Abstract
Background: The association between human papillomavirus (HPV) and overall survival (OS) in oropharynx cancer (OPC) was retrospectively examined in TAX 324, a phase III trial of sequential therapy for locally advanced head and neck cancer.
Methods: Accrual for TAX 324 was completed in 2003 and data updated through 2008. Pretherapy tumor biopsies were studied by PCR for human papillomavirus type 16 and linked to OS, progression-free survival (PFS) and demographics.
Results: Of 264 patients with OPC, 111 (42%) had evaluable biopsies; 56 (50%) were HPV+ and 55 (50%) were HPV−. HPV+ patients were significantly younger (54 versus 58 years, P = 0.02), had T1/T2 primary cancers (49% versus 20%, P = 0.001), and had a performance status of zero (77% versus 49%, P = 0.003). OS and PFS were better for HPV+ patients (OS, hazard ratio = 0.20, P < 0.0001). Local–regional failure was less in HPV+ patients (13% versus 42%, P = 0.0006); at 5 years, 82% of HPV+ patients were alive compared with 35% of HPV− patients (P < 0.0001).
Conclusions: HPV+ OPC has a different biology compared with HPV− OPC; 5-year OS, PFS, and local–regional control are unprecedented. These results support the possibility of selectively reducing therapy and long-term morbidity in HPV+ OPC while preserving survival and approaching HPV− disease with more aggressive treatment.
Keywords: chemoradiotherapy, chemotherapy, head and neck cancer, human papillomavirus, oropharynx cancer, randomized trials
introduction
The demographics and prognosis of locally advanced head and neck squamous cell cancer (HNC) has changed dramatically over the last two decades [1–3]. Epidemiologic evidence has revealed a significant increase in the incidence of oropharynx cancer (OPC). Molecular studies of oropharyngeal tumors reveal that this increase is due to an increase in the incidence of tumors that contain human papillomavirus (HPV), most specifically human papillomavirus type 16 (HPV16). There is direct evidence that HPV16 is the molecular cause mechanistically driving the development and viability of the cancer cells [4–6]. Human papillomavirus-related oropharynx cancer (HPVOPC) now accounts for almost 60% of OPC seen in the United States and an increasing fraction of these malignancies in Europe.
Studies in unselected patients suggest that patients with HPVOPC have a better prognosis than patients with HPV, predominantly environmentally related, oropharynx cancer (EROPC) [7–9]. The latter are principally related to smoking exposure [10]. Eastern Cooperative Oncology Group (E)2399, a phase II study of patients considered eligible for resection treated with an aggressive sequential therapy (ST) regimen of induction chemotherapy followed by chemoradiotherapy (CRT) for organ preservation, prospectively evaluated HPV status and reported a significant difference in survival for patients with HPVOPC compared with EROPC [11, 12]. Recently, results of retrospective analyses of survival and HPV status were reported from two international phase III trials comparing CRT regimens in locally advanced HNC [13, 14]. In both trials, there were insufficient patient numbers to report a treatment effect; however, the impact of HPV on survival, regardless of therapeutic assignment, was highly significant. For example, in Radiation Therapy Oncology Group (R)01-29, HPVOPC overall survival (OS) and progression-free survival (PFS) at 3 years were 82% and 74% compared with 57% and 43% for EROPC, respectively.
We retrospectively evaluated tumor HPV16 status, survival and demographics in subjects with OPC treated in TAX 324, a large international randomized phase III clinical trial. TAX 324 compared survival between ST with docetaxel, cisplatin, and 5-fluorouracil (TPF) and ST with cisplatin and 5-fluorouracil (PF) in patients with locally advanced HNC [15]. The data demonstrate a significant difference in survival outcome and patterns of failure between patients with HPVOPC and EROPC and significant differences in demographic characteristics in the populations. These novel results extend the data on survival to encompass a minimum of 5 years follow-up time in a large prospectively selected ST-treated population, important information for planning for new trials for HPVOPC.
methods
subjects
Patients diagnosed with OPC treated on the TAX 324 study were identified. The details of eligibility, therapy administration and survival for patients entered on TAX 324 are provided in prior publications [15]. Surgical pathology slides from initial tumor biopsies before therapy were prospectively collected for various biomarker studies, as has been previously reported. There were 264 patients with OPC identified in the study population and 111 (42%) of these had analyzable tissue for HPV determination. These 111 patients constitute the HPV study population for this analysis. Comparisons were made with the entire set of patients with OPC, as well as those patients without tissue available among the OPC cases. Smoking data were unavailable in this retrospective analysis. Long-term survival data were collected during the minimum 5-year follow-up time extension analysis of TAX 324 and were Institutional Review Board approved, as previously described [16]. The current analysis is based on the existing data as of 8 December 2008.
