Colonic mucosal bft detection was significantly higher in tumor-bearing patients than in colonoscopy control patients and universal in late-stage colorectal cancer (CRC). bft-2 was the most frequent isotype identified, and multiple bft isotypes were more often present in CRC patients.
Keywords: enterotoxigenic Bacteroides fragilis, mucosal microbiota, colorectal cancer, Bacteroides fragilis toxin
Abstract
Background. Enterotoxigenic Bacteroides fragilis (ETBF) produces the Bacteroides fragilis toxin, which has been associated with acute diarrheal disease, inflammatory bowel disease, and colorectal cancer (CRC). ETBF induces colon carcinogenesis in experimental models. Previous human studies have demonstrated frequent asymptomatic fecal colonization with ETBF, but no study has investigated mucosal colonization that is expected to impact colon carcinogenesis.
Methods. We compared the presence of the bft gene in mucosal samples from colorectal neoplasia patients (cases, n = 49) to a control group undergoing outpatient colonoscopy for CRC screening or diagnostic workup (controls, n = 49). Single bacterial colonies isolated anaerobically from mucosal colon tissue were tested for the bft gene with touch-down polymerase chain reaction.
Results. The mucosa of cases was significantly more often bft-positive on left (85.7%) and right (91.7%) tumor and/or paired normal tissues compared with left and right control biopsies (53.1%; P = .033 and 55.5%; P = .04, respectively). Detection of bft was concordant in most paired mucosal samples from individual cases or controls (75% cases; 67% controls). There was a trend toward increased bft positivity in mucosa from late- vs early-stage CRC patients (100% vs 72.7%, respectively; P = .093). In contrast to ETBF diarrheal disease where bft-1 detection dominates, bft-2 was the most frequent toxin isotype identified in both cases and controls, whereas multiple bft isotypes were detected more frequently in cases (P ≤ .02).
Conclusions. The bft gene is associated with colorectal neoplasia, especially in late-stage CRC. Our results suggest that mucosal bft exposure is common and may be a risk factor for developing CRC.
The anaerobe Bacteroides fragilis is a common colonic symbiote with an affinity for mucosal colonization but is also known to comprise only a small proportion of the fecal microbiota (approximately 0.5%–1%) [1, 2]. There are 2 molecular subtypes, nontoxigenic B. fragilis (NTBF) and enterotoxigenic B. fragilis (ETBF). Nearly 30 years ago, ETBF was implicated as causing diarrheal illnesses affecting livestock [3] and humans [4]. ETBF is now established as a cause of diarrheal disease in all age groups globally, with most reports focusing on young children [5]. Limited data also support an association of ETBF with active inflammatory bowel disease (IBD) [6, 7] and colorectal cancer (CRC) [8, 9]. Similar to other enteric pathogens, asymptomatic ETBF colonization is detected in children and adults with carriage rates as high as 40% in fecal samples from healthy adults [10].
ETBF pathogenicity is due to the B. fragilis toxin (BFT), a 20 kDa zinc-dependent metalloprotease toxin with 3 isotypes (BFT-1, BFT-2, and BFT-3) [11]. Sequence analysis indicates that the bft gene is unique and, since cloned in 1995 [12], only identified in B. fragilis. In vitro BFT binds to a specific colonic epithelial receptor activating Wnt and NF-κB signaling pathways with increased cell proliferation, epithelial release of proinflammatory mediators, and induction of DNA damage [5, 13–16]. In vivo ETBF, but not NTBF, induces BFT-dependent acute and chronic colitis in C57BL/6 mice [11, 17]. In multiple intestinal neoplasia (MinApc+/−) mice, a model for human CRC, ETBF promotes interleukin 17 (IL-17)–dependent carcinogenesis [8]. These data suggest that ETBF is a candidate etiologic agent in human sporadic CRC.
To further address the role of ETBF in the pathogenesis of human CRC, characterizing mucosal exposure to BFT is critical because long-term mucosal exposure is hypothesized to contribute to colon neoplastic transformation. Herein, we present novel data on the detection of the bft gene, the critical virulence determinant of ETBF, in mucosal samples from colorectal neoplasia patients (cases) compared with individuals undergoing outpatient colonoscopy (controls).
MATERIALS AND METHODS
Patient Population
Adult patients with colorectal neoplasia (cases; 43 = CRC, 6 = adenomas) undergoing primary colorectal surgical resections at Johns Hopkins Hospital (JHH) were studied between May 2010 and September 2012. Only tissue not needed for pathologic diagnosis was collected. Individuals undergoing outpatient colonoscopy (controls) at JHH between August 2011 and February 2013 for routine CRC screening or a diagnostic workup (eg, for anemia) were also studied.
