Abstract
OBJECTIVES:
Seeds are excellent sources of proteinase inhibitors, some of which may have satietogenic and slimming actions. We evaluated the effect of a trypsin inhibitor from Tamarindus indica L. seeds on weight gain, food consumption and cholecystokinin levels in Wistar rats.
METHODS:
A trypsin inhibitor from Tamarindus was isolated using ammonium sulfate (30–60%) following precipitation with acetone and was further isolated with Trypsin-Sepharose affinity chromatography. Analyses were conducted to assess the in vivo digestibility, food intake, body weight evolution and cholecystokinin levels in Wistar rats. Histological analyses of organs and biochemical analyses of sera were performed.
RESULTS:
The trypsin inhibitor from Tamarindus reduced food consumption, thereby reducing weight gain. The in vivo true digestibility was not significantly different between the control and Tamarindus trypsin inhibitor-treated groups. The trypsin inhibitor from Tamarindus did not cause alterations in biochemical parameters or liver, stomach, intestine or pancreas histology. Rats treated with the trypsin inhibitor showed significantly elevated cholecystokinin levels compared with animals receiving casein or water.
CONCLUSION:
The results indicate that the isolated trypsin inhibitor from Tamarindus reduces weight gain by reducing food consumption, an effect that may be mediated by increased cholecystokinin. Thus, the potential use of this trypsin inhibitor in obesity prevention and/or treatment should be evaluated.
Keywords: Tamarind, Satiety, Slimming, Obesity, CCK
INTRODUCTION
Obesity is considered an epidemic in the globalized world. As the cost of treating obesity and its associated diseases is high (1,2), investments in natural products that may reduce dietary intake and weight gain are needed. Thus, advances in scientific technical knowledge involving quality, safety and efficacy, which are inherent to herbal medicines, have placed the area of bioactive natural products in a position of great interest in recent years. In the treatment of obesity, factors such as the high cost and the side effects of drugs, including those presented by allopathic anorectics, have increased the demand for natural products with slimming actions (3).
In this context, vegetable- and fruit-derived proteinase inhibitors have been studied in animal models for their effects on reducing energy consumption and/or food intake. These studies confirm that endogenous cholecystokinin (CCK) is important in the control of food intake. Furthermore, protease inhibitors may have therapeutic potential to reduce food intake by stimulating increases in serum CCK (4–7).
The tamarind fruit is often consumed in Brazil and almost all of its parts are used. Although many therapeutic effects, such as laxative, digestive and anti-diabetes effects are locally attributed to this fruit (8,9), few studies have tested the effect of this fruit or its extracts in vitro or in vivo.
Our group previously isolated a trypsin inhibitor from tamarind seeds (TTI). This inhibitor is 20 kDa in size and presented a non-competitive inhibition mechanism. TTI inhibited the larval growth of Rhyzophertha dominica, Anthonomus grandis and Ceratitis capitata by inhibiting digestive enzymes and did not show inhibition either to the serine proteases elastase and chymotrypsin or to the cysteine proteases papain and bromelain (10). Thus, trypsin inhibitors have been isolated and their heterologous actions related to health benefits, such as anti-inflammatory, gastroprotective and satiety control effects (7,12,13), have been extensively investigated (11).
Nevertheless, it remains unclear whether these extracts present effects in reducing food consumption and weight gain and if these effects are CCK dependent. Thus, this study investigated the effect of TTI in food consumption and weight gain in rats. We also analyzed CCK levels in the studied animals. Our results show a potential effect of TTI in reducing food consumption and weight gain, possibly mediated by increased CCK levels. This study shows that isolated TTI may be a potential phytotherapeutic candidate for preventing/treating obesity.
MATERIALS AND METHODS
Materials
Chemicals and Reagents: CELM Kit® (São Paulo, Brazil); Kit transferases from Labtest Diagnostic (Paraná, Brazil); Kit Phoenix Pharmaceuticals Inc. (Burlingame, USA); Soybean trypsin inhibitor, Kunitz type, from Sigma (St. Louis, MO).
Tamarind fruit seeds
Tamarind fruits were obtained from markets in Natal, a city from the Rio Grande do Norte state in northeastern Brazil. Tamarind seeds were obtained after peeling and removing the pulp using knives.
Isolation of the tamarind seed trypsin inhibitor
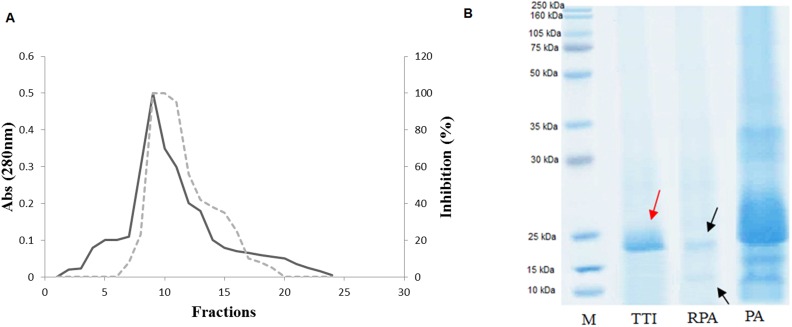
Isolation of the tamarind seed trypsin inhibitor followed the methodology described by Araújo et al. (10), with modifications. The modifications occurred in the step to obtain TTI; once the fraction was saturated with 30–60% ammonium sulfate from the crude extract, Tamarindus indica L. seeds were precipitated with acetone 1∶2 (v/v). The fraction obtained after precipitation was isolated using Trypsin-Sepharose 4B affinity chromatography, subjected to dialysis for approximately 24 h against Tris-HCl buffer (50 mM, pH 7.5), labeled TTI and then subjected to 12% polyacrylamide gel electrophoresis in the presence of SDS. All isolation steps were monitored and subjected to trypsin inhibition assays using the specific substrate BApNA (N-benzoyl-DL-arginine-p-nitroanilide) (14) and protein quantification (15). Figure 1) shows the steps of the TTI isolation.
