Abstract
Resveratrol (3,4′,5-trihydroxystilbene) is a plant-derived polyphenolic trans-stilbenoid, which exerts multifaceted antiaging effects. Here, we propose a novel delivery system for resveratrol, which significantly increases its cellular uptake into aged cells. Combination of resveratrol with a positively charged lipid component to “conventional” liposomes converts these lipid vesicles to a robust fusogenic system. To study their cellular uptake and cellular effects, we treated primary cerebromicrovascular endothelial cells isolated from aged F344xBN rats with resveratrol encapsulated in fusogenic liposomes (FL-RSV). To demonstrate effective cellular uptake of FL-RSV, accumulation of the lipophilic tracer dye, DiR, and resveratrol in cerebromicrovascular endothelial cells was confirmed using flow cytometry and confocal microscopy and high-performance liquid chromatography electrochemical detection. Treatment of aged cerebromicrovascular endothelial cells with FL-RSV activated Nrf2 (assessed with a reporter gene assay), significantly decreased cellular production of reactive oxygen species (assessed by a flow cytometry-based H2DCFDA fluorescence method), and inhibited apoptosis. Taken together, encapsulation of resveratrol into novel fusogenic liposomes significantly enhances the delivery of resveratrol into aged cells, which subsequently results in rapid activation of cellular Nrf2-driven antioxidant defense mechanisms. Our studies provide proof-of-concept for the development of a novel, translationally relevant interventional strategy for prevention and/or control of oxidative stress-related pathophysiological conditions in aging.
Key Words: Endothelial, Fusogenic liposomes, Oxidative stress, Polyphenol.
Recent studies provide strong evidence that treatment of laboratory rodents with resveratrol (3,4′,5-trihydroxystilbene), a plant-derived polyphenolic phytoalexin, exerts multifaceted health benefits, including significant antiaging effects. Feeding resveratrol was shown to improve health and survival of mice with metabolic syndrome (1,2), mimic the effects of caloric restriction (3), and to exert significant vasoprotective effects in aged mice and mice with accelerated vascular aging (4–6). Epidemiological studies confirm that Mediterranean diets that are rich in resveratrol are associated with significantly reduced risk of cardiovascular disease in elderly humans as well (7,8).
Despite advances in our understanding of the cytoprotective and antiaging actions of resveratrol, its potential therapeutic use in many age-related diseases is hampered by its physicochemical properties, mainly its low aqueous solubility and its relatively low bioavailability. Here, we propose a novel delivery system for resveratrol, which may significantly increase its diffusion into tissues and enhance cellular uptake in studies on models of aging.
The solubility, bioavailability, and cellular uptake of polyphenolic compounds can be increased by encapsulating them in liposomes (9,10). Conventional phospholipid-based liposomes are spherically closed lipid bilayers enclosing an aqueous core, which can be used for encapsulation of lipophilic drug into the lipid bilayers and for hydrophilic drugs into the aqueous core. Resveratrol can be successfully encapsulated in conventional liposomes (9–13). Studies show that uptake of conventional liposomes into cells exhibits a strong preference for either clathrin-dependent or clathrin-independent endocytotic uptake. However, the efficiency of endocytotic uptake is limited, often below 1%. Thus, in order to achieve biologically active concentrations of resveratrol in tissues, the efficiency of liposome-based cellular delivery of resveratrol needs to be increased.
Recent findings by Csiszar and coworkers (14) led to the development of an innovative approach to enhance the delivery of resveratrol into aged cells by induction of membrane fusion between liposomes and cellular plasma membranes. Positively charged lipids can polarize the delocalized π electrons of highly aromatic molecules inducing temporal dipoles within liposome bilayers. These dipoles are thought to promote local instabilities in the molecular lipid arrangements by reducing the fusion barrier and allowing intermediate fusion formation. This fusion arrangement would lead to subsequent adherence of the liposome and the opposing cell membrane. Support for this mechanism has been provided by previous studies using liposomes containing a variety of synthetic aromatic compounds, which robustly enhance fusion of liposomes with cellular membranes (14). These results encouraged us to propose that a wide range of naturally occurring aromatic polyphenolic compounds such as resveratrol could likewise induce effective membrane fusion between positively charged lipid bilayers and cellular plasma membranes.
The present study was undertaken to test the hypothesis that addition of resveratrol which contains delocalized conjugated π electrons coupled with positively charged lipid components to “conventional” liposomes converts them to a robust fusogenic system (14,15). These liposomes significantly enhance the delivery of resveratrol into aged cells by induction of membrane fusion between liposomes and the plasma membrane of cells. Our delivery system is schematically presented in Figure 1.
Figure 1.
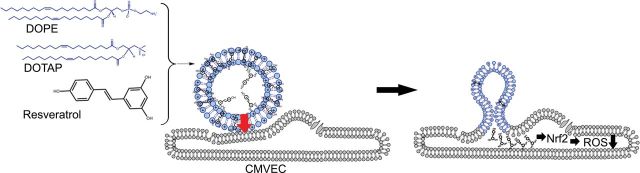
Proposed model for enhanced membrane fusion between resveratrol-containing fusogenic liposomes and aged CMVECs. The synergistic interaction of the three components, the neutral lipid DOPE, the positively charged lipid DOTAP, and the aromatic resveratrol, results in an effective fusogenic mixture. Earlier studies of Brittes and coworkers (83) demonstrated that resveratrol is deeply located in the lipid bilayer, and only 10% of resveratrol molecules is found in the aqueous phase. The deep membrane location of resveratrol can be explained by its high lipophilicity, which is in turn related with the high partition coefficients determined for this compound. The positively charged lipids can polarize the delocalized π electrons of the highly aromatic structure of resveratrol inducing temporal dipoles in the liposome bilayers. These dipoles likely promote local instabilities in the molecular lipid arrangements (arrow), which will lead to subsequent fusion of the liposome and the opposing cell membrane. The model predicts that even short-term treatment of cells with resveratrol encapsulated in fusogenic liposomes will (i) deliver resveratrol in cells in sufficient quantities to activate Nrf2, an important cytosolic target of resveratrol, which will (ii) confer antioxidant effects, significantly attenuating age-related oxidative stress in CMVECs. CMVEC = cerebromicrovascular endothelial cell; DOPE = 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP = 1,2-dioleoyl-3-trimethylammonium-propane.