Exclusion Criteria
Cases who received preoperative radiation and/or chemotherapy or with a history of CRC or IBD were excluded [18–20]. Similarly, controls with a history of CRC, IBD, or chemotherapy within 2 years of their procedure were excluded.
Antibiotic Exposure
A subset of cases received preoperative mechanical bowel preparation (MBP) without or with oral antibiotics, most often neomycin and erythromycin (MBP-No Abx vs MBP-Abx) (Table 1). Preoperative intravenous antibiotics were administered to all cases (cefotetan or clindamycin/gentamicin) within 1 hour of skin incision. In January 2012, JHH protocols changed to comply with newly emerging surgical infection prophylaxis guidelines [21] advocating MBP-Abx prior to all colorectal surgical procedures for surgical site infection prophylaxis. History of antibiotic use within 12 months preceding colonoscopy was assessed by questionnaire. Oral antibiotics were not part of colonoscopy MBP.
Table 1.
Characteristics of Cases and Controls
| Characteristics | All Cases (n = 49) | No Abx Cases (n = 26) | Controls (n = 49) |
|---|---|---|---|
| Age, y, median (IQR)a | 62 (52–76) | 64 (52.2–75.2) | 62 (49–66) |
| Male sexb | 22 (44.9) | 11 (42.3) | 20 (40.8) |
| Racec | |||
| White | 38 (77.6) | 20 (76.9) | 18 (36.7) |
| African American | 8 (16.3) | 4 (15.4) | 26 (53.1) |
| Other | 3 (6.1) | 2 (7.7) | 5 (10.2) |
| Bowel preparation | |||
| No prep | 20 (41) | 20 (76.9) | 0 |
| MBP-No Abx | 6 (12) | 6 (26.1) | 49 (100) |
| MBP-Abx | 23 (47) | 0 (0) | 0 |
| Indication for procedure | |||
| Screening | NA | NA | 30 (61.2) |
| Diagnostic workup | NA | NA | 19 (38.8) |
| Colorectal tumor | 49 (100) | 26 (100) | NA |
| Histologic diagnosis | |||
| Total tumors | 51 | 28 | NA |
| Tubular adenoma | 5 (10.2) | 3 (10.7) | 11 (22.4)d |
| Tubulovillous adenoma | 3 (5.9)e | 2 (7.1)e | NA |
| Adenocarcinoma | 43 (84.3)f | 23 (82.1)f | NA |
| Stage Ig | 7 (16.3) | 4 (17.4) | NA |
| Stage IIg | 12 (27.9) | 7 (30.4) | NA |
| Stage IIIg | 11 (25.6) | 7 (30.4) | NA |
| Stage IVg | 13 (30.2) | 5 (21.7) | NA |
| Tumor size, cm, median (IQR) | 4.5 (1.8) | 4.4 (1.9) | NA |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: Abx, antibiotics; IQR, interquartile range (defined as quartile 3 - quartile 1); MBP-Abx, mechanical bowel preparation with oral antibiotics; MBP-No Abx, mechanical bowel preparation without oral antibiotics; NA, not applicable.
a t test, P = .248 (all cases) and P = .367 (No Abx cases), compared to controls.
b Fisher exact test, P = .838 (all cases) and P = 1.00 (No Abx cases), compared to controls.
c χ2 test of independence, P = .002 (all cases) and P < .001 (No Abx cases), compared to controls.
d Eleven of the 49 controls had tubular adenomas removed during colonoscopy.
e One patient with 2 tubulovillous adenomas.
f One patient with both an adenocarcinoma and tubulovillous adenoma.
g The age distribution was similar between stage I/II and stage III/IV cases (χ2 test, P = .763).
Study Approval
This study was approved by the JHH Institutional Review Board. All samples were obtained in accordance with the Health Insurance Portability and Accountability Act.
Sample Collection
Mucosal tissue punches (4, 5 or 8 mm) from paired tumor and grossly normal tissue (Supplementary Figure 1) were harvested from the surgical specimens. Tissue pairs proximal to or from the hepatic flexure were defined as right colon while specimens distal to the hepatic flexure were defined as left colon. Colonoscopy biopsies were obtained from the right (cecum or ascending) and/or left (descending or sigmoid) colon using 2.8-mm disposable biopsy forceps (Boston Scientific Corporation). Surgical specimens were exposed to air for up to 45 minutes prior to tissue collection; colonoscopy biopsies were exposed to air ≤30 seconds.
Tissue Processing
Sample pairs from cases or controls were processed by 1 or both approaches as follows:
Broth Single Colony Method
Tissue samples were placed in peptone yeast glucose bile broth and then in an anaerobic chamber (Anaerobe Systems) at 37°C. Turbid broth (25 µL) was then inoculated on Brucella blood agar (BRU) (nonselective medium; Anaerobe Systems) and Bacteroides bile esculin agar (BBE) (Bacteroides selective; Becton Dickinson) to obtain single colonies (approximately 48–72 hours). BRU colonies were reisolated on BBE to select Bacteroides species. From each sample, 8–16 BBE isolates were expanded on tryptic soy agar with 5% sheep blood (TSA) (Anaerobe Systems) and tested for the bft gene.