Figure 1.
Trypsin-Sepharose 4B affinity chromatography of the tamarind seed trypsin inhibitor (TTI). A) Elution profile of the retained acetone-precipitated fraction (RPA). Adsorbed proteins were monitored at 280 nm (-). The inhibitory activity on trypsin (---) was assayed using 100 µL of TTI. B) Denaturing electrophoresis on a 12% polyacrylamide gel after staining with Coomassie Brilliant Blue R-250. M, Molecular mass markers; PA, precipitated with acetone; RPA, fraction obtained after precipitation with acetone isolated by Trypsin-Sepharose affinity chromatography and TTI, tamarind trypsin inhibitor, isolated by Trypsin-Sepharose affinity chromatography and subjected to dialysis. The arrows indicate 14 kDa for RPA and 20 kDa for TTI.
Animal study design
In this study, two animal experiments were conducted. All experimental procedures were approved by the Animal Ethics Committee of the Federal University of Rio Grande do Norte state in Brazil (protocol No. 011/2010).
Experiment I
This first experiment was performed to assess the effect of TTI on food consumption, weight gain and TTI digestibility in rats. Male Wistar rats (n = 24) aged 3 months and weighing 100 to 150 g were kept in cages in a vivarium at 23±2°C with a 12-hour light-dark cycle and humidity between 45 and 55%. After 3 days of adaptation in metabolic cages, the rats were divided into 4 groups according to the diets received for 11 days: 1) Standard diet, AIN-93G (S, n = 6); 2) Standard diet, AIN-93G+1 mL of water (SW, n = 6); 3) Protein-free diet (PF, n = 6); 4) AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL (25 mg/kg TTI, n = 6). Water and TTI were given by oral gavage. Rats were weighed daily using a calibrated scale. Food consumption was registered daily, as described below. Feces were individually collected on the 4th, 7th and 10th days, identified and kept at -20°C for digestibility analysis. The feces were dried in a greenhouse with circulating air at 105°C for 24 h, cooled, weighed and crushed in a multiprocessor to measure the total nitrogen levels (16). The apparent and true digestibilities were then calculated (17).
On day 11, the rats were anesthetized with 2% xylazine and 5% ketamine and then euthanized. Blood was collected by cardiac puncture and stored in Falcon tubes. Serum was separated by centrifugation at 3000 g for 10 min and used for the measurement of glucose (GL), triacylglycerol (TG), total cholesterol (COL) and high-density lipoprotein (HDL) (Kit CELM®, São Paulo, Brazil). The blood samples were also subjected to enzymatic assays for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Labtest transferases diagnostic kit, Paraná, Brazil). Organs from euthanized rats were assessed for histopathological alterations as described below (18).
Experiment II
This experiment was performed to assess the effect of TTI on short-term satiety and CCK production in rats. Male Wistar rats (n = 36) aged 3 months and weighing 100 to 150 g were kept in cages in a vivarium at 23±2°C with a 12-hour light-dark cycle and humidity between 45 and 55%. All rats received standard diets (AIN-93G) ad libitum during this experiment (19). After 3 days of adaptation in metabolic cages, the rats were divided into 5 groups according to the treatment received by oral gavage: 1) Standard diet, AIN-93G+1 mL of water (SW, n = 6); 2) AIN-93G+25 mg/kg Casein in 1 mL water (25 mg/kg C, n = 6); 3) AIN-93G+25 mg/kg Soybean trypsin inhibitor in 1 mL water (25 mg/kg IS, n = 6); 4) AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL water (25 mg/kg TTI, n = 6); and 5) AIN-93G+50 mg/kg tamarind trypsin inhibitor in 1 mL water (50 mg/kg TTI, n = 6).
Casein is a protein without inhibitory activity that was used to discard the hypothesis that the effect of TTI could be an unspecific protein effect. Soybean trypsin inhibitor was used to compare the satietogenic effect of TTI once it was well documented that soybean trypsin inhibitor promotes satiety (6,20). Two different TTI concentrations were used to verify whether the observed TTI effects were dose dependent.
In experiment II, the rats were also weighed daily using a calibrated scale. Food consumption was registered 1 h, 2 h and 16 h after treatment, as described below. On day 11, blood samples were taken 1 h after treatment for CCK analysis, as described below. Next, rats were anesthetized with 2% xylazine and 5% ketamine and then euthanized. The blood was collected by cardiac puncture and stored in Falcon tubes. Gamma glutamyl transferase was evaluated by measuring the blood dosage (21). Total protein was measured using the biuret method (22). Albumin was measured using bromocresol green (BCG). The globulin content was calculated as the difference between the total protein and albumin and then the albumin/globulin ratio was calculated. C-reactive protein was detected by agglutination immune reaction using commercially available kits (BioClin).
