Abstract
Abnormal skin findings are identified in over 90% of human immunodeficiency virus (HIV)-infected persons globally. A prospective cohort study of HIV-infected patients with skin complaints commencing antiretroviral therapy (ART) in northern Tanzania was undertaken. Consecutive HIV-infected subjects presenting with skin complaints, who met criteria for ART initiation, were recruited at a Tanzanian Regional Dermatology Training Center. A single dermatologist evaluated all subjects; baseline skin biopsies were performed, and CD4+ cell counts and plasma HIV RNA levels were measured. All subjects received a fixed-dose combination of stavudine, lamivudine, and nevirapine. A total of 100 subjects were enrolled; 86 subjects completed six months of follow-up. Median baseline CD4+ cell counts and plasma HIV RNA levels were 120 cells/μl and 5.2 log10 copies/ml. The most common dermatologic condition was papular pruritic eruption (47%). The median baseline score on the Burn Scale was 38%. After six months, 10 subjects had achieved the complete resolution of skin abnormalities. In those without complete resolution, the median Burn Scale score improved to 7%. Five patients developed new eruptions by month 3, which in two cases were attributed to drug reactions. In the 86 subjects remaining on ART after six months, the median CD4+ cell count had increased to 474 cells/μl, and plasma HIV RNA levels were <400 copies/ml in 85 (99%) subjects. Patients with HIV infection with skin complaints experienced marked clinical improvements following ART initiation.
Introduction
Cutaneous symptoms and signs are nearly ubiquitous among human immunodeficiency virus (HIV)-infected patients; 90% will develop at least one cutaneous manifestation associated with HIV infection during the clinical course of their disease.1 Given the concentration of CD4+ cells within the skin, including CD4+ lymphocytes and Langerhans cells, it is not surprising that the skin plays a critical role in the immunopathogenesis and clinical presentation of HIV infection.2 The spectrum of associated dermatologic complications includes co-infections caused by bacteria, viruses, dermatophytes and other fungi, mycobacteria, malignancies such as Kaposi’s sarcoma, and other miscellaneous conditions.3 In HIV-infected persons, these dermatologic complications may present with increased severity, an atypical presentation and appearance, and a prolonged course, or may be unresponsive to conventional therapies.4,5 In general, many of the skin complications of progressive HIV disease are associated with lower CD4+ cell counts.5
The spectrum of dermatologic complications may also differ between resource-rich and resource-limited settings (RLSs); for example, papular pruritic eruption (PPE) is a common manifestation in RLSs but not in resource-rich areas, and its occurrence is associated with CD4+ cell counts of <200 cells/μl.6,7 Extensive observations have defined a spectrum of anticipated skin findings in each setting. Because many cutaneous complications occur in patients with lower CD4+ cell counts, it is logical to predict that the initiation of antiretroviral therapy (ART) has the potential to improve skin conditions if CD4+ cell counts increase on treatment. Observations in patients beginning ART in resource-rich settings have identified significant improvements in cutaneous findings, in addition to dramatic improvements in HIV-related morbidity and mortality.8,9
However, the vast majority of HIV-infected persons who require ART reside within RLSs, where the costs, delivery, and monitoring of ART have historically represented significant challenges. With international support, ART is becoming increasingly available in RLSs, and an estimated eight million persons in these circumstances are currently receiving treatment.9 One study describing cutaneous responses to first-line ART initiation among patients with PPE in Uganda documented significant improvements in skin findings.10 Generic fixed-dose combinations of stavudine, lamivudine, and nevirapine are commonly used as first-line regimens in RLSs. Although this ART regimen has demonstrated outstanding efficacy in improving HIV-related outcomes in RLSs, nevirapine can cause drug-associated skin rashes in up to 20% of treated persons.11 A study conducted by Kumarasamy et al.12 reported that ART caused rashes in 8% of patients, including Stevens–Johnson syndrome in 0.4%. Nevirapine was implicated as the likely causative factor in 85% of patients taking this drug who developed rashes.12 In addition, ART is frequently initiated simultaneously with co-trimoxazole prophylaxis, which may also cause skin rashes in up to 6% of users.11,12
Finally, examples of immune reconstitution syndrome (IRS) have been described in patients beginning ART in RLSs, and the skin may be an important site for localization of an IRS.13 To address these gaps in knowledge regarding the initiation of ART in RLSs, we prospectively studied a cohort of 100 HIV-infected persons with cutaneous complaints and findings, in whom ART was initiated, in northern Tanzania.
