Abstract
Complex mental activity induces improvements in cognition, brain function, and structure in animals and young adults. It is not clear to what extent the aging brain is capable of such plasticity. This study expands previous evidence of generalized cognitive gains after mental training in healthy seniors. Using 3 MRI-based measurements, that is, arterial spin labeling MRI, functional connectivity, and diffusion tensor imaging, we examined brain changes across 3 time points pre, mid, and post training (12 weeks) in a randomized sample (n = 37) who received cognitive training versus a control group. We found significant training-related brain state changes at rest; specifically, 1) increases in global and regional cerebral blood flow (CBF), particularly in the default mode network and the central executive network, 2) greater connectivity in these same networks, and 3) increased white matter integrity in the left uncinate demonstrated by an increase in fractional anisotropy. Improvements in cognition were identified along with significant CBF correlates of the cognitive gains. We propose that cognitive training enhances resting-state neural activity and connectivity, increasing the blood supply to these regions via neurovascular coupling. These convergent results provide preliminary evidence that neural plasticity can be harnessed to mitigate brain losses with cognitive training in seniors.
Keywords: aging, brain plasticity, CBF, cognitive training, MRI
Introduction
The world's aging population is growing disproportionately; the lifespan is being extended dramatically. Research is needed to determine whether progress can be made in lengthening human cognitive span to more closely match extended life expectancy. Efforts focused on discovering ways to strengthen cognitive capacity to reason, to make informed decisions, and support living independently may be particularly beneficial (Hertzog et al. 2009). Extensive evidence has documented continuous age-related cognitive declines, even in the absence of a diagnosed dementia (Cepeda et al. 2001; Mahncke et al. 2006; Mattay et al. 2006; Kennedy et al. 2009; Cappell et al. 2010). Concomitantly, age-related brain losses are represented in structural shrinkage, loss of white matter integrity, and reduced functional connectivity, preferentially affecting frontal and temporal networks (Kennedy et al. 2009; Cappell et al. 2010; Hafkemeijer et al. 2012). Until recently, age-related cognitive declines were viewed as a consequence of living longer rather than a brain condition to be mitigated or solved.
One key issue that warrants serious consideration is the extent to which brain plasticity can be induced following strategy-based cognitive training. The mechanisms of brain plasticity (i.e., functional and structural changes) that support cognitive gains with training remain poorly understood. Advances in magnetic resonance imaging (MRI) are elucidating a broad spectrum of neural mechanisms that underpin brain changes—whether in decline or gain (Draganski and May 2008). Studies to date have largely characterized brain changes that represent aging brain in decline or precede onset of dementia (Kennedy et al. 2009; Lu et al. 2011). For instance, a reduction in global cerebral blood flow (CBF) has been reported with increased age as measured by pseudocontinuous arterial spin labeling (pCASL) MRI (Lu et al. 2011). Also, age-related reductions in functional and structural connectivity have been reported, as measured by functional connectivity MRI (fcMRI) and diffusion tensor imaging (DTI) MRI respectively (Kennedy et al. 2009; Hafkemeijer et al. 2012). The aforementioned findings have been linked to cognitive declines manifested as early as 3 years prior to cognitive decline (Hafkemeijer et al. 2012; Schlee et al. 2012). Whereas evidence is still equivocal, cognitive training has been shown to induce benefits which have been measured predominantly by cognitive performance (Ball et al. 2002; Mahncke et al. 2006; Draganski and May 2008; Willis and Schaie 2009; Landau et al. 2012). A growing trend suggests that strategy-based cognitive training, in particularly, may have a beneficial impact on preventing and potentially reversing age-related brain decline (Valenzuela et al. 2007; Boyke et al. 2008; Anand et al. 2011). In a prior study, Anand et al. (2011) identified improved ability on synthesized thinking and generalized gains to frontally mediated processes of switching and verbal fluency in cognitively healthy adults, mean age 75 years, using the same strategy-based cognitive training as incorporated in the present study. However, few studies have incorporated direct measures of change in brain function and structure. The studies that exist show training-related brain changes in only one aspect of measurement (e.g., structural/functional connectivity or activation patterns) (Nyberg et al. 2003; Mozolic et al. 2010; Brehmer et al. 2011).