Assessment of food consumption and body weight evolution
Food consumption was analyzed daily in all experiments by calculating the difference between the diet provided (before consumption) and the diet consumed using a calibrated scale with 0.01 mg precision (Tecnal). In experiment I, this calculation was performed as follows: food consumption per day (g) = diet provided (g) - diet consumed (g). The results for each day are expressed as the mean food consumption (g) in each group. In experiment II, the results are expressed as the percentage (%), considered 100% of the average of the usual pattern of food consumption of the rats before testing (3 days of adaptation). Thus, after testing, the percentage decrease in consumption (%) was obtained. In experiment I, weights were also assessed daily using a calibrated scale with 0.01 mg precision (Tecnal). The results are expressed as the mean weight gain per group.
Assessment of histopathological alterations
In experiment I, after the animals were euthanized, the thoracic-abdominal cavity was opened. The intestines, pancreas, stomach and liver were removed and fixed with paraffin in blades using a standardized method (18).
Plasmatic CCK evaluation
In experiment II, plasmatic CCK was measured 1 h after treatment by an immunoassay using commercially available kits according to the manufacturer's instructions (Phoenix Pharmaceuticals Inc., Burlingame, USA).
Statistical analysis
Weight gain and food consumption were analyzed using multifactor ANOVA to find possible differences between groups. When significant differences were detected, Tukey's post-hoc test was used. CCK and the biochemical parameters (GL, TG, total cholesterol, HDL, LDL, ALT, AST, GGT, albumin and C-reactive protein) were analyzed and compared between the different groups using Student's t-test and multifactorial ANOVA with Tukey's post-hoc test. Data were analyzed for normality and homoscedasticity through the Kolmogorov-Smirnov test and Levene's test, respectively. All data were analyzed using the Statistica 7 software (Stat Soft, Tulsa, OK, USA).
RESULTS AND DISCUSSION
TTI reduced weight gain and food consumption in rats
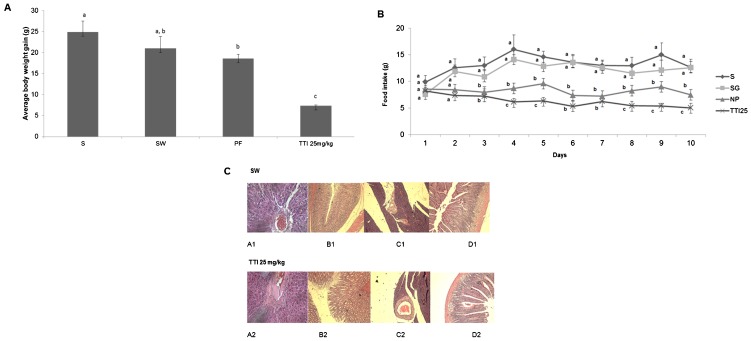
As shown in Figure 2A), weight gain was significantly lower in the animals treated with TTI in experiment I. By treating one group with Standard diet (S) and another with AIN-93G+1 mL of water given by gavage (SW), our data reject the hypothesis that the gavage itself could reduce weight gain. Interestingly, 25 mg/kg TTI treatment caused a reduction in weight gain that was higher than the protein-free diet.
Figure 2.
Wistar rats were subjected to oral gavage with the standard diet AIN-93G (S, n = 6), standard diet AIN-93G+1 mL of water (SW, n = 6), protein-free diet (PF, n = 6), or AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL water (25 mg/kg TTI, n = 6) for 11 days. A) The evolution of body weight, in grams (g). The results are expressed as the mean ± standard deviation of each group. Different letters indicate p<0.05 using one-way ANOVA and Tukey's post-hoc test. B) The average food intake in grams per day (g/day). The results are expressed as the mean ± standard deviation of each group. C) Assessment of the histopathological alterations in the SW and TTI groups. A1 and A2: liver sections (40× magnification); B1 and B2: stomach sections (10× magnification); C1 and C2: pancreatic sections (10× magnifications); D1 and D2: bowel sections (10× magnification).
The food consumption data corroborates weight gain data. Figure 2B) shows that animals treated with 25 mg/kg TTI had the lowest food consumption compared with the other studied groups.
Mclaughlin et al. (23) analyzed the effect of the synthetic trypsin inhibitor N,N-dimethyl-4-(4-guanidino-benzyloxy)-phenyl ethyl methane-sulfate (DGPM) in obese and thin Zucker rats. The administration of DGPM (25 to 200 mg/kg) in rats after 6 h of fasting decreased food consumption in a dose-dependent manner. A decrease in the mean size of the meals was observed in both obese and thin rats. Nevertheless, this effect was higher among the obese rats. Furthermore, the administration of 100 mg/kg twice a day for 7 days decreased food consumption and body weight in obese rats but not in thin rats. This shows a specific prominent action of DGPM in obese rats (23). In our study, we did not evaluate obese rats.
As observed by Mclaughlin et al. (23) using DGPM, the effects of TTI could possibly be potentiated in obese models. Nevertheless, our data for normal Wistar rats are consistent, thus indicating that further studies using obese models should be performed to verify if the effects observed are more prominent in an obese model.
A recent study evaluated the effect of concentrated potato protease inhibitors (PPIC), potato protease inhibitor II and casein at concentrations of 100 mg/kg administered for 10 days by oral gavage on food consumption and weight gain in Wistar rats (180-200 g) (24). The authors found that casein and PPIC significantly reduced food consumption. Similarly to our results, the use of PPIC caused a reduction in weight gain of 11%.
