mineka, 2001).
Additionally, the more robust fear memories observed in the Keypad groups in the present study may also be the result of rehearsal. Although this study was not a controlled study of rehearsal per se, the act of reporting one's US-expectancy on each trial is similar to mentally rehearsing the CS–US contingencies (e.g., (Joos, Vansteenwegen, Vervliet, & Hermans, 2013). Rehearsal has been shown to strengthen and sustain conditioned fear responses to a rehearsed CS+as compared to a non-rehearsed CS+. According to this interpretation, the groups without a Keypad did not have the opportunity to mentally rehearse the CS–US associations as they anticipated and executed their expectancy ratings (whereas the Keypad group did) and thereby did not show sustained fear memory expression across the experimental sessions.
4.4. The effect of US-expectancy measures on fear extinction during reconsolidation
The putative reconsolidation update mechanism has been proposed as a means of disrupting the reconsolidation of an acquired CS–US association (fear memory) by reactivating a previously reinforced CS+ through the presentation of a single, isolated, unrein-forced CS+ trial followed by Extinction Training. Monfils et al. (2009) suggested the lateral amygdala as a potential anatomical locus for the retrieval + extinction effects on fear return. Schiller et al. (2010) and Oyarzun et al. (2012) employed a single method for assessing fear responses (skin conductance) in their studies that produced very similar effects to those reported by Monfils et al. (2009). A possible explanation for the failure of subsequent groups to replicate the retrieval + extinction blockade of fear return may be the recruitment of neural systems that extend beyond the amygdala, which has been suggested as a principal neurobiological substrate for this phenomenon (Agren, Engman, et al., 2012), to involve areas such as hippocampus and frontal cortices (Weike et al., 2007) as a result of including on-line US-expectancy measures. In other words, cortical representations of the CS-US association may have elicited amygdala activation and, as such, strengthened the original fear memory (Olsson & Phelps, 2007).
The results of the present study in the groups that did not have on-line US-expectancy measures indicate some effects of reactivation of a previously reinforced CS+ during an isolated retrieval trial. Fear-potentiated startle to the reactivated CS+a was lower than that which was observed to the non-reactivated CS+b at the outset of Extinction Training. This reduction was not observed in the Keypad/Retrieval group and interpretation of this finding is masked by the decreased fear learning, and loss of fear expression signal throughout the experimental sessions, that was seen in the No Keypad groups. An additional effect of reactivation observed in the current study was the lack of reinstatement of fear-potentiated startle in the Keypad/Retrieval group. In fact, the only group to show reinstatement in our study was the Keypad/No Retrieval group. While the No Keypad/Retrieval group also did not show reinstatement, it is unclear whether this effect is due to the absence of the Keypad rather than the presence of the reactivation cue, given that the No Keypad/No Retrieval group also did not show reinstatement. Notably, this lack of reinstatement was not specific to the reactivated CS+a, but extended to the CS+b. The potential to reduce the reinstatement of learned fear to similar (potentially generalized) cues has compelling clinical implications and certainly warrants further study.
It has become clear to investigators as the retrieval + extinction paradigm is increasingly studied that there are boundary conditions that exist under which reconsolidation does not occur (Nader & Hardt, 2009). As described by Golkar et al. (2012), the inability to replicate the disruption of reconsolidation by Extinction Training within the reconsolidation time window has been most often discussed in terms of boundary conditions such as acquisition memory strength.
With regard to acquisition memory strength, one issue to consider with regard to the discrepancies reported using the retrieval + extinction paradigm is the schedule of reinforcement utilized during fear acquisition (as discussed by Auber et al., 2013). Our current study used 100% reinforcement on the CS+ trials during fear acquisition similar to Soeter and Kindt (2011a, 2011b). The studies by Schiller et al. (2010) and Oyarzun et al. (2012), however, employed a partial reinforcement schedule (37.5%). It is possible that the lack of a disruption of reconsolidation (as evidenced by spontaneous recovery observed in all groups of the present study) may be due to stronger conditioning and subsequent resistance to reconsolidation update. This possibility is supported by animal work showing that an increased number of reinforced trials down-regulates cellular and molecular mechanisms in the amygdala that are necessary to open a reconsolidation window thereby preventing the potential for disruption (Wang, de Oliveira, & Nader, 2009). However, recent findings by Agren, Engman, et al. (2012) and Agren, Furmark, et al. (2012) showed that reconsolidation could be disrupted using retrieval + extinction methods and 100% reinforcement during fear acquisition. Thus, it remains unclear whether acquisition strength contributes to the discrepant results reported regarding the disruption of reconsolidation.
