Abstract
Human immunodeficiency virus (HIV)-infected individuals with latent Mycobacterium tuberculosis infection have substantially higher rates of progression to active tuberculosis than HIV-uninfected individuals with latent tuberculosis. To explore HIV-induced deficits in M. tuberculosis–specific CD8+ T-cell functions, we compared interferon γ production, degranulation, and proliferation of CD8+ T cells in response to M. tuberculosis peptides (ESAT-6/CFP-10) between HIV-infected (median CD4+ T-cell count, 522 cells/µL; interquartile range, 318–585 cells/µL) and HIV-uninfected individuals with latent tuberculosis from South Africa. We found that M. tuberculosis–specific degranulation and proliferative capacities were impaired in the HIV-infected group. Thus, our results suggest that HIV coinfection compromises CD8+ T-cell functions in latent tuberculosis.
Keywords: latent tuberculosis, CD8+ T cells, HIV, degranulation, proliferation
In 2013 the World Health Organization reported 8.7 million incident cases of active tuberculosis worldwide and 1.4 million attributable deaths [1]. In addition, an estimated one-third of the world's population harbor Mycobacterium tuberculosis in an asymptomatic state called latent M. tuberculosis infection, or latent tuberculosis. Although most healthy individuals with latent tuberculosis are able to control infection, various coexistent conditions, including silicosis, diabetes, renal disease, malnutrition, and immunosuppressive medications (ie, steroids and tumor necrosis factor α inhibitors), hasten the progression to active tuberculosis disease [2]. Human immunodeficiency virus (HIV) is among the most important risk factors to date, because the 10% lifetime risk of progression to active tuberculosis climbs to a 5%–10% annual risk with HIV coinfection [3].
Natural history HIV cohorts have highlighted the importance of CD4+ T cells in controlling tuberculosis. As CD4+ T cells decline, the incidence and extrapulmonary dissemination of tuberculosis rises [4, 5]. During acute HIV infection, M. tuberculosis–specific CD4+ T cells are preferentially depleted in peripheral blood [6, 7]. Although M. tuberculosis–specific polyfunctional CD4+ T-cell responses are largely preserved in HIV-infected individuals with latent tuberculosis, the degree of HIV viremia correlates inversely with their capacity to produce interleukin 2 [8]. Interestingly, even with preservation of CD4+ T-cell counts, the risk of developing active tuberculosis is higher among HIV-infected individuals than among their HIV-uninfected counterparts [9]. This led us to hypothesize that defective M. tuberculosis–specific CD8+ T-cell immunity may also contribute to the heightened risk of tuberculosis progression in HIV-infected individuals with latent tuberculosis.
Relatively little is known about M. tuberculosis–specific CD8+ T-cell immunity in humans. Murine studies have demonstrated a 10-fold increase in M. tuberculosis in the lungs of infected mice after antibody-mediated depletion of CD8+ T cells or with infection of CD8+ T cell–deficient mice [10]. Among macaques with latent tuberculosis coinfected with simian immunodeficiency virus, those with fewer M. tuberculosis–specific (interferon [IFN] γ+ CD8+ T cells progressed more rapidly to active tuberculosis [11]. Studies of individuals with latent tuberculosis treated with tumor necrosis factor α inhibitors highlight the importance of M. tuberculosis–specific CD8+ T-cell cytolytic granule production in maintaining latency [12]. Although defects in HIV-specific degranulation and T-helper 1 responses have been reported to be associated with uncontrolled HIV viremia [13], little is known about the effects of HIV infection on M. tuberculosis–specific CD8+ T-cell immunity.
To identify potential HIV-induced defects in CD8+ T-cell immunity in latent tuberculosis, we sought differences in M. tuberculosis–specific CD8+ T-cell functions in HIV-infected and HIV-uninfected individuals with latent tuberculosis from tuberculosis-endemic regions in South Africa. Specifically, we evaluated the effect of HIV coinfection on M. tuberculosis–specific CD8+ T-cell IFN-γ production, degranulation, and proliferative capacity.