Nevertheless, our results show a more powerful effect of TTI with a smaller dose (25 mg/kg) and the reduction in weight gain was higher (approximately 70%). Furthermore, we found a considerable effect on food consumption once food consumption was reduced by 47%.
TTI treatment produced no effects on in vivo digestibility
The satisfactory use of proteins for nutrition is dependent on the protein composition, its amino acid bioavailability, digestibility and the presence or absence of toxicity and anti-nutritional factors (25,26). Thus, analyzing the digestibility of 25 mg/kg TTI was important to assess whether the reduction effects on weight gain and food consumption was due to the existence of anti-nutritional factors that could affect overall protein absorption.
Table 1 shows the digestibility results for the groups of animals in experiment I. There were no significant differences between true digestibility and apparent digestibility in the different groups studied. This excludes the hypothesis that TTI could cause digestibility alterations that interfere with the protein digestion/absorption process in treated animals.
Table 1.
True digestibility and apparent digestibility in Wistar rats subjected to the standard diet AIN-93G (S, n = 6), AIN-93G+1 mL of water (SW, n = 6), AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL water (25 mg/kg TTI, n = 6) and protein-free diet (PF, n = 6) for 11 days. The results are expressed as the mean ± standard deviation in each group. Different lower case letters indicate p<0.05 between groups. Different upper case letters indicate p<0.05 between true digestibility and apparent digestibility. One-way ANOVA followed by Tukey's post-hoc test or Student's t-test was used.
| Treatment | Urine nitrogen (%) | Fecal nitrogen (%) | True Digestibility (%) | Apparent Digestibility (%) |
| S | 1.12 | 1.75 | 90.70±1.22aA | 96.36±0.91aB |
| SW | 1.15 | 1.69 | 91.10±1.02aC | 96.68±2.03aD |
| TTI | 1.18 | 1.68 | 89.88±0.94aE | 92.58±1.38aE |
| PF | 0.18 | 0.39 | - | - |
Not all trypsin inhibitors have the effect of reducing weight gain without interfering with protein digestibility. Some studies, using Wistar rats fed soybean trypsin inhibitor, found a reduction in weight gain and food consumption (27–29). However, soybean trypsin inhibitor also reduced digestibility compared with casein, which was not the case in our study.
TTI treatment did not alter the histopathology of specific organs
Because oral administration of plant extracts may cause in vivo damage to organs (27–30), in experiment I, we analyzed the effect of 25 mg/kg TTI on the histopathology of the intestines, pancreas, stomach and liver. Figure 2C) shows that there were no alterations in the liver parenchyma of treated animals: the stroma was preserved, the arterioles, venules and capillaries were filled with normal red blood cells, the membranes and nuclei were preserved and no signs of cell death could be observed.
Animals treated with TTI also presented normal stomach mucosa, intact gastric glands, no inflammatory infiltration and intact submucosal, muscular and serous layers (Figure 2C). The pancreas also showed no alterations, exhibiting intact tissue, normal Langerhans islets and no size alterations or cytoarchitectural distortion (Figure 2C). The intestines also presented normal architecture and layers, with no signs of inflammatory infiltration (Figure 2C).
These results are in accordance with those of other studies that have found no histopathological variations in diverse organs after treatment with trypsin inhibitors (20,31). Unlike lectins (27), trypsin inhibitors do not seem to interact with intestinal mucosal cells.
TTI produced no effects on the general biochemical parameters of treated animals
The results of the biochemical analysis of the effect of 25 mg/kg TTI treatment in experiment I is shown in Table 2. Glucose, total cholesterol, LDL, HDL and triglycerides were not different between TTI-treated animals and controls. As expected, glucose and triglycerides were significantly lower in the animals that received the protein-free diet, possibly due to an adaptation of metabolism because the feeding was not balanced. The same effect was observed in experiment II (Table 3), in which TTI at 25 mg/kg and 50 mg/kg doses also did not cause significant alterations in glucose, total cholesterol, HDL or triglycerides compared with the other groups.
Table 2.
Biochemical parameters in Wistar rats subjected to the standard diet AIN-93G (S, n = 6), standard diet AIN-93G+1 mL of water (SW, n = 6), protein-free diet (PF, n = 6) and AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL water (25 mg/kg TTI, n = 6) for 11 days. The results are expressed as the mean ± standard deviation of each group. Different letters indicate p<0.05 between groups; one-way ANOVA followed by Tukey's post-hoc test was used.
| Groups Parameters | S | SW | PF | TTI 25 mg/kg |
| Glucose | 151.2±7.2a | 162.3±6.7a | 93.0±1.0b | 158.1±9.8a |
| Total cholesterol | 51.4±6.2a | 59.7±4.3a | 54±6.0b | 41±3.0a |
| LDL | 19.8±6.2a | 32.7±3.3b | 18.0±3.0a | 24.3±3.5a |
| HDL | 24.2±2.2a | 23.8±0.8a | 20.0±0.8b | 24.6±1.2a |
| Triglycerides | 26.6±1.4a | 18.5±6.7a | 19.5±5.8b | 21.3±4.7a |
| ALT | 24.4±4.6a | 26.8±5.1a | 13.7±0.3b | 21.1±1.0a |
| AST | 82±8.0a | 91±7.1ab | 102±7.0b | 79±6.2a |
Table 3.