Previous studies demonstrated that the NF-E2–related factor 2 (Nrf2)/antioxidant response element (ARE) pathway is a key intracellular target of resveratrol (16–20), which when activated confers significant antiaging, antioxidative, and antiapoptotic effects. Thus, our current studies also sought to determine whether treatment with resveratrol encapsulated in fusogenic liposomes can activate Nrf2 and exert antioxidative and antiapoptotic effects in aged cells. We chose to study primary cerebromicrovascular endothelial cells (CMVECs) derived from aged F344xBN rats. These cells retain the aging phenotype in vitro and thus exhibit significant oxidative stress and increased rates of apoptosis.
Methods
Establishment and Characterization of Primary CMVEC Cultures
Male 24-month-old Fischer 344 x Brown Norway (F344xBN) rats were obtained from the National Institute on Aging. The animals were disease free with no signs of systemic inflammation and/or neoplastic diseases. All animals were maintained according to National Institutes of Health guidelines and use protocols were approved by the Institutional Animal Care and Use Committees of the University of Oklahoma Health Sciences Center. To establish a primary CMVEC culture, animals were euthanized with CO2 and brains were removed aseptically. Isolated brains were rinsed in ice cold phosphate-buffered saline (PBS), minced into ≈1-mm sections, and washed twice in ice cold 1× PBS by low-speed centrifugation (50g, 2–3 minutes). Diced tissue sections were digested in a solution of collagenase (800 U/g tissue), hyaluronidase (2.5 U/g tissue), and elastase (3 U/g tissue) in 1 mL PBS/100mg tissue for 45 minutes at 37°C in rotating humid incubator. The digested tissue was passed through a 100-µm cell strainer to remove undigested tissue sections. The single cell lysate was centrifuged for 2 minutes at 70g. After removing the supernatant carefully, the pellet was washed twice in cold PBS supplemented with 2.5% fetal calf serum, and the suspension centrifuged at 300g, for 5 minutes at 4°C.
To create an endothelial cell–enriched fraction, the cell suspension was centrifuged through a gradient using an OptiPrep solution (Axis-Shield, PoC, Norway). Briefly, cell pellets were resuspended in Hanks’ balanced salt solution and mixed thoroughly with 40% iodixanol (final concentration: 17% [w/v] iodixanol solution; ρ = 1.096g/mL (21)). Two milliliters of Hanks’ balanced salt solution were layered on top and tubes were centrifuged at 400g for 15 minutes at 20°C. Endothelial cells, which banded at the interface between Hanks’ balanced salt solution and the 17% iodixanol layer, were collected. The endothelial cell–enriched fraction was incubated for 30 minutes at 4°C in the dark with anti-CD31/PE (BD Biosciences, San Jose, CA) and anti-MCAM/FITC (BD Biosciences). After washing the cells twice with MACS Buffer (Milltenyi Biotech, Cambridge, MA), anti-FITC magnetic bead–labeled and anti-PE magnetic bead–labeled secondary antibodies were used for 15 minutes at room temperature. Endothelial cells were collected by magnetic separation using the MACS LD magnetic separation columns according to the manufacturer’s guidelines (Milltenyi Biotech). The endothelial fraction was cultured on fibronectin coated plates in endothelial growth medium (Cell Application, San Diego, CA) for 10 days.
Endothelial cell were phenotypically characterized by flow cytometry (GUAVA 8HT; Merck Millipore, Billerica, MA). Briefly, antibodies against five different endothelial specific markers were used (anti-CD31-PE, anti-erythropoietin receptor-APC, anti-VEGF R2-PerCP, anti-ICAM-fluorescein, and anti-CD146-PE), and isotype-specific antibody–labeled fractions served as negative controls. All antibodies were purchased from R&D Systems (R&D Systems, Minneapolis, MN).
Preparation of Fusogenic Liposomes
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-3-trimethylammonium-propane, chloride salt (DOTAP), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL), whereas the fluorescent lipophilic tracer, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR), was purchased from Life Technology (Eugene, OR). To prepare fusogenic lipid stock solution, DOPE, DOTAP, and DiR were mixed in chloroform/ethanol (10/1) in a molar ratio of DOPE/DOTAP/DiR 1/1/0.005 (Figure 1). To prepare conventional liposomes, DPPC was dissolved in chloroform/ethanol (10/1) with the fluorescent tracer DiR in a molar ratio of 2/0.005. Resveratrol (purchased from Sigma Aldrich, St Louis, MO) was dissolved in ethanol. Lipid components and resveratrol were mixed with the following molar ratios: total lipids/resveratrol = 2/0 (control), 2/0.3, 2/0.6, 2/1.5, and 2/3mol/mol at 1mg/mL total lipid concentration. After gentle homogenization of the mixtures, the organic solvent was evaporated under vacuum for 0.5 hours. Then, lipids and resveratrol were dispersed in 20 mmol/L 2-(4-(2-hydroxyethyl)-1-piperazinyl)-ethanesulfonic acid buffer (pH 7.4) in a total lipid concentration of 2mg/mL. The solution was homogenized for 2 minutes by intensive stirring and for 20 minutes in an ultrasonic bath.
Membrane Fusion Experiments
Increased membrane fusion between liposomes and cells results in a robust buildup of DiR fluorescence localized to the cell membranes. To monitor incorporation of DiR fluorescence in cell membranes in imaging studies, aged CMVECs were plated on fibronectin-coated glass surface (20,000–30,000 per dish, [Ø = 3.5 cm]) in Rat Brain Endothelial Cell Medium (Cell Application) with 10% fetal bovine serum (Sigma Aldrich). After 24 hours, cells were treated with resveratrol-loaded fusogenic liposomes for 15 minutes at a lipid concentration of 50 µg/mL and washed subsequently. The calculated resveratrol concentrations applied here were the following: 0 (control), 1, 10, 20, 50, and 100 µmol/L. In control experiments, conventional liposomes were administrated at the same concentration and temperature as fusogenic liposomes. Liposomal uptake was evaluated by monitoring the incorporation of DiR fluorescence (excitation: 633nm, emission: LP 650nm) in the cell membrane at a resveratrol concentration of 100 µmol/L using a Leica SP2 MP confocal laser scanning microscope.
To assess uptake of resveratrol-containing fusogenic liposomes by endothelial cells in situ, isolated arterial segments were incubated with the liposomes for 15 minutes. The vessels were then embedded in OCT (optimal cutting temperature) medium and cryosectioned. Liposomal uptake was evaluated by confocal microscopy in unstained sections.
To assess the dose dependency of resveratrol-induced membrane fusion, approximately 500,000 cells were incubated with resveratrol-loaded fusogenic liposomes for 15 minutes at 37°C. Subsequently, the fusion mixture was replaced by fresh medium. Liposomal uptake was assessed by measuring the incorporation of DiR into the cells using the flow cytometer Guava EasyCyte 8HT (Millipore, Hayward, CA).