This investigation addressed whether neuroimaging methodologies of resting-state brain mechanisms could be utilized to characterize coherent patterns of brain change following cognitive training (Nyberg et al. 2003; Mozolic et al. 2010). Review of fcMRI studies suggests that resting-state functional connectivity may be informative in clinical research not only at a group level but also at an individual level as an index of change (Biswal et al. 2010). Assessment of training-induced brain alterations at rest is informative given that the brain's resting energy needs (20% of the body's energy) are much greater than task-evoked neural activity (representing only 5% of the total energy use) (Raichle and Mintun 2006; Fox and Greicius 2010). Resting-state studies reportedly have 3 times the signal to noise ratio compared with conventional task-based activation studies (Fox and Greicius 2010). Additionally, cognitive training has been shown to induce significant increases in resting-state functional connectivity in young adults when compared with nonintervention controls (Takeuchi et al. 2012). Evidence, while equivocal, seems to suggest that a higher level of resting connectivity across different brain networks and CBF may be associated with higher cognitive performance (Hampson et al. 2006; Xu et al. 2007; Takeuchi et al. 2012); whereas lower connectivity has been linked to lower cognitive function (Li et al. 2002; Sorg et al. 2007). In addition to the potential to achieve increases in functional connectivity and CBF in brain resting-state, cognitive training may serve to enhance other energy-consuming neural components such as increased concentration of neurotransmitter receptors, greater rate of turnover of cellular proteins, enzymes, member lipids, and greater axoplasmic transport, according to the “energy budget” of the brain (Attwell and Laughlin 2001).
The key purposes of this study were to elucidate the neurobiology of resting-state brain changes associated with complex mental training in cognitively healthy seniors when compared with a wait-list control group. This research advances prior evidence of cognitive gains from complex mental training by careful study of changes in brain function and structure (Anand et al. 2011; Vas et al. 2011). The study examined training-induced brain changes and timing across a broad array of sensitive brain measurements at rest; specifically, using CBF measured by pCASL MRI, functional connectivity of gray matter using fcMRI and measures of structural connectivity by employing DTI MRI to measure changes in the integrity of white matter tracts. We proposed that complex cognitive training would result in higher CBF and functional connectivity, in 2 separate but coordinated brain networks, central executive network (CEN) and default mode network (DMN), as well as related structural changes in cognitively healthy older adults. Finally, we were interested in the correspondence between significant brain blood flow and cognitive changes.
Materials and Methods
Participants
A total of 37 cognitively normal adults (mean age = 62.9 ± 3.6; 56–71 years of age) were randomized to 2 different groups: wait-list control and cognitive training. All participants underwent Telephone Interview of Cognitive Status-Modified (TICS-M) to screen for dementia, Montreal Cognitive Assessment (MoCA) to detect early cognitive impairment, Beck Depression Inventory-II (BDI) to screen for depressive symptoms, and complete medical, physical, and laboratory assessments by a physician to ensure good general health. The criteria for inclusions were no history of neurological or psychiatric conditions, normal IQ range, native English speakers, and minimum of high school diploma. Exclusionary criteria included: MR scanning contraindications, cognitive status (TICS-M < 28 and MoCA < 26), depression indication (BDI > 14), left-handedness, increased body mass (BMI > 40,). Written informed consent was obtained from all subjects in accordance with the Institutional Review Board (IRB) of our academic institutions: The University of Texas at Dallas, the University of Texas Southwestern Medical Center, and the Cooper Institute.
Complex Cognitive Training Program
The training group underwent an evidenced-based, manualized cognitive training program referred to as gist reasoning (Anand et al. 2011; Vas et al. 2011). Gist reasoning training is strategy-based rather than content specific and entails a systematic use of 3 cognitive processes including strategic attention, integrated reasoning, and innovation to process all types of data. The gist reasoning training involved top-down cognitive control of complex information that is maintained, manipulated and synthesized into abstracted meanings (Anand et al. 2011). Cognitive control processes entailed in gist reasoning have been associated with frontal lobe networks and nodes within both the CEN and DMN (Nichelli et al. 1995; Chapman et al. 2005; Chen et al. 2006). Specifically, the program trained individuals to continually synthesize meanings and goals (i.e., gist reasoning) integral to information encountered in everyday life across a multitude of contents (e.g., medical information, investment information, movies, lectures, newspaper articles, travel highlights). Training also involved practice of innovative thinking by generating diverse interpretations as well as a wide variety of ways to approach or solve a task at hand, whether work or leisure related. Participants were taught to consolidate and incorporate the 3 cognitive processes as often as possible within the context of their own life activities and goals, whether during training, in real life, or in one's own internal thought processes, to train a habit of thinking about information and tasks at hand. The training was delivered by a trained expert in small groups (n ≤ 5) of one 1-h session per week (hours = 12). Additionally, each participant worked individually at home without supervision for 2 additional 1-h sessions per week for 12 weeks (hours logged = 24). Record logs of time and assignment completion were kept for the individual work with feedback from trainers on performance.
