Abstract
Individual differences in striatal dopamine (DA) signaling have been associated both with individual differences in executive function in healthy individuals and with risk for psychiatric disorders defined by executive dysfunction. We used resting-state functional connectivity in 50 healthy adults to examine whether a polymorphism of the dopamine transporter gene (DAT1), which regulates striatal DA function, affects striatal functional connectivity in healthy adults, and whether that connectivity predicts executive function. We found that 9/10 heterozygotes, who are believed to have higher striatal DA signaling, demonstrated stronger connectivity between dorsal caudate (DC) and insular, dorsal anterior cingulate, and dorsolateral prefrontal regions, as well as between ventral striatum and ventrolateral prefrontal cortex, than 10/10 homozygotes. Across subjects, stronger DC-seeded connectivity predicted superior N-back working memory performance, while stronger ventral striatum-seeded connectivity predicted reduced impulsivity in everyday life. Further, mediation analysis suggested that connectivity strength mediated relationships between DAT1 genotype and behavior. These findings suggest that resting-state striato-frontal connectivity may be an endophenotype for executive function in healthy individuals.
Keywords: DAT1, fMRI, functional connectivity, impulsivity, working memory
Introduction
Optimal dopamine (DA) signaling is critical for the function of striato-thalamo-cortical loops that allow the striatum to dynamically gate and/or update information represented in prefrontal cortex (PFC), which enable executive functions such as impulse control and working memory (WM) (Miller and Cohen 2001; Hazy et al. 2007). Communication within these loops can be assessed by temporal correlation of blood oxygen level–dependent signal across brain regions (termed “functional connectivity”) measured during a task-free resting state (Di Martino et al. 2008). Previous work has shown that pharmacologic manipulations of DA affect resting-state striato-frontal functional connectivity (Kelly et al. 2009; Cole et al. 2013). Genetic regulation of DA signaling can also impact striato-frontal connectivity, as connectivity during tasks varied by a polymorphism of the DA receptor D2 gene (Cohen et al. 2007; Stelzel et al. 2010). However, it is unproven whether resting-state striato-frontal connectivity is also under genetic influence.
A polymorphism of the DA transporter (DAT1) gene is a likely candidate to modulate striato-frontal connectivity. DAT1 codes for the DA transporter (DAT) protein, which regulates DA signaling in striatum by re-uptaking released DA (Madras et al. 2005). In vitro studies have demonstrated that lower DAT expression was observed for the “9-repeat” than the “10-repeat” allele (Fuke et al. 2001; Mill et al. 2002; VanNess et al. 2005), resulting in elevated DA signaling for the 9-repeat allele due to reduced clearance (Madras et al. 2005), though in vivo findings have been mixed (Heinz et al. 2000; Jacobsen et al. 2000; Krause et al. 2006). Whether these differences reflect alterations in tonic or phasic activity, or both, is not known. Inheritance of 2 rather than one 10-repeat alleles (i.e., 10/10 genotype) has been associated with worse executive function, including lower WM (Stollstorff et al. 2010), reduced benefits of WM training (Brehmer et al. 2009), and increased impulsivity (Gizer and Waldman 2012). Further, 10/10 subjects had reduced striatal activation (Stollstorff et al. 2010) and less segregated cortico-cortico connectivity networks that predicted increased impulsivity (Gordon et al. 2012a). Although DAT expression is highest in the striatum but weak in PFC (Hall et al. 1999; Madras et al. 2005; Sasaki et al. 2012), 10/10 subjects also had reduced PFC activation during WM (Bertolino et al. 2006, 2009; Caldú et al. 2007; Stollstorff et al. 2010), suggesting that DAT1 effects on striatal DA function affect PFC, likely via striato-frontal connections. Finally, the 10/10 genotype has been reliably (though weakly) associated with attention-deficit hyperactivity disorder [ADHD, (Yang et al. 2007)], a disorder characterized both by executive dysfunction such as increased impulsivity and reduced WM (Van De Voorde et al. 2010) and by reduced task-evoked striato-frontal connectivity (Rubia et al. 2009; Cubillo et al. 2010). In sum, separate lines of research have indicated that the DAT1 gene affects DA function; that DA function influences striato-frontal functional connectivity; that DA function and striato-frontal connectivity both influence WM and control of impulsivity; and that DA function, striato-frontal connectivity, WM, and control of impulsivity are all altered in ADHD. However, it is unknown whether DAT1 genotype affects striato-frontal functional connectivity in healthy adults, and whether that connectivity predicts WM and impulsivity.
We examined DAT1-related differences in seed-based striatal resting-state functional connectivity in healthy young adults. We predicted that 9/10 subjects would demonstrate stronger striato-frontal connectivity than 10/10 subjects. Further, circuits involving discrete subdivisions of the striatum may have specific and separable effects on cognition, as it is known that 1) the dorsal caudate (DC) is critical for WM, as it is strongly active during WM performance (Moore et al. 2013), while DC lesions disrupt WM function (White 2009), and 2) ventral striatum is critical for inhibitory control, as it is active during Go/NoGo (Menon et al. 2001) and Stop-signal (Boehler et al. 2010) response inhibition tasks. Thus, we predicted that increased DC-seeded connectivity would be associated with faster and more accurate WM performance, while increased ventral caudate-seeded connectivity would be associated with reduced trait-level impulsivity.
Materials and Methods
Subjects
Twenty-four 9/10 carriers (mean ± SD, age = 20.42 ± 0.85 years, 7 males) and 26 10/10 carriers (age = 20.42 ± 0.96 years, 8 males) participated in this study for payment after being drawn randomly from a larger pool of Georgetown University undergraduates aged 18–22 years who had been genotyped for DAT1. Genotype groups did not differ in either age (t(48) = 0.16, P = 0.88) or gender (χ2 = 0.015, P = 0.90). The overall sample was 78% white, 14% Asian, 4% African American, and 4% mixed race, and was 10% Hispanic or latino and 90% not Hispanic or latino. These distributions did not differ by genotype group for race (χ2 = 6.14; P = 0.10) or ethnicity (Fisher's exact test 2 sided P = 0.66).