HPV assessment
HPV status of the tumors was assessed as previously described [17]. In brief, DNA was extracted from pretreatment pathology slides of the patients. After DNA was quantified, samples were subjected to HPV16-specific E6 and E7 viral oncoprotein PCR using established primers. Samples had to be positive for both E6 and E7 to be scored as positive, whereas ambiguous or discordant samples were rerun with a second set of overlapping non-nested primers. Cases that remained ambiguous were excluded. A set of repeat reactions were also run for validation. There was insufficient study material to carry out p16 immunohistochemistry. PCR and in situ hybridization have equivalent sensitivity. PCR can be carried out on previously stained slides. Hence, E6/E7 PCR was used to assess HPV16 status [18].
statistics
Collected data were analyzed and documented using descriptive statistics, such as frequency counts and percentages, medians, and rates with the corresponding 95% confidence intervals (CIs). Fisher’s exact test and likelihood ratio test for chi-square (r × c) contingency tables were applied to compare frequency distribution between patients’ subgroups.
OS was calculated from randomization to the date of death from any cause or date last known alive. PFS was calculated from randomization to progression or death without documentation of progression. Patients without progression had their follow-up censored at the date of last disease assessment. The method of Kaplan and Meier was used to characterize OS and PFS. The log-rank test was used to compare time-to-event end points between patients’ subgroups. Cox proportional hazards regression was applied to model OS and PFS and estimate the hazard ratios (HRs) with the corresponding 95% CIs.
The hypothesis testing was carried out as two tailed. Results were considered statistically significant if the nominal P value was <0.05. Statistical analysis was conducted using S-PLUS (v.8.0; TIBCO Software Inc., Palo Alto, CA).
results
demographic differences between HPVOPC and EROPC patients
Of the 501 patients entered on TAX 324, 264 (53%) were identified as having OPC. Of these 264 subjects, 119 had tissue prospectively collected and 111, or 42% of all OPC cases, were analyzable for HPV16 status and constitute the study population. The demographic data and test results for group comparisons are shown in Table 1. Comparisons of the cases with analyzable tissue with those without such tissue are also shown. Survival and treatment assignment after 5 years minimum follow-up time in TAX 324 have been previously reported [16]. There are 56 (50%) patients identified as HPV+ (HPVOPC) and 55 (50%) as HPV− (EROPC). Both HPVOPC and EROPC cases are evenly divided with regard to treatment assignment and sex. As previously reported, there are significantly fewer patients identified as black in the HPVOPC population (P = 0.03) [17].
Table 1.
Demographics of patients with oropharynx cancer treated on TAX 324
| HPV+ (N = 56, 50%), n (%) | HPV− (N = 55, 50%), n (%) | HPV tested (N = 111), n (%) | No HPV status (N = 153), n (%) | P valuea | P valueb | |
| Treatment | ||||||
| TPF | 28 (50) | 26 (47) | 54 (49) | 78 (51) | 0.85 | 0.80 |
| PF | 28 (50) | 29 (53) | 57 (51) | 75 (49) | ||
| Sex | ||||||
| Male | 45 (80) | 44 (80) | 89 (80) | 130 (85) | 1.0 | 0.32 |
| Female | 11 (20) | 11 (20) | 22 (20) | 23 (15) | ||
| Ethnicity | ||||||
| White | 54 (96) | 46 (84) | 100 (90) | 130 (86) | 0.03 | 0.22 |
| Black | 1 (2) | 7 (13) | 8 (7) | 11 (7) | ||
| Other | 1 (2) | 2 (4) | 3 (3) | 12 (8) | ||
| Age (years), median (range) | 54 (39–71) | 58 (41–78) | 55 (39–78) | 55 (38–78) | 0.02 | 1.0 |
| Operability | ||||||
| Unresectable | 14 (25) | 25 (46) | 39 (35) | 65 (42) | 0.04 | 0.005 |
| Low curability | 16 (29) | 16 (29) | 32 (29) | 60 (39) | ||
