Abstract
Objectives
Biofilm formation by Candida albicans poses an important therapeutic challenge in human diseases. Typically, conventional antifungal agents encounter difficulty in treating and fully eradicating biofilm-related infections. Novel therapeutic approaches are needed to treat recalcitrant Candida biofilms. Farnesol is a quorum-sensing molecule, which induces apoptosis, inhibits Ras protein pathways and profoundly affects the morphogenesis of C. albicans. We therefore investigated the interactions between farnesol and different classes of antifungal agents.
Methods
The combined antifungal effects of triazoles (fluconazole), polyenes (amphotericin B) and echinocandins (micafungin) with farnesol against C. albicans biofilms were assessed in vitro. Antifungal activity was determined by the XTT metabolic assay and confocal microscopy. The nature and the intensity of the interactions were assessed using the Loewe additivity model [fractional inhibitory concentration (FIC) index] and the Bliss independence (BI) model.
Results
Significant synergy was found between each of the three antifungal agents and farnesol, while antagonism was not observed for any of the combinations tested. The greatest synergistic effect was found with the farnesol/micafungin combination, for which the BI-based model showed the observed effects as being 39%–52% higher than expected if the drugs had been acting independently. The FIC indices ranged from 0.49 to 0.79, indicating synergism for farnesol/micafungin and farnesol/fluconazole and no interaction for farnesol/amphotericin B. Structural changes in the biofilm correlated well with the efficacies of these combinations. The maximum combined effect was dependent on the farnesol concentration for micafungin and amphotericin B.
Conclusions
Farnesol exerts a synergistic or additive interaction with micafungin, fluconazole and amphotericin B against C. albicans biofilms, thus warranting further in vivo study.
Keywords: C. albicans, antifungals, combinations
Introduction
Biofilm-related infections caused by Candida albicans pose a major threat to public health as these infections are refractory to current treatment modalities and thus constitute a reservoir for continued infection.1 Therefore, it is crucial to include the exploration of novel drugs and the use of compounds that may enhance the efficacy of traditional antifungal agents in the therapeutic armamentarium targeting biofilm-related infections.
Quorum-sensing molecules play an essential role in biofilm growth and regulation. These molecules have a unique mechanism of action as they are continually produced during cell growth in amounts proportional to cell mass, leading to a coordinated expression of quorum-sensing target genes. In eukaryotic cells, quorum-sensing was unknown until the discovery of farnesol,2 an acyclic sesquiterpene alcohol generated by the dephosphorylation of farnesyl pyrophosphate during the mevalonate biosynthetic pathway in mammalian and yeast cells.3,4 Exogenous farnesol has been shown to block the yeast-to-filamentous transition and biofilm formation in a concentration- and time-dependent manner, with an optimal effect at high concentrations (>100 μM) and at the earliest stages of biofilm development.5–7
Given the anti-biofilm effects of farnesol against Candida spp., we hypothesized that it could augment the efficacy of conventional antifungal agents. To that end, we aimed in this study to investigate the in vitro effects of farnesol in combination with different classes of different antifungal agents, including triazoles (fluconazole), polyenes (amphotericin B) and echinocandins (micafungin), against C. albicans biofilms.
Materials and methods
Organisms
C. albicans SC5314, a well-characterized biofilm-producing strain, was used throughout this study.8 Candida was maintained in 25% glycerol and 75% peptone at −80°C and was subcultured on Sabouraud dextrose agar (BBL, Becton, Dickinson and Co. Sparks, MD, USA). The day before the experiment, C. albicans was grown overnight in yeast-nitrogen-base broth (pH 7, supplemented with 50 mM glucose; Baltimore, Bioworks) in an orbital shaker at 37°C under aerobic conditions. Before their use for biofilm formation, blastoconidia were suspended in 0.15 M PBS (pH 7.2; Growcells, Irvine, CA, USA), resuspended in RPMI 1640 supplemented with l-glutamine buffered with MOPS (Sigma-Aldrich, St Louis, MO, USA) and adjusted to a cell density of 1 × 106 blastoconidia/mL. All experiments were performed at least in triplicate on three separate days.
Biofilm formation
Biofilms were grown in vitro on the surface of polystyrene, flat-bottom 96-well microtitre plates (Corning Inc., Corning, NY, USA) as previously described.8 Briefly, 100 μL of the standardized C. albicans suspension (1 × 106 blastoconidia/mL) in RPMI 1640 was allowed to adhere and form biofilms at 37°C for 48 h. Following biofilm formation, the medium was aspirated and non-adherent cells were removed by washing twice with sterile PBS.8
Antifungal agents
Fluconazole (Sigma-Aldrich, St Louis, MO, USA), deoxycholate amphotericin B (Sigma-Aldrich, St Louis, MO, USA) and micafungin (Fujisawa Healthcare Inc., Deerfield, IL, USA) were used in this study. The antifungal agents were obtained in lyophilized powder form. Stock solutions of fluconazole (2 mg/mL), amphotericin B (10 mg/mL) and micafungin (5 mg/mL) were prepared in sterile distilled water (for amphotericin B and micafungin) with 10% DMSO (for fluconazole) and preserved according to the manufacturer's instructions. Farnesol (Sigma Chemical Co., St Louis, MO, USA) obtained as 3 M stock solution was diluted to a 30 mM solution in 100% methanol.6 The working concentrations of all the antifungal agents used were prepared in RPMI 1640. The drug-free control contained 1% methanol.