Because ADHD has previously been linked to the 10-10 DAT1 genotype, to striato-frontal connectivity reductions, and to worse WM and control of impulsivity, the possible presence of undiagnosed ADHD in the sample of healthy adults could artificially increase the strength of any observed relationships between these variables. Thus, to ensure that the subjects did not meet criteria for ADHD, all subjects were screened for ADHD using the recommended cutoff score of 24 on either the inattention or the hyperactivity/impulsivity portions of the Adult ADHD Self-report Rating Scale (Kessler et al. 2005). No subjects were above this cutoff; one was at the cutoff for inattention (and scored 8 for hyperactivity/impulsivity, well below the cutoff for that scale), but no results changed if this subject was excluded from analysis. Subjects were also screened by verbal self-report of any current or past psychiatric or neurological diagnosis, and no subjects reported any such diagnosis. Additional exclusion criteria included self-reports of 1) use of psychotropic medication (e.g., stimulants, SSRIs); 2) overt neurological injury or disease, seizure disorder, psychiatric diagnosis; 3) contraindications for MRI, for example, presence of metal, pregnancy. All subjects gave informed consent in accordance with guidelines of the Georgetown University Institutional Review Board. Thirty-nine of the 50 subjects tested here were examined in a previous report examining DAT1 effects on functional connectivity (Gordon et al. 2012a), though all subjects returned for new scans; and, thus, there were no fMRI data overlap between this report and the previous one.
Genotyping
DNA was extracted from Oragene saliva kits (DNA Genotek, Inc., Ottawa, Ontario, Canada). The 40 bp variable number tandem repeat (VNTR) polymorphism in the 3′ UTR of DAT1 was genotyped by PCR as previously described (Daly et al. 1999) using the following primers; Forward: 5′-TGTGGTGTAGGGAACGGCCTGAG-3′ Reverse: 5′-CTTCCTGGAGGTCACGGCTCAAGG-3′. PCR was performed using the Accuprime Taq DNA polymerase system (Invitrogen) with the following PCR program: 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 68 °C for 1 min. The PCR products were then run out on a 2% agarose gel stained with ethidium bromide. A 100 bp DNA ladder was then used to identify the various repeat alleles by size: 7-repeat (360 bp), 8-repeat (400 bp), 9-repeat (440 bp), 10-repeat (480 bp), and 11-repeat (520 bp). Genotyping was successful for 153 of the 158 subjects in the original sample. Observed genotype frequencies in the sample were 10/10–60.1%; 9/10–33.3%; 9/9–5.9%; other, 0.6%.
Behavioral Testing
All behavioral testing was conducted at the time of genotyping, ∼2 months before scanning.
Trait Measure of Impulsivity
Subjects completed the Barratt Impulsiveness Scale (BIS) version 11 (Patton et al. 1995), on which a higher score signifies more impulsive everyday behavior.
Working Memory Task
Subjects performed a computerized N-back task consisting of 6 72 s N-back blocks which alternated between 2- and 3-back conditions. Each N-back block consisted of 24 serially presented consonants appearing for 500 ms, with an intertrial interval of 2500 ms. Each block was preceded by a screen informing the subject of the N-back condition. Subjects were instructed to press the space bar when the current letter matched the letter n trials ago (e.g., for the 2-back condition, subjects see: R V N W N – button-press for N). Targets were present on 21% of trials. Neither condition contained sequences of stimuli that were targets in the other condition. Stimuli were presented using E-Prime (Psychology Software Tools, Inc., Pittsburg, PA, USA).
For each condition, accuracy was calculated as % targets identified minus % false alarms. Reaction time (RT) was calculated as the average reaction time to successfully identified targets.
fMRI Data Acquisition
Subjects were scanned for 5:04 min during the resting state, in which they were told to relax with eyes closed and to not think of anything in particular. Imaging was performed on a Siemens Trio 3T scanner (Erlangen, Germany). One hundred fifty-two whole-brain images were acquired using a gradient echo pulse sequence (37 slices, TR = 2000 ms, TE = 30 ms, 192 × 192 mm FOV, 90° flip angle, voxel dimensions 3 mm isotropic). The first 4 images of the resting run were discarded to allow for signal stabilization. Additionally, a high-resolution T1-weighted structural scan (magnetization prepared rapid gradient echo, MPRAGE) was acquired with the parameters: TR/TE = 2300/2.94 ms, TI = 900 ms, 90° flip angle, 1 slab, 160 sagittal slices with a 1.0 mm thickness, FOV = 256 × 256 mm2, matrix = 256 × 256, resulting in an effective resolution of 1.03 mm isotropic voxels.
Image Preprocessing
Using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Version 7.10 Mathworks, Inc., Sherborn, MA, USA), images were corrected for translational and rotational motion by realigning to the first image of the scan. Across all runs, all subjects demonstrated <2.0 mm of translational motion in any one direction (max translation = 1.25 mm) and <1.0° of rotation around any one axis (max rotation = 0.54°). Images were then slice-time corrected, normalized to an echo planar imaging template, resampled every 2 mm, and smoothed using a Gaussian kernel with full-width at half-maximum of 8 mm. A band-pass filter was applied to the data in order to restrict signal variation to frequencies between 0.01 and 0.1 Hz. Application of a filter in this fashion has become standard in the field as a way to help eliminate noise from non-neuronal sources (Van Dijk et al. 2010), based on observations that the majority of the power of resting-state fluctuations fall within this range (Biswal et al. 1995; Cole et al. 2010; Niazy et al. 2011).
Finally, because even small amounts of motion (<2 mm) can affect measures of functional connectivity (Power et al. 2012; Van Dijk et al. 2012), we employed the “scrubbing” technique from Power et al. (2012) to eliminate contributions of motion. For each time point, framewise displacement (FD) was calculated as the distance moved relative to the previous time point, and scans with FD > 0.5 mm were removed from further analysis using custom MATLAB scripts. The percent of scans removed by scrubbing was very low on average (mean ± SD: 3.43 ± 6.70%), and the number of scans removed did not differ by genotype (9/10: 3.24 ± 6.64%; 10/10: 3.61 ± 6.87%; t(48) = 0.20, P = 0.75) and was not correlated with any N-back or impulsivity behavioral measure (absolute value of all r(48)s < 0.17; all P’s > 0.25). Forty-seven of the 50 subjects retained at least 85% of their scans. The remaining 3 subjects (1 9/10; 2 10/10) retained 77.7%, 70.9%, and 68.9% of their scans, representing at least 3:45 min of scanning time in all subjects.