Direct Single Colony Method
Mucosal tissue samples collected in anaerobic transport medium (ATM) (Anaerobe Systems) were washed twice with 0.016% dl-dithiothreitol in saline prior to pestle homogenization in sterile phosphate-buffered saline in an anaerobic chamber. Homogenized tissue dilutions (100–10−6, 25 µL each) were inoculated on BRU and BBE agar and 8–16 colonies per sample were tested for the bft gene and expanded on TSA as above. Colony-forming unit (CFU) counts were obtained from BBE agar.
Unless otherwise stated, the data from the 2 processing methods were combined for presentation because the results did not differ (78.3% concordant; P = 1.00 [McNemar test]; data not shown). On average, a total of 32 colonies per patient were analyzed for the bft gene for both cases and controls (see also “Results” section).
bft Polymerase Chain Reaction Analysis
Colonies from TSA plates were boiled and supernatant was used as DNA template for touch-down polymerase chain reaction (PCR) amplification evaluating 368-bp and/or 281-bp regions of the bft gene. PCR reactions used Platinum PCR SuperMix (Life Technologies Corporation) and 1 µM of forward and reverse primers according to protocol on a thermocycler (Applied Biosystems) (Supplementary Table 1) [22, 23]. PCR products were evaluated on 1.5% low-melting agarose gels and stained with ethidium bromide.
bft Isotype Identification
PCR products from bft-positive bacterial colonies were purified using the QIAquick PCR Purification Kit protocol (Qiagen) and sequenced (Genewiz, Inc) to determine bft isotypes. Sequences were screened with BLASTN against the National Center for Biotechnology Information nucleotide database, and isotype was verified at 99% identity and coverage.
Statistical Analysis
Patients were considered bft-positive if at least 1 bacterial colony from any tissue sample was bft-positive. Patient characteristics were compared using unpaired t test, Fisher exact test, or χ2 test as appropriate. The prevalence of bft between cases and controls and among tissue groups was compared using, as appropriate, McNemar, χ2, or Fisher exact test. CFU counts between cases and controls were analyzed using the Mann–Whitney U test, and bft isotypes between a subset of cases and controls with χ2 test. All statistical tests were performed with GraphPad Prism 5.0 and GraphPad InStat 3.05 and P < .05 defined as significant.
RESULTS
Patient Characteristics
A total of 49 cases with 8 adenomas and 43 adenocarcinomas (1 patient, 2 adenomas; 1 patient, CRC and adenoma) and 49 controls were studied (Table 1, Supplementary Table 2). Median age (62 years) and sex distribution were similar between cases and controls. Cases were significantly more often white, compared with controls who were more often African American (P = .002). Of the 51 tumor samples analyzed from cases, 47.1% (24/51) were from the left colon. Most controls (n = 30 [61.2%]) were undergoing CRC screening, with 91.8% having both right and left colon biopsies obtained (Supplementary Table 2).
Among cases, 23 patients (46.9%) had MBP-Abx within 24 hours prior to their operation, 20 (41%) had no MBP, and 6 (12%) had MBP-No Abx (Table 1). Among controls, 12 (24.5%) reported a history of antibiotic exposure within 12 months prior to their procedure, with 58.3% (7/12) reporting antibiotics within 3 months of colonoscopy. Only 2 patients reported taking antibiotics at the time of colonoscopy.
bft Is More Prevalent in Mucosal Samples From Cases Than Controls
Analysis of single bacterial colonies was pivotal in establishing the role of enterotoxigenic Escherichia coli as an etiology of diarrheal disease [24]. Thus, we analyzed individual Bacteroides colonies from BBE plates for the bft gene to detect bft positivity. Because antibiotic exposure could confound bft detection, bft positivity between cases with and without MBP-Abx was compared. Our initial analysis revealed a marked effect of MBP-Abx on bacterial recovery. Among MBP-Abx cases (n = 23), 70% of either tumor or paired normal colon samples did not culture any bacteria compared with no samples from cases without antibiotics (n = 26; Table 1) and, similarly, median Bacteroides species. CFUs were significantly lower in samples from MBP-Abx cases (8.0 CFU/sample; interquartile range [IQR], 2–6.6 × 101) vs cases without antibiotics (5.0 × 102 CFU/sample; IQR, 3 × 101–5 × 103) (Mann–Whitney U test, P < .001; Supplementary Figure 2). Consistent with these data, the number of bft-positive cases was significantly lower in those who received MBP-Abx (43.5% vs 88.5%; P = .002). In contrast, bft positivity was similar in controls regardless of reported antibiotic exposure (Table 2). The 2 controls who reported current antibiotic use were both bft-positive. Three (6.1%) controls did not have any bacterial growth; none reported antibiotic exposure.