Susceptibility testing
Planktonic MICs were determined according to the CLSI (formerly NCCLS) M27-A2 method.9 MICs were determined as the lowest drug concentration at which a prominent (for fluconazole, micafungin and farnesol) or complete (for amphotericin B) decrease in turbidity was observed, corresponding to an ∼50% reduction in growth and complete inhibition of growth, respectively.10–12
Biofilm MICs were determined after incubation with the antifungal compounds for 24 h as the minimum antifungal drug concentrations that caused ≥50% reduction in the metabolic activity of the biofilms compared with controls.12
Biofilm formation and the anti-biofilm activity of the antifungal agents were assessed by XTT (0.25 mg/mL) and menadione (25 μM) assay. XTT conversion was measured spectrophotometrically (Magellan CE PR3100 TSC; Bio-Rad Laboratories, Redmond, WA, USA) at 450/620 nm. Mature biofilms were incubated for 24 h at 37°C with the antifungal agents, washed with PBS and then incubated for an additional 30 min to allow the conversion of XTT into its formazan derivative.
The metabolism inhibitory effect of the drugs was calculated using colour absorbance (A) as 100% × (Awell − Abackground) / (Adrug-free well − Abackground) where the background was measured from a fungal-free well (the control, considered to be 100%).13
Combination antifungal treatment of biofilms
Mature biofilms were incubated for 24 h at 37°C with farnesol alone and in combination with fluconazole, amphotericin B or micafungin including drug-free controls using a two-dimensional (8 × 12) chequerboard microdilution method in sterile 96-well flat-bottom microtitration plates.13 The choice of the appropriate range of drug concentrations was based on the biofilm MIC findings for the individual antifungal agent. The antifungal agents were prepared in serial 2-fold dilutions and their final concentrations ranged from 0.586– to 300 μM for farnesol, 8 to 512 mg/L for fluconazole, 0.5 to 32 mg/L for amphotericin B and 0.0312 to 2 mg/L for micafungin. The combined effects of each of the antifungal agents with farnesol were quantified by the XTT metabolic reduction assay.
Analysis of farnesol and antifungal agent interactions
The nature of the in vitro interactions between farnesol and each of the antifungal agents was assessed using two different models: the Loewe additivity model14 using the fractional inhibitory concentration (FIC) index, and the Bliss independence model.15
The FIC index is expressed with the following equation:
where and are the MICs of drugs A and B when acting alone, and and are the concentrations of drugs A and B in combination, respectively, in all of the wells corresponding to an MIC (isoeffective combinations). Among all ∑FICs calculated for all isoeffective combinations, the FIC index was determined as the ∑FICmin (the lowest ∑FIC) or the ∑FICmax (the highest ∑FIC). Off-scale MICs were converted to the next highest or lowest doubling concentration. Finally, the median and the range of FIC indices of the replicates were determined. The MICs of the drugs alone and of all the isoeffective combinations were determined as the lowest drug concentrations showing <50% reduction in the metabolic activity of an untreated control.16
The ∑FICs were interpreted as synergistic when ∑FIC ≤ 0.5, as antagonistic when ∑FIC > 4 and as showing no interaction when ∑FIC > 0.5–4.17 The FIC index corresponded to the ∑FICmin unless the ∑FICmax was higher than 4. In the latter case, both the ∑FICmin and the ∑FICmax were reported.16 The FICs among the different treatment groups were compared by analysis of variance followed by a post-test for linear trends. Differences with two-sided P < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (version 20; SPSS).
According to the Bliss model, the expected theoretical percentage of growth (Eind) (compared with an antifungal agent-free control) describing the effect of the combination of two antifungal agents was calculated using the following equation: Eind = EA × EB, where EA and EB are the experimental percentages of growth when each antifungal agent acts alone. For each combination of x mg/L of antifungal agent A with y mg/L of antifungal agent B in each of the independent replicate experiments, the experimental observed percentage of growth, Eobs, was subtracted from Eind. When the ΔE (ΔE = Eind − Eobs) was positive and its 95% CI did not include 0, significant synergy was claimed for the specific combination of x mg/L of antifungal agent A and y mg/L of antifungal agent B. When the ΔE was negative without its CI overlapping 0, statistically significant antagonism was claimed. In any other case where the 95% CI of ΔE would include 0, the conclusion was Bliss independence. The ΔE was calculated for the combination of each concentration of the two drug combinations. The sum and the mean of all statistically significant synergistic (ΔE ± 95% CI > 0) and antagonistic (ΔE ± 95% CI < 0) combinations are reported. The ΔEs that were significantly different from 0 of all combinations were constructed as a three-dimensional plot, with an interaction surface plot, the peaks above and below the 0 plane indicating synergistic and antagonistic interactions, respectively, while the 0 plane indicated indifferent interactions.18–20
In an attempt to summarize the whole interaction surface in a value, we calculated the sum percentages of all statistically significant synergistic (∑SYN) or antagonistic (∑ANT) interactions for our three different combinations. Interactions with <100% were considered weak, those with 100%–200% were considered moderate and those with >200% were considered strong.13,20
Confocal laser scanning microscopy (CLSM)
CLSM was used to visualize the structural effects of antifungal agents alone and in combination with farnesol. Candida biofilms formed on the surface of 5 mm diameter glass-bottom multiwell culture plates (MatTek Corp., Ashland, MA, USA) were then treated with fluconazole, amphotericin B or micafungin alone and in combination with farnesol as described above. After treatment, the supernatant was removed and the biofilms were incubated with fluorescent stains. Namely, 150 μL of PBS containing FUN-1 (0.2 μL from 10 mmol/L stock; Molecular Probes) and concanavalin A-Alexa Fluor 647 conjugate (0.75 μL from 5 mg/mL stock; Invitrogen) was added to each well containing biofilms and was incubated for 45 min at 37°C. FUN-1 is converted into orange-red or yellow-orange fluorescent intravacuolar compounds by metabolically active cells,21 whereas the concanavalin A-Alexa Fluor 647 conjugate binds to a-mannopyranosyl and a-glucopyranosyl residues of cell wall polysaccharides and emits green fluorescence. Stained biofilms were visualized by CLSM. Imaging was performed with a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Microimaging) equipped with a 40X C-Apochromat (numerical aperture, 1.2) objective. Image z-stacks with 0.22 μm x-y pixel size, 1.0 μm z-axis step size and 2.0 optical slice thickness were collected.
