Abstract
The HLA class II (DRB1 and DQB1) associations with sarcoidosis have been studied by several groups but often without consistent results. In this paper, we consider the hypothesis that observed inconsistencies relate to distinct, genetically encoded disease phenotypes which differ in prevalence between centres. We therefore typed HLA-DRB1 and DQB1 in 340 UK, 139 Dutch and 163 Japanese sarcoidosis patients and, respectively, 354, 218 and 168 healthy controls from these populations. We applied consistent phenotyping and genotyping and investigated associations between HLA class II alleles and distinct disease phenotypes within and between ethnic groups. DRB1*01 and DQB1*0501 are protective against all manifestations of sarcoidosis. Lung-predominant sarcoidosis is associated with DRB1*12 and *14. Löfgren's syndrome is a common sarcoidosis phenotype in the Dutch and is strongly associated with the DRB1*0301 allele. This phenotype is not seen among the Japanese in whom DRB1*0301 is absent. The same allele is protective for UK uveitis. Sarcoid uveitis is common in Japan. The DRB1*04–DQB1*0301 haplotype is a risk factor for this disease manifestation in Japanese and UK subjects but protective for sarcoidosis overall. We show that distinct sarcoidosis phenotypes have similar genetic associations across ethnic groups. The disease case mix differs between centres and may be explained by different ethnic allelic frequencies.
INTRODUCTION
The accumulated lifetime risk of sarcoidosis is ∼1.3% for women and 1.0% for men (1). The lungs are most commonly affected by this Th1-driven granulomatous disease, with fibrosis causing significant morbidity and death (2,3). Sarcoidosis clearly has a very strong genetic component; the familial risk ratio λ's score estimates of 36–73 (4) are significantly higher than in many autoimmune disorders such as rheumatoid arthritis and Type I diabetes mellitus. A single published microsatellite screen (5), albeit at very low density, showed the HLA region to contain the marker with the highest LOD score. Further, a non-caseating granulomatous disease, chronic beryllium disease (CBD), which is phenotypically very similar to sarcoidosis, has one of the strongest HLA associations known, with 80–90% of patients carrying DPB1* Glu 69 as well as a weaker, but independent association with HLA DRB1 alleles (6–11). It is unclear why strong and consistent major histocompatibility complex (MHC) associations are not found with non-CBD sarcoidosis across ethnic groups, despite several clear associations between HLA class II alleles HLA DRB1 and DQB1 (12–23). The prime aim of this study is to address this question.
Full rigorous meta-analysis of HLA data in sarcoidosis is not possible because of significant changes in methodology and nomenclature over the last 20 years which have lead to an enormous increase in system complexity. Allowing for these changes, we considered a number of non-exclusive reasons to explain the apparent inconsistency in the published literature. Firstly, there may indeed be interethnic differences in sarcoidosis susceptibility. Secondly, a gene in the MHC, other than the class II alleles, may be implicated. Thirdly, there may be differences in environmental exposure between regions (24,25). Alternatively, we considered that some of the apparent differences might relate to different phenotypes of disease between ethnic groups and differences in methodology between studies.
In order to test our hypothesis, we established databases for the UK and Dutch patients that used identical case definitions. Patients from both populations were followed regularly for 4 years. We also studied a totally different ethnic group (Japanese). Patient definitions were harmonized by a single clinician who worked in all three centres. We applied the same detailed genotyping methodology to all samples and rigorously audited both laboratory and clinical data. We selected control populations that came from the same catchment areas as the patients as far as possible. We utilized data mining tools and cluster analysis techniques mainly for audit purposes but also to aid clinical judgement in the assignment of possible subgroups of patients to generate hypotheses.
RESULTS
Controls
The carriage frequencies of DRB1 and DQB1 alleles in all cohorts are provided in Supplementary Material, S1–3. As expected, the Japanese data were very different from those of the other groups. Among the Caucasian control populations, there were marginal differences between the UK and Dutch groups. Therefore, we analyzed the three groups separately.
Study subjects
Table 1 shows the frequencies of clinical phenotype in the three sarcoidosis groups. The majority of Japanese study participants had uveitis, often in combination with neurological or cardiac involvement. This was not the case in the Caucasian sarcoidosis cohorts. Löfgren's syndrome was common among Dutch patients, rare in the UK cohort and absent in the Japanese. In contrast, chest radiological stage IV disease was more common in the UK cohort (data not shown). This probably reflects the fact that the Dutch cohort is from a secondary care hospital while the UK patients are from a tertiary centre which is likely to attract patients with chronic fibrotic disease. Initial data mining suggested that uveitis as well as Löfgren's syndrome might be a distinct clinical and genetic entity, and this hypothesis was further investigated.
Table 1.
Frequencies of clinical phenotype in UK, Dutch and Japanese sarcoidosis
| UK (N = 340) (%) | Dutch (N = 139) (%) | Japan (N = 163) (%) | |
|---|---|---|---|
| Uveitis | 70 (20.6) | 9 (6.5) | 119 (73.0) |
| Cardiac | 9 (2.6) | 4 (2.9) | 38 (23.3) |
| Neurological | 34 (10.0) | 2 (1.4) | 28 (17.2) |
| Uveitis only | 61 (17.9) | 9 (6.5) | 67 (41.1) |
| Uveitis and cardiac | 2 (0.6) | 0 (0.0) | 27 (16.6) |
| Uveitis and neurological | 7 (2.1) | 0 (0.0) | 18 (11.0) |
| Uveitis, neurological and cardiac | 0 (0.0) | 0 (0.0) | 7 (4.3) |
| Neurological only | 27 (7.9) | 2 (1.4) | 4 (2.5) |
| Cardiac only | 7 (2.1) | 4 (2.9) | 3 (1.8) |
| Löfgren's syndrome | 3 (0.9) | 47 (33.8) | 0 (0.0) |
| Erythema nodosum (excluding Löfgren's syndrome) | 25 (7.4) | 2 (1.4) | 0 (0.0) |
| Skin disease (excluding EN) | 49 (14.4) | 6 (4.3) | 49 (30.1) |
| Joint (all) | 34 (10.0) | 30 (21.6) | 8 (4.9) |
| Joint (excluding Löfgren's syndrome) | 34 (10.0) | 4 (2.9) | 8 (4.9) |
| Lung-predominant sarcoidosis | 233 (68.5) | 78 (56.1) | 37 (22.7) |
Lung-predominant sarcoidosis excludes Löfgren's syndrome and uveitis, neurological or cardiac sarcoidosis.
HLA associations
HLA alleles positively and negatively associated with sarcoidosis differed between patient cohorts. Figure 1 shows the odds ratio of DRB1 alleles in UK, Dutch and Japanese populations. Table 2 summarizes the significant associations between sarcoidosis and DRB1 and DQB1 alleles in all three groups. (Further details are provided in Supplementary Material, S1–3). There were some associations common to two or more cohorts but others that were unique to a particular group.
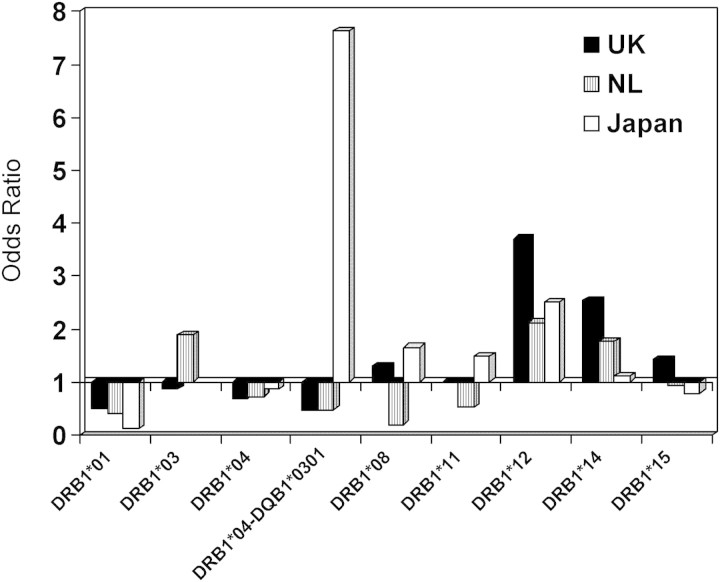
Figure 1.