Biochemical parameters in Wistar rats fed the standard diet, AIN-93G+1 mL of water (SW, n = 6), AIN-93G+25 mg/kg casein in 1 mL water (25 mg/kg C, n = 6), AIN-93G+25 mg/kg soybean trypsin inhibitor in 1 mL water (25 mg/kg IS, n = 6); AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL water (25 mg/kg TTI, n = 6), and AIN-93G+50 mg/kg tamarind trypsin inhibitor in 1 mL water (50 mg/kg TTI, n = 6) for 11 days. The results are expressed as the mean ± standard deviation of each group. Different letters indicate p<0.05 between groups; one-way ANOVA followed by Tukey's post-hoc test was used.
| Groups Parameters | SW | C 25 mg/kg | IS 25 mg/kg | TTI 25 mg/kg | TTI 50 mg/kg |
| Glucose | 212±35.9a | 212±64.8a | 220±38.2a | 212±58.0a | 205±57.5a |
| Total cholesterol | 75.3±9.8a | 81.9±12a | 88.8±10.5a | 76.2±2.1a | 83.5±8.4a |
| LDL | 26.1±8.1a | 33.4±1.4a | 39±12.6a | 32.3±9.3a | 40.3±6.7a |
| HDL | 24.1±3.8a | 32.8±6.3a | 33.2±6.9a | 27.6±3.3a | 34.0±6.5a |
| Triglycerides | 82.2±19.0a | 102.5±33.1a | 75.0±33.8a | 59.0±25.5a | 64.7±25.0a |
| ALT | 78.4±15.5a | 60.4±24.7a | 67.5±10.1a | 57.5±9.8a | 59.7±5.6a |
| AST | 137.6±16.9a | 158.8±26.6a | 101.5±35.2a | 149.6±36.4a | 154±49.4a |
| Gamma GT | 5.5±1.2a | 7.0±1.9a | 10.6±4.0a | 9.7±3.5a | 8.9±2.8a |
| Albumin | 4.5±0.3a | 4.58±0.8a | 5.0±0.5a | 4.5±0.1a | 4.55±0.1a |
| Globulin | 4.2±0.1a | 3.3±0.8b | 4.0±0.18ab | 3.9±0.5ab | 3.1±0.2b |
| ALB/GLO Ratio | 1.1±0.1a | 1.4±0.2b | 1.3±0.17ab | 1.2±0.2ab | 1.47±0.0b |
| Total protein | 8.6±0.7ab | 7.9±1.4ab | 8.9±0.4a | 8.3±0.7ab | 7.6±0.3b |
| C-reactive Protein | NR | NR | NR | NR | NR |
NR: Non-reactive.
Interestingly, 25 mg/kg TTI treatment did not decrease glucose levels after decreasing food consumption. This absence of an effect on glucose levels was also observed in experiment II, as shown in Table 3.
The liver enzymes ALT and AST were also similar between the 25 mg/kg and 50 mg/kg TTI-treated and control groups in both experiments I and II (Tables 2 and 3), respectively), which may indicate that the use of TTI did not cause liver damage. Furthermore, gamma GT, analyzed in experiment II, did not differ between the studied groups. This is in agreement with the liver histopathological analysis, which showed no significant alterations, suggesting that TTI may be harmless to this tissue (Figure 2C).
To the best of our knowledge, this is the first study to assess gamma GT in Wistar rats treated with trypsin inhibitors. Nevertheless, Garthoff (20), in studies using pigs given a partially purified trypsin inhibitor preparation, found that GGT levels were not changed.
To corroborate the digestibility analyses, we also evaluated the effect of TTI on total protein, albumin and globulin levels in experiment II. As shown in Table 3, albumin levels did not differ between the groups studied, showing that none of the treatments interfered with the nutritional status of the animals. Globulin was significantly lower in the 50 mg/kg TTI-treated and casein-treated groups compared with the group fed a standard diet (Table 3).
Globulin changes may indicate adaptation to stress. Adapted animals tend to have normal values, whereas stressed animals have increased levels (32,33). Thus, based on the small decrease found in the 25 mg/kg and 50 mg/kg TTI- and casein-treated groups, it is not likely that the animals were stressed.
As globulin levels were different in the 50 mg/kg TTI- and casein-treated groups compared with the control groups, the albumin/globulin ratio was also significantly different in these groups. Nevertheless, the total protein levels were similar between the studied groups.
Other studies using trypsin inhibitors have found no alterations in glucose, cholesterol, triglycerides, total protein, globulin and albumin levels in pig (20), mice (34) and rat (24,35) models.
To assess the possible induction of inflammation by 25 mg/kg and 50 mg/kg TTI, we also analyzed C-reactive protein in the animals from experiment II. No alterations were found and all groups presented similar C-reactive protein concentrations (Table 3).