MATERIALS AND METHODS
Study Subjects and Sample Collection
Men and women aged ≥18 years were recruited in the Western Cape province of South Africa at the South African Tuberculosis Vaccine Initiative field site in the Worcester area, located 110 km outside Cape Town (Supplementary Table 1). Individuals were clinically evaluated for evidence of active tuberculosis disease (ie, cough, weight loss, fever, and night sweats) at study entry. Those with prior diagnosis and/or symptoms of active tuberculosis and those previously treated for tuberculosis were excluded. Peripheral blood was collected in Vacutainer tubes. To evaluate for latent tuberculosis, we assayed Peripheral blood mononuclear cells (PBMCs) from HIV-infected individuals, using QuantiFERON-TB Gold In-Tube (QFT; Cellestis) and intracellular staining for IFN-γ followed by flow cytometry. To assess PBMCs from HIV-uninfected individuals, we used intracellular staining for IFN-γ followed by flow cytometry. Individuals positive for QFT or intracellular IFN-γ were defined as having latent tuberculosis, and those with negative results were excluded. The Alere Determine HIV Combo test was used to screen for HIV-1 and HIV-2 infection. PBMCs were isolated using Ficoll-Hypaque (Sigma-Aldrich) in South Africa and cryopreserved at −180°C anywhere from 4 months to 4 years post-collection. Oregon Green (OG) proliferation assays using fresh PBMCs were conducted in Cape Town, South Africa, at the South African Tuberculosis Vaccine Initiative laboratories. The rest of the cell stimulations and flow cytometry were performed at the Emory Vaccine Center in Atlanta, Georgia, using cryopreserved PBMCs shipped from South Africa to the Emory Vaccine Center.
Proliferation Assay
Freshly isolated PBMCs were labeled with Cell Trace OG 488 carboxylic acid diacetate succinimidyl ester (OG; Life Technologies) and incubated for 6 days with ESAT-6/CFP-10 peptide pools, Staphylococcus enterotoxin B (SEB), or left nonstimulated. Cells were stained with LIVE/DEAD Fixable Violet Dead Cell Stain (Vivid; Life Technologies), fixed with FACS Lysing Solution (BD Biosciences), and stained with anti-CD4 Qdot605 (S3.5; Life Technologies), anti-CD3 allophycocyanin-H7 (SK7), and anti-CD8 peridinin chlorphyll protein (PerCP)-cyanine (Cy) 5.5 (SK-1) from BD Biosciences.
PBMC Stimulation and Flow Cytometry
Thawed PBMC were rested overnight at 37°C and 5% carbon dioxide in Roswell Park Memorial Institute 1640 medium (Lonza) with 10% fetal bovine serum, 2 mmol/L glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin. The lymphocyte viability was 75%–95%, as determined by the Countess Automated Cell Counter (Invitrogen Life Technologies). The PBMCs were prestained with CD107a-phycoerythrin Cy7 (BD Biosciences) for 1 hour, after which 2 × 106 PBMCs were each subjected to (1) no stimulation, (2) stimulation with ESAT-6/CFP-10 peptide pools of 15 mer overlapping with 11 amino acids (10 µg/mL; Genemed Synthesis), or (3) SEB (5 µg/mL), as described elsewhere [14]. A costimulatory CD28/49d cocktail (1 µg/mL; BD Biosciences), brefeldin A, and monensin (10 µg/mL) were added at the time of stimulation, followed by incubation for 18 hours. The PBMCs were stained with the LIVE/DEAD kit (Invitrogen Life Technologies) and surface stained with CD4–peridinin chlorophyll (BD Biosciences) and CD8-BV570 (BioLegend). Cells were permeabilized with a Cytofix/Cytoperm Kit (BD Biosciences) and stained with IFN-γ-Pacific blue (BD Biosciences) and CD3-allophycocyanin-Cy7 (BioLegend), fixed using 1% paraformaldehyde, and samples were acquired on an LSR-II system (BD Biosciences).