4.5. Limitations
There are a number of limitations in the current study that should be addressed in future investigations. First, in the No Keypad groups, it is difficult to assess the potential contribution of decreased attention to the reduced fear expression signal. Incorporation of other measures such as skin conductance response or eye-tracking in the study might assess attention and increase consistency with other studies in the literature. Next, we based the experimental design on our previous studies on extinction (Norr-holm et al., 2008; Norrholm, Anderson, et al., 2011; Norrholm, Jovanovic, et al., 2011) that used 6 blocks, with 4 trials of each type per block. However, it is possible that this large number of trials (i.e., 96 trials), coupled with the absence of active responding on a keypad, results in a “floor effect” in the No Keypad groups. A shorter extinction session may provide a stronger fear expression signal across Days 2 and 3 of testing. Finally, the four groups had relatively small sample sizes (ranging from 10 to 20 per group) which may have been underpowered to detect significant effects. However, some of the smaller groups did show significant effects, indicating that there was sufficient power. In addition, the small effect sizes in the groups with non-significant effects suggested that these differences would not be significant even with larger sample sizes.
5. Conclusions: an update to the disruption of reconsolidation through update mechanisms
In conclusion, the present study was aimed at extending the reconsolidation update mechanism (retrieval + extinction) that has putatively been shown to prevent the return of fear in some studies while having no reported effect in others. The data we now report suggest that even with the inclusion of US-expectancy measures (and the associated recruitment of higher brain circuits) fear-potentiated startle paradigms that capture the acquisition, maintenance, extinction, recovery, re-extinction, and reinstatement of conditioned fear (see Fig. 4, panel C) are well-suited to advance the study of the retrieval + extinction mechanism and may shed further light on its neurobiological mechanism of action and, ultimately, it's potential utility as a non-pharmacological intervention for the treatment of anxiety disorders.
Acknowledgments
We thank Alexander McCarthy, Ilana Olin, Sharon Ashley McCullough, Jeremy Whitley, and James A. Chitty for their assistance in the preparation of this manuscript. This work was funded in part by the Brain and Behavior Foundation (formerly NARSAD; S.D.N. and T.J.), the Department of Defense (DOD)/Congressionally Directed Medical Research Program (CDMRP, Award # W81XWH-08-2-0170) (PI, S.D.N.), the Emory University Research Committee, a PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (S.D.N.), and the VA Merit Program (B.B.).
Footnotes
Financial disclosures
Drs. Norrholm, Jovanovic, and Bosshardt report no financial disclosures. Mr. Anderson, Mr. Kwon, and Mr. Warren report no financial disclosures. Dr. Bradley receives grant support or has received awards from the VA Merit Award Program, the American Foundation for Suicide Prevention, and the American Psychoanalytic Association Psychoanalytic Research Fund.
References
- Agren T, Engman J, Frick A, Bjorkstrand J, Larsson E-M, Furmark T, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337(6101):1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- Agren T, Furmark T, Eriksson E, Fredrikson M. Human fear reconsolidation and allelic differences in serotonergic and dopaminergic genes. Translational Psychiatry. 2012;2(e76):1–6. doi: 10.1038/tp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference. methodological issues or boundary conditions? Psychopharmacology (Berl) 2013;226:631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddez Y, Baeyens F, Luyten L, Vansteenwegen D, Hermans D, Beckers T. Rating data are underrated: Validity of US expectancy in human fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44:201–206. doi: 10.1016/j.jbtep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time and memory retrieval in the interference paradigms of Pavlovian conditioning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral process in extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton MD, Bolles RC. Role of contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108(1):4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Craske MG. Anxiety disorders: Psychological approaches to theory and treatment. Westview Press; Boulder, CO: 1999. [Google Scholar]
- Davis M. Neurobiology of fear responses: The role of the amygdala. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Behavioural Brain Research. 1993;58(1–2):175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Beckers T. A review of recent developments in research and theories on human contingency learning. Quarterly Journal of Experimental Psychology. 2002;55B:289–310. doi: 10.1080/02724990244000034. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidation, or, how stable is the engram. Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. Journal of Neuroscience. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ. What might the psychobiology of posttraumatic stress disorder teach us about future approaches to pharmacotherapy? Journal of Clinical Psychiatry. 2000;61:44–51. [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, et al. Tools for translational neuroscience. PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress Anxiety. 2011;28(12):1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Bellander M, Olsson A, Ohman A. Are fear memories erasable?-reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Frontiers in Behavioral Neuroscience. 2012;6(80):1–10. doi: 10.3389/fnbeh.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology (Berl) 2008;199(3):421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, et al. Selective erasure of a fear memory. Science. 