MRI Acquisition
MRI investigations were performed on a 3 Tesla MR system (Philips Medical System, Best, The Netherlands). A body coil was used for radiofrequency (RF) transmission and an 8-channel head coil with parallel imaging capability was used for signal reception. We used different MRI techniques to investigate changes at rest: a pCASL sequence was used to measure CBF (Aslan et al. 2010), fcMRI was used to assess functional connectivity of the brain (Raichle et al. 2001), and DTI MRI to provide an assessment of structural connectivity between brain regions via white matter tracts (Mori and Barker 1999). Additionally, a high-resolution T1-weighted image was acquired as an anatomical reference. The details of imaging parameters and their processing techniques are provided below:
Imaging parameters for pCASL experiments were: single-shot gradient-echo EPI, field-of-view (FOV) = 240 × 240, matrix = 80 × 80, voxel size = 3 × 3 mm2, 27 slices acquired in ascending order, slice thickness = 5 mm, no gap between slices, labeling duration = 1650 ms, time interval between consecutive slice acquisitions = 35.5 ms, TR/TE = 4020/14 ms, SENSE factor 2.5, number of controls/labels = 30 pairs, RF duration = 0.5 ms, pause between RF pulses = 0.5 ms, labeling pulse flip angle = 18°, bandwidth = 2.7 kHz, echo train length = 35, and scan duration 4.5 min. The post labeling delay was 1525–2448 ms for slice #1 through 27, respectively. Using the current protocol and labeling location, our previous technical study had measured an arterial transit time of 938 ms in young individuals (Liu et al. 2011). Therefore, even considering that the arterial transit time in elderly individuals can be 20% longer and be more variable, the post labeling delay used is expected to be sufficient for the labeled bolus to reach the imaging slices. The sequence parameters for fcMRI were FOV = 220 × 220, matrix = 64 × 64, slice thickness = 4 mm, no gap between slices, voxel size = 3.44 × 3.44 × 4 mm3, 36 axial slices, TR/TE = 2000/30 ms, flip angle = 70°, 120 image volumes, and scan duration = 4 min. The DTI sequence parameters were single-shot spin-echo EPI, FOV = 224 × 224 mm2, matrix = 128 × 128, slice thickness = 3 mm (includes 1 mm slice gap), voxel size = 1.75 × 1.75 × 3 mm3, 50 slices, TR/TE = 4410/51 ms, SENSE factor 2.5, 30 gradient-encoding directions with a b value of 1000 s/mm2, and scan duration = 3.7 min. The high-resolution T1-weighted image parameters were magnetization prepared rapid acquisition of gradient-echo (MPRAGE) sequence, TR/TE = 8.3/3.8 ms, shot interval = 2100 ms, inversion time = 1100 ms, flip angle = 12°, 160 sagittal slices, voxel size =1 × 1 × 1 mm3, FOV = 256 × 256 × 160 mm3, and duration 4 min.
MR Data Processing
PCASL image series were realigned to the first volume for motion correction (SPM5's realign function, University College London, UK). An in-house MATLAB (Mathworks, Natick, MA, USA) program was used to calculate the difference between averaged control and label images. Then, the difference image was corrected for imaging slice delay time to yield CBF-weight image, which was normalized to the Brain template from Montreal Neurological Institute (MNI). This procedure was carried out using a nonlinear elastic registration algorithm, Hierarchial Attribute Matching Mechanism for Elastic Registration (HAMMER, University of Pennsylvania, PA, USA). The HAMMER algorithm detects and corrects for region-specific brain atrophy which is commonly seen in elderly subjects. Last, the absolute CBF was estimated by using Alsop and Detre's equation in the units of mL blood/min/100 g of brain tissue (Alsop and Detre 1996). This method is represented by the following equation:
where fpCASL is the blood flow value at voxel (x,y,z) obtained from pCASL in ml blood/min/100 g brain; α is the labeling efficiency (0.86); λ is the blood-brain partition coefficient (0.98 mL/g); δ is the arterial transit time of blood from the tagging plane to the imaging slice (2 s); w is the delay between the end of labeling and the start of acquisition (1.525 s); T1 is the brain tissue T1 (1.165 s); T1a is the T1 of arterial blood (1.624 s); T1RF is the T1 in the presence of off-resonance irradiation (0.75 s); is the value of equilibrium magnetization of brain tissue, which was obtained from manual ROI drawing of midaxial slice of the control image and accounting for the saturation recovery of the magnetization (T1 = 1.165 s, recovery time = labeling time + post labeling delay of this slice).
