Abstract
Background
Advanced glycation end products and their cell-bound receptors are thought to mediate the adverse effects of vascular disease through oxidative stress, inflammation and endothelial dysfunction. We examined the association between the soluble form of receptor for advanced glycation end products (sRAGE) and kidney disease.
Methods
In this case-cohort study nested within the Atherosclerosis Risk in Communities (ARIC) study, baseline sRAGE levels were measured in a cohort random sample of participants without kidney disease (n= 1218), and among participants who developed incident chronic kidney disease (CKD) [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and ≥25% eGFR decline, n = 151] and end-stage renal disease (ESRD) [entry in the US Renal Data System (USRDS) registry, n = 152].
Results
Baseline sRAGE levels were inversely related to baseline eGFR (r = −0.13). After adjusting for age, sex and race, one interquartile range higher log10-transformed sRAGE was associated with development of CKD [odds ratio: 1.39; 95% confidence interval (95% CI) 1.06–1.83; P = 0.02] and ESRD (hazard ratio: 1.97; 95% CI 1.47–2.64; P < 0.001). These associations were not significant after eGFR adjustment.
Conclusions
High sRAGE levels are associated with incident CKD and ESRD risk, but not after adjustment for kidney function at baseline. Future studies are needed to investigate specific mechanisms underlying the association of sRAGE with kidney disease risk.
Keywords: advanced glycation end product receptor, chronic kidney failure, risk factors
INTRODUCTION
Advanced glycation end products (AGEs), which are produced by the Maillard reaction, and their cell-bound receptors (RAGE) are thought to mediate the adverse effects of dysglycemia and vascular disease [1–4]. Activation of the AGE–RAGE complex contributes to reactive oxygen species generation, inflammation and endothelial dysfunction, thereby contributing to chronic disease progression [5–7]. The soluble form of RAGE (sRAGE) is a 48-kDa, positively charged cleavage product of RAGE, which retains the ligand binding site but lacks the transmembrane and signaling domains [8, 9]. sRAGE has gained attention due to its ability to bind the various ligands of RAGE, including high mobility group box 1, S100/calgranulin and β-amyloid protein in addition to AGEs [10]. By binding to AGEs and other ligands without initiating the signaling cascade, sRAGE is thought to neutralize the detrimental effects of AGE–RAGE complex activation [10, 11]. Due to this purported protective role, sRAGE has even been proposed as a therapeutic agent targeting vascular inflammation for the prevention of cardiovascular disease [11, 12].
Whereas there is growing literature on sRAGE and its associations with diabetes and cardiovascular disease, the role of this marker in the development of kidney disease is not well characterized. Prior studies show that sRAGE levels are elevated among patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) relative to individuals without kidney disease or with less severe disease [13–16]. These studies also report that sRAGE levels are inversely correlated with kidney function in cross-sectional analyses [13, 14, 16]. In an analysis of 230 older women, a higher sRAGE level at baseline was associated with a greater likelihood of developing CKD after 12 months [n = 32 incident CKD cases; odds ratio (OR): 1.32; 95% confidence interval (95% CI) 1.01–1.74; P = 0.05] [16]. Additional studies with longer follow-up, larger sample sizes, more diverse populations and with adjustment for estimated glomerular filtration rate (eGFR) are needed to confirm this isolated finding.
We hypothesized that higher plasma concentrations of sRAGE at baseline would be accompanied by decreased kidney function and associated with increased risk of incident CKD and ESRD in a community-based population.
MATERIALS AND METHODS
Study design
The Atherosclerosis Risk in Communities (ARIC) study is a community-based cohort study of 15 792 individuals recruited from four US communities (Washington County, MD; Forsyth County, NC; Jackson, MS; Minneapolis, MN), which has been previously described [17]. Briefly, ARIC study participants were initially enrolled in 1987–89 and additionally examined at follow-up study visits (1990–92, 1993–95, 1996–98, 2011–13) to collect information on demographics, health history, lifestyle characteristics, anthropometrics and biological specimens (12-h fasting blood samples, spot urine samples). Blood samples were centrifuged within 30 min of venipuncture and stored at −70°C until analysis. We report here the results of a nested case-cohort study within the ARIC study.