Results
The planktonic MICs of fluconazole, amphotericin B and micafungin were 4, 0.125 and 0.25 mg/L, respectively, and that of farnesol was 300 μM. The biofilm MICs of fluconazole, amphotericin B and micafungin, as evidenced by a decrease in Candida metabolic activity in an XTT colorimetric assay, were >256, 4 and 0.25 mg/L, respectively, and that of farnesol was >600 μM (the results are the mean values from four experiments performed on different days).
Table 1 summarizes the in vitro interactions between farnesol and each of the antifungal agents as determined by the FIC index. The mean FIC indices of the metabolic inhibitory effects of each combination were 0.50 for the farnesol/fluconazole combination, 0.79 for the farnesol/amphotericin B combination and 0.49 for the farnesol/micafungin combination, indicating a synergistic interaction for the farnesol/fluconazole and farnesol/micafungin combinations and no interaction for the farnesol/amphotericin B combination. No value of ∑FIC > 4, indicative of antagonism, was observed for any of the farnesol combinations tested. There was an overall reduction in the MICs of all the agents tested in combinations compared with the MICs when they were tested alone. Namely, the median MIC of farnesol tested alone was 450 μM, while in combination with fluconazole this reduced to 150 μM. The MIC (median value) of fluconazole alone was 1024 mg/L and in combination with farnesol was 64 mg/L. For the farnesol/amphotericin B combination, the MIC (median value) of farnesol alone was 600 μM and in combination was 14 μM, and for amphotericin B the respective median values were 1.5 mg/L (alone) and 1 mg/L (in combination). For the farnesol/micafungin combination, the MIC (median value) of farnesol alone was 600 μM and in combination was 300 μM, and for micafungin the respective median values were 4 mg/L (alone) and 0.25 mg/L (in combination).
Table 1.
In vitro interactions by FIC indices of farnesol in combination with antifungal agents against C. albicans biofilms
| Antifungal combination | Number of experimentsa | Type of interaction | ∑FIC mean ± SEM | ∑FICmin (range) |
|---|---|---|---|---|
| Farnesol and fluconazole | 3 | synergistic | 0.50 ± 0.02 | 1.00–0.15 |
| Farnesol and amphotericin B | 3 | no interaction | 0.79 ± 0.02 | 1.03–0.25 |
| Farnesol and micafungin | 5 | synergistic | 0.49 ± 0.02 | 1.00–0.14 |
aPerformed in triplicate.
The interaction parameters of the Bliss independence model are summarized in Table 2 and the interaction surface plots of farnesol combined with each of the three antifungal agents are shown in Figures 1–3. A synergistic interaction is depicted as a net positive direction of ΔE. The magnitude of the synergistic interactions is directly related to positive ΔE value. The different tones in three-dimensional plots represent different percentile bands of synergy.
Table 2.
In vitro interactions by Bliss independence analysis of farnesol in combination with antifungal agents against C. albicans biofilms
| Antifungal combination | Number of experimentsa | Type of interaction | Mean % ΔE value (range) | Mean % SEM (range) | ∑SYN% (n) |
|---|---|---|---|---|---|
| Farnesol and fluconazole | 3 | synergistic | 38.03 (23.10–48.86) | 7.11 (3.19–9.8) | 1217.1 (32) |
| Farnesol and amphotericin B | 3 | synergistic | 16.23 (6.88–30.86) | 2.56 (1.27–4.16) | 438.2 (27) |
| Farnesol and micafungin | 5 | synergistic | 43.72 (38.74–51.96) | 8.21 (6.28–10.33) | 480.9 (11) |
ΔE, the experimental observed percentage of growth subtracted from the calculated percentage of growth according to the Bliss independence model theory (see the text).
∑SYN, the sum of percentages of all synergistic interactions.
n, the number of separate combinations with statistically significant synergistic interactions.
aPerformed in triplicate.
Figure 1.
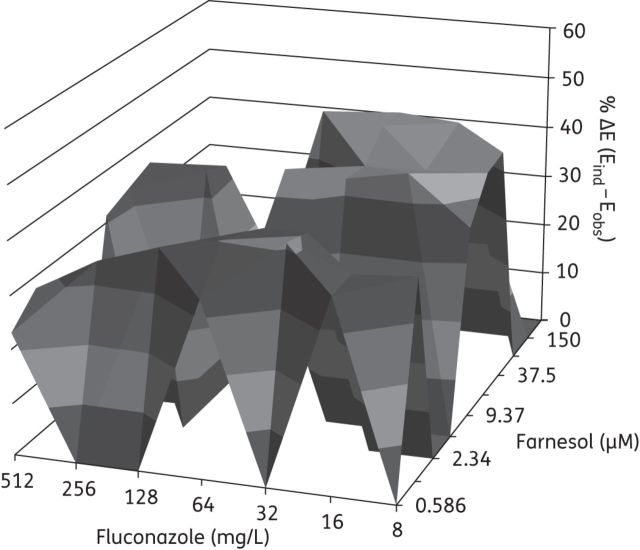
In vitro interaction between farnesol (0.586–300 μM) and fluconazole (8–512 mg/L) against C. albicans biofilms based on the Bliss independence no interaction model. The x-axis and y-axis represent the concentrations of farnesol and fluconazole and the z-axis represents the % ΔE (see the text). The zero plane (ΔE = 0) represents indifferent interactions whereas volumes above (ΔE > 0) represent statistically significant synergistic interactions. The magnitude of the synergistic interactions is directly related to positive ΔE value. The different tones in the three-dimensional plots represent different percentile bands of synergy. The % ΔE ± SEM was 38.0% ± 7.1% for the farnesol/fluconazole combination (synergistic interaction). The combination of farnesol and fluconazole against C. albicans biofilms resulted in a 1217.1% sum of statistically significant interactions (∑SYN).