Materials and methods
Subjects were recruited at the Kilimanjaro Christian Medical Center (KCMC) Regional Dermatology Training Center (RDTC) and Mawenzi Regional Hospital Infectious Diseases Clinics in Moshi, Tanzania. Approximately 4000 HIV-infected persons receive care in these two clinics, and 25 patients per month begin ART. Eligibility criteria included a positive rapid HIV antibody test, age of ≥18 years, skin complaints, and fulfillment of the Tanzanian National Ministry of Health Guidelines for initiation of ART (World Health Organization [WHO] stage IV disease, WHO stage III disease with a CD4+ cell count of <350 cells/μl, or any WHO stage with a CD4+ cell count of <200 cells/μl). All subjects provided written informed consent, and the study was approved by the KCMC Research Ethics Committee.
Study visits were conducted at the KCMC RDTC, at which a single dermatologist evaluated all subjects at entry and during follow-up. A careful history was taken, and a subjective Likert scale questionnaire documenting skin complaints was completed. A standardized clinical examination was performed using the Burn Scale to assess the proportions of affected skin. Punch skin biopsies were obtained from affected areas at study entry. Biopsy specimens were examined by hematoxylin and eosin staining; potassium hydroxide (KOH) and periodic acid Schiff (PAS) stains were performed when indicated. Blood samples were sent for confirmation of HIV infection using two rapid test methods, Capillus™ HIV-1/HIV-2 (Trinity Biotech Plc, Bray, Ireland) and Determine™ HIV-1/2 (Abbott Laboratories, Inc., Abbott Park, IL, USA).14 All CD4+ cell counts were measured using a Partec cycle flow counter analyzer (Partec GmbH, Münster, Germany). Plasma HIV-1 RNA was quantified using the Abbott m2000 RealTime HIV-1 system (Abbott Laboratories, Inc.), which has a lower limit of detection of 75 copies/ml).15,16 Digital photographs of affected areas were taken at the time of recruitment and at subsequent follow-up visits, or at any time in the event of new eruptions. Photographs were taken to assess improvement or worsening of the cutaneous condition(s). The Burn Scale Rule of 9s (when applicable) was used to assess the extent of the area affected. Burn Scale scores were considered to indicate improvement if the score reached ≤10%, moderate improvement if the score reached >10% and <40%, and no improvement if the score remained at ≥40% from entry to completion of the study. Subjective improvement indicators were given scores on a Likert scale and classified as indicating that the patient’s condition had: improved sufficiently; improved but not sufficiently; remained unchanged; deteriorated slightly; or worsened (Table 1). Karnofsky scores were also utilized as a performance measure. All measures of responses to treatment are presented for only those subjects continuing ART in an on-treatment analysis.
Table 1.
Classification of level of improvement according to objective indicators
| Improved, sufficiently | Patients in whom 5–7 assessment indicators (subjective Likert scale, Burn Scale score Rule of 9s, clinical improvement as documented by photography, Karnofsky score, CD4+ cell count, plasma HIV RNA level, eruption of new lesions) show improvement |
| Improved, not sufficiently | Patients in whom 2–4 assessment indicators show improvement |
| No improvement/same condition | Patients in whom 0 or 1 assessment indicator show improvement |
| Slight deterioration | Patients in whom 2–4 assessment indicators show no improvement |
| Worsened | Patients in whom 5–7 assessment indicators show no improvement |
All subjects began a fixed-dose combination of stavudine 40 mg, lamivudine 150 mg twice daily, and nevirapine 200 mg once daily for the first two weeks, followed by stavudine 40 mg, lamivudine 150 mg, and nevirapine 200 mg twice daily. Stavudine dosing was adjusted by weight; subjects weighing <60 kg received 30 mg twice daily. Efavirenz was substituted for nevirapine in suspected nevirapine toxicity and zidovudine for stavudine in suspected stavudine toxicity. All subjects received trimethoprim-sulfamethoxazole prophylaxis 800/160 mg three times per week, which was discontinued without substitution if suspected toxicity developed.