| Organ preservation | 26 (46) | 14 (26) | 40 (36) | 28 (18) | ||
| Nodal stage | ||||||
| N0–N1 | 13 (23) | 18 (33) | 31 (28) | 39 (25) | 0.30 | 0.67 |
| N2–N3 | 43 (77) | 43 (77) | 80 (72) | 114 (75) | ||
| T stage | ||||||
| T1–T2 | 28 (49) | 11 (20) | 39 (35) | 42 (27) | 0.001 | 0.22 |
| T3–T4 | 28 (51) | 44 (80) | 72 (65) | 110 (72) | ||
| TX | 0 | 0 | 0 | 1 (1) | ||
| Clinical stage | ||||||
| III | 10 (18) | 5 (9) | 15 (14) | 19 (12) | 0.27 | 0.85 |
| IV | 46 (82) | 50 (91) | 96 (86) | 133 (87) | ||
| NA | 1 (1) | |||||
| WHO PS | ||||||
| 0 | 43 (77) | 27 (49) | 70 (63) | 85 (56) | 0.003 | 0.31 |
| 1 | 13 (23) | 28 (51) | 41 (37) | 66 (43) | ||
| NA | 2 (1) |
Exact two-sided P value for testing null hypothesis of no difference in parameter’s distribution between HPV+ and HPV− patients.
Exact two-sided P value for testing null hypothesis of no difference in parameter’s distribution between patients with HPV status assessed and those without evaluable tissues.
HPV, human papillomavirus; T, tumor; WHO PS, World Health Organization performance status.
HPVOPC cases are significantly younger, 54 compared with 58 years (P = 0.02), than the EROPC cases, despite a highly selected population for a phase III trial. HPVOPC cases were also significantly more likely to be selected for therapy for reasons related to organ preservation. This may have been due to the likelihood of low volume primary site disease, which may be a biologic characteristic of HPVOPC. HPVOPC cases had twice as many T1-/T2-staged primary cancers as EROPC (49% versus 20%, P = 0.001). There are no significant differences in nodal status or stage between the two populations, reflecting that the majority of patients in both groups had N2 or N3 nodal stage disease. This also may reflect a selection bias in the study, which was targeted for inclusion of very advanced poor prognosis patients. Performance status (PS) was also significantly different between the two populations, despite selection for good PS for patients for enrollment in this trial. Thus, 77% of HPVOPC patients were PS zero compared with 49% of the EROPC patients (P = 0.003).
outcome differences between HPVOPC and EROPC patients
Results for OS, PFS and site of failure for the 111 patients analyzed for HPV16 status, independent of treatment arm, are shown in Table 2. HPVOPC- and EROPC-surviving patients were followed for a median of 83 months and 82 months, respectively. At the time of analysis, 79% of HPVOPC patients were still alive, and their PFS rate was 73% compared with 31% OS and 29% PFS for the EROPC patients (both P < 0.0001). OS and PFS functions are shown in Figure 1A and B, respectively. The median OS time for the EROPC patients is 21 months (95% CI 13–49 months), while median survival has not been reached in the HPVOPC group after almost 7 years median follow-up. There is an 80% reduction in mortality in HPVOPC compared with EROPC (HR = 0.2, 95% CI 0.10–0.38; P < 0.0001).
Table 2.
A comparison of survival, PFS and site of failure at the time of analysis for HPV+ and HPV− patients entered on TAX 324
| HPV+ (N = 56), n (%) | HPV− (N = 55), n (%) | P valuea | |
| Median follow-up in months (95% CI) | 83 (77–93) | 82 (68–86) | NS |
| Survival status | |||
| Alive | 44 (79) | 17 (31) | <0.0001 |
| Dead | 12 (21) | 38 (69) | |
| PFS status | |||
| No progression | 41 (73) | 16 (29) | <0.0001 |
| Progressionb | 15 (27) | 39 (71) | |
| Local–regional failure | 7 (13) | 23 (42) | 0.0006 |
| Distant metastases | 3 (5) | 6 (11) | NS |
| Bothc | 1 (2) | 2 (4) | NS |
| Total disease failures | 9 (16) | 27 (49) | 0.0002 |
| Died without recurrence | 5 (9) | 12 (22) | 0.07 |
Exact two-sided P value for testing null hypothesis of no difference in parameter’s distribution between HPV+ and HPV− patients.