The whole-brain blood flow values were calculated by averaging all the voxels in the brain. In voxel-based analyses (VBA), the individual CBF maps were spatially smoothed (with full-width half-maximum [FWHM] of 4 mm) to account for small differences in sulci/gyri location across subjects. For cluster extent inference, we used a program based on AlphaSim, called 3dClustsim in AFNI (NIMH Scientific and Statistical Computing Core, Bethesda, MD, USA), which controls false-positive activation clusters over the set of all activation clusters throughout the whole-brain volume. We refer to this procedure in Results as familywise error correction (FWE corrected). For cluster inference, we tested the volume of clusters which is conditional on 2 criteria: smoothness of the voxel map and cluster-defining threshold. We estimated the smoothness to be 10 mm FWHM (inherent smoothness plus additional smoothness applied—described above) and set the cluster-defining threshold to the 99.5th percentile of t-statistic distribution. Then, the minimum cluster size of 238 voxels (1904 mm3) yielded a FWE-corrected significance level of 0.05.
Functional connectivity images were analyzed by using AFNI (NIMH Scientific and Statistical Computing Core, Bethesda, MD, USA) and in-house MATLAB scripts. The dataset was preprocessed with slice timing correction, motion correction (realignment), removal of the linear trend, transformation to standard Talairach space (matrix = 61 × 73 × 61, resolution = 3 × 3 × 3 mm3), and smoothing by a Gaussian filter with a FWHM of 6 mm. Next, the whole-brain functional connectivity was analyzed by parcellating the images into 70 anatomical regions per Automated Talairach Atlas Labels in AFNI software (Lancaster et al. 2000). Each region's signal time course was band-pass filtered (0.01–0.1 Hz) to keep only the appropriate frequency fluctuations. Both white matter and cerebrospinal fluid signals were regressed out using averaged signals from the white matter and the ventricles for each ROI. The cross-correlation coefficients (cc) between any possible pair of ROI time series were calculated (70 × 70 Matrix) for each subject at each time period. Next, the correlation matrix was transformed to a z-score matrix and then the upper triangular part of the matrix was average to calculate the whole-brain functional connectivity (Wang et al. 2009).
In the network analysis of functional connectivity, the preprocessed images were band-pass filtered (0.01–0.1 Hz) on a voxel-by-voxel basis to keep only the appropriate frequency fluctuations. Next, the signals in white matter and cerebrospinal fluid were regressed out using averaged signals from the white matter and the ventricles from each voxel time series. The fcMRI data were analyzed using a seed-based approach by choosing bilateral posterior cingulate [±10 −56 −12] and dorsolateral prefrontal [±45 +16 +45] cortices based on MNI coordinates (size = 0.73 cm3) (Sridharan et al. 2008; Xu et al. 2011). The cross-correlation coefficient between these seed voxels and all other voxels was calculated to generate a correlation map. Then, the correlation maps were transformed to a z-score map using Fisher's inverse hyperbolic tangent transformation. An ROI analysis was performed based on 2 known DMN regions: posterior cingulate cortex (PCC) and middle frontal cortex (MFC) and 2 CEN regions: dorsolateral prefrontal cortex (composed of BA 9 and 46) and inferior parietal cortex. The functional ROIs were defined as follows: first, each region's anatomical region was defined based on Talairach Daemon database in AFNI. Then, a functional ROI was defined by choosing the top 200 voxels at each time point (i.e., T1, T2, and T3) and the intersection (i.e., common voxels) of the masks was calculated. Last, to assess the overlap between fcMRI and CBF regions, the fcMRI functional ROIs were then applied to each participant's CBF map.
DTI images were realigned and corrected for eddy current distortions using DTIstudio's AIR program (The Johns Hopkins University, Baltimore, MD). Next, tensor fitting and fractional anisotropy (FA) calculations were performed. The whole-brain FA average was calculated by thresholding the FA images at 0.25 and then averaging all the remaining voxels in the brain. In the tractography analysis, white matter tracts were constructed with minimum FA of 0.25 and maximum turning angle of 70°. The uncinate fasciculus (UF) tract was delineated via 2 techniques: manual and automatic tractography. In manual tractography, the left and right UF were delineated by drawing manual region-of-interests (ROI) per Wakana et al. (2007) method. In the automatic tractography, the CBF clusters were coregistered to each subject's native DTI space and used as an ROI to delineate fiber tract. Specifically, these regions were dilated twice in 26 directions to ensure the clusters were expanded into the white matter tissue. Last, an “AND” operation between the 2 clusters was performed and the resultant fiber was the left UF from the left middle temporal and left superior medial frontal gyri.
