Abstract
Background:
Slowed gait is an important health indicator in older adults but a single identifiable cause is often lacking. We assessed whether a summary index measuring impairments across multiple physiologic systems was associated with slowed gait in older individuals.
Methods:
Data from the Cardiovascular Health Study (n = 3,010) were used to assess associations between baseline physiologic index (measuring vasculature, brain, kidneys, lungs, and glucose metabolism; range 0–10 with 0–2 points/system and lower score indicating higher function) and annual gait speed (m/s) over 6 years. Participants with complete data on the physiologic index and at least two gait speed measures were included. Mean gait speed and 95% confidence intervals (CI) by category of index were calculated using mixed effects models.
Results:
Those with scores of three or higher on the index had significantly slower gait speed at baseline compared to those with scores of 0–2 (7–10: mean speed = 0.83 m/s, 95% CI: 0.80, 0.84; 0–2: mean speed = 1.01 m/s, 95% CI: 0.99, 1.03). Those with higher indices also had faster decline in gait speed compared to those with lower scores after adjustment for demographic and health characteristics (7–10: change in speed = −0.020 m/s/year, 95% CI: −0.024, −0.016; 0–2: change in speed= −0.010 m/s/year, 95% CI: −0.014, −0.006).
Conclusions:
Greater impairment across five organ systems was associated with slower gait speed and greater declines in gait speed over 6 years. Impairments accumulated over multiple physiologic systems may make older adults more vulnerable to slow gait speed.
Key Words: Gait, Physical function, Epidemiology.
Slowed gait speed is common in older adults and is associated with increased risk for disability, cognitive impairment, and earlier death (1–3). In some cases, a single identifiable cause of gait slowing is evident, but more often the underlying cause is unclear. While physical function can be adversely affected by a number of clinically overt diseases, declines in function retain an independent association with age (4). Subclinical, asymptomatic, and undiagnosed changes to physiologic systems are common in older adults and may represent a pathway by which older age is associated with gait speed decline independent of clinical disease. Older adults may be particularly vulnerable to functional limitations when deficits occur across multiple organ systems (4–8). Multi-system impairments are particularly important as many contributors to aging are not organ specific and age-related changes in one system may accelerate deterioration in other systems (9). A single index that captures the full range of subclinical to clinically overt disease across several physiologic systems could provide a more complete measure of the multi-system biologic dynamic that may affect gait speed in older age (10). To our knowledge, no previous analyses have assessed the association of gait speed with a summary index of subclinical and clinical disease across multiple systems.
The physiologic index was developed to summarize disease burden across multiple organ systems, including vasculature, lungs, kidneys, brain, and glucose metabolism (10). It uses non-invasive tests to capture a wide range of disease from subclinical to clinically overt. The physiologic index predicts mortality and disability onset independent of age and multimorbidity counts and is associated with, but distinct from, frailty (10,11). Thus, the physiologic index may be a useful tool to examine the impact of multisystem disease on gait speed in older adults.
We used the Cardiovascular Health Study (CHS) to assess associations of the physiologic index with gait speed over 6 years of follow-up. We hypothesized that individuals with higher index scores, indicative of greater multi-system disease burden, would have slower gait speed and greater declines in gait speed compared to those with lower scores and that these associations would be independent of age and clinical disease. Further, we hypothesized that the physiologic index would account for a greater proportion of the variance between age and gait speed than would a count of clinical diseases.
Methods
Study Subjects
Participants were from the CHS, a community-based study of 5,888 adults aged 65 years and older in four regions of the United States (12). Recruitment from Medicare eligibility lists and age-eligible household members was conducted in 1989/1990 and supplemented by minority recruitment in 1992/1993. Participants were eligible if they did not have cancer under active treatment, were not wheelchair- or bedbound, and did not plan to move out of area within 3 years (12). Participants (n = 3,010) were included in these analyses if they had complete data on components of the physiologic index at the 1992/1993 study visit and at least two measures of gait speed between the 1992/1993 and 1998/1999 visit. The 2,878 excluded participants were 354 who had died before the 1992/1993 visit, 1,918 who were ineligible for magnetic resonance imaging (MRI), 307 missing another component of the index, and 299 missing five or more gait speed measures.