TTI increased short-term satiety and CCK levels
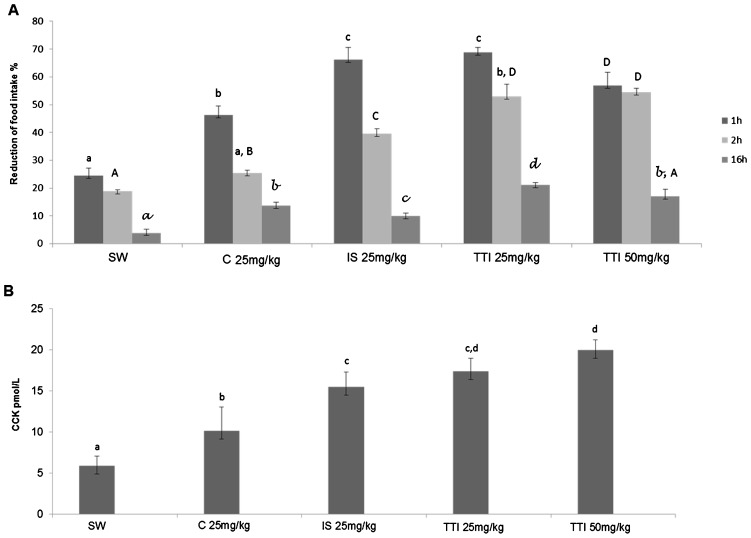
In experiment II, as shown in Figure 3A), food consumption was significantly reduced after the 1st hour in mice treated with TTI at 25 mg/kg and 50 mg/kg doses, with values of 68.87±1.80% and 56.80±4.80%, respectively, compared with that of the mice fed a standard diet (mean reduction of 26.41±2.86%) and of the animals that received casein (mean reduction of 46.30±3.27%). The same effect of food consumption reduction was observed after the 2nd hour of TTI treatment. Nevertheless, only TTI treatment at 50 mg/kg (54.50±1.32%) resulted in a significant difference between animals fed standard diet (18.86±0.54%) and animals given casein (25.74±1.00%).
Figure 3.
Wistar rats were subjected to oral gavage using the AIN-93G standard diet +1 mL of water (SW, n = 6), AIN-93G+25 mg/kg casein in 1 mL water (25 mg/kg C, n = 6), AIN-93G+25 mg/kg soybean trypsin inhibitor in 1 mL water (25 mg/kg IS, n = 6), AIN-93G+25 mg/kg tamarind trypsin inhibitor in 1 mL water (25 mg/kg TTI, n = 6) and AIN-93G+50 mg/kg tamarind trypsin inhibitor in 1 mL water (50 mg/kg TTI, n = 6) for 11 days. A) Food consumption (%) after 1 h, 2 h and 6 h of gavage in the studied groups. B) Plasmatic CCK levels after 1 h of gavage in the studied groups. The results are expressed as the mean ± standard deviation of each group. Different letters indicate p<0.05 using one-way ANOVA and Tukey's post-hoc test.
After 16 h of TTI treatment, a reduction in food consumption was again observed. The 25 mg/kg TTI-treated group displayed significantly reduced food consumption (21.17±0.89%) compared with the reduction observed in the standard diet-fed (3.89±1.29%), casein-treated (13.67±1.25%) and soybean trypsin inhibitor-treated (9.89±1.11%) groups.
Taken together, the results from our study show that TTI at the studied doses may cause a reduction in food intake during the 1st and 2nd hours after administration and that this reduction may last until 16 h after treatment.
To evaluate whether the effect of TTI (25 mg/kg and 50 mg/kg) on reducing food consumption was CCK associated, we evaluated plasmatic CCK levels in experiment II. As shown in Figure 3B), CCK levels after 1 h of treatment were significantly lower in the animals that received a standard (5.92±1.15 pmol/L) and casein diet (10.14±2.9 pmol/L) compared with the groups that received trypsin inhibitors. Among these groups, those treated with TTI at 25 mg/kg and 50 mg/kg presented the highest CCK levels (17.41±1.60 and 20±1.22 pmol/L, respectively). Interestingly, CCK levels in the animals that received TTI at 50 mg/kg were significantly higher than those in the animals treated with soybean trypsin inhibitor (15.51±1.82 pmol/L), an inhibitor with a well-known satiety effect (36,37).
The effect of CCK on human satiety was first described by Pi-Sunyer et al. (38) using exogenous CCK infusion. Ballinger et al. (39) reported a 20% reduction in caloric intake after a physiological infusion of CCK-8. In another study, the effect of CCK-33 at physiological concentrations on satiety was studied in slim and obese individuals. In this study, CCK also promoted satiety and no differences in nutritional status were observed (39). This is an interesting finding because it indicates that natural molecules with the potential to induce CCK secretion may be used to promote satiety, thereby preventing or treating human obesity.
For the above reasons, trypsin inhibitors have been studied. So far, few studies have been performed in humans. Hill et al. (5) evaluated the effect of a potato proteinase inhibitor (POT II) on food intake in 11 lean individuals. A double-blind study was carried out and the authors observed that the use of POT II 5 minutes before lunch significantly reduced energy consumption by 17.5%. In neonatal rats administered soybean trypsin inhibitor (1 mg in 230 mL saline), an 87% increase in CCK plasmatic concentrations was observed (36). Komarnytsky et al. (24) also reported that the oral administration of a potato-derived protease inhibitor concentrate (PPIC) was effective in reducing food intake and weight gain in healthy rats by increasing circulating CCK levels through a trypsin-dependent mechanism.
Another study evaluated the effect of a potato extract that included trypsin inhibitor proteins (Protein) in satiety and CCK production by enteroendocrine cells. In this study, soybean trypsin inhibitor was used as a positive control and water was used as a negative control. The evaluation was performed up to 6 h after administration. The results showed that although soybean trypsin inhibitor and protein reduced food intake, protein but not soybean trypsin inhibitor dose-dependently induced CCK secretion in STC-1 cells (6).