Figure 3.
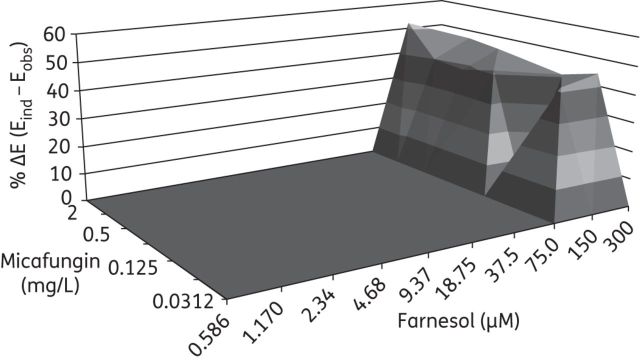
In vitro interaction between farnesol (0.586–300 μM) and micafungin (0.03–2 mg/L) against C. albicans biofilms based on the Bliss independence no interaction model. The x-axis and y-axis represent the concentrations of farnesol and micafungin and the z-axis represents the % ΔE (for more information see the legend for Figure 1). The % ΔE ± SEM was 43.7% ± 8.2% for the farnesol/micafungin combination (a synergistic interaction). The combination of farnesol and micafungin against C. albicans biofilms resulted in a 480.9% sum of statistically significant interactions (∑SYN).
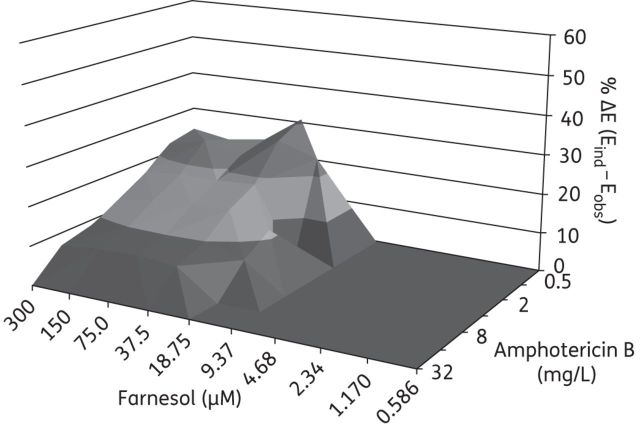
The treatment of C. albicans biofilms with farnesol and fluconazole resulted in synergistic interactions when a range of fluconazole concentrations (8 to 512 mg/L) were combined with farnesol in concentrations ranging from 0.586 to 75 μM (Figure 1). Combination treatment with farnesol and amphotericin B showed synergistic interaction over a range of amphotericin B concentrations (0.5 to 16 mg/L) and at higher farnesol concentrations (18.75–300 μM) (Figure 2). Finally, the treatment of C. albicans biofilms with farnesol and micafungin showed synergistic interactions only at high farnesol concentrations (150–300 μM) and over a range of micafungin concentrations (0.0625–2 mg/L) (Figure 3). Comparing the magnitude of these interactions (related to the ΔE value) for all tested antifungal agents in combination with farnesol, it was shown that micafungin had a greater synergistic effect (43.7%) than farnesol/fluconazole (38.0%, P = 0.04) or farnesol/amphotericin B (16.2%, P < 0.001).
Figure 2.
In vitro interaction between farnesol (0.586–300 μM) and amphotericin B (0.5–32 mg/L) against C. albicans biofilms based on the Bliss independence no interaction model. The x-axis and y-axis represent the concentrations of farnesol and amphotericin B and the z-axis represents the % ΔE (for more information see the legend for Figure 1). The % ΔE ± SEM was 16.2% ± 2.6% for the farnesol/amphotericin B combination (a synergistic interaction). The combination of farnesol and amphotericin B against C. albicans biofilms resulted in a 438.2% sum of statistically significant interactions (∑SYN).
The sums of all statistically significant synergistic are presented in Table 2. All combinations were strong (>200%); the highest value (1217.1%) was observed for the farnesol/fluconazole combination while for the farnesol/amphotericin B and farnesol/micafungin combinations the overall interaction surfaces were comparable (438.2% and 480.9%, respectively).
CLSM visualization of C. albicans biofilms treated with farnesol in combination with antifungal agents
The structural features of C. albicans biofilms after 24 h of treatment with farnesol alone or in combination with antifungal agents were demonstrated by CLSM. In order to optimally study the structural effects of combination therapy on C. albicans biofilms, the concentrations tested were those of the combinations that yielded the highest interaction, i.e. the combinations with the highest % ΔE value.
There was no apparent distortion in the biofilm architecture in the farnesol-treated biofilms at the low concentration (37.5 μM) while at the higher concentration (150 μM) there was a structural distortion, with the biofilm appearing more scant compared with untreated (control) biofilms, with a loss of elongated hyphal elements and a predominance of yeast-like forms (Figure 4).
Figure 4.