Associations between HLA-DRB1 alleles and overall sarcoidosis in UK, Dutch and Japanese populations. In all three groups, DRB1*12 shows a positive association and DRB1*01 a negative association. DRB1*0301, DRB1*1401/02 and DRB1*0401–DQB1*0301 show different associations in the three groups.
Table 2.
Summary of significant associations between sarcoidosis and HLA class II alleles in UK, Dutch and Japanese populations
| Alleles | Sarcoidosis (%) | Control (%) | P-value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| UK | N = 340 | N = 354 | ||||
| Risk | DRB1*12 | 8.8 | 2.5 | 0.001 | 3.71 | 1.73–7.94 |
| DRB1*1401/2 | 13.8 | 5.9 | 0.001 | 2.54 | 1.49–4.36 | |
| DQB1*0503/4 | 13.2 | 5.6 | 0.001 | 2.55 | 1.47–4.41 | |
| DRB1*10 | 2.4 | 0.0 | 0.003 | n/a | ||
| DRB1*1501 | 36.2 | 28.5 | 0.04 | 1.42 | 1.03–1.96 | |
| Protection | DRB1*01 | 12.4 | 21.8 | 0.001 | 0.51 | 0.34–0.76 |
| DRB1*0401–DQB1*0301 | 10.3 | 17.5 | 0.008 | 0.54 | 0.35–0.84 | |
| DQB1*0301 | 34.4 | 43.2 | 0.02 | 0.69 | 0.51–0.94 | |
| Dutch | N = 139 | N = 218 | ||||
| Risk | DQB1*0201 | 40.3 | 25.2 | 0.004 | 2.00 | 1.27–3.16 |
| DRB1*03 | 39.6 | 25.7 | 0.008 | 1.90 | 1.20–2.99 | |
| DQB1*0503/4 | 12.2 | 5.5 | 0.04 | 2.40 | 1.11–5.18 | |
| Protection | DRB1*01 | 11.5 | 23.9 | 0.006 | 0.42 | 0.23–0.76 |
| Japanese | N = 163 | N = 168 | ||||
| Risk | DRB1*0803 | 29.4 | 17.3 | 0.01 | 2.00 | 1.19–3.38 |
| DQB1*0301 | 28.2 | 17.3 | 0.02 | 1.88 | 1.11–3.19 | |
| DRB1*12 | 14.7 | 6.5 | 0.03 | 2.46 | 1.17–5.21 | |
| DRB1*0401 | 4.3 | 0.6 | 0.03 | 7.49 | 0.91–61.60 | |
| DRB1*0401–DQB1*0301 | 4.3 | 0.6 | 0.03 | 7.49 | 0.91–61.60 | |
| Protection | DRB1*01 | 1.2 | 9.5 | 0.001 | 0.12 | 0.03–0.52 |
| DQB1*0501 | 1.8 | 10.1 | 0.002 | 0.17 | 0.05–0.58 | |
| DRB1*1403 | 2.5 | 7.7 | 0.04 | 0.3 | 0.10–0.94 |
The P-values in this table are uncorrected. P < 0.001 is considered significant after correction for the number of alleles (n = 28) and is shown in bold.
Similarities among all three groups
DRB1*01 was significantly decreased in all three groups (UK 12.4 versus 21.8%, P = 0.001, OR = 0.5; Dutch 11.5 versus 23.9%, P = 0.006, OR = 0.4; Japanese 1.2 versus 9.5%, P = 0.001, OR = 0.12). DRB1*12 was increased in UK (8.8 versus 2.5%, P = 0.001, OR = 3.7) and Japanese (14.7 versus 6.5%, P = 0.03, OR = 2.5). DRB1*12 was increased in Dutch patients (2.9 versus 1.4%, P = 0.4, OR = 2.1), although the number of carriers of this allele was too small to make statistically significant comparison.
Differences between Caucasian and Japanese cohorts
In UK and Dutch patients, we observed that both DQB1*0503/4 (UK 13.2 versus 5.6%, P = 0.001, OR = 2.6; Dutch 12.2 versus 5.5%, P = 0.04, OR = 2.4) and DRB1*14 (UK 13.8 versus 5.9%, P = 0.001, OR = 2.5; Dutch 11.5 versus 6.9%, P = 0.2, OR = 1.8) were increased in sarcoidosis, compared with controls. Both were in linkage disequilibrium in these ethnic groups. In contrast, in Japanese patients, where DRB1*14 can also be linked to DQB1*0301, DRB1*14 was decreased, especially when occurring on this non-Caucasian haplotype; DRB1*14–DQB1*0301 (2.5 versus 7.7%, P = 0.04, OR = 0.3).
In addition, in Caucasian patients with sarcoidosis, we observed a trend towards a reduced frequency of DRB1*04 (UK 28.8 versus 36.7%, P = 0.03, OR = 0.7; Dutch 21.6 versus 27.5%, P = 0.3, OR = 0.73), and especially in the DRB1*04–DQB1*0301 haplotype (UK 12.1 versus 22.3%, P = 0.001, OR = 0.5; Dutch 4.3 versus 8.7%, P = 0.2, OR = 0.47). In marked contrast, this same DRB1*0401–DQB1*0301 haplotype was increased in Japanese sarcoidosis cases (4.3 versus 0.6%, P = 0.03, OR = 7.5).
Differences between the three groups
In the Japanese patients, DRB1*0803 (29.4 versus 17.3%, P = 0.01, OR = 2.0) was associated with sarcoidosis overall. This allele (as opposed to other DRB1*08 alleles) is not found in Caucasian populations. In contrast, DRB1*03 and DQB1*0201 were absent from the Japanese population. These alleles were associated with Dutch sarcoidosis overall (DRB1*03 39.6 versus 25.7%, P = 0.008, OR = 1.9; DQB1*0201 40.3 versus 25.2%, P = 0.004, OR = 2.0), but not with disease in the UK. In the UK cohort alone, DRB1*10 (2.4 versus 0%, P = 0.003) and DRB1*15 (36.2 versus 28.5%, P = 0.04, OR = 1.4) were associated with disease.
We identified potential disease subsets using data mining and examined genetic associations with these below.
Löfgren's syndrome
No cases of Löfgren's syndrome occurred in Japanese patients and only three cases were identified from the UK cohort. We therefore only included Dutch Löfgren's syndrome cases for this analysis. Within the Dutch cohort, the haplotype DRB1*03–DQB1*0201 was strongly associated with Löfgren's syndrome (occurring in 80.9% of cases) compared both with controls (25.2%, P < 0.0001, OR = 12.5) and patients with other manifestations (non-Löfgren's syndrome) of sarcoidosis (18.5%, P < 0.0001, OR = 18.6). As found with sarcoidosis overall, DRB1*01 was protective for Löfgren's syndrome compared with controls (8.5 versus 23.9%, P = 0.02, OR = 0.30). Carriage of DRB1*11 was also protective (4.3 versus 17.9%, P = 0.02, OR = 0.2) (Table 3). The DRB1*12 and DRB1*14 alleles, which were increased in sarcoidosis in the complete cohorts, were not found among Löfgren's syndrome patients.
Table 3.
Summary of significant associations between Löfgren's syndrome and HLA DRB1 and DQB1 alleles in Dutch populations
| Alleles | Löfgren's syndrome | Control | P | OR | 95% CI | |
|---|---|---|---|---|---|---|
| N = 47 (%) | N = 218 (%) | |||||
| Risk | DRB1*03 | 80.9 | 25.2 | <0.0001 | 12.5 | 5.69–27.52 |
| DQB1*0201 | 80.9 | 25.2 | <0.0001 | 12.5 | 5.69–27.52 | |
| Protection | DRB1*11 | 2.2 | 17.9 | 0.005 | 0.10 | 0.01–0.76 |
| DQB1*0301 | 10.6 | 28.4 | 0.01 | 0.3 | 0.11–0.79 | |
| DRB1*0101 | 8.7 | 23.9 | 0.03 | 0.3 | 0.10–0.89 | |
| Löfgren's syndrome | No Löfgren's syndrome | P | OR | 95%CI | ||
| N = 47 (%) | N = 92 (%) | |||||
| Risk | DRB1*03 | 80.9 | 18.5 | <0.0001 | 18.6 | 7.59–45.69 |
| DQB1*0201 | 80.9 | 19.6 | <0.0001 | 17.4 | 7.12–42.3 | |
| Protection | DRB1*1401/2 | 0.0 | 17.4 | 0.001 | N/A | |
| DQB1*0503/4 | 0.0 | 18.5 | 0.001 | N/A | ||
| DQB1*0602 | 10.6 | 26.1 | 0.05 | 0.34 | 0.12–0.95 |
The P-values in this table are uncorrected. P < 0.001 is considered significant after correction for the number of alleles (n = 28) and is shown in bold.