Neurocognitive Measures
A battery of neurocognitive measures was administered at 3 time periods, that is, baseline/pretraining (T1), midtraining week 6 (T2), and at end of training, week 12 (T3) for both control and training groups. Assessment of changes in trained functions included 3 randomized versions of the Test of Strategic Learning (TOSL) to measure the ability to synthesize global meanings. This measure was developed and tested to systematically quantify participants' capacity to abstract gist meanings from complex input (Anand et al. 2011; Vas et al. 2011). Measurements of untrained cognitive functions included tests of executive function, memory, and complex attention. Measures of executive function included: Daneman and Carpenter (working memory), “Trails B–Trails A” (switching), and WAIS-III Similarities (concept abstraction). Immediate memory was assessed with trial one of the California Verbal Learning Test-II (CVLT II) - 2 versions used alternatively over time intervals. Finally, complex attention was evaluated with the Delis Kaplan Executive Function System (DKEFS) Color Word Interference (selective attention) and Backward Digit Span. The results were Bonferroni corrected within neuropsychological domain and 1-tailed because of anticipated improvements in the neurocognitive measures of the training group.
Statistical Analysis
A general statistical linear model was applied to assess the contribution of cognitive training on neurocognitive, CBF, functional connectivity, and structural connectivity measures. The model included sessions (T1, T2, and T3), group status (cognitive training and control) and the interaction between these factors. Two variance components—one due to variability across subjects, and one due to variability in the same subject over time—were included to account for the different levels of variability and estimated by residual maximum likelihood (REML). We were primarily interested in how the groups differed across the training sessions, and we hypothesized that the cognitive training group would show an increase in mean measures (except Trails B–Trails A; since it is a timed measure and lower score represent faster performance), either by T2 or T3, relative to the control group. This hypothesis led to 2 orthogonal polynomial interaction contrasts: linear and quadratic. The linear interaction contrast tested whether the mean change between the groups increased monotonically from T1 to T3, and the quadratic interaction contrast tested whether the mean change between groups increased maximally at T2 before decreasing (either back to baseline or only partially) at T3.
Neural Correlate Analysis
Pearson correlations were tested to examine the relationship between the group mean change in voxel-wise CBF and the group mean change in neurocognitive measures. The results were reported as 1-tailed based on the anticipated changes in the positive direction in both domains.
Results
Participant Characteristics
All 19 control participants (CN) completed the neuropsychological assessments and all 18 cognitive training participants (CT) completed the training and neuropsychological assessments at each time point. However, several participants either did not complete all 3 time points of each MR protocol or had gross movement of >3 mm and >3°. As a result, the final MRI data analyses were conducted on the majority of the participants, shown in Table 1. No significant differences in age, gender, Intelligence Quotient (IQ), Montreal Cognitive Assessment (MoCA), Telephone Interview of Cognitive Status-Modified (TICS-M) were noted between groups (P > 0.05).
Table 1.
Subject characteristics and total number of subjects per group, assessments, and MRI technique (mean ± SD)
| Control | Cognitive training | |
|---|---|---|
| Age (years) | 64.0 ± 3.6 | 61.8 ± 3.3 |
| Gender (M/F) | 5/14 | 8/10 |
| IQ | 120.9 ± 10.5 | 121.6 ± 8.0 |
| MoCA | 28.2 ± 1.4 | 27.9 ± 1.4 |
| TICS-M | 29.6 ± 2.0 | 29.4 ± 2.2 |
| Participants (n) | ||
| Cognitive exams | 19 | 18 |
| pCASL MRI | 18 | 13 |
| fcMRI | 16 | 15 |
| DTI | 17 | 14 |
Notes: IQ, Intelligence Quotient; MoCA, Montreal Cognitive Assessment; TICS-M, Telephone Interview of Cognitive Status-Modified.