Resveratrol Determination Using High-Performance Liquid Chromatography Electrochemical Detection
To demonstrate efficient uptake of resveratrol encapsulated in liposomes by aged CMVECs, intracellular concentrations of resveratrol were measured using a model 584 HPLC system equipped with an eight-channel coulometric array detector (ESA, Inc., Chelmsford, MA). Following treatment with resveratrol encapsulated in fusogenic liposomes or conventional liposomes (for 15 minutes), cells were scraped from plates (~2 × 105), suspended in PBS, and centrifuged (5 minutes, 1000g, Eppendorf microcentrifuge) to remove residual media. This process was repeated and pelleted cells were snap frozen in liquid nitrogen and stored at −80°C until analysis. For resveratrol analysis, frozen cell pellets were suspended in 40 µL of water and vortexed. During vortexing, 10 µL of 25% metaphosphoric acid were added to precipitate proteins followed by 50 µL of 100% acetonitrile. Cell lysates were centrifuged in a refrigerated microfuge 15,000 r.p.m. for 7 minutes. The supernatant fractions were removed and precipitated proteins were dissolved in 100 µL of 0.1 N NaOH and saved for protein determinations by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co., Rockford, IL). Aliquots of the supernatant fraction were injected directly into a 5 µL sample loop of the Rheodyne injector port for resveratrol determination using high-performance liquid chromatography equipped with electrochemical detection. All buffers and solutions are filtered through a 0.22-µm mesh nylon filter prior to use and purged with nitrogen gas for approximately 10 minutes. Standards are prepared fresh from resveratrol powder and working concentrations from 1, 2.5, 5, 10, and 20 µmol/mL were prepared in 50% acetonitrile. Concentrations of resveratrol in cell extracts, liposomes, and standards were separated using an ESA column (narrow-bore MD-150 × 3.2mm, 3-µm particle size) and eluted with a mobile phase (26% acetonitrile containing 75mM citric acid, 25mM ammonium acetate, pH = 2.64 adjusted with ammonium hydroxide, if necessary) at a flow rate of 0.6mL/min. The eight-channel CoulArray detectors are set at 100, 200, 320, 380, 440, 500, 560, and 620 mV, respectively. Trans-resveratrol elutes at 5.35 minutes, which can vary slightly with pH and solvent conditions. Peak areas are analyzed using ESA, Inc. software, and concentrations of resveratrol are reported as nmol/mg protein.
Transient Transfection, Nrf2 Reporter Gene Assays
The effect of treatment with resveratrol (10 µmol/L) encapsulated in fusogenic liposomes on transcriptional activity of Nrf2 was tested in aged CMVECs by a reporter gene assay, as described (20,22,23). We used an ARE reporter comprised of tandem repeats of the ARE transcriptional response element upstream of firefly luciferase (SA Biosciences, Frederick, MD) and a renilla luciferase plasmid under the control of the cytomegalovirus promoter (as an internal control). All transfections in vascular smooth muscle cells were performed using the Amaxa Nucleofector Technology (Amaxa, Gaithersburg, MD), as we have previously reported (24–26). Firefly and renilla luciferase activities were assessed after 24 hours using the Dual Luciferase Reporter Assay Kit (Promega, Madison, WI) and a Tecan Infinite M200 plate reader.
Measurement of Cellular Reactive Oxygen Species Production
The oxidative stress theory of aging is one of the most frequently invoked theories to explain the process of aging (27) and thereby the pathogenesis of age-related diseases (28–39). Although the validity of the generalized theory is ardently debated, there is a consensus that oxidative stress plays a key role in vascular aging (35,39–46). To assess the effects of resveratrol encapsulated in fusogenic liposomes on reactive oxygen species (ROS) production in aged endothelial cells, we measured cellular peroxide production using the cell-permeable oxidative fluorescent indicator dye CM-H2DCFDA (5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester; Invitrogen, Carlsbad, CA) as we previously reported (39,46). In brief, approximately 500,000 cells were incubated with resveratrol-loaded fusogenic liposomes (for 15 minutes at 37°C) and then washed with warm PBS and incubated for 24 hours. In control experiments, CMVECs were treated with resveratrol encapsulated in conventional liposomes (for 24 hours). Following the treatment period, cells were incubated with CM-H2DCFDA (10 µM, at 37°C, for 30 minutes). CM-H2DCFDA fluorescence was assessed by flow cytometry as previously reported (39,46) using the flow cytometer Guava easyCyte 8HT (Millipore). Liposomal uptake was confirmed by recording the cellular DiR signal.
To assess the time course of the antioxidant effects of resveratrol, cellular hydrogen peroxide (H2O2) production in aged CMVECs was measured post-FL-RSV (10−5 mol/L) treatment fluorometrically using the Amplex Red/horseradish peroxidase assay as described (40,47). The rate of H2O2 generation was assessed by measuring resorufin fluorescence by a Tecan Infinite M200 plate reader. A calibration curve was constructed using H2O2 and the production of H2O2 in the samples was calculated as pmol H2O2 released per minute. Hoechst 33258 fluorescence, representing cellular DNA content, was used for normalization. Data are expressed as arbitrary units relative to control samples.
Caspase 3/7 Activity Assay
To assess the effects of resveratrol encapsulated in fusogenic liposomes on oxidative stress-induced apoptosis in young and aged endothelial cells, we measured caspase-3/7 activity (a useful measure of apoptotic cell death) in CMVECs using the Caspase-Glo 3/7 assay kit (Promega) as previously reported (45,46,48,49). As apoptosis is an important physiological endpoint in aging research (50,51), young and aged CMVECs were pretreated with FL-RSV (10−5 mol/L) than exposed to 3 × 10−4 mol/L H2O2. Untreated cells with or without H2O2 served as positive and negative controls, respectively. In 96-well plates, 50 µl sample was mixed for 30 seconds with 50 µl Caspase-Glo 3/7 reagent and incubated for 2h at room temperature. Lysis buffer with the reagent served as blank. Luminescence of the samples was measured using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC). Luminescent intensity values were normalized to the sample protein concentration.
Data Analysis
Statistical analyses of data were performed by analysis of variance and all data expressed as means ± standard error of the mean (30). A p value less than .05 was considered statistically significant. Data are expressed as means ± standard error of the mean.