Table 2.
Case and Control bft Status by Single Bacteroides Colony Analysis
| Patients | Casesa |
Controls |
P Valued | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | No Abxb | MBP-Abx | P Valuec | Total | No Abx | Abx | P Valuec | ||
| Total | |||||||||
| No. | 49 | 26 | 23 | 49 | 37 | 12 | |||
| No. bft+ (%) | 33 (67.3) | 23 (88.5) | 10 (43.5) | .002 | 33 (67.3) | 24 (64.9) | 9 (75.0) | .726 | .054 |
| Left-sided | |||||||||
| No. | 24 | 14 | 10 | 49 | 37 | 12 | |||
| No. bft+ (%) | 18 (75.0) | 12 (85.7) | 6 (60.0) | .192 | 26 (53.1) | 21 (56.7) | 5 (41.7) | .508 | .033 |
| Right-sided | |||||||||
| No. | 27 | 12 | 15 | 45 | 34 | 11 | |||
| No. bft+ (%) | 16 (59.3) | 11 (91.7) | 5 (33.3) | .005 | 25 (55.5) | 17 (50.0) | 8 (72.7) | .297 | .040 |
| P valuee | .372 | 1.00 | .414 | .838 | .638 | .214 | |||
Case or control bft positivity is based on combined direct and broth single colonies from BBE plates (Supplementary Materials and Methods); all P values were calculated with Fisher exact test. Note that left-sided and right-sided cases does not equal total cases; 2 patients had both a left and right sided tumor and are represented in both categories (see Table 1).
Abbreviations: Abx, antibiotics; BBE, Bacteroides bile esculin agar; MBP-Abx, mechanical bowel preparation with oral antibiotics; MBP-No Abx, mechanical bowel preparation without oral antibiotics.
a Case specimens include analysis of patients with adenomas (n = 6) or carcinomas (n = 43) and their paired normal tissues (Supplementary Methods). P values were calculated using Fisher exact test. For controls, exposure to antibiotics was determined by questionnaire (Supplementary Materials and Methods).
b No Abx cases includes MBP-No Abx and No Prep (see Table 1)
c MBP-Abx vs No Abx groups for cases; Abx vs No Abx groups for controls.
d Total controls vs No Abx cases.
e Left-sided vs right-sided.
Because antibiotic exposure did not modify bft results in controls, we next compared all MBP-No Abx cases to all controls. This analysis suggested that cases were more often bft-positive than controls (88.5% vs 67.3%, respectively; P = .054; Figure 1A; Table 2). Importantly, bft positivity did not differ by race in cases (white, 85.0% vs African-American, 100.0%; P = 1.00) and controls (white, 60.0% vs African-American, 73.1%, P = .492). Further stratification by left vs right colon tumors revealed a significant association of bft detection in cases compared to corresponding left vs right control biopsies (P = .033 and P = .040, respectively; Figure 1A, Table 2). All 3 cases with surgically removed tubulovillous adenomas were bft-positive whereas among controls, bft-positive status did not differ between individuals with (n = 11 [54.5%]) or without (n = 38 [71.1%]) small tubular adenomas removed at colonoscopy (Fisher exact test, P = .466; Supplementary Table 2B).
Figure 1.