MRI Measurements
CBF was measured by pCASL MRI in both control and cognitive groups. The global CBF at T1 for both control and cognitive training groups were similar; 47.2 mL/100 g/min and 47.0 mL/100 g/min, respectively (P = 0.98). The cognitive training group's global CBF increased by 7.9% from T1 to T2 and remained elevated (7.9%) at T3. To evaluate which brain regions may have contributed to the CBF increase, we conducted a voxel-wise analysis. Figure 1 shows the VBA results between control and cognitive training groups, testing whether the CBF differences increase monotonically from T1 to T3 due to cognitive training or whether the CBF differences peak at T2. The cognitive training group showed a significant increase in blood flow at T3 in left middle temporal, left superior medial, left inferior frontal gyri compared with the control group. Additionally, the cognitive training group showed a peak increase at T2 in the inferior temporal gyrus, precuneus, and posterior cingulate gyrus compared with the control group. The control group did not show any significant changes at T2 or T3 in CBF compared with the cognitive training group. Table 2 summarizes these findings with a FWE rate maintained at 0.05 (P < 0.05, cluster volume ≥1904 mm3).
Figure 1.
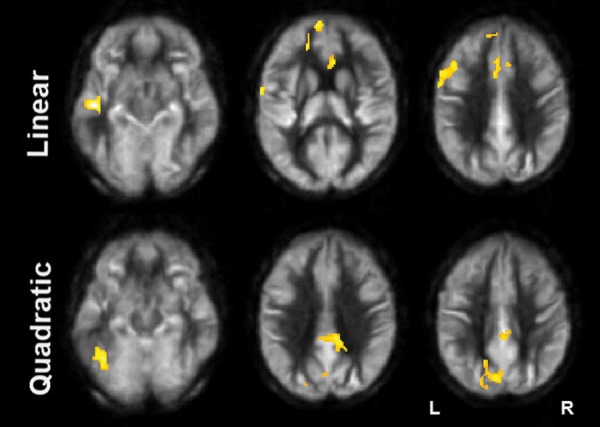
Results of CBF voxel-based comparison superimposed on an average CBF map of all participants for linear and quadratic interaction contrasts at P < 0.05 (FWE corrected) and k ≥ 1904 mm3. Note: The regions experiencing a linear increase are located in the frontal lobe while the regions experiencing a quadratic pattern of CBF increase are located in the posterior.
Table 2.
CBF regions that showed significant blood flow increase at rest in Cognitive Training compared with Control group
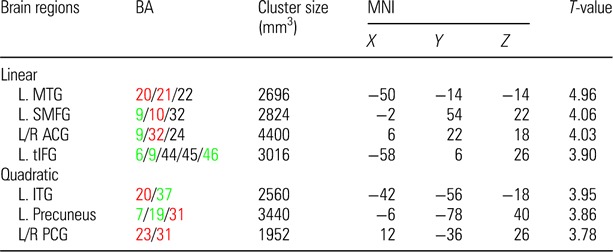 |
Notes: MTG, middle temporal gyrus; SMFG, superior medial frontal gyrus; ACG, anterior cingulate gyrus; tIFG, triangular part of inferior frontal gyrus; ITG, inferior temporal gyrus; PCG, posterior cingulate gyrus; L/R, left/right.
The colored Brodmann Areas (BA) represent DMN (red) and CEN (green) regions based on prior research.
Based on the increased regional CBF findings in Table 2, we characterized 2 distinct brain networks: default mode network (DMN) and central executive network (CEN) (Fox et al. 2005; Sridharan et al. 2008). Both DMN and CEN networks have been identified as integrated functional hubs that mediate higher order cognitive control processes such as embodied in gist reasoning. The components of these networks, respectively, were combined to summarize the relationship between regional blood flow and functional connectivity. Figure 2A shows the average functional connectivity maps (i.e., z-score maps) in the DMN and CEN for the cognitive training group, in which is seen qualitatively that functional connectivity increases over the training sessions—monotonically (T1-to-T3) in the DMN and with a maximum increase at T2 in the CEN. Moreover, the functional connectivity changes in DMN and CEN mirrored the blood flow changes in the same regions (Fig. 2B). Specifically, DMN's functional connectivity and CBF both increased monotonically (T1-to-T3) in the cognitive training group relative to controls (P = 0.04 and P = 0.01, respectively, Table 3). Also, CEN's functional connectivity and CBF showed similar peak increases at T2 in the cognitive training group relative to controls (P = 0.03 and P = 0.0005, respectively, Table 3). The whole-brain functional connectivity of the cognitive training group did not show significant temporal changes compared with the control group (Table 3).
Figure 2.