Nested case-cohort study population
To be eligible for the case-cohort study, all participants were required to have attended the baseline visit (ARIC study visit 2, 1990–92; n = 14 348, 90.9% of the ARIC cohort), a measured serum creatinine value (n = 14 292, 99.6% of participants who attended the baseline visit) and normal kidney function at baseline, defined as eGFR ≥60 mL/min/1.73 m2 (n = 13 983, 97.8% of those with measured serum creatinine). Of these participants, we selected a random sample of the cohort to serve as the comparison group (cohort random sample: n = 1218) and all incident cases of CKD and ESRD for measurement of sRAGE in plasma samples collected at baseline. The primary case group was all participants who developed CKD (n = 151). The secondary case group consisted of all participants who developed ESRD (renal replacement therapy; n = 152).
Definition of incident chronic kidney disease
CKD status was defined as eGFR <60 mL/min/1.73 m2 and ≥25% decline in eGFR at follow-up (ARIC study visit 4, 1996–98) relative to baseline. The incident CKD case group consisted of 151 participants, including 21 participants selected for the cohort random sample (cases: n = 151; noncases: n = 1197; total sample: N = 1348).
Definition of incident end-stage renal disease
Incident ESRD cases (renal replacement therapy) were identified through the US Renal Disease System (USRDS) registry by entry date between baseline and 31 December 2005. This registry captures patients receiving dialysis or transplant as reported on the Centers for Medicare and Medicaid Services Medical Evidence Form-2728, which is filed within 45 days of renal replacement therapy initiation. There were 152 ESRD cases including five cases that overlapped with the cohort random sample (cases: n = 152; noncases: n = 1213; total sample: N = 1365). In a sensitivity analysis, we used an alternative outcome definition incorporating untreated as well as treated kidney failure and results were similar to that for ESRD defined by entry into the USRDS registry.
Measurement of sRAGE
Baseline plasma levels of sRAGE were measured with a commercially-available ELISA kit (Quantikine® Human RAGE Immunoassay, R&D Systems, Minneapolis, MN). The intra- and interassay coefficients of variation for the assay were 2.8 and 9.6%, respectively. The sRAGE assay has been shown to be robust to numerous adverse pre-analytical conditions including multiple freeze-thaw cycles and delayed centrifugation [18, 19]. Study and laboratory staff were blinded to kidney disease case status for measurement of sRAGE and other covariates.
Measurement of covariates
Creatinine was measured by the modified kinetic Jaffé method and calibrated to the National Institute of Standards and Technology standard [20]. Serum cystatin C was measured using a particle-enhanced turbidimetric assay (Gentian, Moss, Norway). eGFR was calculated with CKD Epidemiology Collaboration equations using creatinine only (eGFRCr), cystatin C only (eGFRCys) and both creatinine and cystatin C (eGFRCr-Cys) [21, 22]. Serum levels of β2 microglobulin and β-trace protein were measured using particle-enhanced immune-nephelometric N Latex assays (Siemens Diagnostics, IL). C-reactive protein was measured from stored plasma samples using a latex-particle-enhanced immunoturbidimetric assay on the Roche Modular P800 Chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Hemoglobin A1c was measured from stored whole blood samples using high-performance liquid chromatography, and standardized to the Diabetes Control and Complications Trial hemoglobin A1c assay [23]. Plasma high-density lipoprotein cholesterol levels were measured enzymatically after precipitation with dextran sulfate–magnesium [24]. Three seated measurements of blood pressure were taken by a certified technician using a random-zero sphygmomanometer after 5 min of rest, and the mean of the second and third readings was used for analysis. Body mass index was calculated as weight (kg)/height (m)2 using measurements taken during the study clinical examination. Participants reported their date of birth, sex, race, current smoking status and physician-diagnosed diabetes and use of hypoglycemic medication or insulin through a structured interview with a trained interviewer.