Data were double-entered in a Microsoft Access database and transferred to SPSS Version 16.0 (SPSS, Inc., Chicago, IL, USA) for analysis. Baseline characteristics were analyzed using descriptive statistics and summarized in frequency tables; median values and interquartile ranges (IQRs) are presented. The Wilcoxon rank-sum test was used to test the equality of medians at baseline and after six months of follow-up.
Results
A total of 100 subjects were recruited from October 2006 to June 2007. Women (n = 59, 59%) represented the majority of study participants. The mean age of study subjects was 37 years (range: 16–66 years). The median duration of cutaneous symptoms was 90 days (IQR: 60–180 days). The most common dermatologic findings were PPE (47%), followed by eczema (13%), pruritis (9%), xerosis (7%), tinea infections (5%), herpes zoster (4%), Kaposi’s sarcoma (4%), molluscum contagiosum (4%), and plain warts (3%) (Table 2). The median Burn Scale score at baseline was 38% (IQR: 3–80%). All subjects demonstrated clinically advanced HIV disease; 95% of subjects had WHO stage III disease, and 5% had WHO stage IV disease. The median CD4+ cell count at baseline was 120 cells/μl (IQR: 2–202 cells/μl), and 37% of patients had CD4+ cell counts of <100 cells/μl. The median plasma HIV RNA level at baseline was 5.2 log10 copies/ml (IQR: 2.6–6.6 log10 copies/ml), and 64% of subjects had RNA levels of >5.0 log10 copies/ml.
Table 2.
Baseline characteristics and dermatologic manifestations in patients with human immunodeficiency virus (HIV) infection (n = 100)
| Sex: female, n (%) | 59 (59) |
| Age, years, median (IQR) | 37 (30–45) |
| CD4+ cell count, cells/μl, median (IQR) | 120 (2–202) |
| Plasma RNA, log10 copies/ml, median (IQR) | 5.2 (2.6–6.6) |
| Dermatologic manifestations, n (%) | |
| Pruritic papular eruption | 47 (47) |
| Eczema | 13 (13) |
| Pruritis | 9 (9) |
| Xerosis | 7 (7) |
| Tinea | 5 (5) |
| Burn scale score,%, median (IQR) | 38% (3–80) |
IQR, interquartile range.
A total of 86% of subjects completed six months of follow-up on ART. Reasons for loss to follow-up included abscondment (n = 8), non-adherence to drugs (n = 3), death (n = 2), and adverse drug reactions (n = 1). All 86 of the subjects who completed the study remained on a fixed-dose combination of stavudine, lamivudine, and nevirapine. Of the two patients who died, one had advanced Kaposi’s sarcoma with gastrointestinal involvement, and the other developed severe anemia. Two patients had suspected drug reactions involving the skin: one had Stevens–Johnson syndrome attributed to nevirapine, and the second developed a reaction attributed to trimethoprim-sulfamethoxazole.