Functional Connectivity Calculation
Striatal Seed Creation
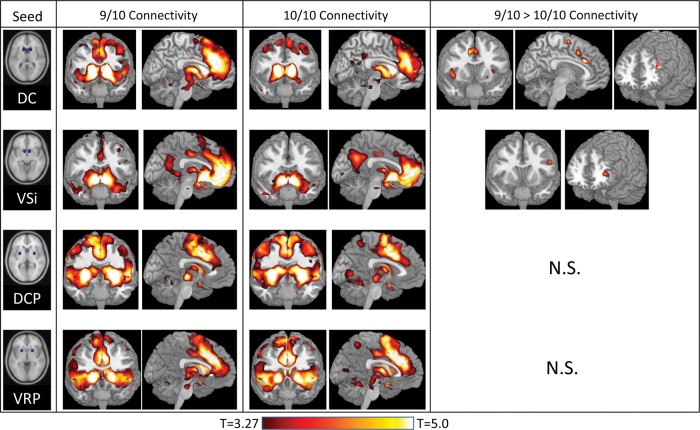
Using Marsbar (Brett et al. 2003), bilateral striatal seeds were created as spheres of radius 6 mm centered around 4 of the 6 left/right mirrored coordinates used by Di Martino et al. (2008) to delineate striatal resting-state functional connectivity networks. These bilateral seeds are shown in the left column of Figure 1 and were in the head of the DC, centered around the Montreal Neurological Institute coordinates [±13 15 9]; in the inferior ventral striatum (VSi), centered around [±9 9 −8]; in the ventral rostral putamen (VRP), centered around [±20 12 –3]; and in the dorsal caudal putamen (DCP), centered around [±28 1 3]. Seed time courses were calculated as the average time course of all voxels in each seed. Di Martino et al. (2008) also reported connectivity from superior ventral striatum and dorsal caudal putamen, but found that these connectivity patterns were very similar to those in the nearby VSi and DCP, respectively; to avoid redundancy, we excluded these 2 seeds from our analyses.
Figure 1.
t-Tests of connectivities from the 4 bilateral striatal seeds (first column) reveals regions which showed strong connectivity across all subjects with the 9/10 (n = 24; second column) and 10/10 (n = 26; third column) genotypes. Two-sample t-tests (fourth column) reveal regions in which 9/10 individuals had significantly stronger connectivity with the DC and the VSi than 10/10 individuals. No region demonstrated greater connectivity for 10/10 than 9/10 individuals, and no effects of DAT1 were found for DCP- or VRP-seeded connectivity.
Nuisance Signal Identification
To identify the effects of motion and physiological noise (such as respiration and heart rate) that would be common to all voxels, time courses approximating these signals were calculated using Marsbar. Physiological noise time courses were approximated by obtaining signal time courses from white matter and cerebrospinal fluid segmentations of the MPRAGE image (Van Dijk et al. 2010). Motion time courses were obtained as the 6 realignment parameter time courses from the motion correction preprocessing step, expressed as absolute differences from the first time point in each of the 3 translation and rotation directions. Notably, the global signal was not included as a nuisance signal, as recent work suggests that regression of the global signal may reduce the accuracy of connectivity estimates (Chen et al. 2012; Saad et al. 2012).
Voxelwise Intrinsic Functional Connectivity Calculation
For each subject, custom MATLAB scripts were used to conduct partial correlations between each seed's time course and the time courses of every voxel in the brain, while partialling out the motion and physiological noise time courses. The resulting r values were converted to Z-scores using Fisher's transformation in order to increase normality of the distribution, allowing further statistical analysis of correlation strengths. This produced a brain map of the strength of intrinsic connectivity with each seed.
Statistical Analysis
Overall Connectivity
For each seed in each genotype group, we identified overall patterns of both positive and negative connectivity with the seed by entering subjects' individual connectivity maps into a voxelwise 1-sample t-test using SPM8. These connectivity patterns are presented for illustrative purposes; no hypotheses were tested in this analysis. For consistency with the group comparison results (below), results were corrected for multiple comparisons (including both the number of voxels and the number of seed maps tested) at P < 0.05 using Monte-Carlo simulation (Ward 2000), which established the correction threshold at P < 0.001, k = 64.
DAT1 Effects on Connectivity
For each seed, we tested for effects of DAT1 on striatal functional connectivity by entering each subject's connectivity map into a voxelwise 2-sample t-test using SPM8. Results were again corrected for voxel and seed multiple comparisons at P < 0.05 using the same Monte-Carlo-defined threshold as above.
Association Between DAT1-Modulated Functional Connectivity and Executive Function
To determine whether striatal connectivity with any DAT1-modulated region predicted executive function, we examined correlations between DAT1-modulated striatal connectivity and our behavioral measures. Regions of interest (ROIs) were created as the distinct clusters of voxels in which striatal connectivity significantly differed by DAT1 genotype. For each of these ROIs, each subject's mean connectivity strength with the striatal seed was calculated by extracting the connectivity values from each voxel in the ROI and averaging across voxels, resulting in 7 connectivity measures for each subject. For each behavioral measure (Impulsiveness, 2- and 3-back accuracy, and 2- and 3-back RT), we investigated which of these 7 connectivity measures (including 5 DAT1-modulated DC connectivities and 2 DAT1-modulated VSi connectivities) most strongly predicted behavior across subjects. Towards this end, we used SPSS to conduct a hierarchical stepwise multiple regression for each behavioral measure in which the 7 connectivity measures were possible predictors in a regression model explaining the variance in that behavioral measure. Alpha thresholds for entry into the models were set at 0.05, while the thresholds for removal were set at 0.1. Significant associations were further examined using post hoc bivariate correlations.