Those in the analytic sample were younger (p < .001), more likely to be white (p = .002), had fewer diagnosed health conditions (p < .001), and had lower body mass index (p < .001) than the excluded. Comparing those in the analytic sample to those who were excluded but had data on physiologic or gait measures, those included had higher baseline gait speed and better values on all physiologic measures (all p < .001).
Physiologic Index
The physiologic index was constructed as previously described (10). Atherosclerotic vascular disease defined by ultrasound as the mean of maximum left and right internal carotid artery wall intima-media thickness [IMT (13)]. Spirometry using the American Thoracic Society standards measured forced vital capacity [FVC (12)]. Fasting glucose (14) and cystatin C (15), measuring kidney function, were assessed as previously described. Brain MRI was obtained on 1.5T scanners by a standard protocol with interpretation at a central reading center (16). White matter grade indicated cerebral small-vessel disease (17). Missing values at baseline for either cohort were imputed as previously described (18). Missing values in 1992–1993 for members of the original cohort were replaced with the individual mean value from the 1991 to 1992 and 1993 to 1994 visits, if available, and if not, prior values were carried forward.
Construction of the index was based on tertiles of the five measures, with the best values classified as 0 and the worst as 2 (10). Although choice of cut points was arbitrary, 0 generally represented a healthy, normal value, and values of 2 were in the range of individuals with clinically diagnosed disease in that organ system. Individual scores were summed for a total score ranging from 0 to 10. Cut points were: for carotid IMT, 0 (0.60–0.98mm), 1 (0.98–1.71mm), and 2 (1.71–3.94mm); for FVC were sex-specific [women: 0 (2.8–3.8L), 1 (2.1–2.8L), 2 (0.6–2.1L); men: 0 (4.1–6.5L), 1 (3.1 to 4.1L), 2 (0.3–3.1L)]; for cystatin-C 0 (0.6–0.92mg/L), 1 (0.92–1.19mg/L), 2 (1.19–3.5mg/L); and for white matter grade, 0 (0–1), 1 (2), 2 (3–9) on a 0 to 9 ordinal scale. For interpretability, clinical cut points defined by the American Diabetes Association were used for fasting glucose [0 (<100mg/dL), 1 (100–126mg/dL), 2 (>126mg/dL (19))]. Medicated diabetics were included in the highest tertile.
Gait Speed
Yearly assessments of gait speed were conducted over a 15-foot course at usual walking speed starting from a standing position. Time to complete the course (in seconds) was recorded by stopwatch. The average of two trials was converted to meters/second (m/s).
Clinically Diagnosed Diseases
Presence of arthritis of the lower extremities, kidney disease, osteoporosis, and chronic lung disease was self-reported physician diagnoses. Diabetes, CHD, CHF, and stroke were centrally adjudicated as described elsewhere (20). Individuals were considered depressed if they had a score greater than 10 on a modified Center for Epidemiologic Studies Depression Scale [CES-D(21,22)].
Covariates
Demographic and health characteristics known to be associated with gait speed in older adults were assessed as potential confounders (Table 1). Age, gender, and race were self-reported at baseline. Height and weight were measured using standard procedures. Self-rated health was reported as excellent, very good, good, fair, or poor and was recoded as either good (good to excellent) or poor (fair or poor). Smoking status was never, former, or current.
Table 1.