One patient with a second primary was included as a progression event.
Patients with recurrence local–regionally and distantly were counted in both categories.
PFS, progression-free survival; HPV, human papillomavirus; CI, confidence interval; NS, nonsignificant.
Figure 1.
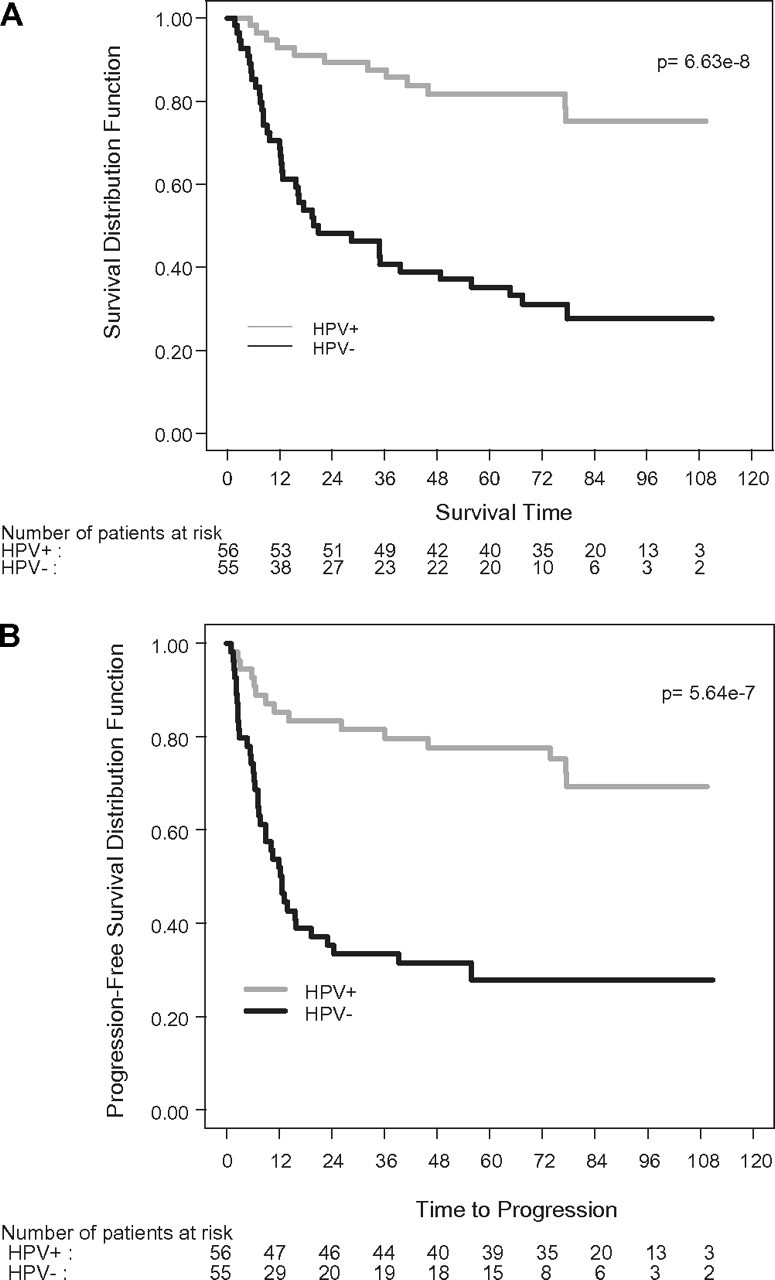
Kaplan–Meier curves for overall survival (A) and progression-free survival (B) for HPV+ and HPV− patients treated on TAX 324. HPV, human papillomavirus.
Analysis of the site of failure, as shown in Table 2, reveals a significant reduction in local–regional failure (13% versus 42%, P = 0.0006) and slightly reduced distant failure in the HPVOPC patients compared with the EROPC patients. There was also a significant difference in total disease failures (16% versus 49%, P = 0.0002) and a borderline improvement in deaths without recurrence (P = 0.07). These data indicate that local–regional control is the major parameter contributing to improved survival and that PS and comorbidities among EROPC patients account for another fraction of mortality.