Results
Increased Uptake of Resveratrol Encapsulated in Fusogenic Liposomes in Aged CMVECs
To assess the cellular uptake of fusogenic liposomes, we detected the accumulation of the lipophilic tracer dye DiR in CMVECs using flow cytometry (Figure 2A) and laser scanning microscopy (Figure 2B). As lipid base, we used a 1/1 (w/w) ratio of a neutral phospholipid (DOPE) and a cationic lipid (DOTAP) combined with a small amount of the red lipophilic tracer DiR. This lipid mixture was not able to fuse with cell membranes resulting in low uptake efficiencies. The addition of resveratrol, which contain large delocalized π electron systems, completely changed the cellular uptake processes. Addition of resveratrol to the liposomes significantly increased the liposomal uptake of aged CMVEC cells. As shown in Figure 2A, 15 minutes of incubation with fusogenic liposomes resulted in significant increase in DiR-stained cells, whereas the cellular uptake of conventional liposomes after 15 minutes incubation was not significant.
Figure 2.
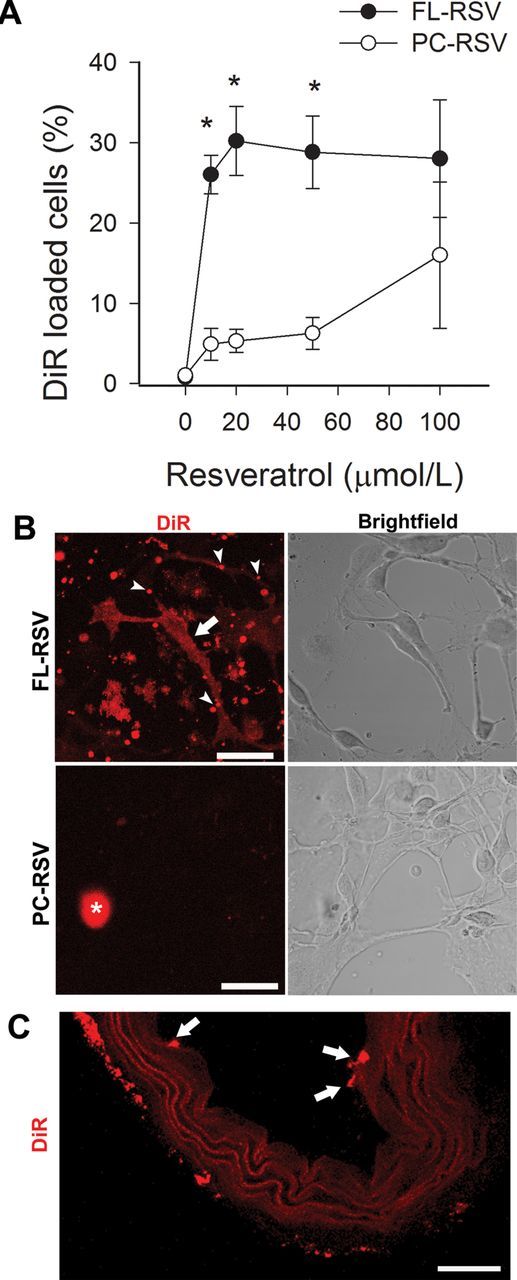
Effective resveratrol delivery to aged CMVECs using fusogenic liposomes (incubation time: 15 minutes). The effective concentration of resveratrol to induce membrane fusion was determined by flow cytometry by analyzing the cellular uptake of the fluorescent tracer DiR. (A) The percentage of DiR-loaded aged CMVECs as a function of applied resveratrol concentration (FL-RSV). Resveratrol delivered by conventional liposomes (PC-RSV) did not increase incorporation of DiR in cells. *p < .05 vs PC-RSV. (B) Liposomal delivery in CMVECs was visualized by detecting the fluorescent tracer DiR (red) using laser scanning microscopy. Resveratrol incorporated in positively charged liposomes (cRSV = 100 µmol/L) induced homogenous DiR signal distribution in the plasma membrane of CMVECs (FL-RSV; upper panel). Resveratrol incorporated in conventional liposomes (PC-RSV; lower panel) was less effective (cRSV = 100 µmol/L). Arrowheads: fusogenic liposomes attached to the cell surface. Arrows: incorporation of DiR in the cell membrane. *Debris. Scale bar: 100 µm. (C) Confocal images showing the effective uptake of resveratrol-containing fusogenic liposomes in endothelial cells of isolated vessel segments (incubation time: 15 minutes). Arrows: incorporation of DiR in endothelial cells (EC). Scale bar: 150 µm. CMVEC = cerebromicrovascular endothelial cell; DiR = 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide.
Analysis of DiR fluorescence by laser scanning microscopy shows increased DiR fluorescence localized to the cell membranes of aged CMVEC cells (Figure 2B), indicating membrane fusion between liposomal and plasma membranes of cells. The cellular uptake of conventional liposomes after 15-minute incubation was not significant (Figure 2B). Analysis of DiR fluorescence in sections of isolated arterial segments show rapid and effective uptake of fusogenic liposomes by endothelial cells in situ as well (Figure 2C).
In order to assess cellular uptake of resveratrol, we used a validated high-performance liquid chromatography method for the quantitative analysis of trans-resveratrol in cellular extracts. As shown in Figure 3, short-term incubation of aged CMVECs with FL-RSV resulted in a significant increase in intracellular trans-resveratrol content. By contrast, resveratrol encapsulated in conventional liposomes was significantly less effective in increasing intracellular trans-resveratrol levels (Figure 3B).
Figure 3.