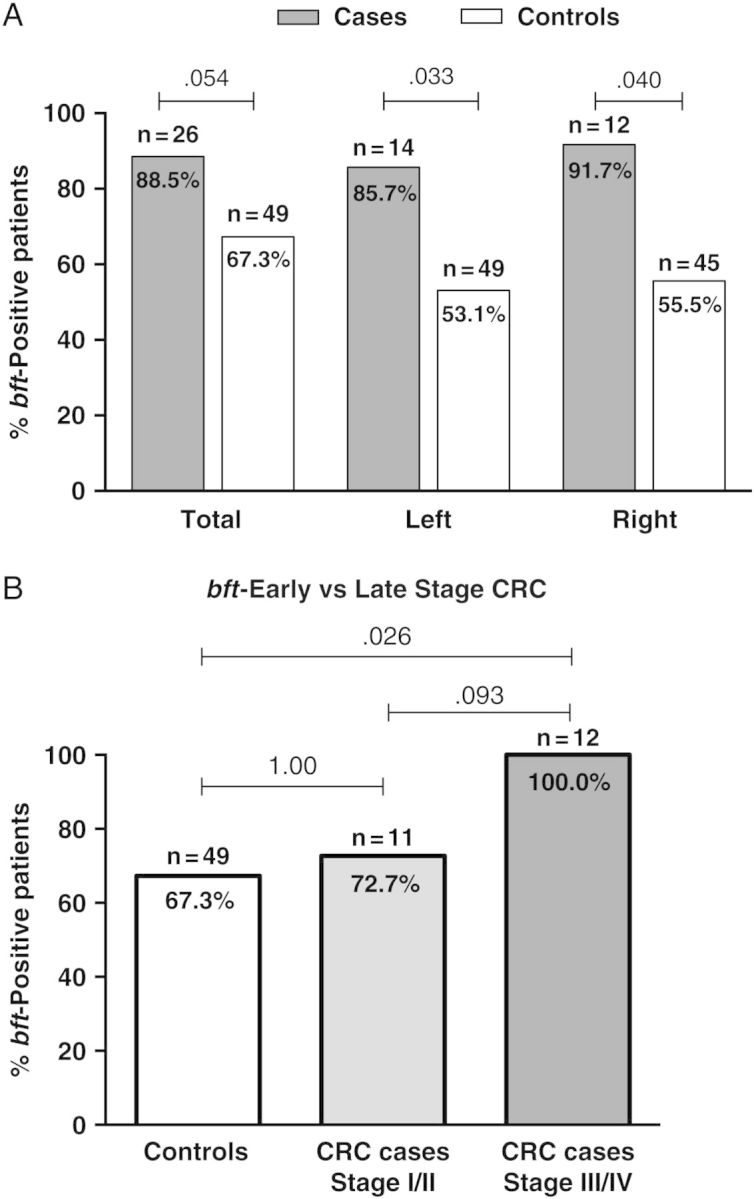
Case and control bft status. A, Overall, cases (n = 26; no antibiotics) were more often bft-positive than controls (n = 49; P = .054). bft positivity was present significantly more often in both left- and right-sided colorectal neoplasia cases compared with left and right colon biopsies from controls (Fisher exact test, P = .033 and P = .040, respectively). B, Patients with colorectal cancer (CRC) (n = 23; no antibiotics) were stratified by tumor stage (early, stage I/II; and late, stage III/IV). All 12 late-stage CRC patients (100%) were bft-positive compared with 67.3% of all controls and 71.1% of controls without adenomas (Fisher exact test, P = .026 and P = .046, respectively). Cases with adenomas were excluded from this analysis.
A potential limitation is the number of colonies that were examined in cases and controls. A median of 32.0 (IQR, 24.7–40.5) colonies from cases and 32 (IQR, 27.0–48.0) from controls were examined (Mann–Whitney U test, P = .857; Supplementary Figure 3A). However, when the single colonies evaluated per tissue sample were corrected for tissue size (mm2), the number of colonies tested in controls was approximately 3.5 times greater than for cases (Mann–Whitney U test, P < .001; Supplementary Figure 3B), suggesting a possible underrepresentation of the results in cases. Despite this potential bias, a significantly higher bft frequency was detected in MBP-No Abx cases compared with controls in both the right and left colon (Figure 1A).
The Majority of Paired Tissue Samples Are Both bft-Positive
Next, we analyzed whether bft detection differed between tumor and normal tissue pairs from MBP-No Abx cases or between right and left colonoscopy biopsy pairs within a single control (Supplementary Figure 4). From cases, 24 tumor/normal tissue pairs were analyzed and, from controls, 35 left/right biopsy pairs. Among the 24 tumor/normal pairs, 13 (54.2%) tissue pairs were both bft-positive. In 7 (29.2%) pairs, only 1 sample (tumor or normal) was bft-positive, whereas in 4 pairs (16.7%) bft was not detected in either sample. Among 35 left/right biopsy pairs from controls, 10 (28.6%) biopsy pairs were both bft-positive; in 11 (31.4%) pairs, only 1 biopsy (left or right) was bft-positive; and in 14 (40.0%) pairs, both were bft-negative. The frequency of bft detection on tumors vs normal tissues in cases or right vs left colon biopsies in controls did not differ (McNemar test, tumor vs normal, P = 1.00; right vs left biopsies, P = 1.00). An analysis of the median percentage of bft-positive colonies revealed a nonsignificant trend (P = .477), with the highest number of bft-positive colonies detected on tumors and lowest on control biopsies (tumor, 18.8%; paired normal, 12.5%; biopsies 6.3%) (Supplementary Figure 5). Thus, individual patient tumor/normal pairs were concordant for bft status in 75% of cases and 67% of controls. Altogether, these data suggest that mucosal bft presence is not limited to tumors but spans a larger portion of the colonic mucosa.