We have previously shown that patients with Löfgren's syndrome, but not with non- Löfgren's syndrome sarcoidosis, have an increased frequency of the CCR2 haplotype 2 (26). This, added to the strong HLA-DR association and the distinctive clinical course, leads us to consider Löfgren's syndrome a clinically related but genetically separate disease from other forms of sarcoidosis. We have therefore performed subsequent analysis with the Löfgren's syndrome patients removed.
Uveitis
Uveitis was very common in the Japanese cohort (73.0%), less common in the UK group (20.6%) and rare in the Dutch cohort (nine patients, 6.5%). Data mining suggested that it might represent a genetically different subset of disease both in Caucasoid and Japanese subjects.
The DRB1*03–DQB1*0201 haplotype (associated with Löfgren's syndrome) was only found in four out of 70 (5.7%) UK uveitis patients compared with 25.7% controls (P < 0.0001, OR = 0.18), suggesting a protective role of the haplotype for this manifestation of sarcoidosis. This haplotype was not present among the Dutch uveitis patients and, as previously noted, was absent from the Japanese population.
DQB1*0503/4 (15.7 versus 5.6%, P = 0.007, OR = 3.1) and DRB1*14 (17.1 versus 5.9%, P = 0.003, OR = 3.3), which were both associated with disease overall, were also increased in UK uveitis patients compared with controls. In the Japanese cohort, DRB1*0803, which is uncommon in Caucasian populations, was associated with sarcoid uveitis (32.8 versus 17.3%, P = 0.004, OR = 2.3) as well as with disease overall. In contrast, the DRB1*0401–DQB1*0301 haplotype, which was ‘protective’ for sarcoidosis overall, was increased in frequency in those UK sarcoidosis patients with uveitis, compared with those without (21.4 versus 7.4%, P = 0.001, OR = 3.4). Carriage of the DRB1*0401 and DQB1*0301 alleles was also increased with uveitis in the Japanese cohort (4.3 versus 0.6%, P = 0.09, OR = 7.33) (Table 4).
Table 4.
Summary of significant associations between uveitis and HLA DRB1 and DQB1 alleles in UK and Japanese populations
| Alleles | Uveitis (%) | Control (%) | P | OR | 95% CI | |
|---|---|---|---|---|---|---|
| UK | N = 70 | N = 354 | ||||
| Risk | DRB1*1401/2 | 17.1 | 5.9 | 0.003 | 3.3 | 1.53–7.03 |
| DRB1*10 | 4.3 | 0.0 | 0.004 | N/A | ||
| DQB1*0503/4 | 15.7 | 5.6 | 0.007 | 3.1 | 1.42–6.83 | |
| Protection | DRB1*03 | 7.1 | 25.7 | <0.0001 | 0.22 | 0.09–0.57 |
| DQB1*0201 | 7.1 | 25.1 | <0.0001 | 0.23 | 0.09–0.59 | |
| Japanese | N = 119 | N = 168 | ||||
| Risk | DRB1*0803 | 32.8 | 17.3 | 0.004 | 2.3 | 1.34–4.07 |
| DRB1*0401 | 4.2 | 0.6 | 0.09 | 7.3 | 0.85–63.53 | |
| DRB1*0401–DQB1*0301 | 4.2 | 0.6 | 0.09 | 7.3 | 0.85–63.53 | |
| Protection | DRB1*0101 | 1.7 | 9.5 | 0.006 | 0.16 | 0.04–0.72 |
| DQB1*0501 | 2.5 | 10.1 | 0.02 | 0.23 | 0.07–0.80 | |
| DRB1*1403 | 1.7 | 7.7 | 0.03 | 0.20 | 0.05–0.92 | |
| DQB1*0604 | 5.0 | 12.5 | 0.05 | 0.37 | 0.15–0.95 | |
| Uveitis (%) | No uveitis (%) | P | OR | 95%CI | ||
| UK | N = 70 | N = 270 | ||||
| Risk | DRB1*0401–DQB1*0301 | 21.4 | 7.4 | 0.001 | 3.41 | 1.64–7.08 |
| DRB1*04 | 42.9 | 25.2 | 0.006 | 2.23 | 1.29–3.85 | |
| Protection | DRB1*03 | 7.1 | 27.0 | <0.0001 | 0.21 | 0.08–0.54 |
| DQB1*0201 | 7.1 | 27.4 | <0.0001 | 0.20 | 0.08–0.53 | |
| Japanese | N = 119 | N = 44 | ||||
| Risk | DQB1*0302 | 19.3 | 2.3 | 0.005 | 10.3 | 1.35–78.77 |
| DRB1*0802 | 10.9 | 0.0 | 0.02 | N/A | ||
| Protection | DQB1*0301 | 22.7 | 43.2 | 0.02 | 0.39 | 0.19–0.81 |
| DRB1*12 | 10.9 | 25.0 | 0.05 | 0.37 | 0.15–0.90 |
The P-values in this table are uncorrected. P < 0.001 is considered significant after correction for the number of alleles (n = 28) and is shown in bold.
Other clinical phenotypes
In Caucasians with sarcoidosis, no clear HLA associations were found for individuals with cardiac involvement (UK, n = 9; Dutch, n = 4), neurosarcoidosis (UK, n = 34; Dutch, n = 2), sarcoidosis-related joint disease (in the absence of Löfgren's syndrome) (UK, n = 34; Dutch, n = 4) or cutaneous involvement [excluding erythema nodosum (EN), UK, n = 49; Dutch; n = 6], although the small numbers may have precluded meaningful analysis.
In contrast, distinct associations were found in the Japanese cohort. Comparing sarcoidosis patients with or without skin disease, there was a significant association with DRB1*15 and with DQB1*0602 (26.5 versus 7.9%, P = 0.003, OR = 4.2; and 22.4 versus 7.0%, P = 0.01, OR = 3.8, respectively). Neurosarcoidosis was significantly associated with DRB1*0803 (46.4%) compared with both controls (17.3%, P = 0.001, OR = 4.2) and sarcoidosis patients without neurological involvement (25.9%, P = 0.05, OR = 2.5). This allele was also significantly increased in cardiac sarcoidosis (36.8%) compared with controls (17.3%, P = 0.01, OR = 2.8). However, 25 out of 28 (89%) neurosarcoidosis and 34 out of 38 (89.5%) cardiac sarcoidosis patients had uveitis. After multiple regression analysis, only neurosarcoidosis remained significantly associated with the DRB1*0803 allele.
Although sarcoid uveitis, cardiac involvement and neurosarcoidosis do not display HLA associations as strong as in Löfgren's syndrome, we judged them to be distinct enough to warrant separation of these patients and those with Löfgren's syndrome from individuals with predominantly lung involvement.
Lung-predominant sarcoidosis (excluding Löfgren's syndrome, uveitis, cardiac and neurological involvement)
In this aspect of our study, we defined patients with lung-predominant sarcoidosis as those with non-Löfgren's syndrome sarcoidosis without uveitis, neurological or cardiac involvement (UK n = 233; Dutch n = 92; and Japanese n = 37). This group also included individuals with cutaneous disease. Table 5 summarizes the significant positive and negative associations between HLA DRB1 and DQB1 alleles and lung-predominant sarcoidosis in the UK, Dutch and Japanese populations.
Table 5.