Statistical analysis
sRAGE quartiles were created based on the distribution of sRAGE in the cohort random sample and, for continuous analyses, sRAGE was log10-transformed to improve normality of its distribution. Descriptive statistics were used to describe the study population overall and to examine differences by sRAGE quartile. Differences in baseline characteristics by sRAGE quartile were tested using analysis of variance and χ2 tests. Pairwise Spearman rank correlation coefficients were calculated in the cohort random sample for sRAGE and other clinical analytes.
Logistic regression models were used to examine the association between sRAGE and incident CKD, since CKD status was assessed at a discrete time point (ARIC study visit 4, 1996–98). Cox proportional hazard regression models were used to examine the association between sRAGE and incident ESRD, incorporating time from baseline (1990–92) until the date of entry into the USRDS ESRD registry or censoring due to death or end of follow-up (31 December 2005). Risk ratios were estimated per one interquartile range increase of log10-transformed sRAGE, which was calculated based on the sRAGE distribution in the cohort random sample. Inverse probability weighting was used to account for random selection of participants from the overall ARIC cohort for the subcohort [25, 26]. Competing risks regression models were used to examine the association between sRAGE and kidney disease, accounting for deaths that occurred before kidney events.
To examine the independent association of sRAGE with incident CKD and ESRD, we compared several multivariable models adjusted for baseline covariates. Model 1 included basic demographic characteristics (age, sex, and race). Model 2 included all variables in Model 1 plus eGFRCr-Cys because this estimate is our best approximation of measured GFR [21]. As a sensitivity analysis, we substituted eGFRCr and eGFRCys for eGFRCr-Cys in separate models, and results were similar. Model 3 included all variables in Model 2 plus additional markers of kidney filtration (β2 microglobulin, β-trace protein), diabetes status due to the known association of diabetes with both sRAGE and kidney disease, and traditional kidney disease risk factors (high-density lipoprotein, body mass index, systolic blood pressure, current smoking status, C-reactive protein). We conducted analyses stratified by racial group, diabetes status, sex and by angiotensin-converting enzyme (ACE) inhibitor use based on knowledge from previous studies. Restricted cubic splines were used to present age, sex and race-adjusted as well as age, sex, race and eGFR-adjusted hazard ratios for ESRD by continuous sRAGE level with knots at 439.6, 733.7, 965.6, 1244.5 and 2034.6 pg/mL, centering at the 25th percentile, and truncation of sRAGE values at the 1st and 99th percentiles. Statistical tests were two-sided, significance was assessed at an α level of 0.05, and analyses were performed using Stata version 12.1 statistical software (StataCorp, LLC, College Station, TX).
RESULTS
Among noncases, 58.1% were women, 21.5% were black and baseline eGFR was 97.2 mL/min/1.73 m2 (Supplementary data, Table S1). Relative to noncases, participants in the CKD and ESRD case groups were more likely to be older, male, black, cigarette smokers, to have diabetes and higher systolic blood pressure levels. At the higher quartiles of plasma concentration of sRAGE, there were more women, fewer blacks and lower levels of hemoglobin A1c, systolic blood pressure, body mass index and C-reactive protein (Table 1). Among participants with sRAGE levels in the higher quartiles, eGFR was lower and kidney filtration markers were higher. Baseline sRAGE levels were inversely related to baseline eGFR (r = −0.13, P < 0.001) and positively correlated with kidney filtration markers (β2 microglobulin: r = 0.09, P = 0.001; β-trace protein: r = 0.17, P < 0.001).
Table 1.