Late-Stage CRC Patients Have Higher bft Detection Than Early-Stage CRC Patients
We examined whether bft prevalence differed by cancer stage in patients with MBP-No Abx CRC (early-stage [stage I/II] vs late-stage [stage III/IV]; Table 1). All late-stage CRC patients (100%) were bft-positive compared with 72.7% of early-stage CRC patients (P = .093; Figure 1B). When compared to the overall bft-positive rate in controls (67.3%) or in controls without tubular adenomas detected on colonoscopy (n = 38 [71% bft+]), bft detection was significantly higher among late-stage CRC patients (P = .026 and P = .046, respectively).
Multiple bft Isotypes Are Present in Mucosal Samples of Cases
From a subset of patients (28 cases [n = 24 CRC; n = 4 adenomas] and 32 controls), single-colony bft PCR-products were purified and sequenced to identify the bft isotype. In total, 103 and 122 bft-positive colonies were sequenced from cases and controls, respectively. Overall, bft-2 was the most commonly identified isotype on the colon mucosa in both cases (41.2%) and controls (57.6%) (P = .226; Table 3). Multiple bft isotypes, most often bft-1 and bft-2, were detected significantly more often in cases (67.8%) than in controls (34.4%) (P = .019; Table 3).
Table 3.
Determination of bft Isotype by Sequence Analysis of Polymerase Chain Reaction Amplicons
| bft Isotype Status | Casesa | Controls |
|---|---|---|
| Patients | 28 | 32 |
| Single isotype | 9 (32.1) | 21 (65.6) |
| bft-1 | 3 (10.7) | 7 (21.9) |
| bft-2 | 6 (21.4) | 13 (40.6) |
| bft-3 | 0 (0.0) | 1 (3.1) |
| Mutiple isotypesb | 19 (67.9) | 11 (34.4) |
| bft-1 and -2 | 16 (57.1) | 9 (28.1) |
| bft-1 and -3 | 1 (3.6) | 0 (0.0) |
| bft-2 and -3 | 1 (3.6) | 1 (3.1) |
| bft-1, -2 and -3 | 1 (3.6) | 1 (3.1) |
| Overall isotype frequencyc | ||
| bft-1 | 21 (43.8) | 17 (38.6) |
| bft-2 | 24 (50.0) | 24 (54.5) |
| bft-3 | 3 (6.3) | 3 (6.8) |
| Single coloniesd | 103 | 122 |
| bft-1 | 38 (24.8) | 32 (21.2) |
| bft-2 | 63 (41.2) | 87 (57.6) |
| bft-3 | 2 (1.3) | 3 (2.0) |
Data are presented as No. (%).
Abbreviation: MBP-Abx, mechanical bowel preparation with oral antibiotics.
a Includes 7 MBP-Abx cases that grew bacteria.
b Cases had multiple bft isotypes detected compared with controls (Fisher exact test, P = .019).
c bft-2 was the most common isotype and did not differ between cases and controls (χ2 test of independence, P = .884). The numbers represent patients with single and multiple isotypes.
d Total single colonies isolated from cases or controls; the distribution of isotypes was not different (χ2 test of independence, P = .226). Numbers represent total isolated colonies that are bft+.
DISCUSSION
Our key finding is that there is a significant association of bft detection in left- and right-sided colon mucosal samples from cases compared with controls with 100% of late-stage CRC identified as bft-positive. This supports prior work where bft detection in stools was significantly higher in hospitalized CRC patients than outpatient controls [9]. Additionally, bft mucosal detection was common in our controls and higher than prior results based on fecal analyses of adults (40%) [10]. The colon mucosal microbial community is either unique or a subset of that detected in feces [25, 26] with Bacteroidetes and, specifically B. fragilis, reported as more abundant in mucosal than luminal samples in CRC patients [27, 28]. In vivo experimental studies show that ETBF is highly carcinogenic and in vitro studies demonstrate potential mechanisms for colon epithelial cell oncogenic transformation [8, 11, 13, 16]. The prevalent mucosal detection of bft suggests that ETBF may be one member of the microbiota contributing to colon carcinogenesis.
Unexpectedly and in contrast to work using fecal samples, where bft-1 detection is most common [5], our results show bft-2 as the most common mucosal isotype. Furthermore, cases significantly more often had multiple bft isotypes compared with controls, an observation not reported before in humans. In previous work, approximately 65% of fecal ETBF strains harbored bft-1 compared with approximately 25% bft-2 and approximately 10% bft-3 [5, 9, 22, 29, 30]. In 1 prior study of CRC patients, subtyping fecal ETBF also revealed predominant bft-1 (87.1%), not significantly different from controls (bft-1, 87.5%) [9]. Notably, BFT-2 has greater potency and biologic activity in vitro and in vivo compared to BFT-1 and, in preliminary data, is more carcinogenic in MinApc+/− mice (Wu and Sears, unpublished data) [5, 31]. Collectively, this suggests that bft-2-expressing stains exhibit enhanced mucosal adherence and carcinogenic potential compared to bft-1 strains, supporting a role for bft in the initiation and/or progression of human CRC.