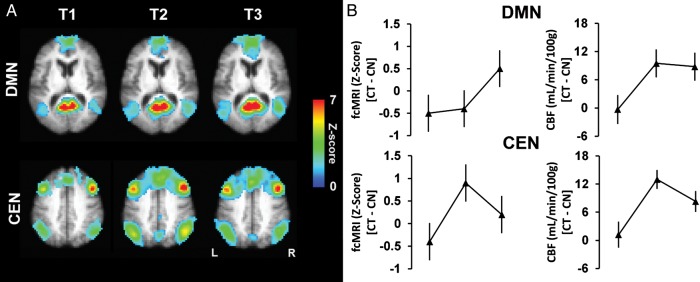
(A) The average functional connectivity maps (i.e., DMN and CEN) of the cognitive training group are overlaid on their average T1-weighted image. For illustration purposes, the z-score maps were arbitrarily thresholded (z-score ≥ 1, k ≥ 50) to qualitatively visualize the change in the intensity and cluster size. (B) Mean change in fcMRI z-scores (left column) and mean change in absolute CBF (right column) are shown for DMN and CEN across time periods. The DMN shows an increase in both mean fcMRI and mean aCBF from T1 to T3 for the cognitive training (CT) group relative to controls (CN). The CEN shows a maximal increase in both mean fcMRI and mean aCBF at T2 for the cognitive training group relative to controls.
Table 3.
Pre (T1), Mid (T2), and Post (T3) neuroimaging results at rest per MR technique (mean ± SEM)
| Control (CN) |
Cognitive training (CT) |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | Linear | Quadratic | |
| WB aCBF | 47.2 ± 1.1 | 44.3 ± 2.0 | 46.8 ± 2.0 | 47.0 ± 2.4 | 50.7 ± 2.3 | 50.7 ± 2.4 | 0.04 | 0.002 |
| WB fcMRI | 0.12 ± 0.02 | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.73 | 0.76 |
| WB FA | 0.425 ± 0.002 | 0.424 ± 0.002 | 0.424 ± 0.002 | 0.425 ± 0.002 | 0.424 ± 0.002 | 0.426 ± 0.002 | 0.37 | 0.75 |
| fcMRI (DMN) | 6.8 ± 0.4 | 7.1 ± 0.4 | 6.6 ± 0.4 | 6.3 ± 0.4 | 6.7 ± 0.4 | 7.1 ± 0.4 | 0.04 | 0.26 |
| aCBF (DMN) | 55.6 ± 2.8 | 49.9 ± 2.7 | 53.2 ± 2.7 | 55.3 ± 3.1 | 59.4 ± 3.0 | 62.0 ± 3.0 | 0.01 | 0.06 |
| fcMRI (CEN) | 4.0 ± 0.4 | 3.8 ± 0.4 | 4.1 ± 0.4 | 3.6 ± 0.4 | 4.7 ± 0.4 | 4.3 ± 0.4 | 0.23 | 0.03 |
| aCBF (CEN) | 47.0 ± 2.5 | 41.2 ± 1.8 | 44.2 ± 2.0 | 48.2 ± 2.8 | 54.2 ± 2.0 | 52.5 ± 2.2 | 0.01 | 0.001 |
| DTI MT (L. UF) | 0.502 ± 0.006 | 0.498 ± 0.006 | 0.495 ± 0.006 | 0.488 ± 0.007 | 0.495 ± 0.007 | 0.503 ± 0.006 | 0.003 | 0.45 |
| DTI MT (R. UF) | 0.470 ± 0.005 | 0.468 ± 0.005 | 0.476 ± 0.005 | 0.481 ± 0.006 | 0.481 ± 0.006 | 0.482 ± 0.006 | 0.53 | 0.42 |
| DTI AT (L. UF) | 0.498 ± 0.007 | 0.493 ± 0.007 | 0.493 ± 0.007 | 0.493 ± 0.008 | 0.494 ± 0.007 | 0.504 ± 0.007 | 0.02 | 0.41 |
Notes: WB, whole brain; aCBF, absolute cerebral blood flow in mL/min/100 g; fcMRI, functional connectivity MRI in z-score; DMN, default mode network; CEN, central executive network; DTI, diffusion tensor imaging; UF, uncinate fasciculus; MT, manual tractography of UF; AT, automatic tractography of UF; and L/R, left/right.
Linear and quadratic refer to orthogonal polynomial contrasts of mean group differences over the 3 training sessions.