Demographic and Health Characteristics of Participants by Category of Physiologic Index: Cardiovascular Health Study 1992/1993
| Total | Category 1 (healthiest) | Category 2 | Category 3 | Category 4 (unhealthiest) | p-Value | |
|---|---|---|---|---|---|---|
| Physiologic index score | 0–2 | 3–4 | 5–6 | 7–10 | ||
| N = 3,010 | N = 463 (15.4%) | N = 1035 (34.4%) | N = 1,008 (33.5%) | N = 504 (16.7%) | ||
| Age, mean (SD) | 74.5 (4.9) | 72.0 (3.5) | 73.6 (4.2) | 75.2 (4.9) | 77.2 (5.6) | <.001 |
| Female gender, n (%) | 1757 (58.4) | 326 (70.4) | 626 (60.5) | 554 (55.0) | 251 (49.8) | <.001 |
| Black race, n (%) | 422 (14.0) | 51 (11.0) | 128 (12.4) | 168 (16.7) | 75 (14.9) | .007 |
| Poor SRH, n (%) | 490 (16.3) | 31 (6.7) | 135 (13.1) | 190 (18.9) | 134 (26.6) | <.001 |
| BMI, mean (SD) | 26.6 (4.4) | 25.5 (4.0) | 26.3 (4.3) | 27.0 (4.4) | 27.6 (4.6) | <.001 |
| Physical activity (kcal/week), mean (SD) | 1531.2 (1765.8) | 1830.3 (1858.0) | 1610.1 (1706.0) | 1493.1 (1838.5) | 1170.2 (1582.6) | <.001 |
| Smoking status | <.001 | |||||
| Never, n (%) | 1369 (46.6) | 233 (51.4) | 525 (52.0) | 429 (43.4) | 182 (37.1) | |
| Former, n (%) | 1302 (44.3) | 186 (41.1) | 406 (40.2) | 458 (46.4) | 252 (51.4) | |
| Current, n (%) | 270 (9.1) | 34 (7.5) | 79 (7.8) | 101 (10.2) | 56 (11.4) | |
| Diabetes, n (%) | 292 (9.7) | 7 (1.5) | 39 (3.8) | 116 (11.6) | 130 (25.8) | <.001 |
| CHD, n (%) | 592 (19.7) | 49 (10.6) | 159 (15.4) | 217 (21.5) | 167 (33.1) | <.001 |
| CHF, n (%) | 125 (4.2) | 5 (1.1) | 25 (2.4) | 40 (4.0) | 55 (10.9) | <.001 |
| Arthritis, n (%) | 910 (31.0) | 124 (27.3) | 310 (30.6) | 295 (30.2) | 181 (37.0) | .009 |
| Osteoporosis, n (%) | 269 (9.4) | 50 (11.2) | 84 (8.5) | 93 (9.7) | 42 (8.8) | .4 |
| Lung disease, n (%) | 812 (27.0) | 103 (22.3) | 283 (27.3) | 273 (27.1) | 153 (30.4) | .04 |
| Kidney disease, n (%) | 29 (1.0) | 1 (0.2) | 7 (0.7) | 12 (1.2) | 9 (1.8) | .05 |
| Stroke, n (%) | 133 (4.4) | 8 (1.7) | 30 (2.9) | 45 (4.5) | 50 (9.9) | <.001 |
| Depression, n (%) | 343 (11.4) | 44 (9.5) | 92 (8.9) | 120 (11.9) | 87 (17.3) | <.001 |
| Gait speed (m/s), mean (SD) | 0.92 (0.23) | 1.01 (0.22) | 0.96 (0.22) | 0.89 (0.21) | 0.83 (0.23) | <.001 |
Note: BMI = body mass index; CHD = coronary heart disease; CHF = congestive heart failure; SD = standard deviation; SRH = self-rated health.
Statistical Analysis
Descriptive statistics were calculated for all covariates by categories of the physiologic index using ANOVA or chi-square tests as appropriate. Mixed effects models with random slopes and intercepts were used to calculate means and 95% confidence intervals (CI) in gait speed and rate of decline in gait speed over follow-up by category of the physiologic index. Since rate of change was dependent on time of follow-up, time was centered in multivariable models to represent the mid-point of follow-up (year 3). Adjusted results included the following a priori identified potential confounders: age, gender, race, height, weight, and smoking status (never, former, current). Age, height, and weight were centered on the mean for the entire sample. Additional analyses included presence of each clinical disease either at baseline or as time-varying covariates, updated annually: diabetes, CHD, CHF, arthritis, stroke, kidney disease, osteoporosis, chronic lung disease, and depression. Interactions with time were tested for all covariates and significant (p ≤ .05) interactions were retained in final models. Additional analyses assessed the association of age with gait speed adjusted for the index or for a count of nine clinical diseases.