Data Analysis
Flow cytometry data were analyzed using FlowJo software (version 9). GraphPad Prism software (version 6.0) was used for statistical analysis. Nonstimulated IFN-γ and CD107a responses were subtracted from all ESAT-6/CFP-10 and SEB-stimulated PBMC responses. The Mann–Whitney U test was used to evaluate differences between HIV-infected and HIV-uninfected individuals with latent tuberculosis. Differences were considered statistically significant at P < .05 (2 tailed).
Ethical Approval
This study was approved by the Emory University Institutional Review Board, the Human Research Ethics Committee of the University of Cape Town, and the Western Cape Department of Health. All subjects provided written informed consent before participation.
RESULTS
Study Participants
To evaluate the effect of HIV infection on M. tuberculosis–specific CD8+ T-cell function, we recruited 20 HIV-infected and 22 HIV-uninfected individuals with latent tuberculosis from the Western Cape province, South Africa (Supplementary Table 1). Among HIV-infected individuals, 75% (15 of 20) were female, 95% (19 of 20) were black, and 5% (1 of 20) were biracial; the mean age was 32 years (range, 21–46 years). The median absolute CD4+ T-cell count was 522 cells/µL (interquartile range, 318–585 cells/µL), with a median viral load of 16 736 HIV RNA copies/mL (interquartile range, 994–44 869 copies/mL). Among the HIV-uninfected individuals, 73% (16 of 22) were female, 41% (9 of 22) were black, 50% (11 of 22) were biracial, and 9% (2 of 22) were Asian. The mean age among the HIV-uninfected group was 29 years (range, 20–46 years). At the time of blood collection, none of the participants reported prior antiretroviral or antituberculosis therapy.
M. tuberculosis–Specific CD8+IFN-γ+ T-Cell Responses in HIV-Infected and HIV-Uninfected Subjects With Latent Tuberculosis
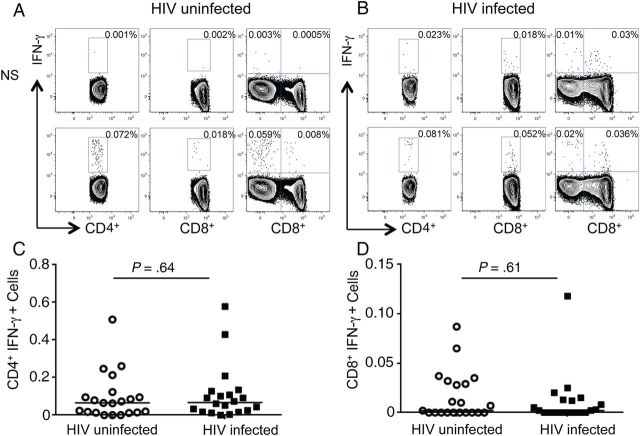
To detect M. tuberculosis antigen-specific CD8+ T-cell responses, we first assessed IFN-γ-production in PBMCs from HIV-uninfected and HIV-infected individuals with latent tuberculosis in response to stimulation with ESAT-6/CFP-10 peptide pools (Figure 1). Regardless of HIV infection status, latently infected individuals had lower frequencies of antigen-specific CD8+IFN-γ+ compared with CD4+IFN-γ+ T cells (Figure 1A and 1C). No differences were noted in the frequencies of M. tuberculosis–specific CD4+IFN-γ+ (Figure 1B) or CD8+IFN-γ+ (Figure 1C) populations between the HIV-infected and HIV-uninfected groups. Thus, M. tuberculosis–specific IFN-γ responses were not altered by coinfection with HIV in individuals with latent tuberculosis. On stimulation with SEB, there were no differences in CD4+ T-cell IFN-γ production between the 2 groups; however, SEB stimulation induced significantly higher IFN-γ production by CD8+ T cells in the HIV-infected than in the HIV-uninfected group (P = .002; data not shown).
Figure 1.