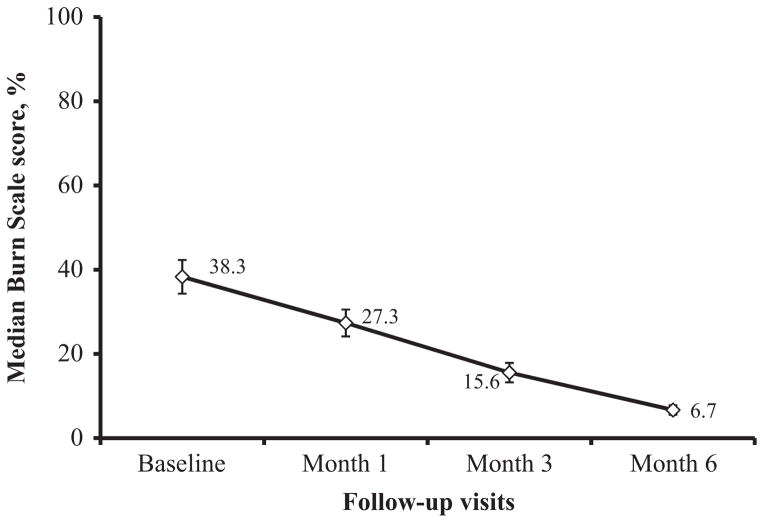
Cutaneous responses were dramatic in many subjects. The median Burn Scale score decreased from 39% at baseline to 27% and 16% at the first and second follow-up visits (Fig. 1). Among the 86 subjects who completed six months of ART, the median Burn Scale score decreased to 7% (IQR: 3–9%). Ten (12%) subjects experienced the complete resolution of dermatologic findings (Burn Scale score: 0%). These patients presented with PPE (n = 3), herpes zoster (n = 2), oral candidiasis (n = 1), molluscum contagiosum (n = 1), tinea (n = 1), hairy leukoplakia (n = 1), and granulomatous candidiasis (n = 1). An example of the complete resolution of dermatologic symptoms in one subject is provided in Figure 2.
Figure 1.
Trends in median scores on the Burn Scale in patients with human immunodeficiency virus (HIV) infection from baseline to 6 months after the initiation of fixed-combination antiretroviral therapy
Figure 2.
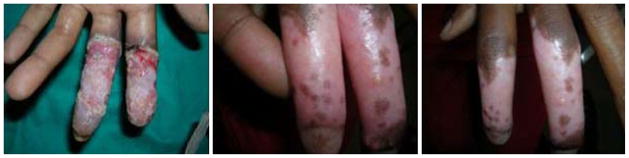
Granulomatous reaction secondary to candidiasis in a patient with human immunodeficiency virus (HIV) infection. Note the improvement from baseline to one, three and 6 months after the initiation of fixed-combination antiretroviral therapy
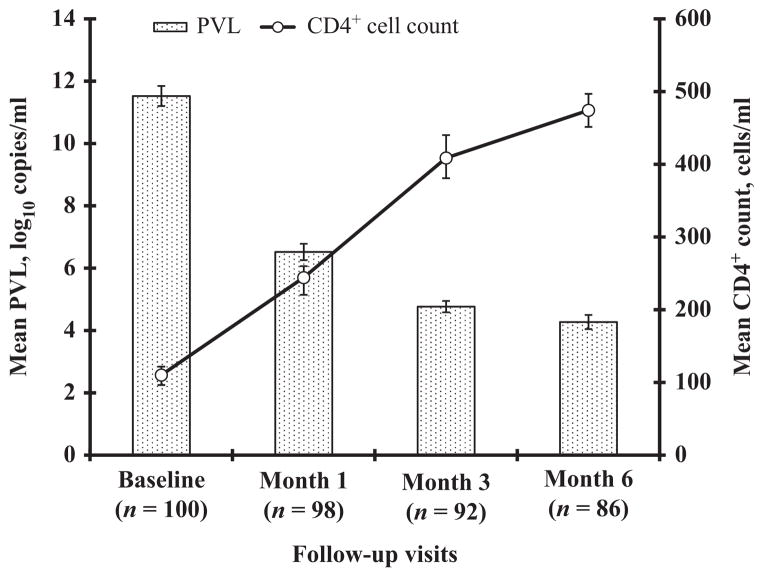
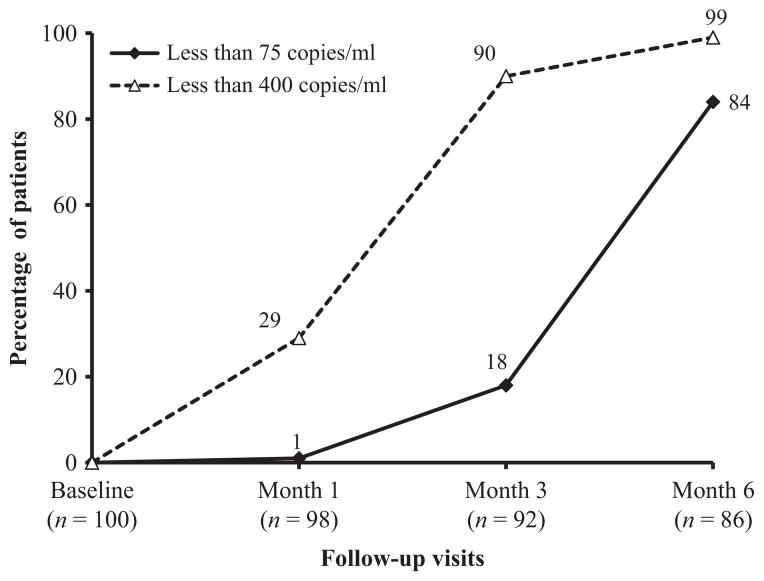
Increases in CD4+ cell counts and responses to ART in plasma HIV RNA levels are shown in Figures 3 and 4. Among the 92 subjects who completed three months of ART, the mean CD4+ cell count increased from 110 cells/μl at baseline to 474 cells/μl, and 18% and 90% achieved plasma HIV RNA levels of <75 copies/ml and <400 copies/ml, respectively. At six months, the numbers of participants with plasma HIV RNA of <75 copies/ml and <400 copies/ml increased to 72 (84%) and 85 (99%), respectively (Fig. 4). Karnofsky status improved from a median baseline score of 70 to a median of 80 following six months of ART.