A sensitivity analysis determined whether removal of any one system altered the results. Due to differences in scale based on number of included systems, these results were analyzed as a per point change on the scale. A second sensitivity analysis substituted the digit symbol substitution test (DSST) for MRI results to assess selection bias by MRI eligibility (23). All analyses used SAS 9.3 (SAS Institute, Cary, NC).
Results
Participants with higher physiologic index scores were older, more likely to be male, and reported worse health compared to those with the lowest score (Table 1). They also had a higher prevalence of all clinical diseases, except osteoporosis (Table 1).
Each component of the index, except glucose, was significantly related to gait speed such that those in the second and third tertiles were significantly slower at baseline than those in the lowest tertile (all p < .04). For glucose, only being in the highest tertile was associated with slower gait speed at baseline (p < .001). Rate of change in gait speed by each component of the index followed a similar pattern of associations.
A significant quadratic term for the index as a continuous variable was observed (p = .001), indicating that associations between the index and gait speed were non-linear. Therefore, further analyses used index categories based on approximate quartiles as previously defined (see Table 1 for distribution)10. Baseline gait speed was inversely associated with physiologic index (p < .001), with those in the lowest (best) category having a baseline mean gait speed of 1.01 m/s (CI: 1.00, 1.03) and those in the highest (worst) category having a mean gait speed of 0.83 m/s (CI: 0.81, 0.85; Table 1).
In addition to baseline differences, the rate of change in gait speed was greater for those in higher categories of the index such that gait speed differences increased over time. There was a significant race by time interaction, indicating that black participants had a faster average decline in gait speed (−0.021 m/s/year) compared to white participants (−0.019 m/s/year; p = .02) in fully adjusted models. Rate of change in gait speed was also greater with older age (p < .001). For simplification, rate of change for white participants of average age are shown. By year 3, those in the best category had a mean gait speed of 1.00 m/s (CI: 0.98, 1.02) and those in the worst category had a mean gait speed of 0.76 m/s (CI: 0.75, 0.78; Table 2). Adjustment for age, demographic, and health characteristics attenuated results, but associations remained significant (Table 2; Figure 1). In fully adjusted models, difference in gait speed at year 3 between those in the highest and lowest index categories (mean difference: −0.13 m/s; CI: −0.15, −0.11) was equivalent to 10 years of age (per year of age = −0.013 m/s; CI: −0.014, −0.012). The difference in rate of change in gait speed per year of follow-up (−0.010 m/s/year; CI: −0.016, −0.005) between these groups was nearly equivalent to an additional year of age.
Table 2.
Mixed Effects Models of the Association Between the Physiologic Index and Gait Speed (m/s) at Year 3 of Follow-Up: The Cardiovascular Health Study (n = 3,010)
| Unadjusted | Adjusted, Without Clinical Diseases† | Fully Adjusted, With Baseline Clinical Diseases‡ | Fully Adjusted, With Time-Varying Covariates§ | |||||
|---|---|---|---|---|---|---|---|---|
| Gait Speed (m/s) (95% CI) | Rate of Change in Gait Speed (m/s/ year) (95% CI) | Gait Speed (m/s) (95% CI) | Rate of Change in Gait Speed (m/s/ year) (95% CI) | Gait Speed (m/s) (95% CI) | Rate of Change in Gait Speed (m/s/ year) (95% CI) | Gait Speed (m/s) (95% CI) | Rate of Change in Gait Speed (m/s/ year) (95% CI) | |
| Physiologic index | ||||||||
| 0–2 | 1.00 | −0.008 | 0.94 | −0.013 | 0.97 | −0.013 | 1.08 | −0.010 |
| (0.98, 1.02) | (−0.011, −0.005) | (0.92, 0.96) | (−0.017, −0.010) | (0.95, 0.99) | (−0.017, −0.009) | (1.05, 1.12) | (−0.014, −0.006) | |
| 3–4 | 0.94** | −0.015* | 0.90** | −0.019* | 0.94* | −0.018* | 1.05** | −0.014 |
| (0.93, 0.95) | (−0.017, −0.012) | (0.89, 0.92) | (−0.021, −0.016) | (0.93, 0.96) | (−0.021, −0.016) | (1.01, 1.09) | (−0.017, −0.012) | |
| 5–6 | 0.86** | −0.020** | 0.85** | −0.022** | 0.89** | −0.022** | 1.00** | −0.017* |
| (0.85, 0.87) | (−0.023, −0.018) | (0.83, 0.87) | (−0.025, −0.020) | (0.87, 0.91) | (−0.025, −0.020) | (0.96, 1.04) | (−0.020, −0.014) | |
| 7–10 | 0.76** | −0.026** | 0.79** | −0.026** | 0.85** | −0.027** | 0.95** | −0.020** |
| (0.75, 0.78) | (−0.029, −0.022) | (0.77, 0.81) | (−0.030, −0.022) | (0.82, 0.87) | (−0.031, −0.022) | (0.91, 0.99) | (−0.024, −0.016) | |
| p for trend | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Notes: CI = confidence interval.