To the best of our knowledge, this is the first in vivo study using TTI, which comes from a very popular fruit in northeastern Brazil. Our data, using TTI at 25 mg/kg and 50 mg/kg doses, possibly demonstrates that the effect of TTI on increasing CCK is not dose dependent. Nevertheless, further kinetic in vivo studies are needed to confirm this hypothesis. Furthermore, this study showed that TTI has an excellent effect on reducing weight gain and increasing satiety. These effects are possibly mediated by stimulating CCK production. To ensure that TTI is a promising phytotherapeutic extract in the prevention and/or treatment of obesity, more studies are needed to understand the mechanisms by which TTI may induce CCK secretion. The TTI signaling pathways and interactions with hormones and adipokines also need to be studied.
ACKNOWLEDGMENTS
This work received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Cientítico e Tecnológico (CNPq) Brazilian research promotion agencies. The authors would like to thank Mauricio Pereira de Sales (in memoriam) for stimulating the development of this work.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.WHO. World Health Organization; Obesity and overweight. [cited 2014 Dec 21]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 2.Wilding JPH. Pathophysiology and aetiology of obesity. Medicine (Baltimore) 2011;39(1):6–10. [Google Scholar]
- 3.Bernardi MM, Spinosa HS, Ricci EL, Reis-Silva M, Silva AC, Dalmolin DP. Perda de peso em ratos alimentados com ração hipercalórica e tratados com o fitoterápico pholianegra® Fac Med Veterinária e Zootec. 2011:2–6. [Google Scholar]
- 4.Chen W, Hira T, Nakajima S, Tomozawa H, Tsubata M, Yamaguchi K, et al. Suppressive Effect on Food Intake of a Potato Extract (Potein Ò) Involving Cholecystokinin Release in Rats. Biosci Biotechnol Biochem. 2012;76(6):1104–9. doi: 10.1271/bbb.110936. [DOI] [PubMed] [Google Scholar]
- 5.Hill A J, Peikin SR, Ryan C A, Blundell JE. Oral administration of proteinase inhibitor II from potatoes reduces energy intake in man. Physiol Behav. 1990;48(2):241–6. doi: 10.1016/0031-9384(90)90307-p. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima S, Hira T, Tsubata M, Takagaki K, Hara H. Potato extract (Potein) suppresses food intake in rats through inhibition of luminal trypsin activity and direct stimulation of cholecystokinin secretion from enteroendocrine cells. J Agric Food Chem. 2011;59(17):9491–6. doi: 10.1021/jf200988f. [DOI] [PubMed] [Google Scholar]
- 7.Serquiz AC. Efeito sacietogênico de um novo inibidor de tripsina da paçoca do amendoim com aumento plasmático de colecistocinina (CCK) Universidade Federal do Rio Grande do Norte. 2012:16–97. p. [Google Scholar]
- 8.College G, Karad MS, Linn T. Anti-Oxidative Effect of Tamarindus Indica in Alloxan Induced Diabetic Rats Preparation of Ethanolic extract of bark of. Int J Res Pharm Biomed Sci. 2011;2(3):1006–9. [Google Scholar]
- 9.Manoharan S, Chellammal A, Linsa Mary A, Vasudevan K, Balakrishnan S, Ranezab Anishkumar P. Antidiabetic efficacy of Tamarindus indica seeds in alloxan induced diabetic rats. Electron J Pharmacol an Thetapy. 2009;2:13–8. [Google Scholar]
- 10.Araujo CL, Bezerra IWL, Oliveira AS, Moura FT, Macedo LLP, Gomes CEM, et al. In vivo bioinsecticidal activity toward ceratitis capitata (fruit fly) and callosobruchus maculatus (cowpea weevil) and in vitro bioinsecticidal activity toward different orders of insect pests of a trypsin inhibitor purified from tamarind tree (Tamarindu) J Agric Food Chem. 2005;53(11):4381–7. doi: 10.1021/jf0502505. [DOI] [PubMed] [Google Scholar]
- 11.Santos EA, Oliveira AS, Rabêlo LMA, Uchôa AF, Morais AHA. Affinity Chromatography as a Key Tool to Purify Protein Protease Inhibitors from Plants. Affinity Chromatography [Internet]. InTech. 2012:211–44. p. Available from: http://www.intechopen.com/books/affinity-chromatography/affinity-chromatography-as-a-key-tool-to-purify-protease-inhibitors-from-plants. [Google Scholar]
- 12.Machado RJA, Monteiro NK V, Migliolo L, Silva ON, Pinto MFS, Oliveira AS, et al. Characterization and pharmacological properties of a novel multifunctional kunitz inhibitor from erythrina velutina seeds. PLoS One. 2013;8(5):e63571. doi: 10.1371/journal.pone.0063571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima VC. Efeito gastroprotetor de isolados proteicos de sementes de Erythrina velutina com ação inibitária sobre a elastase de neutrófilos em modelode úlcera experimental. Universidade Federal do Rio Grande do Norte. 2014:17–87. p. [Google Scholar]
- 14.Kakade M, Simons N, Liener I. An evaluation of natural vs synthetic substrates for measuring the antitryptic of soybean samples.pdf. Cereal Chem. 1969;45(5):518–26. [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Kjeldahl J. A new method for the determination of nitrogen in organic matter. J Anal Chem. 1883;22(366) [Google Scholar]