Figure 3.
Changes in CD4+ count and HIV-1 RNA levels in patients with human immunodeficiency virus (HIV) infection from baseline to 6 months after the initiation of fixed-combination antiretroviral therapy. PVL, plasma viral load
Figure 4.
Percentage of subjects with human immunodeficiency virus (HIV) RNA of <75 copies/ml and <400 copies/ml from baseline to 6 months after the initiation of fixed-combination antiretroviral therapy
In the 10 subjects who achieved the complete resolution of symptoms, the median change in CD4+ cell count was 440 cells/μl (101 cells/μl at baseline and 541 cells/μl at 6 months; P < 0.001), and the median change in HIV-1 RNA was 5.3 log10 copies/ml (190,688 at baseline and 26 copies/ml at 6 months; P < 0.001). In addition to the two patients with drug reactions, another three subjects experienced new or worsened dermatologic findings at six months of follow-up. In the three patients with new eruptions, baseline CD4+ cell counts were 4 cells/μl, 135 cells/μl, and 159 cells/μl, and plasma HIV RNA levels were 3.6 log10 copies/ml, 4.3 log10 copies/ml, and 2.6 log10 copies/ml, respectively. Baseline Burn Scale scores were 12, 72, and 56%, respectively, in these patients.
Discussion
This cohort of HIV-infected persons treated with ART in northern Tanzania experienced marked improvements in cutaneous symptoms. The complete resolution of skin abnormalities in 10 (12%) subjects and the decrease in Burn Scale scores from a median of 38% to 7% over six months may highlight the important role of the skin as an indicator of immunologic health. Similar findings have been reported in populations from more wealthy settings17 and in one previous study in Uganda.10 In parallel, peripheral blood markers of disease progression, including CD4+ cell counts and plasma HIV RNA levels, improved dramatically.