Interferon (IFN) γ production by Mycobacterium tuberculosis–specific CD4+ and CD8+ T cells in human immunodeficiency virus (HIV)-infected (n = 21) and HIV-uninfected (n = 20) individuals with latent M. tuberculosis infection. A, Representative flow cytometry plots for an HIV-uninfected subject (left) and an HIV-infected subject (right) with latent tuberculosis, demonstrating IFN-γ production by CD4+ and CD8+ T cells in response to stimulation with ESAT-6/CFP-10 pooled peptides. B and C, Summary data comparing frequencies of M. tuberculosis–specific CD4+IFN-γ+ (B) and CD8+IFN-γ+ (C) T cells in HIV-uninfected versus HIV-infected individuals with latent tuberculosis. Horizontal lines indicate median frequency of IFN-γ+–producing T-cell subsets. No significant difference was noted between the 2 groups. Abbreviation: NS, nonstimulated cells.
Reduced Frequencies of CD107a+CD8+ T Cells in HIV-Infected Individuals With Latent Tuberculosis
Degranulation activity and release of cytolytic granules in response to antigenic stimulation is indicative of the cytotoxic potential of CD8+ T cells. The lysosome-associated glycoprotein CD107a mobilizes to the cell surface of degranulating CD8+ T cells and serves as a marker of degranulation [15]. To evaluate the cytotoxic potential of M. tuberculosis–specific CD8+ T cells in HIV-uninfected and HIV-infected groups with latent tuberculosis, we assessed the expression of CD107a in ESAT-6/CFP-10–stimulated CD8+ T cells (Figure 2A and 2B). Although CD8+ T cells from HIV-infected individuals with latent tuberculosis had significantly higher levels of CD107a in PBMCs directly ex vivo than HIV-uninfected individuals with latent tuberculosis (P < .001), the proportion of ESAT-6/CFP-10–responsive CD8+ T cells expressing cell surface CD107a was significantly reduced in HIV-infected compared with HIV-uninfected groups (P = .004). The inverse was observed on stimulation with SEB (Figure 2C). Overall, HIV-infected individuals displayed higher proportions of CD107a+CD8+T cells than their HIV-uninfected counterparts (P < .001), suggesting that coinfection with HIV impairs M. tuberculosis–specific CD8+ T-cell degranulation in latent tuberculosis.
Figure 2.
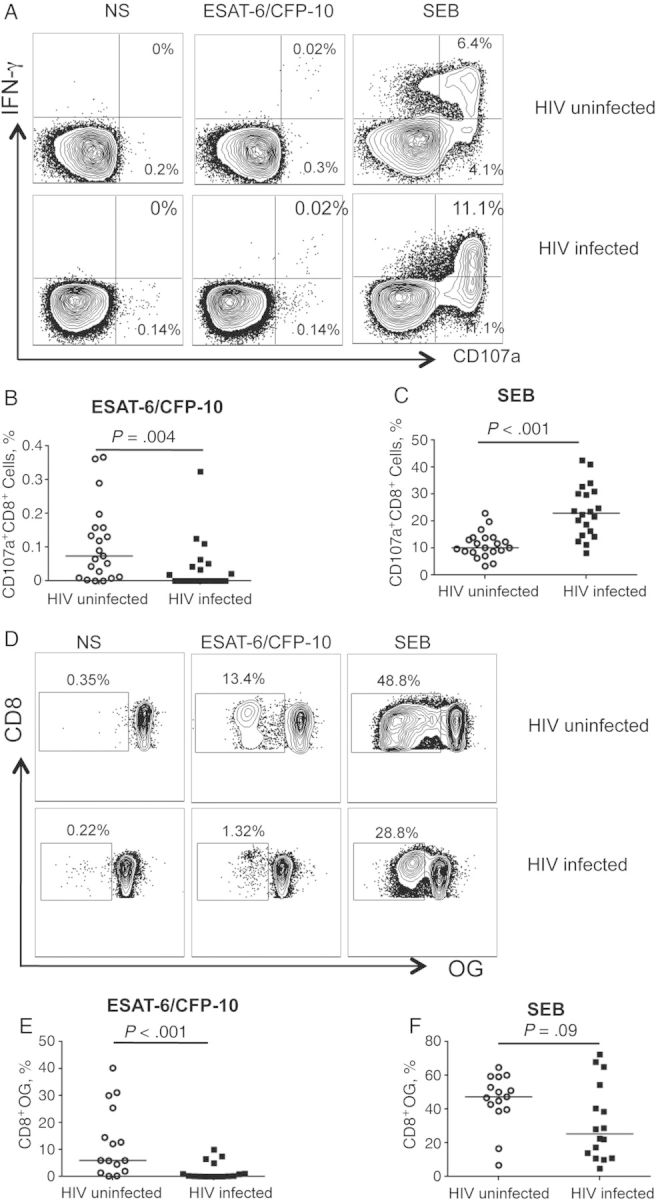