To ensure that any observed associations between striato-frontal connectivity and behavior did not arise solely due to the definition of the ROIs as regions showing effects of genotype on connectivity, we repeated these regressions against behavior using ROIs defined from a previously published independent components analysis (ICA) of an independent group of subjects (from Gordon et al. 2012b). Results from this analysis were very similar to those observed using the original DAT1-modulated ROIs (see Supplementary Materials).
Results
Behavioral Measures
Trait-Level Impulsiveness
Across all subjects, the trait-level measure of Impulsiveness as assessed by the total BIS score was mean ± SD = 55.66 ± 7.39. Genotype groups did not differ in Impulsiveness (9/10: 54.00 ± 6.32; 10/10: 57.19 ± 8.06; t(48) = 1.55, P = 0.13).
Working Memory Performance
Across all subjects, WM accuracy, calculated as % targets hit − % false alarms, was higher in the 2-back condition (91.0 ± 9.5%) than in the 3-back condition (80.0 ± 18.5%; t(49) = 4.47, P < 0.001). Accuracy did not differ between genotype groups in either the 2-back condition (9/10: 92.8 ± 6.8%; 10/10: 89.3 ± 11.3%; t(48) = 1.30, P = 0.20) or the 3-back condition (9/10: 83.3 ± 18.4%; 10/10: 76.8 ± 18.3%; t(48) = 1.26, P = 0.22).
Similarly, across all subjects, average RT for successfully identified targets was faster in the 2-back condition (574.0 ± 151.8 ms) than in the 3-back condition (685.9 ± 241.7 ms; t(49) = 5.83, P < 0.001). However, RT did not differ between genotype groups in either the 2-back condition (9/10: 554.2 ± 139.4 ms; 10/10: 592.3 ± 163.0 ms; t(48) = 0.89, P = 0.38) or the 3-back condition (9/10: 656.8 ± 221.4 ms; 10/10: 712.7 ± 260.4 ms; t(48) = 0.81, P = 0.42).
Striatal Connectivity Networks
Striatal connectivity networks in each group are shown in Figure 1, middle columns. Across groups, common patterns of positive connectivity emerged that were very similar to those observed by Di Martino et al. (2008) using the same seeds. No significant clusters of negative connectivity emerged that were common to both groups.
DC Seed
The bilateral DC seed was strongly connected to perigenual and dorsal anterior cingulate extending into medial PFC and supplementary motor area, bilateral anterior insula extending into bilateral inferior frontal gyrus, and bilateral posterior middle frontal gyrus. Small clusters of high connectivity also emerged in bilateral angular gyrus, bilateral anterior thalamus, bilateral rostral putamen, left middle temporal gyrus, and dorsal pons.
VSi Seed
The bilateral VSi seed was strongly connected to subgenual and anterior cingulate extending into ventral and anteromedial PFC, and posterior cingulate extending into ventral precuneus. Small clusters of high connectivity also emerged in middle cingulate cortex, anteromedial thalamus, bilateral anterior middle temporal gyrus, and bilateral temporal pole.
DCP Seed
The bilateral DCP seed was strongly connected to bilateral insula extending into superior temporal gyrus, bilateral primary motor cortex, middle cingulate extending into supplementary motor area, and bilateral supramarginal gyrus. Small clusters of high connectivity also emerged in bilateral middle frontal gyrus, bilateral inferior frontal gyrus, bilateral central thalamus, bilateral posterior middle temporal gyrus, and bilateral cerebellum.
VRP Seed
The bilateral VRP seed was strongly connected to dorsal anterior and middle cingulate extending into supplementary motor area, bilateral anterior insula, bilateral caudate, bilateral anterior middle frontal gyrus, and bilateral supramarginal gyrus. Small clusters of high connectivity also emerged in bilateral primary motor cortex, bilateral anterior and central thalamus, and bilateral posterior middle temporal gyrus.
Effects of DAT1 Genotype on Striatal Connectivity
For each seed, we examined whether DAT1 genotype groups differed in functional connectivity with that seed. Results are shown in Table 1 and Figure 1, right column.
Table 1.
Clusters demonstrating differences in striatum-seeded connectivity between 9/10 (n = 24) and 10/10 (n = 26) subjects
| Cluster location | Peak coordinates | Peak T value | Cluster size (voxels) |
|---|---|---|---|
| Seed: Dorsal caudate 9/10>10/10 | |||
| Dorsal anterior cingulate | 6, 24, 28 | 5.54 | 530 |
| Right anterior insula | 42, 10, −2 | 4.43 | 164 |
| Supplementary motor area | 6, −6, 66 | 3.90 | 121 |
| Left anterior insula | −36, 18, 6 | 3.71 | 69 |
| Left middle frontal gyrus | −40, 46, 26 | 3.45 | 84 |
| Seed: Inferior ventral striatum 9/10 > 10/10 | |||
| Left posterior inferior frontal gyrus | −52, 12, 28 | 4.91 | 92 |
| Left anterior inferior frontal gyrus | −36, 44, 6 | 4.52 | 201 |
DC Seed
9/10 subjects (n = 24) demonstrated significantly greater functional connectivity with the DC than 10/10 subjects (n = 26) in dorsal anterior cingulate, supplementary motor area, bilateral anterior insula, and left anterior middle frontal gyrus. 10/10 subjects did not demonstrate greater functional connectivity with DC than 9/10 subjects in any region.
VSi Seed
9/10 subjects demonstrated significantly greater functional connectivity with the VSi than 10/10 subjects in 2 clusters within left inferior frontal gyrus, one at the anterior end of the inferior frontal gyrus, just posterior to frontopolar cortex, and one at the posterior end, just anterior to the precentral gyrus. 10/10 subjects did not demonstrate greater functional connectivity with VSi than 9/10 subjects in any region.
Putamen Seeds
No effects of DAT1 were observed on functional connectivity with either the DCP or the VRP seeds.