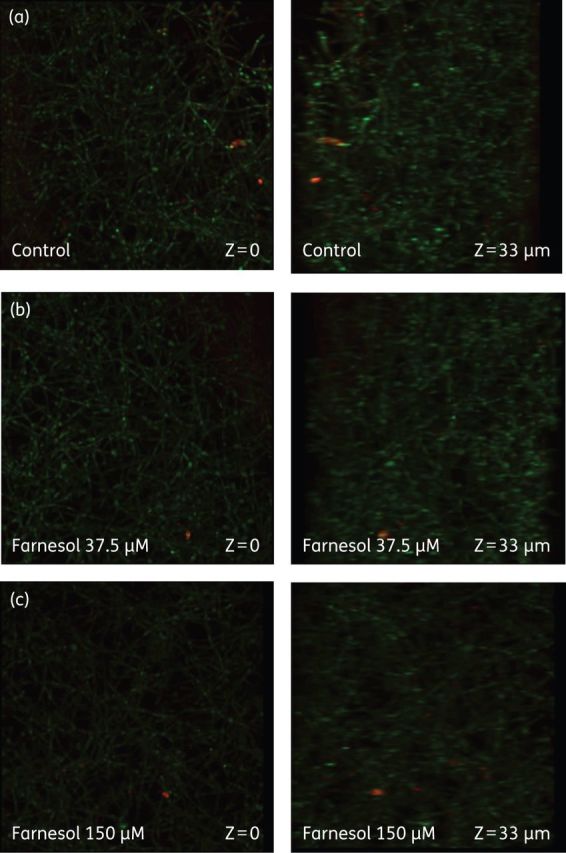
Effect of farnesol on C. albicans biofilms. (a) Untreated biofilm. (b) Biofilm exposed to farnesol at 37.5 μM. (c) Biofilm exposed to farnesol at 150 μM. Concanavalin A-Alexa Fluor 647 conjugate (green stain highlighting the Candida cell walls) and FUN-1 (yellow-orange stain highlighting non-viable cells) were used to directly visualize the effects of antifungal agents on biofilms. Images are single optical sections. The first image in each row depicts the upper layer of the biofilm, while the second panel illustrates a level near the bottom of the biofilm. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Biofilms treated with farnesol in combination with fluconazole showed a mild distortion of their architecture compared with fluconazole-treated biofilms or untreated biofilms; however, the number of non-viable cells, as stained by FUN-1, in the biofilm with the combined treatment was increased (Figure 5a–c). While biofilms exposed to combined treatment with farnesol/amphotericin B were still composed of a network of hyphae and pseudohyphae, the structure was scant compared with untreated biofilms (Figure 5a and e) and the cell viability was reduced compared with amphotericin B-treated biofilms (Figure 5d).
Figure 5.
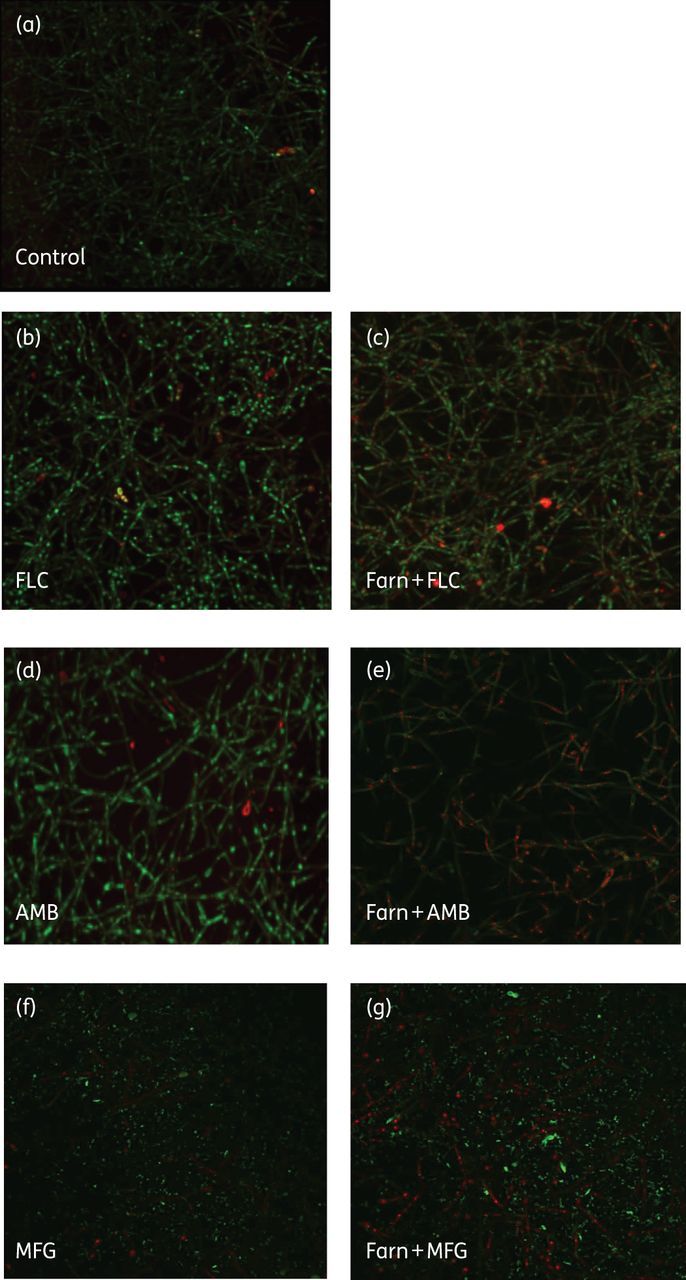
Effect of farnesol combination treatment on C. albicans biofilms. (a) Untreated biofilm. (b) Biofilm exposed to fluconazole at 64 mg/L. (c) Biofilm exposed to combination treatment of farnesol at 37.5 μM plus fluconazole at 64 mg/L. (d) Biofilm exposed to amphotericin B at 0.5 mg/L. (e) Biofilm exposed to combination treatment with farnesol at 37.5 μM plus amphotericin B at 0.5 mg/L. (f) Biofilm exposed to micafungin at 2 mg/L. (g) Biofilm exposed to combination treatment with farnesol at 150 μM plus micafungin at 2 mg/L. Concanavalin A-Alexa Fluor 647 conjugate (green stain highlighting the Candida cell walls) and FUN-1 (yellow-orange stain highlighting non-viable cells) were used to directly visualize the effects of antifungal agents on biofilms. Images are single optical sections. Farn, farnesol; FLC, fluconazole; AMB, amphotericin B; MFG, micafungin. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
More impressive were the images of the combined effect of farnesol and micafungin (Figure 5f and g), in which the biofilm structure was greatly distorted and true hyphae were rarely observed. Collectively, the extent of the morphological changes induced by the farnesol combined treatment was well correlated with the corresponding efficacies of farnesol with antifungal agents against biofilms, with micafungin and farnesol eliciting greater disruption than amphotericin B with farnesol; in addition amphotericin B and farnesol elicited greater disruptions than fluconazole and farnesol. Treatment of C. albicans biofilms with farnesol in combination with each antifungal agent showed that there was an antifungal agent-dependent effect.