OS and PFS rate over time data—as a yearly analysis of events over 5 years—are shown in Table 3. These data are informative in aligning information on OS and PFS for qualitative comparisons among trials focusing on OPC. As can be seen, early PFS at 2 and 3 years predicts later survival for HPVOPC and EROPC patients when treated with ST. PFS is a potential indicator of the durability of survival in both populations of OPC compared with other head and neck sites and the slope of PFS in ST trials can be used to inform long-term survival estimates in a qualitative manner for future studies [19].
Table 3.
A comparison of OS and PFS rates at yearly time points for HPV+ and HPV− patients treated on TAX 324
| HPV+ (N = 56) | HPV− (N = 55) | All (N = 111) | |
| OS rate in % (95% CI)a | |||
| 1 year | 93 (82–97) | 69 (54–79) | 81 (72–87) |
| 2 years | 89 (78–95) | 48 (34–61) | 69 (60–77) |
| 3 years | 87 (75–94) | 41 (28–53) | 65 (55–73) |
| 5 years | 82 (69–90) | 35 (23–48) | 59 (49–67) |
| PFS rate in % (95% CI)a | |||
| 1 year | 85 (73–92) | 52 (38–64) | 69 (59–76) |
| 2 years | 83 (70–91) | 35 (23–48) | 59 (49–68) |
| 3 years | 81 (68–90) | 33 (21–46) | 57 (48–66) |
| 5 years | 78 (64–87) | 28 (17–40) | 53 (43–62) |
P value < 0.0001 for each yearly comparison between HPV+ and HPV− patients.
OS, overall survival; PFS, progression-free survival; HPV, human papillomavirus; CI, confidence interval.
Comparison of the effects of treatment with TPF or PF and HPVOPC and EROPC in the 111 subjects on survival revealed no statistical differences between TPF and PF due to the small numbers of patients in each arm and concomitant loss of statistical power for an unplanned analysis. As can be seen in Figure 2 and supplemental Table S1 (available at Annals of Oncology online), with <30 patients treated within each study arm, neither regimen was significantly superior in either the HPVOPC or the EROPC population for survival. In contrast, in the complete data for 5-year survival analysis of TAX 324, published separately, OS in OPC patients was significantly better with TPF compared with PF [16].
Figure 2.
Kaplan-Meier curves of survival according to HPV status (HPV+ or HPV−) and treatment arm (TPF or PF). See supplemental Table S1 (available at Annals of Oncology online) for statistical comparisons. HPV, human papillomavirus.
discussion
It has become evident over the last 10 years that the biology and demographics of HNC has changed drastically due to increasing numbers of patients with HPVOPC. We can now divide HNC into two distinct diseases with different biology, HPVOPC and EROPC [7, 9, 20]. Understanding the biology and therapeutic outcomes of these two populations is critical as more specific therapies are developed and trials designed. In this study, we report the demographics and long-term survival of patients with OPC based on tumor HPV status. Survival was significantly better for patients with HPVOPC than those with EROPC. This survival advantage resulted in an 80% reduction in mortality at the 5-year analysis among HPVOPC compared with EROPC, primarily from improved local–regional control and to a lesser extent from fewer non-OPC-related deaths. Demographics were different within the protocol-selected population of patients on this phase III trial. Thus, HPVOPC patients tended to be predominantly white, had smaller primary tumors, were younger, and had better PS.
This study confirms and extends data on demographics and survival previously reported [13, 21]. There are advantages and limitations in this analysis in applying the data and understanding the demographics of the two populations compared with epidemiologic studies and other retrospective clinical series. Because this is a protocol-driven trial with well-defined inclusion and exclusion criteria, there is a general selection bias for younger healthier patients. Also, specific demographic factors, youth and PS, are not a matter of intrinsic tumor biology but of incidence. As the epidemic of HPVOPC continues, age may become less of a defining difference as the median age of the HPVOPC subpopulation stabilizes. Worse PS in EROPC may also be related to an increased rate of smoking and associated comorbidities compared with the HPVOPC population. PS is not a biologic correlate of the disease but rather might be best considered a behavioral association that impacts on survival. Smoking on the other hand, as suggested in R01-29, may have a relationship with survival and with tumor biologic behavior through additional tumor mutations, leading to increased aggressiveness of the malignancy and inferior responses to available curative-intent therapies [21]. This notion would be supported by data showing increased local–regional failure and distant metastases in association with increased smoking in HPVOPC.