Baseline demographics, clinical characteristics and measures of kidney function by quartile of soluble receptor for advanced glycation end products (sRAGE) in the cohort random sample (N = 1218)a
| sRAGE, pg/mL |
|||||
|---|---|---|---|---|---|
| Quartile 1: 119.4–708.8 (n = 305) | Quartile 2: 708.9–965.2 (n = 305) | Quartile 3: 965.3–1262.6 (n = 304) | Quartile 4: 1262.7–4650.4 (n = 304) | P-valueb | |
| Age, years | 56.6 (5.8) | 56.6 (5.5) | 56.7 (5.8) | 56.5 (5.5) | 0.9 |
| Female | 51.5% (157) | 56.1% (171) | 55.9% (170) | 68.4% (208) | <0.001 |
| Black | 45.3% (138) | 22.0% (67) | 12.2% (37) | 6.6% (20) | <0.001 |
| Diagnosed diabetes | 5.9% (18) | 6.9% (21) | 5.9% (18) | 3.3% (10) | 0.25 |
| Hemoglobin A1c, % | 5.9 (1.1) | 5.7 (1.2) | 5.6 (0.9) | 5.5 (0.8) | <0.001 |
| Hemoglobin A1c, mmol/mol | 41 (12.0) | 39 (13.1) | 38 (9.8) | 37 (8.7) | <0.001 |
| Systolic blood pressure, mmHg | 124.6 (17.0) | 120.9 (16.3) | 119.2 (18.2) | 116.7 (18.7) | <0.001 |
| ACE inhibitor medication use | 6.9% (21) | 6.9% (21) | 6.3% (19) | 3.3% (10) | 0.18 |
| History of coronary heart disease | 8.0% (24) | 4.7% (14) | 9.4% (28) | 2.3% (7) | 0.001 |
| Current smoker | 15.4% (47) | 21.3% (65) | 20.4% (62) | 16.1% (49) | <0.001 |
| Body mass index, kg/m2 | 30.2 (5.7) | 28.0 (5.2) | 27.5 (5.0) | 25.8 (4.3) | <0.001 |
| High-density lipoprotein, mg/dLc | 49.8 (16.2) | 49.4 (14.6) | 49.1 (16.5) | 52.8 (16.3) | 0.01 |
| C-reactive protein, mg/L | 5.68 (7.5) | 3.65 (5.6) | 3.51 (5.8) | 2.97 (4.5) | <0.001 |
| β2 Microglobulin, mg/L | 1.83 (0.39) | 1.85 (0.34) | 1.87 (0.35) | 1.91 (0.35) | 0.04 |
| β-Trace protein, mg/L | 0.49 (0.17) | 0.55 (0.34) | 0.58 (0.15) | 0.60 (0.16) | <0.001 |
| Cystatin C, mg/L | 0.84 (0.14) | 0.85 (0.14) | 0.86 (0.15) | 0.87 (0.15) | 0.10 |
| eGFR, mL/min/1.73 m2 | 99.9 (15.0) | 96.8 (13.0) | 96.1 (14.1) | 95.0 (12.8) | <0.001 |
aMean (standard deviation) or % (n).
bAnalysis of variance for continuous variables, χ2 for categorical variables.
cTo convert high-density lipoprotein from mg/dL to mmol/L, multiply by 0.02586.
eGFR, estimated glomerular filtration rate; sRAGE, soluble receptor for advanced glycation end products.
After adjusting for age, sex and race, higher baseline concentration of sRAGE was statistically significantly associated with an increased risk of kidney disease [Table 2; CKD OR: 1.39, 95% CI 1.06–1.83, P = 0.02; ESRD hazard ratio (HR): 1.97, 95% CI 1.47–2.64, P < 0.001]. Effect estimates were substantially attenuated after accounting for eGFR (CKD OR: 1.22, 95% CI 0.92–1.62, P = 0.16; ESRD HR: 1.08, 95% CI 0.87–1.34, P = 0.49). In the fully adjusted model, plasma levels of sRAGE were significantly associated with CKD (OR: 1.37; 95% CI 1.01–1.87; P = 0.04), but not ESRD (HR: 1.01; 95% CI 0.74–1.37; P = 0.9). Accounting for the competing risk of death prior to a kidney disease event resulted in quantitative results and interpretation similar to that from the standard survival analysis.