- 17.Amaya H, Acevedo E, Bressani R. Efecto del recalientamiento sobre la disponibilidad de hierro y valor nutritivo de la proteina del frijol negro (Phaseolus vulgaris) cocido. Arch Latinoam Nutr. 1991;16(2):222–37. [PubMed] [Google Scholar]
- 18.Junqueira L, Junqueira L. Rio de Janeiro: Guanabara Koogan; 1983. Técnicas básicas de citologia e histologia. [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 20.Garthoff LH, Henderson GR, Sager AO, Sobotka TJ, Gaines DW, O′Donnell MW, et al. Pathological evaluation, clinical chemistry and plasma cholecystokinin in neonatal and young miniature swine fed soy trypsin inhibitor from 1 to 39 weeks of age. Food Chem Toxicol. 2002;40(4):501–16. doi: 10.1016/s0278-6915(01)00121-1. [DOI] [PubMed] [Google Scholar]
- 21.Szasz G. A Kinetic Photometric Method for Serum gamma-glutamyl transpeptidase. Clin Chem. 1968;15(2):124–36. [PubMed] [Google Scholar]
- 22.Gornall A, Bardawill C, David M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–66. [PubMed] [Google Scholar]
- 23.McLaughlin CL, Peikin SR, Baile C A. Trypsin inhibitor effects on food intake and weight gain in Zucker rats. Physiol Behav. 1983;31(4):487–91. doi: 10.1016/0031-9384(83)90071-9. [DOI] [PubMed] [Google Scholar]
- 24.Komarnytsky S, Cook A, Raskin I. Potato protease inhibitors inhibit food intake and increase circulating cholecystokinin levels by a trypsin-dependent mechanism. Int J Obes. 2011;35(2):236–43. doi: 10.1038/ijo.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araújo AH, Cardoso PCB, Pereira RA, Lima ML, Oliveira AS, Miranda MRA, et al. In vitro digestibility of globulins from cowpea (Vigna unguiculata) and xerophitic algaroba (Prosopis juli.ora) seeds by mammalian digestive proteinases: a comparative study. Food Chem. 2002;78:143–7. [Google Scholar]
- 26.Lima L, Araújo A, Oliveira A, Pereira R, Miranda MR, Sales M. Comparative digestibility and the inhibition of mammalian digestive enzymes from mature and immature cowpea (Vigna unguiculata (L.) Walp.) seeds. Food Control. 2004;15(2):107–10. [Google Scholar]
- 27.Brune MFSS, Pinto M de O, Peluzio M do CG, Moreira MA, Barros EG de. Avaliação bioquímico-nutricional de uma linhagem de soja livre do inibidor de tripsina Kunitz e de lectinas. Ciências e Tecnol Aliment. 2010;30(3):657–63. [Google Scholar]
- 28.Liener IE. Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr. 1994;34(1):31–67. doi: 10.1080/10408399409527649. [DOI] [PubMed] [Google Scholar]
- 29.Peace RW, Sarwar G, Touchburn SP, Botting HG. Effects of soybean trypsin inhibitors and dl-ethionine on growth and serum parameters in young rats. Nutr Res. 1991;11(10):1197–208. [Google Scholar]
- 30.Rackis JJ. Food and Nutrition Press, Inc.; 1982. Proteae Inhibitors: Physiological Properties and nutritional significance; pp. 203–37. p. [Google Scholar]
- 31.Sato N, Suzuki S, Kanai S, Ohta M, Jimi A, Noda T, et al. Different effects of oral administration of synthetic trypsin inhibitor on the pancreas between cholecystokinin-A receptor gene knockout mice and wild type mice. Jpn J Pharmacol. 2002;89(3):290–5. doi: 10.1254/jjp.89.290. [DOI] [PubMed] [Google Scholar]
- 32.Walker HK, Hall WD, Hurst J. Clinical Methods: The History, Physical, and Laboratory Examinations. JAMA: The Journal of the American Medical Association. 1990 [PubMed] [Google Scholar]
- 33.González FHD. Ferramentas de diagnóstico e monitoramento das doenças metabólicas. Ciência Anim Bras. 2009:1–22. [Google Scholar]
- 34.Huang G, Chang H, Chen H, Lu T, Chang Y, Sheu M, et al. Effects of trypsin inhibitor on plasma antioxidant activity and lipid levels in mice from sweet potato roots. J Sci Food Agric. 2008;14(88):2556–62. [Google Scholar]
- 35.Roy DM, Schneeman BO. Effect of soy protein, casein and trypsin inhibitor on cholesterol, bile acids and pancreatic enzymes in mice. J Nutr. 1981;111(5):878–85. doi: 10.1093/jn/111.5.878. [DOI] [PubMed] [Google Scholar]
- 36.Weller A, Corp ES, Tyrka A, Ritter RC, Brenner L, Gibbs J, et al. Trypsin inhibitor and maternal reunion increase plasma cholecystokinin in neonatal rats. Peptides. 1992;13(5):939–41. doi: 10.1016/0196-9781(92)90052-5. [DOI] [PubMed] [Google Scholar]
- 37.Liddle R A, Goldfine ID, Williams J A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984;87(3):542–9. [PubMed] [Google Scholar]
- 38.Pi-Sunyer X, Kissileff HR, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol Behav. 1982;29(4):627–30. doi: 10.1016/0031-9384(82)90230-x. [DOI] [PubMed] [Google Scholar]
- 39.Ballinger A, McLoughlin L, Medbak S, Clark M. Cholecystokinin is a satiety hormone in humans at physiological post-prandial plasma concentrations. Clin Sci (Lond) 1995;89(4):375–81. doi: 10.1042/cs0890375. [DOI] [PubMed] [Google Scholar]