2009;323(1492):1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Joos E, Vansteenwegen D, Vervliet B, Hermans D. Repeated activation of a CS–US-contingency memory results in sustained conditioned responding. Frontiers in Psychology. 2013;4(305) doi: 10.3389/fpsyg.2013.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2011;62(2):695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan E. Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry. 2005;57(12):1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience. 2011;5:44. doi: 10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos A, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research. 2009;167(1–2):151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Sakoman AJ, Kozaric-Kovacic D, Mestrovic AH, Duncan EJ, Davis M, et al. Acute stress disorder versus chronic posttraumatic stress disorder: Inhibition of fear as a function of time since trauma. Depress Anxiety. 2013;30(3):217–224. doi: 10.1002/da.21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: A test of extinction as updating mechanism. Biological Psychology. 2013;92:43–50. doi: 10.1016/j.biopsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation: Maintaining memory relevance. Trends in Neurosciences. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp OV. Human fear learning: Contemporary procedures and measurement. In: Craske MG, Hermans D, Vansteenwegen D, editors. Fear and learning: From basic processes to clinical implications. American Psychological Association; Washington, D.C.: 2006. pp. 37–52. [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicted on basic, neurally mapped mechanisms of Pavlovian learning: The case for conditioned overgeneralization. Depress Anxiety. 2012;29:257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research. 2008;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko N. a. B., et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It's not what you thought it was. American Psychologist. 2006;61(1):10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Monfils M-H, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36(4):567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nature Reviews Neuroscience. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nature Reviews Neuroscience. 2000;1(3):216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Anderson KM, Olin IW, Jovanovic T, Kwon C, Warren VT, et al. Versatility of fear-potentiated startle paradigms for assessing human conditioned fear extinction and return of fear. Frontiers in Behavioral Neuroscience. 2011;5:77. doi: 10.3389/fnbeh.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T. Tailoring therapeutic strategies for treating posttraumatic stress disorder symptom clusters. Neuropsychiatric Disease and Treatment. 2010;6:517–532. doi: 10.2147/NDT.S10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T. Translational fear inhibition models as indicies of trauma-related psychopathology. Current Psychiatry Reviews. 2011:7. [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biological Psychiatry. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, et al. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning and Memory. 2006;13(6):681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, et al. Timing of extinction relative to acquisition: A parametric analysis of fear extinction in humans. Behavioral Neuroscience. 2008;122(5):1016–1030. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nature Neuroscience. 2007;10(9):905–912. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Oyarzun JP, Lopez-Barroso D, Fuentemilla L, Cucurell D, Pedraza C, Rodriguez-Fornells A, et al. Updating fearful memories with Extinction Training during reconsolidation: A human study using auditory aversive stimuli. PLoS ONE. 2012;7(6):1–9. doi: 10.1371/journal.pone.0038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford University Press; 1927. [Google Scholar]
- Razran G. Conditioning and perception. Psychological Review. 1955;62:83–95. doi: 10.1037/h0046875. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychlogy: Animal Behavior Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. Journal of Psychotherapy. 2002;56(1):59. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Strengthening the shaky trace through retrieval. Nature Reviews Neuroscience. 2000;1(3):212–213. doi: 10.1038/35044575. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils M-H, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Phelps EA. Does reconsolidation occur in humans? Frontiers in Behavioral Neuroscience. 2011;5(24) doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Raio CM, Phelps EA. Extinction Training during the reconsolidation window prevents recovery of fear. Journal of Visualized Experiments. 2012;66(e3893) doi: 10.3791/3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: Erasing fear from memory. Neurobiology of Learning and Memory. 2010;94(1):30–41. doi: 10.1016/j.nlm.2010.03.004. http://dx.doi.org/10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: Pharmacological and behavioral manipulation. Learning and Memory. 2011a;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiology of Learning and Memory. 2011b;96:263–271. doi: 10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nature Reviews. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Kindt M, Vansteenwegen D, Hermans D. Fear generalization in humans: Impact of verbal instructions. Behaviour Research and Therapy. 2010;48:38–43. doi: 10.1016/j.brat.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Wang SH, de Oliveira AL, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nature Neuroscience. 2009;12(7):905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44:170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Wolpe J, Rowan VC. Panic disorder: A product of classical conditioning. Behaviour Research and Therapy. 1988;26:441–450. doi: 10.1016/0005-7967(88)90138-6. [DOI] [PubMed] [Google Scholar]