Table 2.
Association of baseline plasma levels of soluble receptor for advanced glycation end products (sRAGE) with incident kidney diseasea
| Incident chronic kidney disease |
Incident end-stage renal disease |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | HR (95% CI) | P-value | |
| Model 1b | 1.39 (1.06–1.83) | 0.02 | 1.97 (1.47–2.64) | <0.001 |
| Model 2c | 1.22 (0.92–1.62) | 0.16 | 1.08 (0.87–1.34) | 0.49 |
| Model 3d | 1.37 (1.01–1.87) | 0.04 | 1.01 (0.74–1.37) | 0.9 |
CI, confidence interval; HR, hazard ratio; OR, odds ratio; sRAGE, soluble receptor for advanced glycation end products.
aEstimates are expressed per one interquartile range (0.251 pg/mL) increase of log10-transformed sRAGE. Interquartile range was calculated based on sRAGE distribution in the cohort random sample.
bModel 1: Age, sex, race.
cModel 2: Variables in Model 1 + baseline estimated glomerular filtration rate.
dModel 3: Variables in Model 2 + β2 microglobulin, β-trace protein, diabetes, high-density lipoprotein, body mass index, systolic blood pressure, current smoking status, C-reactive protein.
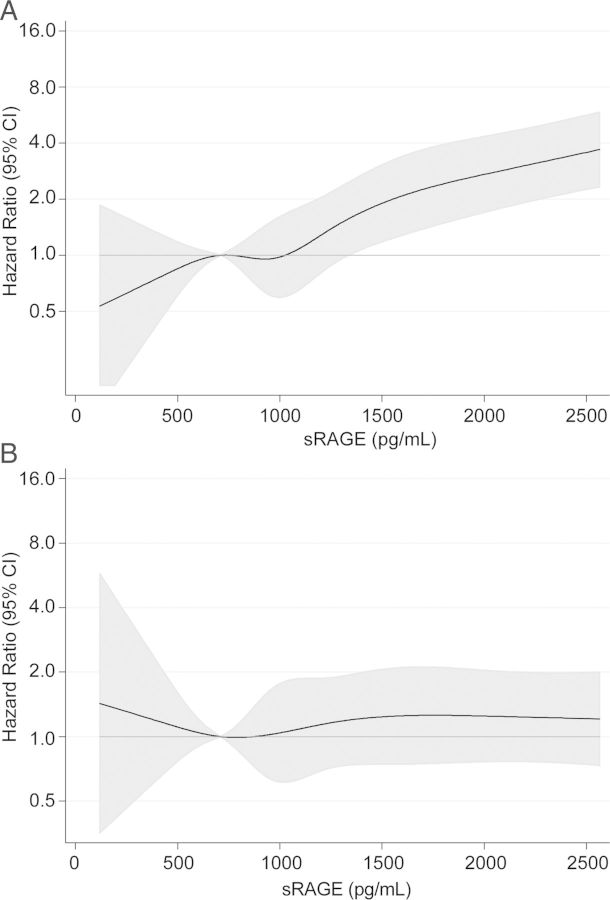
With higher levels of sRAGE, the age-, sex- and race-adjusted risk of ESRD increased approximately linearly (Figure 1A). Additional adjustment for eGFR resulted in a nearly flat line, representing no association between sRAGE and ESRD (Figure 1B).
FIGURE 1:
Hazard ratios and 95% confidence intervals (CI, shaded area) for incident end-stage renal disease by plasma level of soluble receptor for advanced glycation end products (sRAGE) (A) adjusted for age, sex and race and (B) adjusted for estimated glomerular filtration rate, age, sex and race.
sRAGE was not significantly associated with kidney disease outcomes among persons with diabetes, whereas there were statistically significant positive associations between sRAGE and kidney disease outcomes among participants without diabetes in some models (Supplementary data, Table S2). The diabetes–sRAGE interaction term was statistically significant in fully adjusted models for ESRD, but not CKD (Supplementary data, Table S2; Model 2 and Model 3).