Impaired degranulation and proliferation of Mycobacterium tuberculosis–specific CD8+ T cells in human immunodeficiency virus (HIV)-infected compared with HIV-uninfected individuals with latent M. tuberculosis infection. A, Degranulation activity in M. tuberculosis–specific CD8+ T cells was assessed by expression of cell surface CD107a. Representative flow cytometry plots for an HIV-uninfected and an HIV-infected individual with latent tuberculosis demonstrate CD107a expression by CD8+ T cells in nonstimulated (NS) cells (left), on stimulation with ESAT-6/CFP-10 peptide pools (middle) and Staphylococcus enterotoxin B (SEB) (right). B, C, Summary data comparing frequencies of CD107a+CD8+ T cells between HIV-uninfected (n = 21) and HIV-infected (n = 19) groups on stimulation with ESAT-6/CFP-10 (B) or SEB (C). Horizontal lines indicate median frequency of CD107a+CD8+ T cells. D, Proliferative capacity of M. tuberculosis–specific CD8+ T cells was measured after a 6-day stimulation of freshly isolated peripheral blood mononuclear cells with CFP-10 and ESAT-6 peptide pools. Representative flow cytometry plots for an HIV-uninfected and an HIV-infected individual with latent tuberculosis demonstrate proliferation of CD8+ T cells on stimulation with ESAT-6/CFP-10 (middle) or SEB (right). The cells shown are gated on VIVIDloCD3+CD8+ lymphocytes. The percentage on each plot indicates the percentage of proliferating CD8+ T cells after subtraction of background proliferation in the uninfected control condition. E, F, Summary data comparing frequencies of proliferating CD8+ T cells between HIV-uninfected (n = 15) and HIV-infected (n = 16) individuals on stimulation with ESAT-6/CFP-10 (E) or SEB (F). Horizontal lines indicate median frequency of proliferating CD8+ T cells. Differences in the frequencies of CD107a+CD8+ T cells or proliferating CD8+ T cells between HIV-uninfected and HIV-infected groups were assessed using the Mann–Whitney test; differences were considered statistically significant at P < .05 (2 tailed). Abbreviations: IFN, interferon; OG, Oregon Green; VIVID, violet dead cell stain.
Impairment of M. tuberculosis–Specific CD8+ T Cell Proliferation With HIV Coinfection
To explore whether HIV coinfection is associated with defective proliferative capacities of M. tuberculosis–specific CD8+ T cells, we compared the in vitro proliferation of CD8+ T cells in response to stimulation with ESAT-6/CFP-10 peptide pools (Figure 2D and 2E). The proliferative capacity of ESAT-6/CFP-10–specific CD8+ T cells was significantly lower in the HIV-infected group (P < .001) than in the HIV-uninfected group. In contrast, there were no significant differences in SEB-induced proliferation between the 2 groups (Figure 2F; P = .09). These data indicate that coinfection with HIV impairs the proliferative capacities of M. tuberculosis–specific CD8+ T cells in latent tuberculosis.