Summary of significant associations between lung-predominant sarcoidosis and HLA DRB1 and DQB1 alleles in UK, Dutch and Japanese populations
| Alleles | Lung-predominant sarcoidosis (%) | Control (%) | P | OR | 95% CI | |
|---|---|---|---|---|---|---|
| UK | N = 233 | N = 354 | ||||
| Risk | DRB1*12 | 9.9 | 2.5 | <0.0001 | 4.2 | 1.91–9.25 |
| DRB1*1401/2 | 14.6 | 5.9 | 0.001 | 2.7 | 1.53–4.80 | |
| DQB1*0503/4 | 14.2 | 5.6 | 0.001 | 2.8 | 1.54–4.93 | |
| DRB1*10 | 2.1 | 0.0 | 0.01 | N/A | ||
| Protection | DRB1*0401–DQB1*0301 | 5.6 | 17.5 | <0.0001 | 0.3 | 0.15–0.52 |
| DRB1*01 | 12.4 | 21.8 | 0.006 | 0.5 | 0.32–0.81 | |
| DQB1*0301 | 32.2 | 43.2 | 0.009 | 0.6 | 0.44–0.88 | |
| DRB1*04 | 23.2 | 36.7 | 0.001 | 0.5 | 0.36–0.76 | |
| Dutch | N = 78 | N = 218 | ||||
| Risk | DQB1*0503/4 | 20.5 | 5.5 | <0.0001 | 4.4 | 1.99–9.86 |
| DRB1*1401/2 | 19.2 | 6.9 | 0.004 | 3.2 | 1.49–6.96 | |
| Protection | DRB1*01 | 12.8 | 23.9 | 0.058 | 0.5 | 0.23–0.98 |
| Japan | N = 37 | N = 168 | ||||
| Risk | DRB1*12 | 27.0 | 6.5 | 0.001 | 5.3 | 2.05–13.65 |
| DQB1*0301 | 40.5 | 17.3 | 0.004 | 3.3 | 1.52–7.05 | |
| DRB1*1401-2 | 27.0 | 8.9 | 0.006 | 3.8 | 1.54–9.58 | |
| DQB1*0503/4 | 21.6 | 8.3 | 0.04 | 3.0 | 1.17–7.89 | |
| DQB1*0302 | 0.0 | 18.5 | 0.002 | N/A | ||
| Protection | DRB1*01 | 0.0 | 9.5 | 0.08 | N/A | |
| DQB1*0501 | 0.0 | 10.1 | 0.047 | N/A |
The P-values in this table are uncorrected. P < 0.001 is considered significant after correction for the number of alleles (n = 28) and is shown in bold.
As was the case with overall sarcoidosis, DRB1*12 was increased in UK and Japanese patients with lung-predominant sarcoidosis compared with controls (9.9 versus 2.5%, P < 0.0001, OR = 4.2 in UK; 27.0 versus 6.5%, P = 0.001 OR = 5.3 in Japanese, respectively). Similarly, DRB1*01 was decreased in lung-predominant sarcoidosis in all three groups compared with controls (12.4 versus 21.8%, P = 0.006, OR = 0.5 in UK; 12.8 versus 23.9%, P = 0.06, OR = 0.5 in Dutch; 0 versus 9.5%, P = 0.08 in Japanese). Although the DRB1*14 alleles in Caucasians were associated with disease overall, this did not prove to be the case in Japanese patients. However, they were found to be associated with lung-predominant sarcoidosis in all three cohorts (14.6 versus 5.9%, P = 0.001, OR = 2.7 in UK; 19.2 versus 6.9%, P = 0.004, OR = 3.2 in Dutch; 32.4 versus 16.1%, P = 0.04, OR = 2.5 in Japanese, respectively) (Fig. 2). A significant ethnic difference between HLA associations with overall sarcoidosis was observed with the DRB1*0401–DQB1*0301 haplotype. While this was protective for disease overall in the UK, it was a risk factor in the Japanese cohort. The DRB1*0401–DQB1*0301 haplotype remained highly significantly protective in UK patients with lung-predominant disease (5.6 versus 17.5%, P < 0.0001, OR = 0.3). This haplotype, which is a risk factor for uveitis, was present in only two cases in lung-predominant sarcoidosis in Japanese.
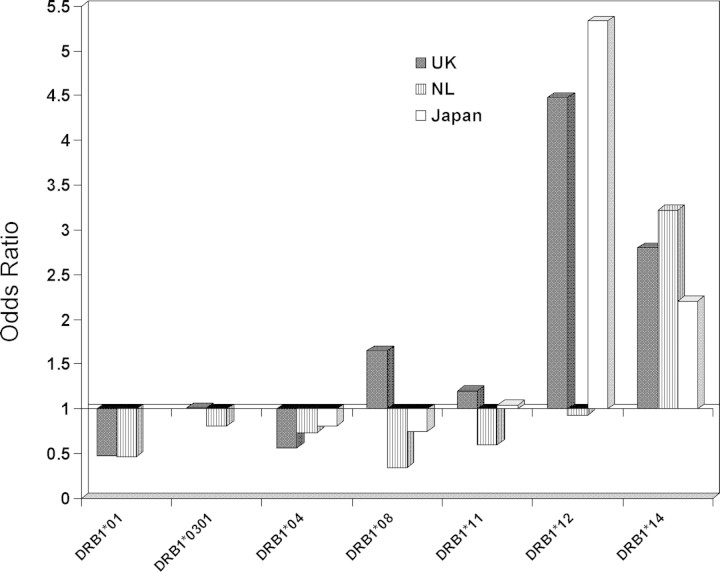
Figure 2.
Associations between HLA-DRB1 alleles and lung-predominant sarcoidosis in UK, Dutch and Japanese populations. DRB1*1401/2 shows a positive association and DRB1*01 a negative association in all three groups. DRB1*12 shows a positive association in UK and Japanese populations. None of these groups had an association with DRB1*11.
Thus, analysis of lung-predominant sarcoidosis alone discloses similar HLA associations in all three cohorts and may explain some of the discrepancies seen in the published literature. Logistic regression analysis showed that DRB1*12 and DRB1*14 are independent susceptibility markers in both UK and Japanese cohorts. In the Dutch group, where the number of individuals with DRB1*12 is small, DRB1*14 alone is a susceptibility marker. DRB1*12 and DRB1*14 are also associated with overall sarcoidosis in these populations. As illustrated in Figures 2 and 3, the DRB1*03 association with Dutch sarcoidosis is entirely dependent on those patients with Löfgren's syndrome and disappears in lung-predominant (non-Löfgren's syndrome) patients. Although other researchers have found an association between overall sarcoidosis and DRB1*11, no association was found in our study between these alleles and overall disease or with any subgroup of sarcoidosis.
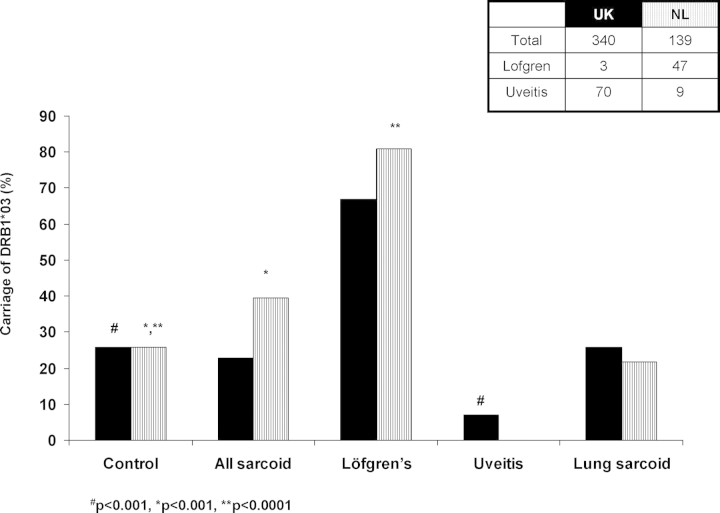
Figure 3.
Carriage frequencies of DRB1*0301 in UK and Dutch sarcoidosis patients and controls. In the Dutch cohort, overall sarcoidosis (P < 0.001) and Löfgren's syndrome (P < 0.0001) were significantly increased compared with controls. In the UK cohort, sarcoidosis with uveitis was significantly less common than in the control groups (P < 0.001). However, after excluding Löfgren's syndrome and uveitis, there were no significant differences between lung-predominant sarcoidosis and controls in both the UK and Dutch cohorts.
HLA amino acid epitopes
Again following the CBD–DPB1*Glu69 model (6–11), we evaluated whether the DRB1 allele associations were due to shared amino acid epitopes, by comparing the sequences of these alleles.
We used the SKDM HLA tool (27) to determine significant associations between amino acid epitopes and clinical phenotypes.
Table 6 summarizes the significant positive and negative associations (showing only corrected P-value <0.05) between DRB1 amino acid epitopes and sarcoidosis phenotypes in the UK, Dutch and Japanese populations. (Details are shown in Supplementary Material, Table S4). Using multiple regression analysis, we observed that the primary associations were with DRB1 alleles (data not shown). No amino acid epitopes remained significantly associated with sarcoidosis overall, lung-predominant sarcoidosis, Löfgren's syndrome or sarcoid uveitis.