Plasma levels of sRAGE were not different among participants using ACE inhibitors compared with participants not taking this class of medication (Table 1; ACE inhibitor users: mean sRAGE = 1067.9 pg/mL; ACE inhibitors nonusers: mean sRAGE = 1057.9 pg/mL; P = 0.42). In stratified analyses, the association of sRAGE and kidney outcomes did not differ by use of ACE inhibitors.
There were substantial differences in sRAGE levels by race, as 45.3% of those within the lowest sRAGE quartile were black and only 6.6% of those in the highest sRAGE quartile were black (Table 1; P < 0.001). There were no significant differences in the association of sRAGE and kidney disease outcomes by race (CKD: P for interaction = 0.81; ESRD: P for interaction = 0.29) or sex (CKD: P for interaction = 0.40; ESRD: P for interaction = 0.22).
DISCUSSION
In this biracial, community-based study of middle-aged adults, higher plasma concentrations of sRAGE at baseline were associated with concurrent indicators of worse kidney function as well as increased risk of incident kidney disease during follow-up, after accounting for age, sex and race. However, the association between sRAGE level and kidney disease was not independent of baseline kidney function assessed by eGFR, suggesting a possible direct effect of kidney filtration on circulating serum levels of sRAGE.
We report an increased risk of kidney disease associated with higher levels of sRAGE, which corroborates findings from prior studies focused on kidney disease as the primary outcome [13–16]. Expanding upon existing evidence and leveraging the strengths of the ARIC study, we conducted several subgroup analyses and controlled for numerous parameters known to be related to sRAGE and kidney disease. Importantly, our study is the first to adjust for eGFR in estimating the association between sRAGE and kidney disease. The inclusion of eGFR in multivariable regression models substantially attenuated the effect estimates. In addition, we observed that baseline sRAGE was cross-sectionally associated with worse kidney function, i.e. inversely correlated with eGFR and positively correlated with other kidney filtration markers (β2 microglobulin, β-trace protein). Taken together, these findings suggest that circulating levels of sRAGE may be directly affected by impairment of the kidney to filter endogenous substances appropriately or, conversely, that sRAGE directly impacts kidney function. In contrast with the current kidney disease literature reporting that higher sRAGE levels are associated with adverse outcomes, previous studies have demonstrated that lower levels of sRAGE are associated with increased risk of diabetes, cardiovascular disease and mortality [2, 27, 28]. However, there is some inconsistency in the directionality of the association with vascular disease which may be the result of effect modification by underlying disease status [29, 30].
Findings from stratified analyses in the current study add to the knowledge base on variability of the association between sRAGE and kidney disease within population subgroups. The sRAGE and kidney disease relationship was present among persons without diabetes but not among persons with diabetes in our study. The notable difference in sRAGE distribution by racial group in our study, with lower sRAGE concentrations among blacks, is in agreement with previous reports [2, 27, 28, 30]. In spite of the difference in sRAGE concentrations between racial groups, we found that the association between sRAGE and kidney outcomes was consistent across racial groups. Inhibition of renin–angiotensin system activity through the use of ACE inhibitors is thought to increase production and secretion of sRAGE in plasma [31]. We found that use of ACE inhibitors was more common among kidney disease cases than noncases. However, there was no difference in sRAGE level by ACE inhibitor use and ACE inhibitor use did not impact risk estimation in stratified analysis. Similarly, a cross-sectional study of individuals with ESRD found that sRAGE level was not associated with ACE inhibitor use [14]. However, use of ACE inhibitors in our study was reported at baseline (1990–92) and may not be reflective of current patterns of medication use. Furthermore, the small size of individual strata limits our ability to make definitive conclusions about results in small subgroups.