Associations Between DAT1-Modulated Functional Connectivity and Executive Function
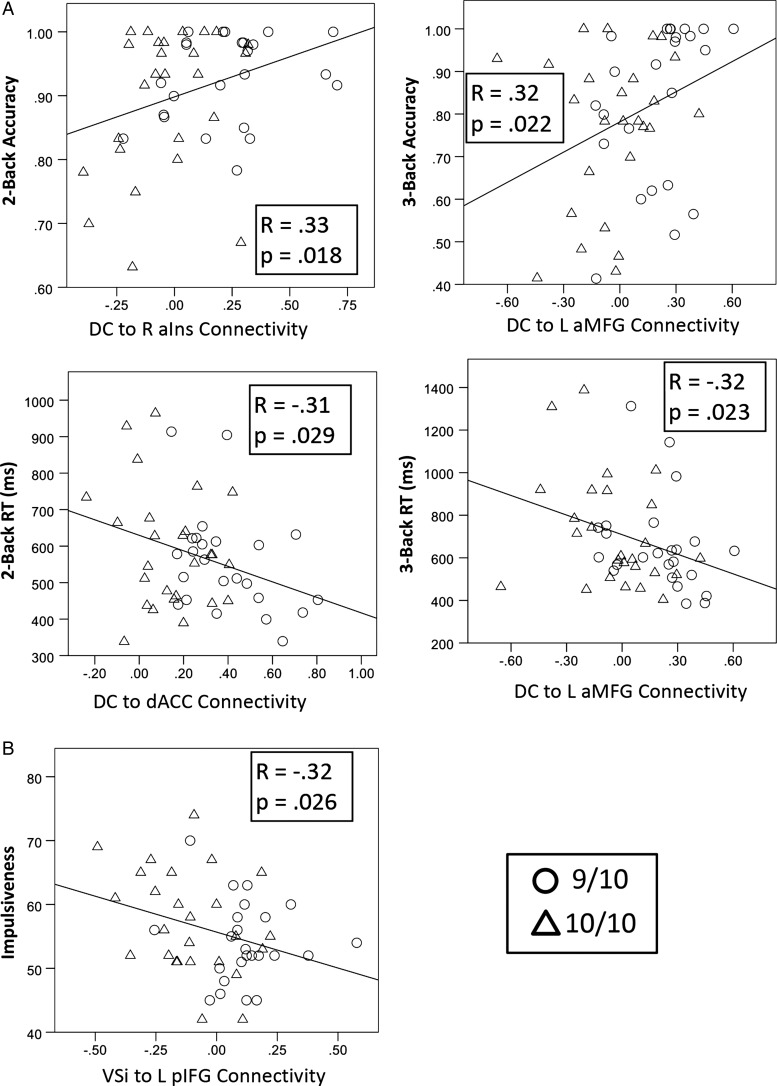
We created ROIs from the 7 clusters showing DAT1 differences above, and within those clusters calculated the average strength of connectivity with the appropriate striatal seed. For each behavioral measure, we then conducted a stepwise multiple regression model to examine whether any of these connectivity values predicted the behavior. Results of the analysis can be seen in Figure 2.
Figure 2.
DAT1-modulated functional connectivity predicted behavior across 9/10 (n = 24) and 10/10 (n = 26) individuals. (A) The strength of functional connectivity from DC to various PFC regions predicted 2- and 3-back accuracies (top row) and reaction times (bottom row). (B) The strength of functional connectivity from VSi to left posterior inferior frontal gyrus predicted self-reported Impulsiveness. RaIns: right anterior insula. L aMFG, left anterior middle frontal gyrus; dACC, dorsal anterior cingulate cortex; L pIFG, left posterior inferior frontal gyrus.
Impulsiveness
Reduced Impulsiveness was predicted by increased connectivity between VSi and posterior inferior frontal gyrus (r(48) = −0.32, P = 0.026).
Working Memory Performance
In the 2-back condition, increased accuracy was predicted by increased connectivity between DC and right anterior insula (r(48) = 0.33, P = 0.018), while faster RT was predicted by increased connectivity between DC and dorsal anterior cingulate cortex (r(48) = −0.31, P = 0.029). In the 3-back condition, both increased accuracy (r(48) = 0.32, P = 0.022) and faster RT (r(48) = −0.32, P = 0.023) were predicted by increased connectivity between DC and left anterior middle frontal gyrus.
In summary, several of the striato-frontal connections that were shown to be stronger in 9/10 than in 10/10 individuals were correlated with behavior. In all cases, increased striato-frontal connectivity—which was present in the 9/10 genotype—predicted superior executive function.
Post hoc Mediation Analysis
Some previous work suggests that DAT1 genotype is associated with executive function and traits through striatal function (Brehmer et al. 2009; Stollstorff et al. 2010; Gizer and Waldman 2012). We investigated whether DAT1 effects on WM and impulsivity may be mediated through the gene's influence over striato-frontal connectivity by testing a mediation model for each of the 5 significant connectivity–behavior relationships observed above, in which we entered both DAT1 genotype and connectivity strength as predictors in a regression testing for effects on behavior. In this model, significant genotype effects on behavior while accounting for connectivity indicate a direct effect, while significant connectivity effects on behavior while accounting for genotype indicate an indirect effect.
These exploratory mediation analyses indicated that the direct effects of DAT1 genotype on the behavioral measures was not significant (all F’s(1,47) < 0.25, all P’s > 0.6). By contrast, multiple significant indirect effects were observed: connectivity between DC and right anterior insula predicted 2-back accuracy (F(1,47) = 4.04, P = 0.05); connectivity between DC and dorsal anterior cingulate predicted 2-back RT (F(1,47) = 4.37, P = 0.043); and connectivity between DC and left anterior middle frontal gyrus predicted 3-back RT (F(1,47) = 4.85, P = 0.033). Further, the other 2 indirect effects were marginally significant: connectivity between DC and left anterior middle frontal gyrus predicted 3-back accuracy (F(1,47) = 3.85, P = 0.056) and connectivity between VSi and left posterior inferior frontal gyrus predicted impulsivity (F(1,47) = 2.85, P = 0.098). Together, these post hoc analyses support the notion that DAT1-behavior relationships may be mediated through the genotype's effects on striato-frontal functional connectivity.
Discussion
This study is the first to demonstrate that resting-state striato-frontal functional connectivity is affected by a polymorphism of the DA transporter gene (DAT1), and the first to demonstrate that DAT1-affected resting-state connectivity predicts executive function. As hypothesized, individuals with one 9-repeat DAT1 allele, which has been linked to increased DA signaling via reduced synaptic DA clearance (Madras et al. 2005), demonstrated greater striato-frontal connectivity than those with 2 10-repeat alleles, and this increased striato-frontal connectivity was associated with superior WM function and reduced trait-level impulsivity. Post hoc mediation analyses suggested that differences in striato-frontal connectivity may be an indirect pathway by which DAT1 genotype influences WM function.