DISCUSSION
Coinfection with HIV substantially increases the risk of tuberculosis disease progression, morbidity, and mortality and helps shed light on the mechanisms involved in the maintenance of latent tuberculosis and on perturbations of immune control. Through this cross-sectional study, we identified HIV-induced defects in M. tuberculosis–specific CD8+ T-cell functionality that may contribute to the heightened risk of progression to active tuberculosis disease in the setting of HIV coinfection.
The frequencies of M. tuberculosis–specific CD8+ T cells producing IFN-γ were lower than the corresponding frequencies of M. tuberculosis–specific CD4+IFN-γ+ T cells in both HIV-infected and uninfected individuals, consistent with other studies evaluating M. tuberculosis–specific CD8+ T-cell cytokine production in individuals with latent tuberculosis [8]. Degranulation and proliferative capacities of CD8+ T cells in response to M. tuberculosis were impaired in the HIV-infected compared with the HIV-uninfected group with latent tuberculosis. Interestingly, we also observed reduced proliferative capacities of CD4T cells from HIV-infected compared with HIV-uninfected individuals (C. L. D. and J. R., unpublished observations), suggesting that this may reflect HIV-induced defects in CD4+ T-cell help due to deficiencies in interleukin 2 or in CD40-CD40L interactions [8]. SEB-specific CD107a+CD8+ T-cell frequencies were higher in HIV-infected than in HIV-uninfected individuals, suggesting that HIV immune activation may reduce their degranulation threshold. We also tested responses to a cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF) peptide pool consisting of CD8 epitopes; no significant differences were observed between HIV-infected and uninfected groups to CEF, although not all subjects showed measurable responses to these peptides (data not shown).
Our results provide new insights into the role of M. tuberculosis–specific CD8+ T cells in maintaining latent tuberculosis and suggest that HIV coinfection may perturb immune control by impairing the capacity of antigen-specific CD8+ T cells to contain latent infection. The relatively small sample size of this cross-sectional study precluded examination of the effects of potential confounders (eg, CD4+ T-cell count and viral load) on immunological function. Furthermore, the lack of long-term follow-up of the latently infected subjects did not allow us to determine whether any of them developed tuberculosis after study enrollment. Nevertheless, despite these limitations, our data underscore the need to further evaluate M. tuberculosis–specific CD8+ T-cell degranulation, proliferation, and cytolytic activity in larger prospective cohorts and determine whether deficits in antigen-specific CD8+ T-cell functions evolve with HIV disease progression by enrolling participants across a broader range of CD4+ T-cell counts and plasma HIV loads.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We acknowledge Surinder Kaur for her assistance with PBMC processing and staining, and we thank the many individuals who participated in the study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant T32AI074492 to A. S. K.), a Center for AIDS Research (CFAR) immunology core grant (grant P30AI050409 to Emory University), an Emory-CFAR R03 developmental grant (to S. M. R. and J. R.), and the National Institutes of Health (NIH) or the NIAID (grant AI083156 to C. L. D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID/NIH.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis report. Geneva: WHO, 2012
- 2.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2000;49(1):51. [PubMed] [Google Scholar]
- 3.McShane H. Co-infection with HIV and TB: double trouble. Int J STD AIDS. 2005;16:95–100. doi: 10.1258/0956462053057576. quiz 1. [DOI] [PubMed] [Google Scholar]
- 4.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 5.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 6.Geldmacher C, Ngwenyama N, Schuetz A, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4T cells after HIV-1 infection. J Exp Med. 2010;207:2869–81. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geldmacher C, Schuetz A, Ngwenyama N, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198:1590–8. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day CL, Mkhwanazi N, Reddy S, et al. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–9. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? a retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–8. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 10.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8T cells. Eur J Immunol. 2000;30:3689–98. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruns H, Meinken C, Schauenberg P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–77. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersperger AR, Martin JN, Shin LY, et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adekambi T, Ibegbu C, Kalokhe A, Tianwei Y, Ray S, Rengarajan J. Distinct effector memory CD4+ T-cell signatures in latent Mycobacterium tuberculosis infection and BCG vaccination. PLoS One. 2012;7:e36046. doi: 10.1371/journal.pone.0036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.