The population under study demonstrated abnormal skin findings at baseline and was mainly recruited from a dermatology clinic. All clinical diagnoses were confirmed by biopsy, and PPE was the most common diagnosis. These findings differ from a previous description of an HIV-infected cohort in Dar es Salaam, in which fungal skin infections represented the most common diagnosis.18 The reasons for these diagnostic differences are not clear. Our study subjects clearly had progressive HIV disease as documented by WHO Stage III or IV clinical manifestations, low CD4+ cell counts, and elevated plasma HIV RNA levels. Such late presentations of HIV infection are common in northern Tanzania and more broadly across sub-Saharan Africa.18,19
These improvements in skin findings were not specific to any single dermatologic diagnosis and were accompanied by increased Karnofsky status scores. Three patients demonstrated worsened skin disease within two months of the initiation of ART, which indicated IRS; however, these patients had improved by the end of six months of follow-up. The previous study undertaken in Uganda investigated 53 patients with PPE only and found a strong relationship between improvements in skin findings and virologic responses to ART.10 Our study has extended these findings to a larger population with dermatologic diagnoses beyond that of PPE.
All subjects received a fixed-dose combination of stavudine, lamivudine, and nevirapine as their ART regimen in accordance with Tanzanian Ministry of Health guidelines.19 This ART regimen has demonstrated outstanding CD4+ cell count and plasma HIV RNA responses in other cohorts in RLSs.18 Our study subjects showed comparable responses in CD4+ cell counts and plasma HIV RNA levels after six months. We were unable to demonstrate a relationship between improvements in skin findings and the magnitude of CD4+ cell count increases or responses in plasma HIV RNA levels below detectable limits. The lack of such a relationship may reflect differences between peripheral blood and cutaneous compartments, a complex balance of immunologic recovery and immune reconstitution inflammatory syndrome in skin, or our small sample size.
Adverse drug reactions were uncommon in our population despite the use of trimethoprim-sulfamethoxazole and a nevirapine-containing ART regimen. Only two subjects discontinued their medications over the first six months, the period during which adverse drug reactions are most likely to occur. Two subjects died within the first six months, a relatively low percentage in comparison with those in other cohorts receiving ART in RLSs.20 In a study performed in 2008 among HIV-1-infected patients in Mozambique, Tanzania, and Malawi, a mortality rate of 7.5% was observed during the first year of ART therapy.21 The low death rate in the present study may reflect the recruitment of the study cohort from a dermatology clinic rather than a setting in which more severe medical illnesses and complicating opportunistic infections such as tuberculosis would be more common.
That we recruited our subjects from a dermatology clinic may have introduced some selection bias into our study, and its results may not be generalizable to all clinical situations. However, cutaneous complaints are widely identified within HIV-infected populations. All subjects were examined by a single observer, whose baseline diagnoses were confirmed by biopsy results. The observer also used repeat photography to assess changes in skin findings over time. Our subjects had low CD4+ cell counts at baseline, and these results may not necessarily be extrapolated to HIV-infected persons with skin complaints and higher CD4+ cell counts who begin ART. Our sample size was relatively small, which may have precluded our ability to identify associations between improvements in skin findings and the magnitude of CD4+ cell count increases and suppression of plasma HIV RNA levels.
In summary, the benefits of ART include improvements in skin abnormalities among HIV-infected persons. Healthcare providers and patients with cutaneous problems should consider ART as a promising intervention.
Acknowledgments
Funding: None.
Footnotes
Conflicts of interest: None.