The gene–connectivity–behavior linkages we observed within one subject pool extend and integrate previous work separately describing DA–connectivity and connectivity–executive function relationships. First, striato-frontal connectivity was increased by factors enhancing DA signaling, including increased striatal DA synthesis capacity (Klostermann et al. 2013), l-dopa administration (Kelly et al. 2009; Cole et al. 2013), and cabergoline administration (Cohen et al. 2007), and decreased by factors which reduce DA signaling, including haloperidol (Cole et al. 2013) and dietary DA depletion (Nagano-Saito et al. 2008). These associations were observed both during the resting state (Kelly et al. 2009; Cole et al. 2013) and during performance of various tasks (Cohen et al. 2007; Nagano-Saito et al. 2008; Klostermann et al. 2012). The present study extends these findings to show that genetically influenced differences in DA signaling affect resting-state striato-frontal connectivity, particularly between the DC and regions such as bilateral anterior insula, dorsal anterior cingulate, and anterior dorsolateral PFC that well-match a known Cingulo-opercular network (Beckmann et al. 2005; Dosenbach et al. 2007, 2008). Notably, striatum-to-Cingulo-opercular connectivity was influenced by pharmacological DA manipulations (Cole et al. 2013), though modulations of this network were observed using a ventral striatal seed rather than the DC seed observed here. Second, stronger connectivity between striatum and PFC has been associated with both superior WM function and reduced impulsivity. Better N-back performance related to stronger resting-state connectivity between Cingulo-opercular PFC regions and putamen (Tu et al. 2012), as well as to stronger task-induced connectivity between DC and the lateral PFC (Klostermann et al. 2012). Further, better impulse control, as measured by the same behavioral scale used in the current study, was specifically associated with stronger functional connectivity between ventral striatal and Cingulo-opercular regions (Davis et al. 2013). This previous work converges with the current findings that stronger connectivity between striatum and the Cingulo-opercular network predicted superior WM and reduced impulsivity.
By demonstrating linkages between a DA-regulating gene, striato-frontal functional connectivity, and multiple domains of executive function, the present work contributes to a model of executive function enabled by multiple segregated DA-sensitive striato-thalamo-frontal loops. Anatomic studies have established that segregated connectivity loops run from striatum to thalamus to PFC, and return to striatum. These loops exhibit anatomical specificity, such that loops through putamen innervate premotor cortex, while loops through caudate innervate more anterior PFC regions (Alexander et al. 1986; Schmahmann and Pandya 2006). These loops are argued to play a causal role in “gating” the transfer of sensory information into currently maintained PFC representations (Hazy et al. 2007; van Schouwenburg et al. 2010), which may be the mechanism by which PFC selectively maintains or updates information in WM (Miller and Cohen 2001). These loops are also hypothesized to be sensitive to striatal DA signaling, which allows them to “learn” what information to gate, which in turn enables optimum WM function (Hazy et al. 2007). The present findings provide support for the idea of DA-sensitive striato-frontal loops helping to enable WM by demonstrating that functional connectivity between 2 regions connected by these striato-frontal loops, the DC and the PFC, is not only sensitive to genetic influences on striatal DA function, but also predicts WM performance. We note that WM testing was done a month or 2 before scanning; while this is a limitation of the study, it bolsters the notion that the observed gene–connectivity–behavior linkage is relatively stable rather than transient or state-specific.
Further, the present findings extend these concepts to other domains of executive control by demonstrating that genetic influences also affect connectivity between ventral striatum and PFC, which in turn predicts control of impulsive behavior. By demonstrating such relationships in the task-free resting-state, the present findings help link these behaviors specifically with communication within the striato-frontal loops, rather than with any task-specific engagement of regions linked by the loops.
Further, in demonstrating that WM function and impulsivity related to different caudate-PFC circuits, this study indicates that loops linking specific caudate and PFC subregions, which have been associated with discrete executive functions, predict behavior in those executive domains. DC has been characterized as the striatal nucleus most relevant to WM (White 2009; Moore et al. 2013), and (Hazy et al. 2007) argues that DC specifically gates information into WM. Meanwhile, the nucleus accumbens and adjacent ventral striatum is known to be critical for inhibitory control due to its central role in reward processing (Menon et al. 2001; Cropley et al. 2006; Li et al. 2008; Boehler et al. 2010). In PFC, the dorsal cingulate, anterior insula, and dorsolateral PFC have all been linked to WM function (Owen et al. 2005), with the cingulate/insular regions specifically linked to task set maintenance (which may influence performance in easier task conditions, in which maintaining task goals is a limiting factor on performance) and the dorsolateral PFC linked to trial-by-trial processing (which may influence performance in difficult conditions requiring complex information processing) (Dosenbach et al. 2007, 2008). By contrast, the inferior frontal gyrus is critical for response inhibition (Aron et al. 2004). The findings of the present study mirror these distinctions, such that stronger connectivity between DC and cingulate/insular cortex predicted superior WM in easier task conditions, stronger connectivity between DC and dorsolateral PFC predicted superior WM in difficult task conditions, and stronger connectivity between ventral striatum and inferior frontal gyrus predicted improved control of impulsivity. Thus, one compelling interpretation of these findings is that they provide support for the idea that anatomically segregated, DA-sensitive striato-frontal loops contribute to functionally segregated executive processes. However, we also note that the apparent specificity of circuit–behavior relationships observed here may be due in part to the specific behavioral regression approach we employed, which tends to allow only the most significant connectivity–behavior relationships to survive. Thus, while the observed connectivity–behavior relationships emerged because those regions are most robustly related to the behaviors, that fact does not mean they are the only DAT1-modulated regions related to the behaviors. Indeed, it is possible that the behaviors may be moderately correlated with the strength of striatal connectivity with many or all Cingulo-opercular network nodes. Future work focusing on a priori Cingulo-opercular regions may be better able to test this possibility.