Discussion
The dimorphic C. albicans is one of the most important fungal species, causing a spectrum of diseases ranging from superficial mucocutaneous to life-threatening invasive infections with a high mortality rate, particularly in immunocompromised individuals.22 Candida dimorphic switching from a yeast to a hyphal morphology is crucial not only for its virulence and dissemination but also for biofilm development. There is clear epidemiological evidence that a significant proportion of Candida infections, particularly those related to indwelling medical devices such as central venous catheters, joint prostheses, dialysis catheters and cardiovascular devices, as well as other chronic and difficult to treat infections like endocarditis, urinary tract infections and denture stomatitis, are related to biofilm formation.23
The mode of action of farnesol in Candida remains largely unknown; nevertheless, it seems to be complex and in all probability involves several mechanisms including growth-inhibitory and apoptosis-promoting effects.24,25 Recent studies from independent investigators have identified at least five different potential signalling pathways regarding farnesol's effect on the morphogenesis of Candida.26 Collectively, these studies show that farnesol inhibits germ tube formation by suppressing both mitogen-activated protein (MAP) kinase and cAMP-protein kinase A (PKA) pathways, while the downstream effectors in these pathways are initially activated by a Ras protein.27–29 Ras signals play an important role in the fate of C. albicans as they exert apoptotic effects accelerating programmed cell death.30 Moreover, several studies make a compelling case that farnesol has an important role in promoting apoptosis in C. albicans;31,32 however, the mechanisms underlying farnesol-induced antifungal activity remain to be further elucidated.
Whether farnesol functions in vivo as a therapeutic or a virulence-promoting factor is a controversial topic as there is a contradiction between experimental findings. The current data suggest that farnesol might be a virulence factor since its lipophilic properties promote invasiveness into cell membranes. Besides, farnesol appears to play a role in host defence mechanisms, promoting a T-helper2 (Th2)-mediated response, which is a less effective immune response against Candida infections.33,34 However, other in vivo models show that exogenously administered farnesol has a protective effect against local and disseminated candidiasis.35,36
In the present study we investigated the interactions between farnesol and antifungal agents representing different classes, i.e. triazoles (fluconazole), polyenes (amphotericin B) and echinocandins (micafungin), against C. albicans biofilms. We used RPMI 1640 culture medium in all the experiments assessing antifungal activity as RPMI 1640 simulates the conditions of glucose and amino acid concentration encountered in mammalian serum and extracellular fluid. However, the effect of serum proteins such as albumin on farnesol activity remains to be determined. Our findings, based on metabolic inhibitory effects, consistently demonstrated that farnesol exerts a synergistic or additive interaction with all the antifungal agents tested while antagonism was not observed for any of the combinations tested. Furthermore, the structural changes of the biofilms correlated well with the efficacies of the combination treatments. Another finding, which can lead to important assumptions regarding the mechanisms of these interactions, and ultimately to farnesol's mode of action per se, is that the degree of synergy between farnesol and antifungal agents differed significantly between the three compounds while the maximum combined effect was dependent on farnesol concentration for amphotericin B and micafungin. The synergistic interaction between farnesol and antifungal agents has previously been shown with planktonic Candida cells.6,37 However, the concept of combination antifungal treatment would be more applicable against the more therapeutically challenging biofilms.
Farnesol's anti-biofilm activity alone or in combination with antimicrobial agents was originally shown in bacterial biofilms where farnesol had a significant impact on glucan accumulation in Streptococcus mutans biofilms.38 Furthermore, farnesol non-specifically enhanced the permeability of Staphylococcus aureus biofilms, acting synergistically with certain classes of antimicrobials such as aminoglycosides, which cannot penetrate intact bacterial cells in order to reach their target. However, this synergistic interaction was not as effective in completely eradicating S. aureus biofilms.39 Similarly, a synergistic effect was also not observed for farnesol with vancomycin, tetracycline and rifampicin against Staphylococcus epidermidis biofilms; however, the biofilms appeared to be more susceptible to the most lipophilic antibiotic (rifampicin) tested.40 The effect of farnesol against staphylococcal biofilms was further validated by studies by Pammi et al.41 where, using both in vitro and in vivo biofilm models, farnesol was shown to inhibit biofilms of S. epidermidis and exert a synergistic effect with nafcillin and vancomycin at most combination ratios. An evaluation of the confocal imaging revealed a significant decrease in the biovolume, thickness and substratum coverage of farnesol-treated S. epidermidis biofilms.41,42 While it is plausible that the modified experimental procedures are responsible for the discrepancies in these studies, it is more likely that the lipophilic properties of farnesol, which favour its localization in cell membranes, may cause a mechanistic disruption of the cytoplasmic membrane and ultimately destruction of the biofilm structure, rendering the microbial cells accessible to antimicrobial agents.