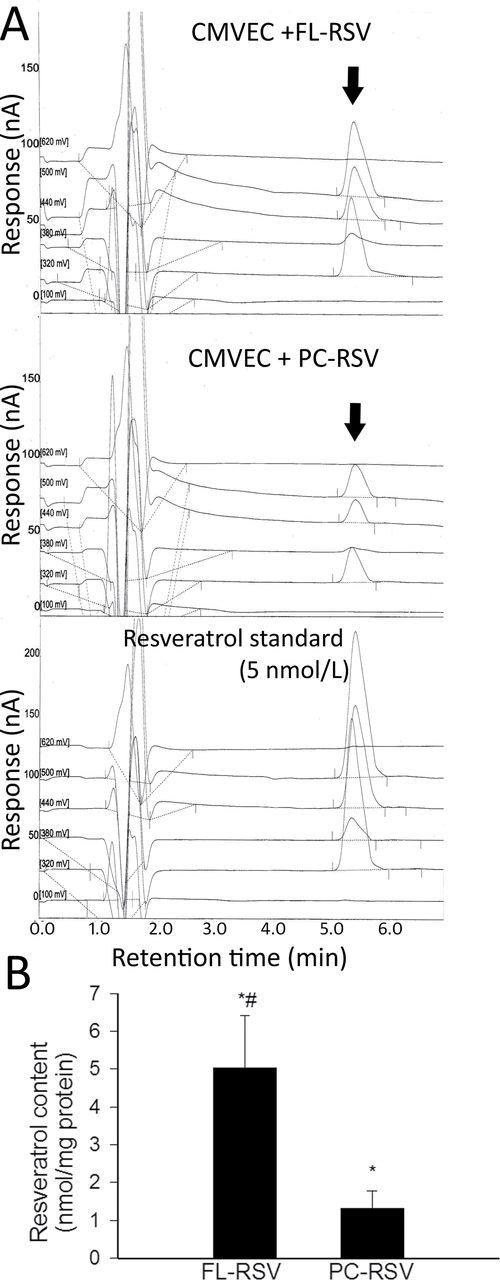
(A) Trans-resveratrol (arrow) content, determined using high-performance liquid chromatography electrochemical detection, in aged CMVECs loaded with FL-RSV (upper panel) or PC-RSV (middle panel). The 5 nmol/L resveratrol standard is shown as reference (lower panel). (B) Normalized cellular resveratrol content upon loading aged CMVECs with FL-RSV or PC-RSV. *p < .05 vs untreated control. # p < .05 vs FL-RSV. Data are mean ± standard error of the mean. CMVEC = cerebromicrovascular endothelial cell.
Resveratrol Encapsulated in Fusogenic Liposomes Increases Transcriptional Activity of Nrf2 in Aged CMVECs
To determine the effect of resveratrol encapsulated in fusogenic liposomes on Nrf2 activation, we transiently transfected aged CMVECs with a Nrf2/ARE-driven reporter gene construct and then treated the cells with resveratrol-containing liposomes for 15 minutes. A significant increase in luciferase activity over the vector control (assessed at 2 hours posttreatment) was noted upon stimulation with resveratrol encapsulated in fusogenic liposomes (Figure 4). By contrast, resveratrol encapsulated in conventional liposomes was significantly less effective (Figure 4).
Figure 4.
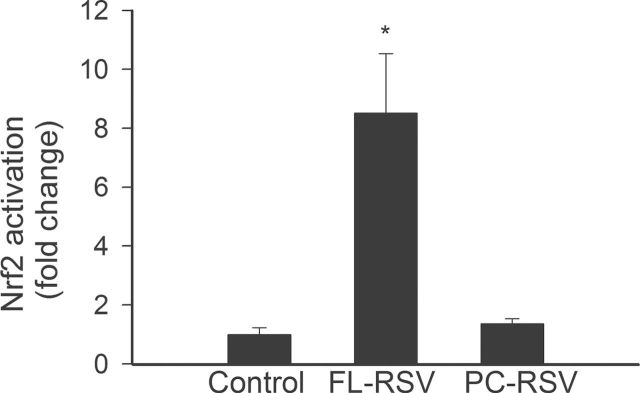
Reporter gene assay showing the effects of short-term incubation with resveratrol encapsulated in fusogenic liposomes or conventional liposomes (FL-RSV and PC-RSV, respectively; for 15 minutes, followed by washout) on Nrf2/ARE reporter activity in cultured primary CMVECs derived from aged rats. Cells were transiently cotransfected with ARE-driven firefly luciferase and cytomegalovirus-driven renilla luciferase constructs followed by liposomal resveratrol treatment. After a 2-hour period, the cells were then lysed and subjected to luciferase activity assay. After normalization, relative luciferase activity was obtained from four to six independent transfections. Data are mean ± standard error of the mean. *p < .05. ARE = antioxidant response element; CMVEC = cerebromicrovascular endothelial cell.
Resveratrol Encapsulated in Fusogenic Liposomes Attenuates Oxidative Stress in Aged CMVECs
Short-term treatment of aged CMVECs with resveratrol encapsulated in fusogenic liposomes elicited significant decreases in cellular ROS production as measured by the fluorescent indicator dye CM-H2DCFDA (Figure 4). By contrast, short-term (15 minutes) treatment of aged CMVECs with resveratrol encapsulated in conventional liposomes was ineffective (data not shown). Only long-term (24 hours) treatment with resveratrol encapsulated in conventional liposomes resulted in a measurable decline in ROS production by aged CMVECs (Figure 5A). This result may reflect the difference in relative uptake of resveratrol by cells treated with fusogenic and conventional liposomes, in that fusogenic liposomes exhibit a greater uptake capacity and increased intracellular titer of resveratrol in short period of time. To assess the time course of antioxidant effects induced by treatment with resveratrol encapsulated in fusogenic liposomes, we measured H2O2 production in aged CMVECs using the Amplex Red/horseradish peroxidase assay. As shown in Figure 5B, aged CMVECs produced significantly more H2O2 than young cells. After treatment with resveratrol encapsulated in fusogenic liposomes, cellular ROS production decreased in a time-dependent manner (Figure 5B), reaching statistical significance at 60 minutes posttreatment and continued to decline up to 24 hours.
Figure 5.