Findings from an exploratory mediation analysis demonstrate that the DAT1 genotype affected WM performance indirectly through effects on striato-frontal connectivity (indirect effects on impulsivity were marginally reliable), which suggests that striato-frontal connectivity may be causally intermediate between DAT1 and executive function. We interpret these results cautiously, as it is not entirely clear whether mediation testing of gene–brain–behavior relationships is appropriate when there is no significant initial gene–behavior association. However, it has been argued that mediation testing is still appropriate in circumstances where the direct relationship (between DAT1 and WM/impulsivity in this case) is likely to exist, but fails to reach significance for power reasons (MacKinnon and Fairchild 2009). This is probably true in the present paper, as 1) the 9/10 DAT1 group did demonstrate numerically superior performance on all of the tested behaviors, though none reached significance, and 2) while direct relationships between DAT1 and these behaviors have been shown in other populations including children (Stollstorff et al. 2010) and ADHD adults (Gizer and Waldman 2012), other studies in healthy adults (Blanchard et al. 2011) or ADHD children (Rommelse et al. 2008) show no effects of the gene on executive function. Confusingly, a few studies have associated the 10-repeat allele with reduced impulsivity (Forbes et al. 2009) or fewer ADHD symptoms (Brown et al. 2011). This may indicate either 1) that the DAT1 polymorphism affects behavior only in children and ADHD individuals (and inconsistently at that), but has no effect on healthy adults, or 2) that there is a noisy relationship between DAT1 and behavior. In the present study, this noisy relationship may be due to many different possible factors, which likely fails to reach significance in the present population of healthy adults because their behavioral performance is so high that it reduces available variance. The finding of an indirect mediating pathway from DAT1 to striato-frontal connectivity to executive function may thus be cautiously interpreted as supporting the presence of causal links between DAT1, striato-frontal connectivity, and executive function.
These results have important implications for interpreting previous work demonstrating effects of the DAT1 gene on PFC function. DAT1 is highly expressed in the striatum but only weakly expressed in PFC (Hall et al. 1999; Madras et al. 2005; Sasaki et al. 2012), indicating that effects of DA signaling driven by DAT1 genotype differences should manifest primarily in striatum, not in PFC. However, previous work has shown that, compared to 9-repeat carriers, 10/10 homozygotes had both reduced PFC activation (Bertolino et al. 2006, 2009; Caldú et al. 2007; Stollstorff et al. 2010) and elevated PFC-based cortico-cortico connectivity (Gordon et al. 2012a) during WM. By demonstrating that DAT1 genotype influences connectivity between striatum and PFC, the present results provide a likely interpretation for those previous findings: that DAT1 genotype differences directly affect striatal DA signaling, which in turn influences connectivity between striatum and PFC, thus indirectly affecting PFC function. This idea—that DAT1 effects on PFC are causally “downstream” of direct effects on caudate—should be investigated in future studies examining effective, or directed, connectivity influences using techniques such as dynamic causal modeling, which can test the hypothesis that the caudate influence over the PFC is different in 9/10 than in 10/10 individuals.
The present findings demonstrating relationships between the 10/10 DAT1 genotype, lower striato-frontal connectivity, and reduced executive function may be highly relevant to the study of ADHD, as previous work has established that each of these 3 factors is linked to ADHD. Inheriting 2 copies of the 10-repeat allele of DAT1 confers risk for ADHD (Yang et al. 2007), a disorder that is defined in part by increased impulsivity and demonstrates reduced WM (Willcutt et al. 2005), and which further demonstrates reduced striato-frontal connectivity (Rubia et al. 2009; Cubillo et al. 2010). Our observation of weak striato-frontal connectivity associated with reduced executive function in individuals who are healthy but have a genetic polymorphism linked to ADHD raises the possibility that resting-state striato-frontal connectivity may be an endophenotype for the reduced executive function observed in ADHD individuals, as it is consistent with primary criteria for an endophenotype as described in (Gottesman and Gould 2003): 1) Association of the endophenotype with a psychiatric disorder (ADHD, as shown in Yang et al. 2007); 2) Heritability of the endophenotype (association with the 10/10 genotype); and 3) State-independence of the endophenotype (its presence in healthy, unaffected individuals). If striato-frontal connectivity does function as an endophenotype in this fashion, it may be relevant not only for the study of ADHD, but also for investigations of variation in executive function within the general population, as this circuit (and genes which alter the circuit) would be expected to influence executive function in any population. The endophenotype status of striato-frontal functional connectivity should be established more definitively by future work investigating whether linkages between DAT1, striato-frontal connectivity, and executive function also exist in ADHD individuals.
Interestingly, DAT1 genotype had strong effects on caudate-seeded connectivity, but no effects on putamen-seeded connectivity. DAT1 is known to be expressed similarly in caudate and putamen (Hall et al. 1999; Sasaki et al. 2012), and pharmacologic DA manipulations affect putamen-PFC connectivity (Cohen et al. 2007; Nagano-Saito et al. 2008). However, other pharmacological work failed to show any DA-related effects on putamen-PFC connectivity (Kelly et al. 2009; Cole et al. 2013; Klostermann et al. 2012). Notably, the studies which observed DA-related effects on putamen-PFC connectivity were conducted during performance of a cognitive task (Cohen et al. 2007; Nagano-Saito et al. 2008), while 2 of the studies which failed to observe putamen-PFC effects were conducted during the resting state (Kelly et al. 2009; Cole et al. 2013), like the present study. We speculate that putamen-PFC connectivities may be affected by differences in DA function only when connectivity is measured during tasks requiring DA release rather than during the resting state.
This study tested specific hypotheses about effects of DAT1 genotype on striato-frontal functional connectivity and executive function because DAT1 has shown small but statistically significant associations with the ADHD phenotype (Yang et al. 2007), which includes disrupted executive function (Willcutt et al. 2005). However, it is known that striato-frontal circuits are also affected by a polymorphism in the DRD2-Taq1A gene (Cohen et al. 2007; Stelzel et al. 2010), and it is very likely that it may also be affected by polymorphisms in other genes that influence striatal DA signaling, such as those coding for other striatal DA receptors (e.g., DRD3) or other enzymes which end DA action (e.g., MAO). These various genes may combine to influence striato-frontal circuits in complex ways, either additive or interactive. By establishing the specific impact of the DAT1 gene on striato-frontal connectivity and behavior, the present findings provide a first step that should ultimately lead towards testing multiple simultaneous gene effects.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by National Institutes of Health (grants R03MH86709 to C.J.V., F31MH088066 to E.M.G., R01NS029525 to J.M.D., and R24HD050846-06 and UL1RR031988 to Children's National Medical Center).