Consistent with the observations in bacteria, the synergistic interaction observed between farnesol and fluconazole against Candida spp. biofilms may be attributable to farnesol's disruptive effect on the fungal cell membrane, which renders the fungal cell membrane permeable to exogenous compounds such as fluconazole.6 However, as has been pointed out, farnesol's mode of action is complex and involves several mechanisms including an inhibition of ergosterol synthesis, which is not surprising given that farnesol is synthesized as part of this pathway. In addition, transcriptomic analysis studies have shown that farnesol affects the expression of genes involved in ergosterol metabolism25 and inhibits fluconazole resistance in Candida biofilms by down-regulating partial gene expression in ergosterol biosynthesis.43
In vitro studies have shown that farnesol is a specific drug efflux pump modulator, reversing the extrusion of specific compounds mediated by selected ATP binding cassette (ABC) drug transporters (such as CaCdr1p, CaCdr2p and ScPdr5p but not CaMdr1p) in planktonic C. albicans.44 However, the efflux pump modulating effect of farnesol does not seem to adequately explain its synergistic effect with fluconazole, ketoconazole, miconazole and amphotericin B in C. albicans cells.44 Instead, the authors suggest that the substantial accumulation of reactive oxygen species (ROS),44 which can lead to apoptotic cell death,45,46 may contribute to farnesol's synergistic effect with antifungal agents. Alternatively, farnesol has been speculated to inhibit fluconazole resistance in C. albicans biofilms by playing a certain role in down-regulating the expression of multidrug resistance (MDR1) genes.43 Thus far, however, the reported effects of farnesol on the drug efflux pump are not straightforward.
Previous investigations have clearly shown that farnesol triggers an apoptotic process in eukaryotic cells via the induction of caspases, the production of ROS and the disruption of mitochondrial integrity, ultimately resulting in cell death.31,47 Elucidating a defined mechanism behind the apoptotic effect of farnesol on C. albicans, Zhu et al.32 provided direct evidence for the involvement of Cdr1p in the extrusion and depletion of the antioxidant glutathione in the cytotoxicity of farnesol through a disruption of intracellular redox homeostasis.
Multiple pathogenic traits of C. albicans including adhesion, biofilm formation and morphogenesis have been associated with the Ras signalling pathway and its downstream components.48 Farnesol activates transmembrane domains of Ras, which in turn inhibit the cAMP-PKA pathway and MAP kinase pathway.27–29 Current evidence indicates that Ras and ROS are two important molecules whose function, while enigmatic, seems to be interrelated.49,50 Ras and ROS are linked in that ROS is a factor that regulates Ras protein levels.49 On the other hand, there is evidence that antifungal agents induce ROS in fungal biofilm cells.51 At this stage it is not possible to draw definitive connections to unravel the mechanisms underlying the interactions of farnesol and antifungal agents. However, we can hypothesize that the synergistic effect observed in our studies may be caused by the antifungal-mediated induction of ROS, which in turn up-regulates Ras proteins, therefore facilitating the action of farnesol in the inhibition of the cAMP-PKA and MAP kinase pathways.45,48–51
In conclusion, the findings from this study demonstrate that farnesol in combination with antifungal agents may have utility as an adjuvant anti-biofilm agent. Elucidating the specific biochemical pathway towards ROS–Ras amplification may lead to new treatment targets to improve the outcome of patients with Candida biofilm-related infections.
Funding
The research project is implemented within the framework of the Action ‘Supporting Postdoctoral Researchers’ of the Operational Program ‘Education and Lifelong Learning’ (Action's Beneficiary: General Secretariat for Research and Technology), and is co-financed by the European Social Fund (ESF) and the Greek State. T. J. W. is a scholar of the Sharp Family Foundation in Pediatric Infectious Diseases.
Transparency declarations
T. J. W. receives research grants to Weill Cornell Medical Center for experimental and clinical antimicrobial pharmacotherapeutics, new diagnostic systems and strategies for augmentation of host defence against life-threatening infections in immunocompromised children and adult patients from Save our Sick Children (SOS) Kids Foundation, Astellas, Novartis, Merck, ContraFect, Cubist and Pfizer. T. J. W. has served as a consultant to Astellas, ContraFect, iCo, Novartis, Pfizer, Methylgene, SigmaTau and Trius. All other authors: none to declare.
Acknowledgements
Assistance from Lee Cohen-Gould Core, Director of the Confocal Scanning Laser Microscopy Core Facility at Weill Cornell Medical College, is gratefully acknowledged.
References
- 1.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–92. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque P, Casadevall A. Quorum sensing in fungi-a review. Med Mycol. 2012;50:337–45. doi: 10.3109/13693786.2011.652201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuranda K, Francois J, Palamarczyk G. The isoprenoid pathway and transcriptional response to its inhibitors in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2010;10:14–27. doi: 10.1111/j.1567-1364.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 4.Edwards PA, Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–85. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- 5.Ramage G, Saville SP, Wickes BL, et al. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–63. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabra-Rizk MA, Shirtliff M, James C, et al. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 2006;6:1063–73. doi: 10.1111/j.1567-1364.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 7.Mosel DD, Dumitru R, Hornby JM, et al. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl Environ Microbiol. 2005;71:4938–40. doi: 10.1128/AEM.71.8.4938-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramage G, Vande Walle K, Wickes BL, et al. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–9. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Second Edition: Approved Standard M27-A2. Wayne, PA, USA: NCCLS; 2002. [Google Scholar]
- 10.Hawser SP, Norris H, Jessup CJ, et al. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–2. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katragkou A, Chatzimoschou A, Simitsopoulou M, et al. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob Agents Chemother. 2008;52:357–60. doi: 10.1128/AAC.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn DM, George T, Chandra J, et al. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–80. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meletiadis J, Mouton JW, Meis JF, et al. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob Agents Chemother. 2003;47:106–17. doi: 10.1128/AAC.47.1.106-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–90. [PubMed] [Google Scholar]