Our study differs in both patient characteristics and microbiology methods compared to previous work. Zitomersky et al detected fecal ETBF in 40% (6/15) of healthy adults (mean age, 42 years) whom had on average 29 Bacteroidales analyzed per stool sample; bft was detected in 56.7% (101/178) of all B. fragilis isolates [10]. Subsequent work by this group, in contrast, identified mucosal ETBF in only 5% of IBD patients (mean age, 15–16 years; n = 63) and controls (mean age, 14 years; n = 31) and approximately 6% (6/104) of all isolates [32]. We studied an older population (median age, 62 years) and analyzed multiple mucosal samples per patient (mean, 28 colonies/patient). One critical methodology difference may be our use of ATM prior to homogenization and plating of samples in an anaerobic hood. This markedly enhanced bft detection (50% bft positivity without ATM vs 89% bft positivity with ATM, P = .024; manuscript in preparation). Overall, mucosal bft detection using our single-colony methodology was notably higher (67.3% of controls and 17.3% of all isolates examined).
In our study, bft was detected in the majority of surgically resected tumors and was uniform in late-stage CRCs, possibly due to enhanced anaerobiosis on larger tumors. This contrasts with Helicobacter pylori gastric colonization that diminishes in gastric cancer compared with earlier disease, as metaplastic tissue appears to be less hospitable for H. pylori [33–35]. Recent data suggest B. fragilis preferentially colonizes colonic epithelial crypts and, thus, may exhibit more stable colonization in CRC through evasion of host immune responses [36]. Crypt accumulation of ETBF strains over time expressing different bft isotypes may enhance carcinogenesis. ETBF induces rapid onset of chronic IL-17–dependent colitis and tumor formation in MinApc+/−mice with foci of persistent Stat3 activation [8, 17] and reactive oxygen species with DNA damage, potent mediators of oncogenesis [16, 37]. We postulate that bft exposure in the human colon may induce chronic, perhaps focal, mucosal inflammation yielding sites prone to DNA mutagenesis and carcinogenesis.
There are several important considerations for interpreting these study results. First, MBP-Abx prior to colorectal surgery significantly reduced bft recovery from surgical specimens and limited, over time, our ability to obtain MBP-No Abx tumor samples due to recently published colorectal surgery antibiotic prophylaxis guidelines. In future studies, samples collected by colonoscopy prior to surgery may help to overcome this potential bias. Second, we analyzed a significantly higher number of colonies per tissue area from controls, and surgical specimens were also exposed to air for longer than control biopsies. These issues could have biased our results toward bft underrepresentation in cases. Last, our data are not as quantitative as those reported by Zitomersky et al, where terminal dilution analysis was performed [10]. Despite these limitations, our cases were still identified to be more often bft-positive than our study controls.
Increasing attention is focused on understanding the contributions of colonic bacteria such as ETBF to colonic dysbiosis and human CRC [38–40]. Although bft is frequently detected and significantly more common in cases compared to controls herein, our results do not define exposure to biologically active BFT toxin. In addition, we did not confirm that bft detection was confined to B. fragilis sensu stricto as in prior work, nor did we define if B. fragilis colonization, independent of bft detection, differed between individuals with CRC and our control population. Many important questions remain to understand the relationship between bft exposure and CRC pathogenesis such as determining if mucosal and fecal B. fragilis and bft detection correlate, whether colonic inflammation correlates with bft detection, and/or a systemic anti-BFT antibody response. Further investigation is warranted to understand if age, sex, race, and/or diet affect bft detection in human populations over time.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Katharine Romans, MS, for her valuable contributions to sample collection.
Financial support. This work was supported by the National Institutes of Health (grant numbers R01 CA151393 to C. L. S. and D. M. P.; K08 DK087856 to E. C. W.; 5T32 CA126607-05 to E. M. H.; and P50 CA062924 [GI SPORE]); the Johns Hopkins Alexander and Margaret Stewart Trust (grant number 300-2344); the Netherlands Organization of Scientific Research (grant number NWO 825.11.031 to A. B.); and the American Society of Colon and Rectal Surgeons (grant number GSRRIG-015 to E. M. H.).