The selection criteria for our study created a bias to include more advanced cases for therapy than might be included in CRT trials for advanced disease. This was further enhanced by the availability of concomitant CRT for the treatment of intermediate-stage patients off protocol as part of standard clinical care in the United States at the time of accrual to the TAX 324 trial. As a result of selection for advanced nodal disease, we identified a biologic difference between HPVOPC and EROPC; primary site (T) stage is significantly lower in the HPVOPC group (versus the EROPC group), while neck nodal (N) stage was identical between the two groups. Data from others also suggest that a relatively greater nodal burden might be expected in the HPVOPC cases compared with the EROPC cases for a given primary stage. While this might be expected in incident cases, it would not be observed in the TAX 324 trial due to the selection criteria that biased the study toward more advanced disease. This would also effect assignment of resectability since smaller primary cancers would be expected to be more amenable to surgery. The impact of biases of patient selection on study entry and outcome is important. For example, while this study of ST encouraged inclusion of patients with large advanced tumors, selection for R01-29 would have been influenced by USA community and academic radiation oncologists, who commonly choose to treat patients with very advanced disease with induction therapy rather than only CRT, particularly in EROPC and in sites such as the larynx and hypopharynx [22, 23]. Inherent community and investigator selection biases for phase III trials occur often and highlight why definitive conclusions on efficacy across treatment paradigms must often be made by randomized phase III trials.
It is apparent from the data presented here from TAX 324 as well as other trials that survival is significantly better for HPVOPC compared with EROPC. In Table 4, comparable data on OS and PFS from TAX 324 and R01-29 and OS data from the HeadStart trial are shown for a qualitative comparison [13, 21]. As can be seen, survival in all three trials is similar at the 3-year analysis time point. PFS is different between R01-29 and TAX 324 in the first 3 years by as much as 7%, in favor of TAX 324. Importantly, in assessing outcomes, CRT trials may also have greater rates of late distant metastases and/or late fatal morbidity not captured by early analyses of CRT trials [19, 24].
Table 4.
A comparison of OS and PFS among phase III trials
| Study, geolocation | Patients tested | Therapeutic regimens | HPV+/tested (%HPV+) | Time interval | Survival (%) HPV+/HPV− |
| RTOG 01-29, United States | 323/433 (75%) | CRT versus RT | 206/323 (65) | 3-year OS | 82 versus 57 |
| 3-year PFS | 74 versus 43 | ||||
| 5-year OS | NA | ||||
| 5-year PFS | NA | ||||
| HeadStart, international | 195/465 (42%) | CRT versus CRT | 54/195 (28) | 2-year OS | 94 versus 77 |
| 2-year PFS | NA | ||||
| 5-year OS | NA | ||||
| 5-year PFS | NA | ||||
| TAX 324, international | 111/264 (42%) | ST versus ST | 56/111 (50) | 3-year OS | 87 versus 41 |
| 3-year PFS | 81 versus 33 | ||||
| 5-year OS | 82 versus 35 | ||||
| 5-year PFS | 78 versus 28 |
OS, overall survival; PFS, progression-free survival; HPV, human papillomavirus; RTOG, Radiation Therapy Oncology Group; CRT, chemoradiotherapy; RT, radiotherapy; ST, sequential therapy; NA, not available.
Improved survival is explained, as shown in this data and the 3- and 2-year data from R01-29 and HeadStart, respectively, by a significant improvement in local–regional control [13, 21]. In those studies, death from second primaries and noncancer causes were also reduced in the HPVOPC group and accounted for about one-third to one-half of the improvement in survival for HPVOPC compared with EROPC. Thus, in purely CRT-utilizing trials, local–regional tumor control appeared to be markedly better as a biologic consequence of the HPV origin of the HPVOPC tumors. In R01-29, the rate of distant metastases was similar between the HPVOPC patients and the EROPC patients, in alignment with the data from many studies that demonstrate that CRT has a very small to negligible impact on distant metastases. Our trial extends and expands the data reported from the E2399 trial, a phase II ST trial. The rate of distant metastases is very low for the EROPC and HPVOPC patients, and the entire survival advantage for HPVOPC in E2399 can be explained by improved local–regional control and reduced noncancer deaths [11].