The mechanism through which sRAGE exerts its influence on tissues and signaling pathways is not fully elucidated. One possibility is that sRAGE levels may reflect the degree of AGE–RAGE activity, which may in turn contribute to adverse health consequences [1, 4, 5]. That is, in the presence of high levels of circulating AGEs, RAGE mRNA expression increases, resulting in upregulation of RAGE production [32]. Given that sRAGE can be a cleaved product of RAGE, the plasma concentration of sRAGE could correspond to the amount of RAGE produced. Another potential explanation is that, similar to AGEs, elevated levels of circulating sRAGE result from decreased renal clearance and subsequent accumulation of sRAGE among those with decreased kidney function [33, 34]. That is, plasma levels of sRAGE may be partially determined by the kidney's ability to filter effectively. sRAGE concentration in the circulation may represent the inflammatory state, and thereby lead to kidney disease pathogenesis [5, 35].
The present study is the largest with the longest follow-up time to investigate sRAGE and kidney disease and our results were consistent across two kidney disease outcomes. This study benefitted from the rigorous measurement of known risk factors for kidney disease and the use of a diverse, community-based study population.
There are several limitations that are important in interpreting these results. Firstly, we only had single measurements of sRAGE obtained from samples in long-term storage. However, the reproducibility of sRAGE measurements in masked duplicate specimens was high (r = 0.99, n = 20), and stable over a 3-year interval within the same participants (r = 0.78, n = 179) [36]. Secondly, only sRAGE levels were captured. The additional assessment of AGE concentration in the circulation may provide a more complete depiction of the mechanism of sRAGE. Thirdly, GFR was not measured directly. However, our findings were robust across different assessments of eGFR, including eGFRCr-Cys, which most closely approximates measured GFR [21]. Analyses of population subgroups are limited by smaller sample sizes than the overall study population, and these findings should be validated in studies with sufficient statistical power to assess subgroup differences. There may be selection bias due to loss-to-follow-up in the analysis of incident CKD in which eligibility was dependent on providing a blood sample at a follow-up visit.
In conclusion, our study demonstrates that high levels of circulating sRAGE are associated with worse kidney function and are a risk marker for the development of both early and late stage kidney disease. Notably, the direction of the association of sRAGE and kidney disease is the inverse of that for other chronic diseases. Furthermore, the observed associations appeared to be largely explained by baseline kidney function suggesting a possible direct effect of kidney filtration on circulating plasma sRAGE levels.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C and HHSN268201100012C). The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions. This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK076770 and also a grant from the American Heart Association to Dr Selvin. Dr Rebholz is supported in part by National Heart, Lung and Blood Institute grant T32HL007024. Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
REFERENCES
- 1.Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Halushka MK, Rawlings AM, et al. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes. 2013;62:2116–2121. doi: 10.2337/db12-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henning C, Smuda M, Girndt M, et al. Molecular basis of Maillard amide-advanced glycation end product (AGE) formation in vivo. J Biol Chem. 2011;286:44350–44356. doi: 10.1074/jbc.M111.282442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukami K, Yamagishi S, Okuda S. Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des. 2014;20:2395–2402. doi: 10.2174/13816128113199990475. [DOI] [PubMed] [Google Scholar]
- 5.Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 6.Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 7.Sadik NA, Mohamed WA, Ahmed MI. The association of receptor of advanced glycated end products and inflammatory mediators contributes to endothelial dysfunction in a prospective study of acute kidney injury patients with sepsis. Mol Cell Biochem. 2012;359:73–81. doi: 10.1007/s11010-011-1001-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Bukulin M, Kojro E, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283:35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 9.Sarkany Z, Ikonen TP, Ferreira-da-Silva F, et al. Solution structure of the soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2011;286:37525–37534. doi: 10.1074/jbc.M111.223438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han SH, Kim YH, Mook-Jung I. RAGE: the beneficial and deleterious effects by diverse mechanisms of actions. Mol Cells. 2011;31:91–97. doi: 10.1007/s10059-011-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 12.Lanati N, Emanuele E, Brondino N, et al. Soluble RAGE-modulating drugs: state-of-the-art and future perspectives for targeting vascular inflammation. Curr Vasc Pharmacol. 2010;8:86–92. doi: 10.2174/157016110790226642. [DOI] [PubMed] [Google Scholar]