References
- 1.Cebes LO, Gonzalez Intxaurraga MA. Dermatoses in the AIDS. Acta Dermatovenerol Alp Panonica Adriat. 2001;10 [Google Scholar]
- 2.Josephine M, Issac E, George A, et al. Patterns of skin manifestations and their relationships with CD4 counts among HIV/AIDS patients in Cameroon. Int J Dermatol. 2006;45:280–284. doi: 10.1111/j.1365-4632.2004.02529.x. [DOI] [PubMed] [Google Scholar]
- 3.Hengge UR, Franz B, Goos M. Decline of infectious skin manifestations in the era of highly active antiretroviral therapy. AIDS. 2000;14:1069–1070. doi: 10.1097/00002030-200005260-00025. [DOI] [PubMed] [Google Scholar]
- 4.Dlova NC, Mosam A. Inflammatory noninfectious dermatoses of HIV. Dermatol Clin. 2006;24:439–448. doi: 10.1016/j.det.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Maurer TA. Perspective – dermatologic manifestations of HIV infection. Top HIV Med. 2005;13:149–154. [PubMed] [Google Scholar]
- 6.Morpeth SC, Crump JA, Shao HJ, et al. Predicting CD4 lymphocyte count < 200 cells/mm3 in an HIV type 1-infected African population. AIDS Res Hum Retroviruses. 2007;23:1230–1236. doi: 10.1089/aid.2007.0053. [DOI] [PubMed] [Google Scholar]
- 7.Nnoruka E, Chukwuka J, Anisuiba B. Correlation of mucocutaneous manifestations of HIV/AIDS with CD4 counts and disease progression. Int J Dermatol. 2007;46:14–18. doi: 10.1111/j.1365-4632.2007.03349.x. [DOI] [PubMed] [Google Scholar]
- 8.Zancanaro PC, McGirt LY, Mamelak AJ, et al. Cutaneous manifestations of HIV in the era of highly active antiretroviral therapy: an institutional urban clinic experience. J Am Acad Dermatol. 2006;54:581–588. doi: 10.1016/j.jaad.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 9.UNAIDS. [Accessed August 27, 2012];The Global AIDS Epidemic – Fact Sheet. 2011 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/201207_FactSheet_Global_en.pdf.
- 10.Castelnuovo B, Byakwaga H, Menten J, et al. Can a response of pruritic papular eruption to antiretroviral therapy be used as a clinical parameter to monitor virologic outcomes? AIDS. 2008;22:269–273. doi: 10.1097/QAD.0b013e3282f313a9. [DOI] [PubMed] [Google Scholar]
- 11.Montaner JS, Hogg R, Raboud J, et al. Antiretroviral treatment in 1998. Lancet. 1998;352:1919–1922. doi: 10.1016/S0140-6736(98)07532-1. [DOI] [PubMed] [Google Scholar]
- 12.Kumarasamy N, Solomon S, Chaguturu SK, et al. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in Southern India. Clin Infect Dis. 2005;41:1525–1528. doi: 10.1086/497267. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson MA. [Accessed December 11, 2011];Clinical implications of immune reconstitution in AIDS. 2005 http://hivinsite.ucsf.edu/InSite?page=kb-03-04-03.
- 14.Mayhood MK, Afwamba IA, Odhiambo CO, et al. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and determine HIV-1/2 rapid HIV antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46:3946–3951. doi: 10.1128/JCM.01045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crump JA, Scott LE, Msuya E, et al. Evaluation of the Abbott m2000rt RealTime HIV-1 assay with manual sample preparation compared with the ROCHE COBAS AmpliPrep/Amplicor HIV-1 Monitor Version 1. 5 using specimens from East Africa. J Virol Methods. 2009;162:218–222. doi: 10.1016/j.jviromet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott LE, Crump JA, Msuya E, et al. Abbott RealTime HIV-1 m2000rt viral load testing: manual extraction versus the automated m2000sp extraction. J Virol Methods. 2011;172:78–80. doi: 10.1016/j.jviromet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calista D, Morri M, Stagno A, et al. Changing morbidity of cutaneous diseases in patients with HIV after the introduction of highly active antiretroviral therapy including a protease inhibitor. Am J Clin Dermatol. 2002;3:59–62. doi: 10.2165/00128071-200203010-00006. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. [Accessed February 24, 2011];Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. 2006 www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
- 19.Ministry of Health and Social Welfare. National AIDS Control Program (NACP) 2. Dar es Salaam: MoHSW; 2005. National guidelines for the clinical management of HIV/AIDS – Tanzania. [Google Scholar]
- 20.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24:555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 21.Rosen S, Fox M, Gill C. Patient retention in antiretroviral therapy programs in sub-Saharan Africa. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]