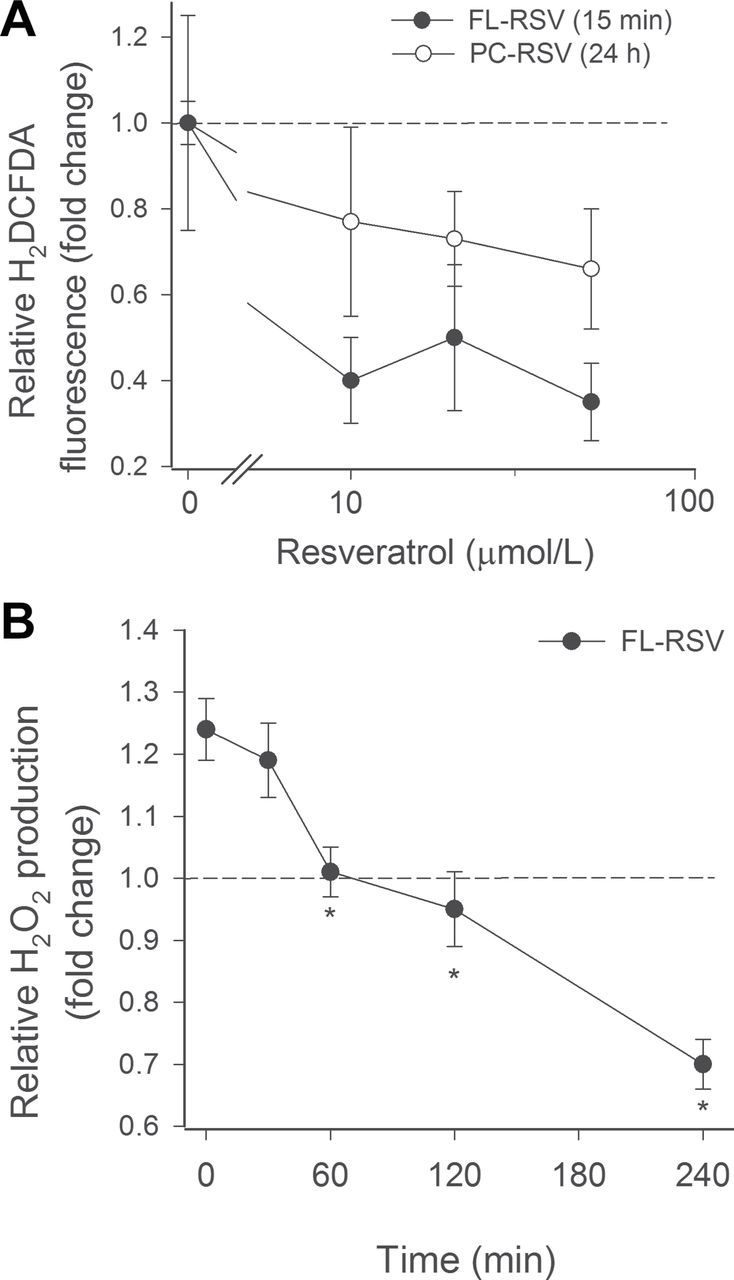
(A) Short-term incubation with resveratrol encapsulated in fusogenic liposomes (FL-RSV; for 15 minutes, followed by washout) attenuates cellular production of free radicals in aged CMVECs (CM-H2DCFDA staining, flow cytometry). The effect of short-term pretreatment with FL-RSV was comparable to the effect of chronic (for 24 hours) treatment with resveratrol encapsulated in conventional liposomes (PC-RSV). Short-term treatment of CMVECs with PC-RSV was ineffective. Data are mean ± standard error of the mean. The concentration-dependent decline in ROS production was significant (p < .05) for RSV, FL-RSV, and PC-RSV as well. (B) Time-dependent decreases in H2O2 production in aged CMVECs after treatment with FL-RSV. Changes in H2O2 production reached statistical significance (*p < .05) at 60 minutes posttreatment and continued to decline up to 24 hours. Cellular H2O2 production was assessed by the Amplex Red/horseradish peroxidase method. Dashed line indicates level of H2O2 production in CMVECs derived from young animals. CMVEC = cerebromicrovascular endothelial cell; ROS = reactive oxygen species.
Resveratrol Encapsulated in Fusogenic Liposomes Exerts Antiapoptotic Effects in Aged CMVECs
To assay another component of the potential antiaging activity of treatment with resveratrol encapsulated in fusogenic liposomes in vitro, we assessed its effects on endothelial apoptosis. Compared with young CMVECs, aged CMVECs exhibit significantly increased basal rate of apoptosis, as shown by measurements of caspase 3/7 activity (Figure 6). Treatment with H2O2 induces apoptosis in both young and aged CMVECs, as shown by significant increases in caspase 3/7 activity (Figure 6). Short-term treatment of aged CMVECs with resveratrol encapsulated in fusogenic liposomes significantly inhibited apoptosis both under basal conditions and after H2O2 treatment (Figure 6).
Figure 6.
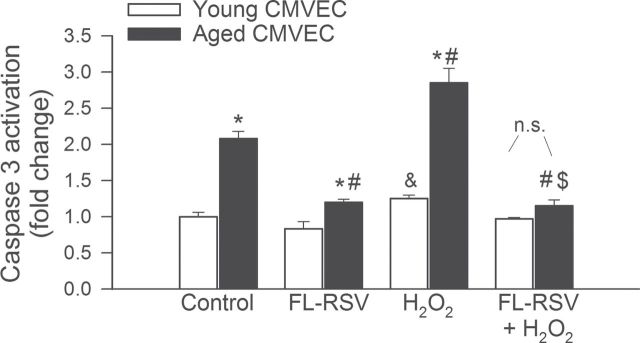
Short-term treatment of aged CMVECs with resveratrol encapsulated in fusogenic liposomes (FL-RSV) significantly inhibits apoptosis both under basal conditions and after H2O2 treatment. Shown are decreases in caspase 3/7 activity (a marker of apoptosis) in aged CMVECs posttreatment with FL-RSV. Compared with young CMVECs, aged CMVECs exhibited significantly increased caspase 3/7 activity (*p < .05). # p < .05 vs untreated aged, $ p < .05 vs aged + H2O2, and & p < .05 vs untreated young. Data are mean ± standard error of the mean (n.s. = nonsignificant). CMVEC = cerebromicrovascular endothelial cell.
Discussion
Our studies report for the first time that resveratrol present within fusogenic liposomes significantly enhances their fusion to cell membranes (Figure 2) and thus can facilitate the cellular uptake of resveratrol into aged cells (Figure 3). The cellular uptake processes of conventional liposomes that are based on a simple composition of neutral phospholipids show a strong preference of endocytotic pathways with a limited efficiency. By contrast, the cellular uptake of fusogenic liposomes that contain positively charged lipids in association with aromatic resveratrol molecules in a distinct molar ratio is far more efficient than that of conventional liposomes. We found that resveratrol induces membrane fusion between liposome and cellular plasma membranes at physiologically relevant concentrations. Interestingly, increases in the amount of resveratrol loaded into liposomes (molar ratios of lipid/resveratrol of 2/1.5–3) were not associated with a corresponding increase in membrane fusion efficiency. It is likely that above a certain resveratrol concentration, complete membrane loading is achieved and the excess resveratrol loaded accumulates in a phase that will not influence membrane fusion. Because resveratrol-containing fusogenic liposomes were rapidly taken up by endothelial cells in situ (Figure 2C), we predict that a lipophilic delivery system using fusogenic liposomes will be an effective approach for studies in vivo.
The highly apolar aromatic structure of resveratrol makes it an especially appropriate candidate for successful incorporation into fusogenic liposomes. In addition to resveratrol, other naturally occurring polyphenolic compounds with antiaging properties (eg, curcumin [(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione]) may also be easily incorporated into positively charged liposomes. We propose that enhanced membrane fusion between fusogenic liposomes and cellular membranes can be facilitated by the content of their aromatic cargo such as resveratrol that will result in increased cellular delivery. Future studies are warranted to demonstrate whether resveratrol and other polyphenolic compounds encapsulated in fusogenic liposomes can effectively penetrate various tissues in vivo as well. We predict that the cerebral microcirculation would be a particularly sensitive targeted delivery with fusogenic liposomes.