Results are shown for the categories of physiologic index. Rate of change in adjusted models represents change for white participants of average age.
**p < .001 and *p < .05 compared to those with physiologic index = 0–2.
†Adjusted for time2, age, age × time, gender, race, race × time, height, weight, and smoking status.
‡Adjusted for time2 and baseline age, age × time, gender, race, race × time, height, weight, smoking status, diabetes, coronary heart disease, congestive heart failure, arthritis, stroke, kidney disease, depression, osteoporosis, and chronic lung disease.
§Adjusted for time2 and baseline age, age × time, gender, race, race × time, height, and time-varying weight, smoking status, diabetes, coronary heart disease, congestive heart failure, arthritis, stroke, kidney disease, depression, osteoporosis, and chronic lung disease.
Figure 1.
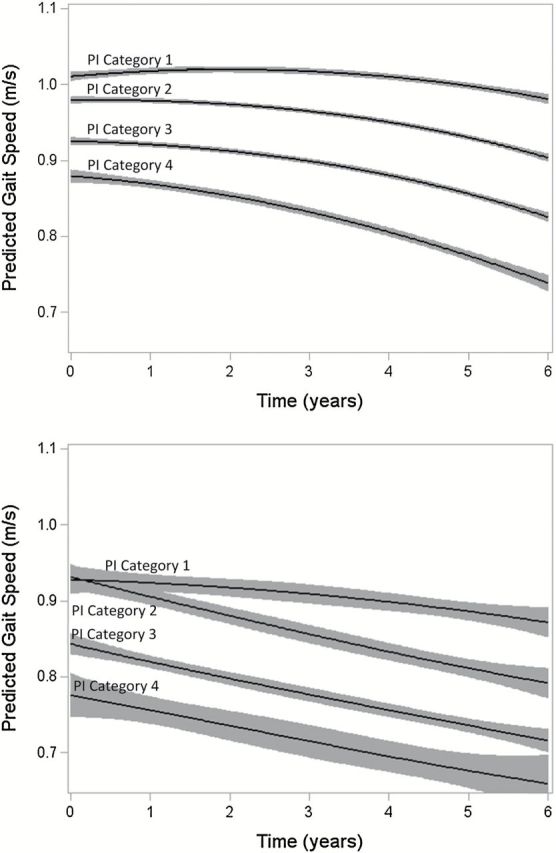
Predicted a gait speed (m/s) with 95% confidence limits by physiologic index (PI) category over 6 years: the Cardiovascular Health Study (n=3010) for participants of average age and (A) white or (B) black race. aAdjusted for time2 and baseline gender, height, and time-varying weight, smoking status, diabetes, coronary heart disease, congestive heart failure, arthritis, stroke, kidney disease, depression, osteoporosis, and chronic lung disease.
In unadjusted models, age was significantly associated with gait speed at year 3 (mean difference/year age = −0.015 m/s; CI: −0.016, −0.014). The physiologic index attenuated the association of age with gait speed by 0.004 m/s (25.9%) whereas a count of clinical diseases attenuated the association by only 0.001 m/s (8.5%) (Supplementary Appendix Table 1).
Sensitivity analyses determined that no system in the index was driving the associations with gait speed and that selection bias was minimal (not shown).