Supplementary Material
Notes
The authors thank Megan Norr for her contributions to subject recruitment and testing. Conflict of Interest: None declared.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci (Regul Ed) 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, Caforio G, Sinibaldi L, Ursini G, Popolizio T, et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J Neurosci. 2009;29:1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Chamberlain SR, Roiser J, Robbins TW, Müller U. Effects of two dopamine-modulating genes (DAT1 9/10 and COMT Val/Met) on N-back working memory performance in healthy volunteers. Psychol Med. 2011;41:611–618. doi: 10.1017/S003329171000098X. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain—conjunction analyses of the stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bellander M, Fürth D, Karlsson S, Bäckman L. Working memory plasticity modulated by dopamine transporter genotype. Neurosci Lett. 2009;467:117–120. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox (abstract) NeuroImage. 2003;16 (CD–ROM) [Google Scholar]
- Brown AB, Biederman J, Valera E, Makris N, Doyle A, Whitfield-Gabrieli S, Mick E, Spencer T, Faraone S, Seidman L. Relationship of DAT1 and adult ADHD to task-positive and task-negative working memory networks. Psychiatry Res. 2011;193:7–16. doi: 10.1016/j.pscychresns.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MÁ, Serra-Grabulosa JM, Junqué C. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Ward BD, Li W, Antuono P, Li S. A method to determine the necessity for global signal regression in resting-state fMRI studies. Magn Reson Med. 2012;68:1828–1835. doi: 10.1002/mrm.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Krohn-Grimberghe A, Elger CE, Weber B. Dopamine gene predicts the brain's response to dopaminergic drug. Eur J Neurosci. 2007;26:3652–3660. doi: 10.1111/j.1460-9568.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- Cole DM, Oei NYL, Soeter RP, Both S, van Gerven JMA, Rombouts SARB, Beckmann CF. Dopamine-Dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23:1509–1516. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8 doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59:898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, FitzGerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4:192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Davis FC, Knodt AR, Sporns O, Lahey BB, Zald DH, Brigidi BD, Hariri AR. Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex. 2013;23:1444–1452. doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E, Brown S, Kimak M, Ferrell R, Manuck S, Hariri A. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Waldman ID. Double dissociation between lab measures of inattention and impulsivity and the dopamine transporter gene (DAT1) and dopamine D4 receptor gene (DRD4) J Abnorm Psychol. 2012;121:1011–1023. doi: 10.1037/a0028225. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Stollstorff M, Devaney JM, Bean S, Vaidya CJ. Effect of dopamine transporter genotype on intrinsic functional connectivity depends on cognitive state. Cereb Cortex. 2012a;22:2182–2196. doi: 10.1093/cercor/bhr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Stollstorff M, Vaidya CJ. Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Hum Brain Mapp. 2012b;33:1536–1552. doi: 10.1002/hbm.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J-C, Farde L, Sedvall G. Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. NeuroImage. 1999;9:108–116. doi: 10.1006/nimg.1998.0366. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc B Biol Sci. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Kelly C, De Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-Dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, et al. The world health organization adult ADHD self-report scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Klostermann EC, Braskie MN, Landau SM, O'Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol Aging. 2012;33:623.e15–623.e24. doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Dresel SH, Krause K-H, La Fougère C, Zill P, Ackenheil M. Striatal dopamine transporter availability and DAT-1 gene in adults with ADHD: no higher DAT availability in patients with homozygosity for the 10-repeat allele. World J Biol Psychiatry. 2006;7:152–157. doi: 10.1080/15622970500518444. [DOI] [PubMed] [Google Scholar]
- Li CR, Yan P, Sinha R, Lee T-W. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ. Current directions in mediation analysis. Curr Dir Psychol Sci. 2009;18:16–20. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/noGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore AB, Li Z, Tyner CE, Hu X, Crosson B. Bilateral basal ganglia activity in verbal working memory. Brain Lang. 2013;125:316–323. doi: 10.1016/j.bandl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazy RK, Xie J, Miller K, Beckmann CF, Smith SM. Spectral characteristics of resting state networks. Prog Brain Res. 2011;193:259–276. doi: 10.1016/B978-0-444-53839-0.00017-X. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, Arias-Vásquez A, Buschgens CJM, Fliers E, Faraone SV, Buitelaar JK, Sergeant JA, Franke B, Oosterlaan J. A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1536–1546. doi: 10.1002/ajmg.b.30848. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad A-M, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Saad Z, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox R. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Ito H, Kimura Y, Arakawa R, Takano H, Seki C, Kodaka F, Fujie S, Takahata K, Nogami T, et al. Quantification of dopamine transporter in human brain using PET with 18F-FE-PE2I. J Nucl Med. 2012;53:1065–1073. doi: 10.2967/jnumed.111.101626. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in D2 receptor density. J Neurosci. 2010;30:14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollstorff M, Foss-Feig J, Cook EH, Stein MA, Gaillard WD, Vaidya CJ. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage. 2010;53:970–977. doi: 10.1016/j.neuroimage.2009.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P-C, Hsieh J-C, Li C-T, Bai Y-M, Su T-P. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. NeuroImage. 2012;59:238–247. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Van De Voorde S, Roeyers H, Verté S, Wiersema JR. Working memory, response inhibition, and within-subject variability in children with attention-deficit/hyperactivity disorder or reading disorder. J Clin Exp Neuropsychol. 2010;32:366. doi: 10.1080/13803390903066865. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schouwenburg MR, Den Ouden HEM, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data. 2000. Available from: URL http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf .
- White NM. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav Brain Res. 2009;199:3–23. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yang B, Chan RCK, Jing J, Li T, Sham P, Chen RYL. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.