Table 6.
Associations with HLA-DRB1 amino acid residues and clinical phenotypes
| Position and amino acid | UK |
Dutch |
Japan |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Association | Pcorr | OR | Pcorr | OR | Pcorr | OR | |||
| Lung-predominant | 1 | 85A | Positive | 0.018 | 3.26 | 0.06 | 5.23 | ||
| 4 | 13G | Positive | 0.037 | 2.64 | |||||
| 4 | 70R | Positive | 0.021 | 2.5 | 0.98 | 2.23 | |||
| 4 | 74E | Positive | 0.025 | 2.23 | 1 | 2.22 | |||
| 4 | 13H | Negative | <0.0001 | 0.4 | |||||
| 4 | 71K | Negative | 0.046 | 0.56 | |||||
| 6 | 30H | Positive | 0.017 | 4.06 | 0.06 | 5.23 | |||
| 6 | 11V | Negative | 0.0022 | 0.45 | |||||
| 7 | 47Y | Negative | 0.0036 | 0.46 | |||||
| 7 | 71K | Negative | 0.046 | 0.56 | |||||
| 9 | 37L38L | Positive | 0.017 | 4.06 | 0.06 | 5.23 | |||
| 9 | 57A60H | Positive | 0.048 | 2.68 | 0.48 | 2.95 | |||
| 9 | 57D | Negative | 0.029 | 0.3 | 0.92 | 0.38 | |||
| Löfgren's syndrome | 1 | 86V | Positive | 0.0085 | 7.6 | ||||
| 4 | 13S | Positive | 0.39 | 4.59 | |||||
| 4 | 26Y | Positive | <0.0001 | 9.29 | |||||
| 4 | 71K | Positive | <0.0001 | 9.07 | |||||
| 4 | 74R | Positive | <0.0001 | 11.66 | |||||
| 7 | 71K | Positive | <0.0001 | 9.07 | |||||
| 9 | 37N | Positive | <0.0001 | 6.8 | |||||
| Uveitis | 4 | 74E | Positive | 0.01 | 3.41 | ||||
| 4 | 70R | Negative | 0.016 | 0.75 | |||||
| 4 | 74R | Negative | 0.03 | 0.24 | |||||
| 9 | 57V | Negative | 0.032 | 0.28 | |||||
P-values on this table are corrected by 65.
When we examined association between HLA-DRB1 pocket residues and clinical phenotype, pocket 4 appeared as the major discriminating functional structure with pocket 6 as a secondary marker for lung-dominant sarcoidosis. Furthermore, pocket 9 showed discrimination between Löfgren's syndrome and lung-dominant sarcoidosis, with a similar trend in Japan for the lung-dominant sarcoidosis. Pocket 7 results are difficult to interpret because the key position, amino acid at 71, is shared with pocket 4.
DISCUSSION
Studies of the genetics of sarcoidosis from different groups have often produced discrepant results. In this paper, we have applied consistent clinical definitions and HLA genotyping across sarcoidosis populations from three countries. We demonstrated clear differences in the frequencies of various phenotypes of sarcoidosis between the cohorts. However, the individual clinical subtypes have similar HLA associations, irrespective of ethnicity. While we can not exclude differences in environmental exposure between populations, the similar HLA associations of each phenotype suggest that the variation in frequency between subtypes of disease may be secondary to differences in HLA allelic frequencies between ethnic groups. A corollary of our observation is that the MHC susceptibility gene for sarcoidosis is an HLA gene, and not another gene in this region. We therefore propose that the disparate reports in the literature can, in significant part, be explained by (i) differences in the ethnicity of studied populations and (ii) clinical heterogeneity among patients. Furthermore, differences in HLA typing and nomenclature between reports may have further confused comparisons. We have shown that two subgroups of sarcoidosis, Löfgren's syndrome and sarcoidosis uveitis, have clearly distinct HLA genetics and should perhaps be considered as separate sarcoidosis groups in the way already defined for chronic beryllium disease. A proposed schematic for the genetic subtypes of sarcoidosis is illustrated in Figure 4.
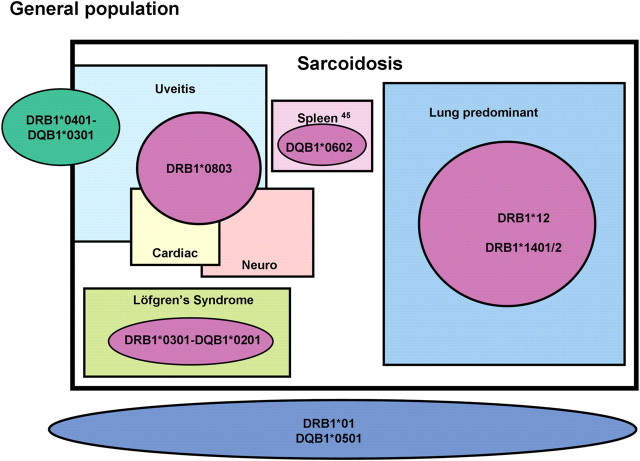
Figure 4.
A proposed schema for the genetic subtypes of sarcoidosis. DRB1*01 and DQB1*0501 are protective for overall sarcoidosis. The DRB1*0401–DQB1*0301 haplotype is protective for sarcoidosis overall but a risk factor for uveitis as shown. Within sarcoidosis, DRB1*0301–DQB1*0201 is associated with Löfgren's syndrome. Lung sarcoidosis is associated with both DRB1*12 and *1401/2. DRB1*0803 is a risk factor not only for uveitis, but also for neurological and cardiac sarcoidosis. Splenomegaly is associated with DRB1*0602 (45).
Most studies report an association between sarcoidosis and DRB1*12(13,15,19,28), and this was confirmed in our work in all three sarcoidosis cohorts. Similarly, there is general agreement with our finding that DRB1*0101 and DQB1*0501 are protective for overall sarcoidosis (13,28). We have shown that the DRB1*0101 and DQB1*0501 alleles are protective against lung-predominant sarcoidosis, Löfgren's syndrome and uveitis. Because the alleles are protective in all subtypes of disease, their effect on the overall cohort is protective regardless of the proportion of patients with each of the distinct subtypes (i.e. differences in case mix) in a particular cohort.
However, genetic associations within the MHC region are complicated by the extensive linkage disequilibrium in this region (29). This is especially strong between DRB1 and DQB1 alleles, with the result that alleles are often inherited together as haplotypes. Association of an allele with a disease can occur if that allele is functionally implicated or if it is on the same haplotype as the functional gene (i.e. the allele is just a marker). This is illustrated by the negative association between DRB1*0101 and DQB1*0501 and sarcoidosis in our patients. These alleles occur together on a haplotype that is common in Caucasian populations. We believe, however, that the primary association is with DRB1*0101, as DQB1*0501 can also occur with DRB1*10 and this haplotype appears to be a risk factor in the UK cohort.
Although associations with DRB1*12, DRB1*0101 and DQB1*0501 are found across ethnic groups, a number of differences occur, which we believe are due to case mix. DRB1*14 has been reported to be a susceptibility marker for sarcoidosis in some studies (13,15,16,22), but not to be associated with disease in others. We have found it to be associated with lung-predominant sarcoidosis in both Caucasians and Japanese (especially when occurring on a haplotype with DQB1*0503/4), but not with uveitis. DRB1*04 has been reported to be under-represented in Caucasian (13,17,19) sarcoidosis cohorts. However, we have shown that while the DRB1*0401–DQB1*0301 haplotype is protective for lung-predominant sarcoidosis and for overall disease in the UK and Dutch populations, it is a risk factor for uveitis in UK and Japan as was reported in the ACCESS (19) study.
The DRB1*03 allele (occurring on a haplotype with −DQB1*0201) has been found to be associated with sarcoidosis in a number of European studies (12,17,18,30), but not in reports from the UK (13), the USA (19) or Japan (15,16). We have shown this haplotype to be strongly associated with Löfgren's syndrome and to be protective against uveitis. These alleles are virtually absent in Japanese populations, perhaps explaining both the absence of Löfgren's syndrome and the high prevalence of uveitis in Japanese patients. The reports from the UK are from a centre with a high proportion of patients with chronic disease and a low frequency of Löfgren's syndrome, as we have again illustrated. In Europe, DRB1*0301 and/or DQB1*0201 plays an additional role in Löfgren's syndrome negative sarcoidosis disease set. Thus, in addition to uveitis protection and Löfgren's syndrome susceptibility, they have a clear role in terms of good prognosis identified by our group (21) and by others (12,31).