- 13.Basta G, Leonardis D, Mallamaci F, et al. Circulating soluble receptor of advanced glycation end product inversely correlates with atherosclerosis in patients with chronic kidney disease. Kidney Int. 2010;77:225–231. doi: 10.1038/ki.2009.419. [DOI] [PubMed] [Google Scholar]
- 14.Kalousova M, Hodkova M, Kazderova M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–411. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Park S, Lee MJ, et al. Plasma levels of soluble receptor for advanced glycation end products (sRAGE) and proinflammatory ligand for RAGE (en-RAGE) are associated with carotid atherosclerosis in patients with peritoneal dialysis. Atherosclerosis. 2012;220:208–214. doi: 10.1016/j.atherosclerosis.2011.07.115. [DOI] [PubMed] [Google Scholar]
- 16.Semba RD, Ferrucci L, Fink JC, et al. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis. 2009;53:51–58. doi: 10.1053/j.ajkd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Brown LF, Fraser CG. Assay validation and biological variation of serum receptor for advanced glycation end-products. Ann Clin Biochem. 2008;45:518–519. doi: 10.1258/acb.2008.008043. [DOI] [PubMed] [Google Scholar]
- 19.Wittwer C, Lehner J, Fersching D, et al. Methodological and preanalytical evaluation of a RAGE immunoassay. Anticancer Res. 2012;32:2075–2078. [PubMed] [Google Scholar]
- 20.Lustgarten JA, Wenk RE. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972;18:1419–1422. [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvin E, Coresh J, Zhu H, et al. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities Study. J Diabetes. 2010;2:118–124. doi: 10.1111/j.1753-0407.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 25.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–1072. [PubMed] [Google Scholar]
- 26.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 27.Lindsey JB, de Lemos JA, Cipollone F, et al. Association between circulating soluble receptor for advanced glycation end products and atherosclerosis: observations from the Dallas Heart Study. Diabetes Care. 2009;32:1218–1220. doi: 10.2337/dc09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson BI, Moon YP, Kalea AZ, et al. Association of serum soluble receptor for advanced glycation end-products with subclinical cerebrovascular disease: the Northern Manhattan Study (NOMAS) Atherosclerosis. 2011;216:192–198. doi: 10.1016/j.atherosclerosis.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nin JW, Jorsal A, Ferreira I, et al. Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes. 2010;59:2027–2032. doi: 10.2337/db09-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colhoun HM, Betteridge DJ, Durrington P, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60:2379–2385. doi: 10.2337/db11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes JM, Thorpe SR, Thallas-Bonke V, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16:2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 32.Linden E, Cai W, He JC, et al. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol. 2008;3:691–698. doi: 10.2215/CJN.04291007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartog JWL, De Vries APJ, Lutgers HL, et al. Accumulation of advanced glycation end products, measured as skin autofluorescence, in renal disease. Ann N Y Acad Sci. 2005;1043:299–307. doi: 10.1196/annals.1333.037. [DOI] [PubMed] [Google Scholar]
- 34.Renard C, Chappey O, Wautier MP, et al. Recombinant advanced glycation end product receptor pharmacokinetics in normal and diabetic rats. Mol Pharmacol. 1997;52:54–62. doi: 10.1124/mol.52.1.54. [DOI] [PubMed] [Google Scholar]
- 35.Kshirsagar AV, Bomback AS, Bang H, et al. Association of C-reactive protein and microalbuminuria (from the National Health and Nutrition Examination Surveys, 1999 to 2004) Am J Cardiol. 2008;101:401–406. doi: 10.1016/j.amjcard.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bower JK, Pankow JS, Lazo M, et al. Three-year variability in plasma concentrations of the soluble receptor for advanced glycation end products (sRAGE) Clin Biochem. 2014;47:132–134. doi: 10.1016/j.clinbiochem.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.