- 15.Bliss CI. 2 X 2 Factorial experiments in incomplete groups for use in biological assays. Biometrics. 1947;3:69–88. [PubMed] [Google Scholar]
- 16.Meletiadis J, Pournaras S, Roilides E, et al. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother. 2010;54:602–9. doi: 10.1128/AAC.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 18.Chatzimoschou A, Katragkou A, Simitsopoulou M, et al. Activities of triazole-echinocandin combinations against Candida species in biofilms and as planktonic cells. Antimicrob Agents Chemother. 2011;55:1968–74. doi: 10.1128/AAC.00959-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drusano GL, D'Argenio DZ, Symonds W, et al. Nucleoside analog 1592U89 and human immunodeficiency virus protease inhibitor 141W94 are synergistic in vitro. Antimicrob Agents Chemother. 1998;42:2153–9. doi: 10.1128/aac.42.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meletiadis J, Verweij PE, TeDorsthorst DT, et al. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol. 2005;43:133–52. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 21.Millard PJ, Roth BL, Thi HP, et al. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol. 1997;63:2897–905. doi: 10.1128/aem.63.7.2897-2905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 23.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo JH, Jetten AM. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2010;287:123–35. doi: 10.1016/j.canlet.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossignol T, Logue ME, Reynolds K, et al. Transcriptional response of Candida parapsilosis following exposure to farnesol. Antimicrob Agents Chemother. 2007;51:2304–12. doi: 10.1128/AAC.01438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruppa M. Quorum sensing and Candida albicans. Mycoses. 2009;52:1–10. doi: 10.1111/j.1439-0507.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- 27.Han TL, Cannon RD, Villas-Boas SG. The metabolic basis of Candida albicans morphogenesis and quorum sensing. Fungal Genet Biol. 2011;48:747–63. doi: 10.1016/j.fgb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Davis-Hanna A, Piispanen AE, Stateva LI, et al. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman E, Alonso-Monge R, Gong Q, et al. The Cek1 MAPK is a short-lived protein regulated by quorum sensing in the fungal pathogen Candida albicans. FEMS Yeast Res. 2009;9:942–55. doi: 10.1111/j.1567-1364.2009.00545.x. [DOI] [PubMed] [Google Scholar]
- 30.Phillips AJ, Crowe JD, Ramsdale M. Ras pathway signaling accelerates programmed cell death in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 2006;103:726–31. doi: 10.1073/pnas.0506405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirtliff ME, Krom BP, Meijering RA, et al. Farnesol-induced apoptosis in Candida albicans. Antimicrob Agents Chemother. 2009;53:2392–401. doi: 10.1128/AAC.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Krom BP, Sanglard D, et al. Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione. PLoS One. 2011;6:e28830. doi: 10.1371/journal.pone.0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarathna DH, Hornby JM, Krishnan N, et al. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect Immun. 2007;75:1609–18. doi: 10.1128/IAI.01182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarathna DH, Nickerson KW, Duhamel GE, et al. Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model. Infect Immun. 2007;75:4006–11. doi: 10.1128/IAI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martins M, Lazzell AL, Lopez-Ribot JL, et al. Effect of exogenous administration of Candida albicans autoregulatory alcohols in a murine model of hematogenously disseminated candidiasis. J Basic Microbiol. 2012;52:487–91. doi: 10.1002/jobm.201100158. [DOI] [PubMed] [Google Scholar]
- 36.Hisajima T, Maruyama N, Tanabe Y, et al. Protective effects of farnesol against oral candidiasis in mice. Microbiol Immunol. 2008;52:327–33. doi: 10.1111/j.1348-0421.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro RA, Teixeira CE, Brilhante RS, et al. Minimum inhibitory concentrations of amphotericin B, azoles and caspofungin against Candida species are reduced by farnesol. Med Mycol. 2013;51:53–9. doi: 10.3109/13693786.2012.692489. [DOI] [PubMed] [Google Scholar]
- 38.Koo H, Hayacibara MF, Schobel BD, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–9. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 39.Jabra-Rizk MA, Meiller TF, James CE, et al. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 2006;50:1463–9. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes F, Leite B, Teixeira P, et al. Farnesol as antibiotics adjuvant in Staphylococcus epidermidis control in vitro. Am J Med Sci. 2011;341:191–5. doi: 10.1097/MAJ.0b013e3181fcf138. [DOI] [PubMed] [Google Scholar]
- 41.Pammi M, Liang R, Hicks JM, et al. Farnesol decreases biofilms of Staphylococcus epidermidis and exhibits synergy with nafcillin and vancomycin. Pediatr Res. 2011;70:578–83. doi: 10.1203/PDR.0b013e318232a984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomes F, Teixeira P, Cerca N, et al. Effect of farnesol on structure and composition of Staphylococcus epidermidis biofilm matrix. Curr Microbiol. 2011;63:354–9. doi: 10.1007/s00284-011-9984-3. [DOI] [PubMed] [Google Scholar]
- 43.Yu LH, Wei X, Ma M, et al. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob Agents Chemother. 2012;56:770–5. doi: 10.1128/AAC.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma M, Prasad R. The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans. Antimicrob Agents Chemother. 2011;55:4834–43. doi: 10.1128/AAC.00344-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa V, Moradas-Ferreira P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into ageing, apoptosis and diseases. Mol Aspects Med. 2001;22:217–46. doi: 10.1016/s0098-2997(01)00012-7. [DOI] [PubMed] [Google Scholar]
- 46.Longo VD, Liou LL, Valentine JS, et al. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch Biochem Biophys. 1999;365:131–42. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- 47.Scheper MA, Shirtliff ME, Meiller TF, et al. Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia. 2008;10:954–63. doi: 10.1593/neo.08444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inglis DO, Sherlock G. Ras signaling gets fine-tuned: regulation of multiple pathogenic traits of Candida albicans. Eukaryot Cell. 2013;12:1316–25. doi: 10.1128/EC.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Svegliati S, Cancello R, Sambo P, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005;280:36474–82. doi: 10.1074/jbc.M502851200. [DOI] [PubMed] [Google Scholar]
- 50.Hlavata L, Aguilaniu H, Pichova A, et al. The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J. 2003;22:3337–45. doi: 10.1093/emboj/cdg314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem. 2014;6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]