Just as DRB1*0301 is rare in Japanese populations, DRB1*0803 occurs very rarely in whites and American Blacks. This allele was found to be associated with sarcoidosis in Japan. It was associated with uveitis and with neurological and cardiac involvement in these patients. However, because the vast majority of patients with neurological or cardiac disease also had uveitis, and because of the small number of cases, our regression analysis could not determine which of the three patterns was the primary association with DRB1*0803. A Japanese group has reported an association between DQB1*0601 and DRB1*1502 and cardiac disease, although the frequency of uveitis in these patients was not stated (32). We were not able to find any association with DRB1*1502 here. However, in Japan, both DRB1*1502 and DRB1*0803 are in LD with DQB1*0601 (33). Therefore, we think that the primary association with cardiac disease is more likely to be with DQB1*0601 than with DRB1*1502. Cardiac involvement and neurosarcoidosis are rare in European populations, as is the DQB1*0601 allele. With the exception of Löfgren's syndrome and uveitis, we were unable to identify significant HLA associations with any extrapulmonary manifestation of sarcoidosis in the Caucasian cohorts.
Although we have managed to explain many of the differences previously reported between studies, we found no association between sarcoidosis or any of its subtypes and DRB1*11. This has been reported to be a susceptibility marker for Indian (22), Polish (30) and American Black and White sarcoidosis patients (19). Possible explanations are that the allele is more frequent in these populations or that it occurs on haplotypes bearing different DQB1 alleles. For example, in African American subjects, DRB1*11 can form a haplotype not only with DQB1*0301 (as in Caucasians), but also with DQB1*0602 (34).
Recently, we reported that immunorecognition of a mycobacterial antigen, ESAT-6, which occurs in 60% of US sarcoidosis patients, was inhibited by the anti-HLA-DR and HLA-DQ antibody. We have also shown that ESAT-6 immunorecognition in these patients was associated with the possession of a DRB1*11 allele (35). These results may suggest that the possession of DRB1*11 is associated with mycobacterium infection, one of the candidate aetiologies of sarcoidosis. A possible explanation for the lack of an association with DRB1*11 for our UK, Dutch and Japanese sarcoidosis patients is that mycobacterial infection was not aetiologically involved in these populations.
We also examined the association between clinical phenotypes of sarcoidosis and HLA-DR and DQ amino acid epitopes. Voorter et al. (36) have reported that DRArg71 and DQPhe9 are associated with sarcoidosis, but we did not find this. DRArg71 occurs in DRB1*1501/*1502 and DQPhe9 in DQB1*0401 and DQB1*0602. We believe that the reported association with these epitopes were because of the association with DRB1*1501/DQB1*0602 and not these particular amino acids. We found the primary associations with DRB1 alleles and not amino acid epitopes.
When we examined the association between HLA-DRB1 amino acid pocket residues and clinical phenotype, pocket 4 showed associations with lung-predominant, Löfgren's syndrome and uveitis in Caucasian patients. In Löfgren's syndrome patients, lysine (K) at position 74, which is in pocket 4 but seen only in DRB1*0301, showed the strongest association. Therefore, this lysine may be an important amino acid for Löfgren's syndrome. However, amino acid residues at pocket 4 also showed both positive and negative associations with uveitis and lung-predominant sarcoidosis in Caucasian. Thus, the peptide binding pocket 4 might have an important role to distinguish clinical phenotype in Caucasian sarcoidosis.
Recently, a new investigative strategy for HLA and disease studies has been described and applied to systemic sclerosis (37). Full application of this complex technique to our data will therefore be untaken as a separate collaborative study.
It remains uncertain which of the linked alleles within a particular haplotype are responsible for the disease association. Some groups have attributed risk to DQB1 alleles, although T-cell cloning work from the Swedish group indicates that DR alleles (predominantly DRB1 but also DRB3) are responsible (38). Other researchers have implicated DQA1 (22). Valentonyte et al. (39) have attributed the association to polymorphisms in the BTNL2 gene which lies in close proximity in the class II MHC region. However, we found a more significant association in our patients with DRB1 (28). The extreme polymorphism and complex and variable linkage in the MHC region make the associations difficult to dissect.
We began this study to examine the notion that sarcoidosis is a collection of diseases with a final common pathway of granulomatous inflammation, but with distinct MHC associations, perhaps related to separate inducing agents. We view CBD as the model of this concept, with granulomatous disease resulting from exposure to a known inducing agent on the background of a defined HLA allele (6–11). However, our hypothesis is only partially proven. Löfgren's syndrome is clearly genetically distinct, with nearly 80% of patients carrying the extended DRB1*0301–DQB1*0201 haplotype, compared with 7% of sarcoid uveitis patients. In the remaining sarcoidosis patients, the haplotype is also associated with milder disease (21,31). There is also a strong case for sarcoid uveitis being a separate genetic group. The high frequency of this complication in Japan and the opposite associations between DRB1*04 and sarcoid uveitis and lung-predominant sarcoidosis may have confused previous ethnic comparisons. Whether differences in case mix and ethnicity explain differences between our findings and those from studies of African Americans and Hispanic populations remains to be shown.
The importance of large numbers of patients is often stressed in genetic association studies. We agree with this but also believe that precise and consistent phenotyping can maximize the ability to identify significant associations in a given cohort. Moreover, accurate and reproducible HLA typing, together with the knowledge of ethnic differences in haplotype structure and frequencies, is vital to interpreting such studies and to reconciling apparent differences between studies that have been conducted in different groups across the world. Further research is needed to find the genes associated with other clinical phenotypes in different ethnic groups. Because of the complexity of the MHC region, more extensive genotyping is also needed across class I and II regions to define haplotypes accurately. The HLA region is too complex for the current generation of microarray or DNA chips to genotype. However, it is likely that future versions will be able to do so soon, perhaps leading to a renewal in interest in this region.
MATERIALS AND METHODS
Subjects
Three hundred and forty UK Caucasian sarcoidosis patients were recruited from the Royal Brompton Hospital, London, UK, and 139 Dutch Caucasian patients from St Antonius Hospital, Nieuwegein, The Netherlands. Data from some of these patients have already been reported (13,21,26,28,40–42), although new samples were added to increase the sample size. One hundred and sixty-three Japanese sarcoidosis patients were recruited consecutively from Central Clinic in Kyoto, Japan. The diagnosis of sarcoidosis was established when clinical and radiological findings were supported by histological evidence of non-caseating epithelioid cell granulomas, after exclusion of other known causes of granulomatosis. Three hundred and fifty-four UK and 218 Dutch control samples were used. These have previously been described (21,26,40–42). The Japanese control population comprised 168 healthy unrelated native Japanese mainly collected from the Kyoto prefecture.
Evaluation of extra-pulmonary disease
At presentation, all patients were assessed for extra-pulmonary disease, e.g. ophthalmic, cardiac, neurological and skin involvement, according to the ATS/ERS/WASOG statement on sarcoidosis (2). All extra-pulmonary manifestations other than skin disease were defined clinically. Forty-six Dutch sarcoidosis patients, three UK patients and none of the Japanese displayed a symptom cluster consisting of fever, erythema nodosum, arthralgia and bilateral hilar lymphadenopathy that was classified as Löfgren's syndrome. Uveitis was diagnosed by ophthalmologists.
Cardiac sarcoidosis was diagnosed in patients with clear evidence of sarcoidosis, cardiological symptoms and relevant abnormalities of ECG. Neurosarcoidosis was diagnosed in patients with clear evidence of sarcoidosis, neurological symptoms and relevant abnormalities of CT or MRI.