Discussion
A significant association between higher physiologic index and slower gait speed and faster decline in gait speed over 6 years was observed in this national cohort of adults aged 65 years and older. This association was attenuated but not eliminated by adjustment for age, demographic factors, health characteristics, and clinical diseases. In fully adjusted analyses, the effect on gait speed of being in the highest index category compared to the lowest was equivalent to 10 years of age. These differences were also on the order of previously reported increments indicative of increased mortality risk (3).
These results indicate that age-related declines in gait speed are more likely to occur when multiple organ systems are impaired, and that there may not be one key system that is primarily associated with maintaining gait speed (4–8). Importantly, the organ systems included in the index were chosen as indicators of physiologic aging and not because of direct associations with functional outcomes (10); however, the associations with gait were robust. Differences were present across the full index range, indicating that no one system was responsible for the observed results. Further, removal of any system did not change the results. In some older adults, slow gait has a relatively rapid onset and the cause is apparent (eg, hip fracture or stroke). However, in the majority of cases, gait slowing develops insidiously over time and cannot be attributed to a specific cause (24). In these cases, it is likely that gait slowing is due to an accumulation of more subtle impairments across multiple systems. Previous cross-sectional studies have demonstrated that multimorbidity in clinical disease is an important correlate of gait speed (6,25). Further, multimorbidity of clinical disease is associated with longitudinal declines in composite physical function measures (4) and presence of multiple geriatric syndromes arising from impairments in multiple systems are associated with increased risk of disability (7).
These results highlight the importance of subclinical and asymptomatic impairments in addition to clinical disease in relation to physical function in older adults. While inclusion of clinical diseases in the model here is likely an over-adjustment, the association between the index and gait speed remained even after adjustment. Further, inclusion of time-varying clinical disease attenuated but did not eliminate the associations, indicating that subclinical impairments are not simply impacting gait speed through an increased risk for development of clinical disease over time. Subclinical impairments in each of the organ systems assessed here have been shown to have negative impacts on gait speed and physical function in healthy older adults (26–30), but have not previously been assessed together.
The physiologic index may be a biomarker of aging (10); this is supported by the relatively strong attenuation of the age-gait speed association by the index compared to clinical diseases. In adjusted analyses, the effect on gait speed of being in the highest category of the index compared to the lowest was equivalent to 10 years of age. Ultimately, slow gait may represent a pathway by which high scores on the index increases risk for mobility limitations and disability (10,23).
Several limitations should be noted. First, all components of the physiologic index were measured only at the 1992/1993 visit; therefore, we were unable to assess gait speed related to changes in the index over time. Gait speed may have slowed in the more healthy individuals due to new onset of physiologic impairment. Improvements in index scores could also have occurred among those with initially high scores. Biases would not have occurred if the physiologic impairments increased at a comparable rate over time across all levels of baseline index scores. However, due to ceiling effects in those with the highest baseline indices, we may have underestimated the associations of the index with gait speed by not updating the index over time. Second, a large number of individuals were excluded due to missing data on either the index or gait speed during follow-up. This resulted in a younger, healthier sample for these analyses and may have reduced generalizability. However, the full range of physiologic index scores were observed in the analytic sample, suggesting that individuals with an adequate distribution of health and functioning were retained.
This study had several strengths. The physiologic index provides a measure of impairment in multiple organ systems without relying on self-reported or clinically diagnosed disease. Gait speed, an objective measure of physical function, was measured at multiple time points over a 6 year follow-up allowing for assessment of long-term trends.