Previous studies showed that the vasoprotective effects of resveratrol are mediated, at least in part, by the activation of the transcription factor Nrf2 (16–20). Under basal, nonactivated conditions, Nrf2 is located in the cytosol where it interacts with Keap1, a cytosolic repressor protein, which limits Nrf2-mediated gene expression. Upon activation by resveratrol, the cytosolic Keap1-Nrf2 complex dissociates and Nrf2 translocates to the nucleus where it triggers expression of numerous ROS detoxifying (52) and antioxidant genes mediated by the ARE in response to cellular oxidative stress. Our studies show that resveratrol encapsulated in fusogenic liposomes results in efficient release of resveratrol when fused to cell membranes and elicits Nrf2 activation (Figure 4), which significantly attenuates cellular ROS production (Figure 5) in aged CMVECs. These findings also demonstrate that the antioxidative effect of short-term incubation with resveratrol encapsulated in fusogenic liposomes (15 minutes, followed by washout) is comparable to that observed with long-term (24 hours) treatment with an resveratrol encapsulated in conventional liposomes. Thus, our studies suggest that rapid fusion of resveratrol-containing fusogenic liposomes with cell membranes would enable the delivery of significant quantities of resveratrol to cells within a short period of time and achieve therapeutically relevant intracellular concentrations of resveratrol to enhance Nrf2-induced gene expression in aged cells. The time course of the decline in cellular ROS production induced by resveratrol treatment is compatible with the idea that resveratrol exerts its antioxidant effects primarily by upregulating cellular antioxidant defenses in aged cells (Figure 5B). It should be noted that resveratrol and its metabolites both in vivo and ex vivo can promote SIRT1-dependent cellular responses (1,2,53–60), at least in part, by upregulating protein expression of SIRT1 (61). In addition, resveratrol may also inhibit oxidative stress by downregulating NADPH oxidases (6). Although it appears that the effective concentrations for resveratrol to induce Nrf2 is lower than the concentrations needed to achieve the aforementioned effects, we predict that improved liposome-mediated delivery of resveratrol to cells will also increase cellular SIRT1 activation and inhibit NADPH oxidases as well.
Several lines of evidence suggest that Nrf2 plays an important role in regulating the aging process (62). Homologues of Nrf2 are evolutionarily highly conserved, and studies on Caenorhabditis elegans demonstrate that knockdown of the worm homolog of Nrf2 shortens life span (63). Studies by Pearson and coworkers (64) were the first to demonstrate that genetic depletion of Nrf2 also affects life span in mice, increasing age-related cancer morbidity and preventing the anticancer effects of caloric restriction. It has also been suggested that age-related decreases in the activity of Nrf2 contribute to the development of various diseases of aging, including cardiovascular diseases (23,65–69) at least in part due to the compromised antiapoptotic function of Nrf2. In light of the aforementioned findings, it is significant that resveratrol encapsulated in fusogenic liposomes elicits significant Nrf2 activation (Figure 4) and confers antiapoptotic effect (Figure 6) in aged endothelial cells. Numerous studies suggest that resveratrol exerts antiaging effects in many species, including rodents (1,5,17,53,57,58,70–76). Importantly, age-related oxidative stress and apoptosis in the cerebrovasculature has been causally linked to the development of cerebromicrovascular diseases (77–79). Resveratrol encapsulated in fusogenic liposomes exhibit efficacy to restore endothelial Nrf2 activation, attenuate oxidative stress, and inhibit apoptosis in the microvasculature and, thus should be considered for preventing or controlling cerebromicrovascular pathologies associated with aging. Other potential targets of resveratrol encapsulated in fusogenic liposomes include skin (80), brain (81), and the gastrointestinal tract. Moreover, resveratrol has been shown to confer diverse antitumor effects. Thus, targeting tumors represents another possible application for resveratrol encapsulated in fusogenic liposomes.
Taken together, encapsulation of resveratrol into novel fusogenic liposomes significantly enhances the delivery of the polyphenolic stilbenoid into aged cells and subsequently results in rapid activation of cellular Nrf2-driven antioxidant defense mechanisms. Our studies provide proof-of-concept for the development of novel, translationally relevant interventional strategy for prevention or control of oxidative stress-related pathophysiological conditions in aging.
Funding
This work was supported by grants from the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., and W.E.S.), the American Federation for Aging Research (to A.C.), the American Heart Association (to A.C., P.T., and Z.U.), the National Center for Complementary and Alternative Medicine (R01-AT006526 to Z.U.), the National Institute on Aging (AG031085 to A.C.; AG038747 to W.E.S.), the Ellison Medical Foundation (to W.E.S.), the Nemzeti Fejlesztési Ügynökség (SROP-4.2.2.a-11/1/KONV-2012-0024 and -0017 and SPOR-4.2.1/b-10/2/KONV-2010-0012), and the Hungarian Scientific Research Fund (OTKA; K 108444).
Acknowledgements
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program. The authors acknowledge the inspiration from early studies by Mr. Artúr Görgey (82), the expert help of Dr. Danuta Sosnowska with the cell cultures, and the helpful discussions with Prof. Rudolf Merkel (Forschungszentrum Jülich).