Sequence-specific primers–polymerase chain reaction
Analysis of genetic polymorphisms in the HLA-DRB1 and DQB1 allele
Genomic DNA was extracted from peripheral blood cells and genotyped for HLA-DRB1 and HLA-DQB1 using allele or allele group sequence-specific primers and polymerase chain reaction (PCR). A total of 28 DRB1 alleles and 13 DQB1 alleles were identified in all study subjects, namely: DRB1*0101/2 *0103, *0301, *04, *0701, *08 *0901, *1001, *1101-4, *1201, *1301, *1302, *1303, *1401/2, *1403, *1501, *1502, *1601 and DQB1*0201, *0202, *0301, *0302, *0303, *0401/2 *0501, *0502, *0503/504, *0601, *0602, *0603 and *0604/6/9. Full details of primers, PCR condition and gel electrophoresis have been described previously (21,43,44). DRB1*04- and DRB1*08-positive Japanese cases were retyped using high-resolution DRB1 typing (DRB1*0401, *0402, *0403, *0404, *0405, *0406, *0407, *0408, *04010, *0801, *0802, *0803).
Analysis of haplotype of HLA-DRB1–QB1 alleles
Major haplotypes were deduced from the results of DRB1 and DQB1 typing by visual scoring and were confirmed using previous HLA studies in Caucasian (29) and Japanese subjects (33).
Data analysis
The full clinical and genetic database was analysed using a data mining programme (Knowledge Studio—Angoss software). This identified database errors as well as possible associations requiring conventional statistical analysis. HLA-DRB1 allele and HLA-DQB1 allele carrier frequencies, i.e. number of individuals carrying at least one copy of the allele, were determined by direct counting for the control, sarcoidosis and the clinical subgroups. Statistical analysis was performed with SPSS version 14 (www.spss.com) using χ2 contingency table analysis with the appropriate number of degrees of freedom and Yates' correction, or Fisher's exact test if the expected cell frequencies were lower than 5. The results are shown as uncorrected P-values and odd ratios in the text and in Tables 2–5. After correcting for multiple comparisons, correcting for the number of possible alleles (Bonferroni's correction), P < 0.001, is considered as significant.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the Asmarley Trust (London, UK).
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr Joy Ross, Dr Dag Rutter, Dr Karin Grim (Imperial College London, UK) and Dr Kunimru, Dr Matuura and Dr Yamazaki for their technical support.
Conflict of Interest statement. The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Hillerdal G., Nou E., Osterman K., Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am. Rev. Respir. Dis. 1984;130:29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Costabel U., Hunninghake G.W. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur. Respir. J. 1999;14:735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 3.Newman L.S., Rose C.S., Maier L.A. Sarcoidosis. N. Engl. J. Med. 1997;336:1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 4.McGrath D.S., Daniil Z., Foley P., du Bois J.L., Lympany P.A., Cullinan P., du Bois R.M. Epidemiology of familial sarcoidosis in the UK. Thorax. 2000;55:751–754. doi: 10.1136/thorax.55.9.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schurmann M., Lympany P.A., Reichel P., Muller-Myhsok B., Wurm K., Schlaak M., Muller-Quernheim J., du Bois R.M., Schwinger E. Familial sarcoidosis is linked to the major histocompatibility complex region. Am. J. Respir. Crit. Care. Med. 2000;162:861–864. doi: 10.1164/ajrccm.162.3.9901099. [DOI] [PubMed] [Google Scholar]
- 6.Amicosante M., Sanarico N., Berretta F., Arroyo J., Lombardi G., Lechler R., Colizzi V., Saltini C. Beryllium binding to HLA-DP molecule carrying the marker of susceptibility to berylliosis glutamate beta 69. Hum. Immunol. 2001;62:686–693. doi: 10.1016/S0198-8859(01)00261-0. [DOI] [PubMed] [Google Scholar]
- 7.Amicosante M., Berretta F., Rossman M., Butler R.H., Rogliani P., Berg-Loonen E., Saltini C. Identification of HLA-DRPhebeta47 as the susceptibility marker of hypersensitivity to beryllium in individuals lacking the berylliosis-associated supratypic marker HLA-DPGlubeta69. Respir. Res. 2005;6:94. doi: 10.1186/1465-9921-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maier L.A., McGrath D.S., Sato H., Lympany P., Welsh K., du B.R., Silveira L., Fontenot A.P., Sawyer R.T., Wilcox E., Newman L.S. Influence of MHC class II in susceptibility to beryllium sensitization and chronic beryllium disease. J. Immunol. 2003;171:6910–6918. doi: 10.4049/jimmunol.171.12.6910. [DOI] [PubMed] [Google Scholar]
- 9.McCanlies E.C., Ensey J.S., Schuler C.R., Kreiss K., Weston A. The association between HLA-DPB1Glu69 and chronic beryllium disease and beryllium sensitization. Am. J. Ind. Med. 2004;46:95–103. doi: 10.1002/ajim.20045. [DOI] [PubMed] [Google Scholar]
- 10.Rossman M.D., Stubbs J., Lee C.W., Argyris E., Magira E., Monos D. Human leukocyte antigen Class II amino acid epitopes: susceptibility and progression markers for beryllium hypersensitivity. Am. J. Respir. Crit. Care Med. 2002;165:788–794. doi: 10.1164/ajrccm.165.6.2104002. [DOI] [PubMed] [Google Scholar]
- 11.Saltini C., Richeldi L., Losi M., Amicosante M., Voorter C., Berg-Loonen E., Dweik R.A., Wiedemann H.P., Deubner D.C., Tinelli C. Major histocompatibility locus genetic markers of beryllium sensitization and disease. Eur. Respir. J. 2001;18:677–684. doi: 10.1183/09031936.01.00106201. [DOI] [PubMed] [Google Scholar]
- 12.Berlin M., Fogdell-Hahn A., Olerup O., Eklund A., Grunewald J. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 1997;156:1601–1605. doi: 10.1164/ajrccm.156.5.9704069. [DOI] [PubMed] [Google Scholar]
- 13.Foley P.J., McGrath D.S., Puscinska E., Petrek M., Kolek V., Drabek J., Lympany P.A., Pantelidis P., Welsh K.I., Zielinski J., du Bois R.M. Human leukocyte antigen-DRB1 position 11 residues are a common protective marker for sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2001;25:272–277. doi: 10.1165/ajrcmb.25.3.4261. [DOI] [PubMed] [Google Scholar]
- 14.Iannuzzi M.C., Maliarik M.J., Poisson L.M., Rybicki B.A. Sarcoidosis susceptibility and resistance HLA-DQB1 alleles in African Americans. Am. J. Respir. Crit. Care Med. 2003;167:1225–1231. doi: 10.1164/rccm.200209-1097OC. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara M., Inoko H., Suzuki K., Ono H., Hiraga Y., Ando H., Naruse T., Ishida T., Ohno S. HLA class II genotyping of sarcoidosis patients in Hokkaido by PCR-RFLP. Jpn J. Ophthalmol. 1996;40:540–543. [PubMed] [Google Scholar]
- 16.Ishihara M., Ishida T., Inoko H., Ando H., Naruse T., Nose Y., Ohno S. HLA serological and class II genotyping in sarcoidosis patients in Japan. Jpn. J. Ophthalmol. 1996;40:86–94. [PubMed] [Google Scholar]
- 17.Martinetti M., Tinelli C., Kolek V., Cuccia M., Salvaneschi L., Pasturenzi L., Semenzato G., Cipriani A., Bartova A., Luisetti M. ‘The sarcoidosis map’: a joint survey of clinical and immunogenetic findings in two European countries. Am. J. Respir. Crit. Care Med. 1995;152:557–564. doi: 10.1164/ajrccm.152.2.7633707. [DOI] [PubMed] [Google Scholar]