These results provide further evidence that multi-system impairments can adversely affect physical function in older adults and extend this evidence beyond clinically manifest disease. Gait speed has been suggested as an indicator of underlying physiologic health in older adults and routine monitoring has been recommended (3,31). Older adults with impairments in multiple physiologic systems may need to be more closely monitored for slowing gait in order to prevent consequences such as mobility limitations and disability. Further, these results indicate that adjustment for overt clinical disease alone in studies of adverse outcomes related to slow gait speed may be insufficient as they do not capture more subtle impairments. Future studies should assess whether improvements in physiologic measures can slow or even reverse age-associated declines in gait speed.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Additional funding for this analysis came from a training grant from the NIA (T32-AG-000181).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
References
- 1. Clouston SA, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts [epub ahead of print]. Epidemiol Rev. 2013. 10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kriegsman DM, Deeg DJ, Stalman WA. Comorbidity of somatic chronic diseases and decline in physical functioning: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57:55–65. 10.1016/S0895-4356(03)00258-0 [DOI] [PubMed] [Google Scholar]
- 5. Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women’s Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. [DOI] [PubMed] [Google Scholar]
- 6. Cesari M, Onder G, Russo A, et al. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Gerontology. 2006;52:24–32. 10.1159/000089822 [DOI] [PubMed] [Google Scholar]
- 7. Rosso AL, Eaton CB, Wallace R, et al. Geriatric syndromes and incident disability in older women: results from the women’s health initiative observational study. J Am Geriatr Soc. 2013;61:371–379. 10.1111/jgs.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duncan PW, Chandler J, Studenski S, Hughes M, Prescott B. How do physiological components of balance affect mobility in elderly men? Arch Phys Med Rehabil. 1993;74:1343–1349. [DOI] [PubMed] [Google Scholar]
- 9. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanders JL, Boudreau RM, Fried LP, Walston JD, Harris TB, Newman AB. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: the Cardiovascular Health Study. J Am Geriatr Soc. 2011;59:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 13. O’Leary DH, Polak JF, Wolfson SK.Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. [DOI] [PubMed] [Google Scholar]
- 14. Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2236–2241. 10.1016/j.jacc.2003.10.074 [DOI] [PubMed] [Google Scholar]
- 15. Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. 10.1681/ASN.2005040384 [DOI] [PubMed] [Google Scholar]
- 16. Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382 [DOI] [PubMed] [Google Scholar]
- 17. Kuller LH, Arnold AM, Longstreth WT.Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. 10.1016/j.neurobiolaging.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 18. Arnold AM, Kronmal RA. Multiple imputation of baseline data in the cardiovascular health study. Am J Epidemiol. 2003;157:74–84. [DOI] [PubMed] [Google Scholar]
- 19. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 20. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. [DOI] [PubMed] [Google Scholar]
- 21. Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. [DOI] [PubMed] [Google Scholar]
- 22. Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. [DOI] [PubMed] [Google Scholar]
- 23. Sanders JL, Boudreau RM, Penninx BW, et al. Health ABC Study. Association of a Modified Physiologic Index with mortality and incident disability: the Health, Aging, and Body Composition study. J Gerontol A Biol Sci Med Sci. 2012;67:1439–1446. 10.1093/gerona/gls123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 25. Tolea MI, Costa PT, Terracciano A, et al. Sex-specific correlates of walking speed in a wide age-ranged population. J Gerontol B Psychol Sci Soc Sci. 2010;65B:174–184. 10.1093/geronb/gbp130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. 10.1111/j.1532-5415.2005.53214.x [DOI] [PubMed] [Google Scholar]
- 27. Buchman AS, Boyle PA, Leurgans SE, Evans DA, Bennett DA. Pulmonary function, muscle strength, and incident mobility disability in elders. Proc Am Thorac Soc. 2009;6:581–587. 10.1513/pats.200905-030RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hiltunen L, Keinänen-Kiukaanniemi S, Läärä E, Kivelä SL. Functional ability of elderly persons with diabetes or impaired glucose tolerance. Scand J Prim Health Care. 1996;14:229–237. [DOI] [PubMed] [Google Scholar]
- 29. Odden MC, Chertow GM, Fried LF, et al. HABC Study. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006;164:1180–1189. 10.1093/aje/kwj333 [DOI] [PubMed] [Google Scholar]
- 30. Elbaz A, Ripert M, Tavernier B, et al. Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke. 2005;36:2198–2202. 10.1161/01.STR.0000181752.16915.5c [DOI] [PubMed] [Google Scholar]
- 31. Universal health outcome measures for older persons with multiple chronic conditions. J Am Geriatr Soc. 2012;60:2333–2341. 10.1111/j.1532-5415.2012.04240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