References
- 1. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. [DOI] [PubMed] [Google Scholar]
- 3. Smith JJ, Kenney RD, Gagne DJ, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. [DOI] [PubMed] [Google Scholar]
- 5. Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–915. [DOI] [PubMed] [Google Scholar]
- 8. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. [DOI] [PubMed] [Google Scholar]
- 9. Caddeo C, Teskac K, Sinico C, Kristl J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int J Pharm. 2008;363:183–191. [DOI] [PubMed] [Google Scholar]
- 10. Kristl J, Teskac K, Caddeo C, Abramović Z, Sentjurc M. Improvements of cellular stress response on resveratrol in liposomes. Eur J Pharm Biopharm. 2009;73:253–259. [DOI] [PubMed] [Google Scholar]
- 11. Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8. [DOI] [PubMed] [Google Scholar]
- 12. Fabris S, Momo F, Ravagnan G, Stevanato R. Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys Chem. 2008;135:76–83. [DOI] [PubMed] [Google Scholar]
- 13. Hung CF, Chen JK, Liao MH, Lo HM, Fang JY. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J Nanosci Nanotechnol. 2006;6:2950–2958. [DOI] [PubMed] [Google Scholar]
- 14. Csiszár A, Hersch N, Dieluweit S, Biehl R, Merkel R, Hoffmann B. Novel fusogenic liposomes for fluorescent cell labeling and membrane modification. Bioconjug Chem. 2010;21:537–543. [DOI] [PubMed] [Google Scholar]
- 15. Kleusch C, Hersch N, Hoffmann B, Merkel R, Csiszár A. Fluorescent lipids: functional parts of fusogenic liposomes and tools for cell membrane labeling and visualization. Molecules. 2012;17:1055–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. [DOI] [PubMed] [Google Scholar]
- 17. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820 doi:10.1093/gerona/glr228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. [DOI] [PubMed] [Google Scholar]
- 19. Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. [DOI] [PubMed] [Google Scholar]
- 20. Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tucsek Z, Gautam T, Sonntag WE, et al. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta . J Gerontol A Biol Sci Med Sci. 2011;66:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. [DOI] [PubMed] [Google Scholar]
- 26. Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. [DOI] [PubMed] [Google Scholar]
- 27. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. [DOI] [PubMed] [Google Scholar]
- 28. Ludlow AT, Spangenburg EE, Chin ER, Cheng WH, Roth SM. Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. J Gerontol A Biol Sci Med Sci. 2014. doi:10.1093/gerona/glt211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sosnowska D, Richardson C, Sonntag WE, Csiszar A, Ungvari Z, Ridgway I A. Heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living noncolonial animal. J Gerontol A Biol Sci Med Sci. 2013. doi:10.1093/gerona/glt201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tucsek Z, Toth P, Sosnowsk D, et al. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol Biol Med Sci. 2013. doi:10.1093/gerona/glt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Almeida M, O’Brien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68:1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinha-Hikim I, Sinha-Hikim AP, Parveen M, et al. Long-term supplementation with a cystine-based antioxidant delays loss of muscle mass in aging. J Gerontol A Biol Sci Med Sci. 2013;68:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leiser SF, Fletcher M, Begun A, Kaeberlein M. Life-span extension from hypoxia in Caenorhabditis elegans requires both HIF-1 and DAF-16 and is antagonized by SKN-1. J Gerontol A Biol Sci Med Sci. 2013;68:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ungvari Z, Sosnowska D, Mason JB, et al. Resistance to genotoxic stresses in Arctica islandica, the longest living noncolonial animal: is extreme longevity associated with a multistress resistance phenotype? J Gerontol A Biol Sci Med Sci. 2013;68:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ungvari Z, Csiszar A, Sosnowska D, et al. Testing predictions of the oxidative stress hypothesis of aging using a novel invertebrate model of longevity: the giant clam (Tridacna derasa). J Gerontol A Biol Sci Med Sci. 2012;68:359–367 doi:10.1093/gerona/gls159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cesari M, Kritchevsky SB, Nicklas B, et al. ; Health ABC study. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2012;67:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behrens MI, Silva M, Salech F, et al. Inverse susceptibility to oxidative death of lymphocytes obtained from Alzheimer’s patients and skin cancer survivors: increased apoptosis in Alzheimer’s and reduced necrosis in cancer. J Gerontol A Biol Sci Med Sci. 2012;67:1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Csiszar A, Podlutsky A, Podlutskaya N, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012;67:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610 doi:10.1093/gerona/gls072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ungvari Z, Philipp EE. Comparative gerontology–from mussels to man. J Gerontol A Biol Sci Med Sci. 2011;66:295–297. [DOI] [PubMed] [Google Scholar]
- 45. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510 doi:10.1093/gerona/glr004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. [DOI] [PubMed] [Google Scholar]
- 48. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pepe M, Mamdani M, Zentilin L, et al. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res. 2010;106:1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ungvari Z, Podlutsky A, Sosnowska D, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68:1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gesing A, Masternak MM, Lewinski A, Karbownik-Lewinska M, Kopchick JJ, Bartke A. Decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis constitute new potential beneficial features of long-lived growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2013;68:639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crane FL, Navas P, Low H, Sun IL, de Cabo R. Sirtuin activation: a role for plasma membrane in the cell growth puzzle. J Gerontol A Biol Sci Med Sci. 2013;68:368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. [DOI] [PubMed] [Google Scholar]
- 54. Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589–1597. [DOI] [PubMed] [Google Scholar]
- 55. Miyazaki R, Ichiki T, Hashimoto T, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. [DOI] [PubMed] [Google Scholar]
- 56. Gracia-Sancho J, Villarreal G, Jr, Zhang Y, García-Cardeña G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 2010;85:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. [DOI] [PubMed] [Google Scholar]
- 58. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. [DOI] [PubMed] [Google Scholar]
- 59. Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol. 2010;50:829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jasper H. SKNy worms and long life. Cell. 2008;132:915–916. [DOI] [PubMed] [Google Scholar]
- 64. Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. [DOI] [PubMed] [Google Scholar]
- 66. Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Labbé A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278. [DOI] [PubMed] [Google Scholar]
- 74. Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tung BT, Rodriguez-Bies E, Ballesteros-Simarro M, Motilva V, Navas P, Lopez-Lluch G. Modulation of endogenous antioxidant activity by resveratrol and exercise in mouse liver is age dependent. J Gerontol A Biol Sci Med Sci. 2013. [DOI] [PubMed] [Google Scholar]
- 76. Strong R, Miller RA, Astle CM, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation. 2008;15:225–236. [DOI] [PubMed] [Google Scholar]
- 78. Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296:H1914–H1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. [DOI] [PubMed] [Google Scholar]
- 80. Detoni CB, Souto GD, da Silva AL, Pohlmann AR, Guterres SS. Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem Photobiol. 2012;88:913–921. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Xu H, Fu Q, Ma R, Xiang J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in parkinsonian rats. J Neurol Sci. 2011;304:29–34. [DOI] [PubMed] [Google Scholar]
- 82. Görgey A. Über die festen, flüchtigen, fetten Säueren des Cocusnussöles. Sitzungsberichte der mathematisch-naturwissenschaftlichen Classe der k Akademie der Wissenschaften in Wien. Vienna, Austria: Akademie der Wissenschaften in Wien; 1848:208–227. [Google Scholar]
- 83. Brittes J, Lúcio M, Nunes C, Lima JL, Reis S. Effects of resveratrol on membrane biophysical properties: relevance for its pharmacological effects. Chem Phys Lipids. 2010;163:747–754. [DOI] [PubMed] [Google Scholar]