Importantly, data from this 5-year minimum follow-up analysis indicate that the 2- and 3-year actuarial PFS rates predict 5-year actuarial OS in HPVOPC and, therefore, can be used to predict long-term survival in ST studies. The data from R01-29 have been limited to the 3-year survival data reported. Long-term survival and PFS in R01-29 are not available yet, and thus, data on the impact of the lower 3-year PFS in HPVOPC, late toxicity and the risk for delayed distant metastases on 5-year OS in the CRT regimen are not available.
Patients with HPVOPC are young and will live for prolonged periods. They are at high risk for long-term toxicity and mortality from therapy. While the long-term consequences of chemotherapy are relatively constrained, high-dose radiotherapy (RT) and CRT substantially impact on local tissues and organ function and result in significant rate of late mortality and morbidity in patients [19,24–27]. Studies are now being designed to reduce the impact of RT and CRT for patients. Identifying appropriate end points and study arms that will allow an early assessment of outcomes will be problematic, particularly for equivalence studies wherein survival differences are small and where prolonged time periods and large patient numbers are necessary to accurately assess outcomes. For ST as given with TAX 324, 3-year PFS may be an appropriate end point. The same may not be possible for CRT [25, 27, 28]. The best example of changing outcomes in CRT trials would be R91-11, in which a premature negative conclusion regarding the efficacy of induction therapy was published with the early analysis [19, 23]. Late toxicity and morbidity, a hallmark of upfront cisplatin-based CRT trials, led to equivalence between induction therapy and CRT for laryngectomy-free survival at 5 years and more importantly a nonsignificant relative 10% improvement in OS in the PF induction arm compared with the CRT arm, which included an every 3-week bolus cisplatin for three cycles during RT.
We can conclude from this study that HPVOPC has a different biology, demographics and OS compared with EROPC and represents a different disease. The OS, PFS, and local–regional control obtained with ST at 5 years and CRT at 2 and 3 years in HPVOPC are unprecedented. The excellent 3-year PFS predicts durable long-term OS in HPVOPC treated with ST. These data support the notion of developing different therapeutic approaches for HPV+ and HPV− OPC. The survival results in HPVOPC achieved in TAX 324 strongly suggest that it might be possible to reduce long-term morbidity in HPVOPC and preserve survival perhaps by better selection and by reducing RT intensity in the context of ST for more advanced cases. We might best approach HPV− disease with novel therapies and more aggressive ST or CRT.
funding
Sanofi-Aventis; Steven Tendrich Fund to M.R.P. These Funds were used for HPV testing of tissues and the statistical analysis of the data.
disclosure
NJS was a senior employee of Sanofi-Aventis at the time of data gathering and writing. MRP received an honorarium from Sanofi-Aventis for an educational speaking event in 2010. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–992. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 3.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 4.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 5.Furniss CS, McClean MD, Smith JF, et al. Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120:2386–2392. doi: 10.1002/ijc.22633. [DOI] [PubMed] [Google Scholar]
- 6.Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101:412–423. doi: 10.1093/jnci/djp017. [DOI] [PubMed] [Google Scholar]
- 7.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 8.Ringstrom E, Peters E, Hasegawa M, et al. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–3192. [PubMed] [Google Scholar]
- 9.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 10.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 11.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 12.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–3977. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 13.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 16.Lorch J, Haddad R, Goloubeva O, et al. Long term results of TAX324, a randomized phase III trial of sequential therapy with TPF versus PF in locally advanced squamous cell cancer of the head and neck. Lancet Oncol. 2011;12:153–159. doi: 10.1016/S1470-2045(10)70279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agoston ES, Robinson SJ, Mehra KK, et al. Polymerase chain reaction detection of HPV in squamous carcinoma of the oropharynx. Am J Clin Pathol. 2010;134:36–41. doi: 10.1309/AJCP1AAWXE5JJCLZ. [DOI] [PubMed] [Google Scholar]
- 19.Forastiere A, Maor M, Weber R, et al. Long term results of Intergroup RTOG 91-11: a phase III trial to preserve the larynx—induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol. 2006 24: 18S (Abst 5517) [Google Scholar]
- 20.McLaughlin-Drubin ME, Munger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143:195–208. doi: 10.1016/j.virusres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–33. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142–152. doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced larynx cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 24.Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102:1188–1198. doi: 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 25.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adelstein D, Li Y, Adams G, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 28.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