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Huang JY, Lee SM, Mazmanian SK. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe. 2011;17:137–41. doi: 10.1016/j.anaerobe.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macfarlane S, Woodmansey EJ, Macfarlane GT. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl Environ Microbiol. 2005;71:7483–92. doi: 10.1128/AEM.71.11.7483-7492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers LL, Firehammer BD, Shoop DS, Border MM. Bacteroides fragilis: a possible cause of acute diarrheal disease in newborn lambs. Infect Immun. 1984;44:241–4. doi: 10.1128/iai.44.2.241-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers LL, Shoop DS, Stackhouse LL, et al. Isolation of enterotoxigenic Bacteroides fragilis from humans with diarrhea. J Clin Microbiol. 1987;25:2330–3. doi: 10.1128/jcm.25.12.2330-2333.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349–69. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–4. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–32. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toprak NU, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–6. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 10.Zitomersky NL, Coyne MJ, Comstock LE. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infect Immun. 2011;79:2012–20. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee K-J, Wu S, Wu X, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708–18. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncrief JS, Obiso R, Barroso LA, et al. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect Immun. 1995;63:175–81. doi: 10.1128/iai.63.1.175-181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A. 1998;95:14979–84. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect Immun. 2004;72:5832–9. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Shin J, Zhang G, Cohen M, Franco A, Sears CL. The Bacteroides fragilis toxin binds to a specific intestinal epithelial cell receptor. Infect Immun. 2006;74:5382–90. doi: 10.1128/IAI.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin AC, Destefano Shields CE, Wu S, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:15354–9. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick E, Rabizadeh S, Albesiano E, et al. Stat3 activation in murine colitis induced by enterotoxigenic Bacteroides fragilis. Inflamm Bowel Dis. 2014;20:821–34. doi: 10.1097/MIB.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11–6. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 19.Lin XB, Dieleman LA, Ketabi A, et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One. 2012;7:e39764. doi: 10.1371/journal.pone.0039764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Vliet MJ, Tissing WJE, Dun CAJ, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49:262–70. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 21.Infectious Diseases Society of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. 2013. Available at: http://www.idsociety.org/Antimicrobial_Agents/#AntimicrobialProphylaxisforSurgery. Accessed 23 January 2013. [DOI] [PubMed]
- 22.Kato N, Liu CX, Kato H, et al. A new subtype of the metalloprotease toxin gene and the incidence of the three bft subtypes among Bacteroides fragilis isolates in Japan. FEMS Microbiol Lett. 2000;182:171–6. doi: 10.1111/j.1574-6968.2000.tb08892.x. [DOI] [PubMed] [Google Scholar]
- 23.Odamaki T, Sugahara H, Yonezawa S, et al. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18:14–8. doi: 10.1016/j.anaerobe.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Galbadage T, Jiang Z-D, DuPont HL. Improvement in detection of enterotoxigenic Escherichia coli in patients with travelers’ diarrhea by increasing the number of E. coli colonies tested. Am J Trop Med Hyg. 2009;80:20–3. [PubMed] [Google Scholar]
- 25.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Env Microbiol. 2002;68:3401–7. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namavar F, Theunissen EB, Verweij-Van Vught AM, et al. Epidemiology of the Bacteroides fragilis group in the colonic flora in 10 patients with colonic cancer. J Med Microbiol. 1989;29:171–6. doi: 10.1099/00222615-29-3-171. [DOI] [PubMed] [Google Scholar]
- 29.Akpınar M, Aktaş E, Cömert F, Külah C, Sümbüloğlu V. Evaluation of the prevalence of enterotoxigenic Bacteroides fragilis and the distribution bft gene subtypes in patients with diarrhea. Anaerobe. 2010;16:505–9. doi: 10.1016/j.anaerobe.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Scotto d'Abusco AS, Del Grosso M, Censini S, Covacci A, Pantosti A. The alleles of the bft gene are distributed differently among enterotoxigenic Bacteroides fragilis strains from human sources and can be present in double copies. J Clin Microbiol. 2000;38:607–12. doi: 10.1128/jcm.38.2.607-612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears CL. The toxins of Bacteroides fragilis. Toxicon. 2001;39:1737–46. doi: 10.1016/s0041-0101(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 32.Zitomersky NL, Atkinson BJ, Franklin SW, et al. Characterization of adherent Bacteroidales from intestinal biopsies of children and young adults with inflammatory bowel disease. PLoS One. 2013;8:e63686. doi: 10.1371/journal.pone.0063686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asaka M, Kimura T, Kato M, et al. Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer. 1994;73:2691–4. doi: 10.1002/1097-0142(19940601)73:11<2691::aid-cncr2820731107>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Yuan Y, Hunt RH. The association between Helicobacter pylori infection and early gastric cancer: a meta-analysis. Am J Gastroenterol. 2007;102:1789–98. doi: 10.1111/j.1572-0241.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 35.Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer. 2007;121:2782–6. doi: 10.1002/ijc.22992. [DOI] [PubMed] [Google Scholar]
- 36.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501:426–9. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 38.Boleij A, Tjalsma H. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc. 2012;87:701–30. doi: 10.1111/j.1469-185X.2012.00218.x. [DOI] [PubMed] [Google Scholar]
- 39.Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiol. 2013;8:445–60. doi: 10.2217/fmb.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–28. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