- 18.Mrazek F., Holla L.I., Hutyrova B., Znojil V., Vasku A., Kolek V., Welsh K.I., Vacha J., du Bois R.M., Petrek M. Association of tumour necrosis factor-alpha, lymphotoxin-alpha and HLA-DRB1 gene polymorphisms with Lofgren's syndrome in Czech patients with sarcoidosis. Tissue Antigens. 2005;65:163–171. doi: 10.1111/j.1399-0039.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossman M.D., Thompson B., Frederick M., Maliarik M., Iannuzzi M.C., Rybicki B.A., Pandey J.P., Newman L.S., Magira E., Beznik-Cizman B., Monos D. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am. J. Hum. Genet. 2003;73:720–735. doi: 10.1086/378097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutherford R.M., Brutsche M.H., Kearns M., Bourke M., Stevens F., Gilmartin J.J. HLA-DR2 predicts susceptibility and disease chronicity in Irish sarcoidosis patients. Sarcoidosis Vasc. Diffuse Lung Dis. 2004;21:191–198. [PubMed] [Google Scholar]
- 21.Sato H., Grutters J.C., Pantelidis P., Mizzon A.N., Ahmad T., Van Houte A.J., Lammers J.W., Van Den Bosch J.M., Welsh K.I., du Bois R.M. HLA-DQB1*0201: a marker for good prognosis in British and Dutch patients with sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2002;27:406–412. doi: 10.1165/rcmb.4782. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S.K., Balamurugan A., Pandey R.M., Saha P.K., Mehra N.K. Human leukocyte antigen-DR alleles influence the clinical course of pulmonary sarcoidosis in Asian Indians. Am. J. Respir. Cell Mol. Biol. 2003;29:225–231. doi: 10.1165/rcmb.2003-0007OC. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi E., Kawakami Y. Analysis of HLA antigen in Japanese patients with sarcoidosis. Nippon Rinsho. 1994;52:1443–1448. [PubMed] [Google Scholar]
- 24.Kreider M.E., Christie J.D., Thompson B., Newman L., Rose C., Barnard J., Bresnitz E., Judson M.A., Lackland D.T., Rossman M.D. Relationship of environmental exposures to the clinical phenotype of sarcoidosis. Chest. 2005;128:207–215. doi: 10.1378/chest.128.1.207. [DOI] [PubMed] [Google Scholar]
- 25.Newman L.S., Rose C.S., Bresnitz E.A., Rossman M.D., Barnard J., Frederick M., Terrin M.L., Weinberger S.E., Moller D.R., McLennan G., et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 26.Spagnolo P., Renzoni E.A., Wells A.U., Sato H., Grutters J.C., Sestini P., Abdallah A., Gramiccioni E., Ruven H.J., du Bois R.M., Welsh K.I. C-C chemokine receptor 2 and sarcoidosis: association with Lofgren's syndrome. Am. J. Respir. Crit. Care Med. 2003;168:1162–1166. doi: 10.1164/rccm.200303-456OC. [DOI] [PubMed] [Google Scholar]
- 27.Kanterakis S., Magira E., Rosenman K.D., Rossman M., Talsania K., Monos D.S. SKDM human leukocyte antigen (HLA) tool: a comprehensive HLA and disease associations analysis software. Hum. Immunol. 2008;69:522–525. doi: 10.1016/j.humimm.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Spagnolo P., Sato H., Grutters J.C., Renzoni E.A., Marshall S.E., Ruven H.J., Wells A.U., Tzouvelekis A., van Moorsel C.H., Van Den Bosch J.M., et al. Analysis of BTNL2 genetic polymorphisms in British and Dutch patients with sarcoidosis. Tissue Antigens. 2007;70:219–227. doi: 10.1111/j.1399-0039.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad T., Neville M., Marshall S.E., Armuzzi A., Mulcahy-Hawes K., Crawshaw J., Sato H., Ling K.L., Barnardo M., Goldthorpe S., et al. Haplotype-specific linkage disequilibrium patterns define the genetic topography of the human MHC. Hum. Mol. Genet. 2003;12:647–656. doi: 10.1093/hmg/12.6.647. [DOI] [PubMed] [Google Scholar]
- 30.Bogunia-Kubik K., Tomeczko J., Suchnicki K., Lange A. HLA-DRB1*03, DRB1*11 or DRB1*12 and their respective DRB3 specificities in clinical variants of sarcoidosis. Tissue Antigens. 2001;57:87–90. doi: 10.1034/j.1399-0039.2001.057001087.x. [DOI] [PubMed] [Google Scholar]
- 31.Grunewald J., Eklund A., Olerup O. Human leukocyte antigen class I alleles and the disease course in sarcoidosis patients. Am. J. Respir. Crit. Care Med. 2004;169:696–702. doi: 10.1164/rccm.200303-459OC. [DOI] [PubMed] [Google Scholar]
- 32.Naruse T.K., Matsuzawa Y., Ota M., Katsuyama Y., Matsumori A., Hara M., Nagai S., Morimoto S., Sasayama S., Inoko H. HLA-DQB1*0601 is primarily associated with the susceptibility to cardiac sarcoidosis. Tissue Antigens. 2000;56:52–57. doi: 10.1034/j.1399-0039.2000.560107.x. [DOI] [PubMed] [Google Scholar]
- 33.Saito S., Ota S., Yamada E., Inoko H., Ota M. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens. 2000;56:522–529. doi: 10.1034/j.1399-0039.2000.560606.x. [DOI] [PubMed] [Google Scholar]
- 34.Carrington C.V., Kondeatis E., Ramdath D.D., Norman P.J., Vaughan R.W., Stephens H.A. A comparison of HLA-DR and -DQ allele and haplotype frequencies in Trinidadian populations of African, South Asian, and mixed ancestry. Hum. Immunol. 2002;63:1045–1054. doi: 10.1016/S0198-8859(02)00437-8. [DOI] [PubMed] [Google Scholar]
- 35.Oswald-Richter K., Sato H., Hajizadeh R., Shepherd B.E., Sidney J., Sette A., Newman L.S., Drake W.P. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1*1101. J. Clin. Immunol. 2010;30:157–166. doi: 10.1007/s10875-009-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voorter C.E., Amicosante M., Berretta F., Groeneveld L., Drent M., van den Berg-Loonen E.M. HLA class II amino acid epitopes as susceptibility markers of sarcoidosis. Tissue Antigens. 2007;70:18–27. doi: 10.1111/j.1399-0039.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 37.Karp D.R., Marthandan N., Marsh S.G., Ahn C., Arnett F.C., Deluca D.S., Diehl A.D., Dunivin R., Eilbeck K., Feolo M., et al. Novel sequence feature variant type analysis of the HLA genetic association in systemic sclerosis. Hum. Mol. Genet. 2010;19:707–719. doi: 10.1093/hmg/ddp521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunewald J., Wahlstrom J., Berlin M., Wigzell H., Eklund A., Olerup O. Lung restricted T cell receptor AV2S3+ CD4+ T cell expansions in sarcoidosis patients with a shared HLA-DRbeta chain conformation. Thorax. 2002;57:348–352. doi: 10.1136/thorax.57.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valentonyte R., Hampe J., Huse K., Rosenstiel P., Albrecht M., Stenzel A., Nagy M., Gaede K.I., Franke A., Haesler R., et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005;37:357–364. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 40.Grutters J.C., Sato H., Pantelidis P., Lagan A.L., McGrath D.S., Lammers J.W., Van Den Bosch J.M., Wells A.U., du Bois R.M., Welsh K.I. Increased frequency of the uncommon tumor necrosis factor −857T allele in British and Dutch patients with sarcoidosis. Am. J. Respir. Crit. Care Med. 2002;165:1119–1124. doi: 10.1164/ajrccm.165.8.200110-0320. [DOI] [PubMed] [Google Scholar]
- 41.Grutters J.C., Sato H., Pantelidis P., Ruven H.J., McGrath D.S., Wells A.U., Van Den Bosch J.M., Welsh K.I., du Bois R.M. Analysis of IL6 and IL1A gene polymorphisms in UK and Dutch patients with sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2003;20:20–27. [PubMed] [Google Scholar]
- 42.Spagnolo P., Renzoni E.A., Wells A.U., Copley S.J., Desai S.R., Sato H., Grutters J.C., Abdallah A., Taegtmeyer A., du Bois R.M., Welsh K.I. C-C chemokine receptor 5 gene variants in relation to lung disease in sarcoidosis. Am. J. Respir. Crit. Care Med. 2005;172:721–728. doi: 10.1164/rccm.200412-1707OC. [DOI] [PubMed] [Google Scholar]
- 43.Bunce M., O'Neill C.M., Barnardo M.C., Krausa P., Browning M.J., Morris P.J., Welsh K.I. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 44.Welsh K., Bunce M. Molecular typing for the MHC with PCR-SSP. Rev. Immunogenet. 1999;1:157–176. [PubMed] [Google Scholar]
- 45.Sato H., Nagai S., du Bois R.M., Handa T., Suginoshita Y., Ohta K., Welsh K.I., Izumi T. HLA-DQB1 0602 allele is associated with splenomegaly in Japanese sarcoidosis. J. Intern. Med. 2007;262:449–457. doi: 10.1111/j.1365-2796.2007.01829.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.