Based on the monotonic blood flow increase in left middle temporal and left superior medial frontal gyri, we identified the white matter structure connecting the 2 regions called left UF. The UF tract was delineated both manually (Wakana et al. 2007) and automatically (shown in Fig. 3). In the manual tractography method, the FA of left UF showed a monotonic increase from T1 to T3 compared with the control group (P = 0.003). The FA of right UF of cognitive training group, however, did not show any significant temporal changes relative to the control group. In the automatic tractography method, the FA of the left UF similarly showed a monotonic increase in the cognitive training group compared with the control group, which signals better white matter integrity (P = 0.02). Whole-brain FA did not show significant differences as a result of training, as shown in Table 3.
Figure 3.
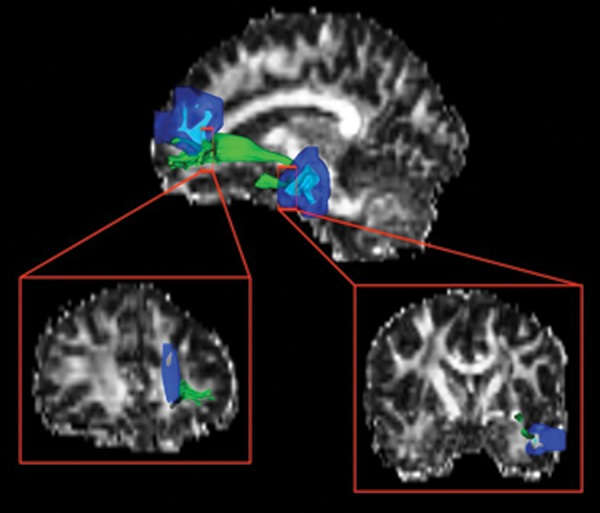
A representative participant's uncinate fasciculus (green) is overlaid on his fractional anisotropy map. The frontal and temporal ROIs (light blue) were expanded twice (dark blue) to ensure expansion into white matter.
Neurocognitive Measures
Table 4 shows the neuropsychological exam results per domain for control and cognitive training groups. No significant differences were noted in the baseline scores (i.e., T1) between the cognitive training and control groups. We found, however, that the cognitive training group significantly improved over time in 2 cognitive domains relative to the control group. The most complex cognitive domain, Strategic Reasoning, that is, ability to synthesize generalized meanings from lengthy textual input, and a measure of Executive Function, Similarities, that is, ability to abstract concepts showed a significant monotonic increase from T1 to T3 (P = 0.002 and P = 0.05, respectively).
Table 4.
Neuropsychological exam results (mean ± SEM)
| Control (CN) |
Cognitive training (CT) |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | Linear | Quad | |
| Strategic reasoning | ||||||||
| TOSL (rs) | 5.8 ± 0.5 | 4.4 ± 0.5 | 4.7 ± 0.3 | 4.7 ± 0.5 | 5.6 ± 0.5 | 5.6 ± 0.3 | 0.002 | 0.07 |
| Executive function | ||||||||
| WAIS-III similarities (ss) | 13.1 ± 0.6 | 13.2 ± 0.5 | 13.8 ± 0.4 | 12.9 ± 0.6 | 13.9 ± 0.5 | 14.9 ± 0.4 | 0.05 | 0.71 |
| Daneman Carpenter (rs) | 2.8 ± 0.2 | 3.1 ± 0.2 | 3.2 ± 0.2 | 2.8 ± 0.2 | 3.3 ± 0.2 | 3.1 ± 0.2 | 0.81 | 0.32 |
| Trails B–Trails A (rs) | 28.5 ± 3.6 | 27.3 ± 4.0 | 31.2 ± 3.3 | 29.9 ± 3.7 | 29.8 ± 4.2 | 27.4 ± 3.4 | 0.42 | 0.66 |
| Memory | ||||||||
| CVLT trial 1 (rs) | 7.5 ± 0.4 | 6.9 ± 0.4 | 7.7 ± 0.6 | 6.6 ± 0.4 | 6.2 ± 0.4 | 8.3 ± 0.6 | 0.12 | 0.39 |
| Complex attention | ||||||||
| DKEFS Color cond3 (ss) | 17.4 ± 0.7 | 14.6 ± 0.6 | 15.3 ± 0.8 | 16.1 ± 0.7 | 15.7 ± 0.6 | 14.7 ± 0.8 | 0.34 | 0.50 |
| Backward digit span (ss) | 8.0 ± 0.5 | 8.5 ± 0.5 | 8.7 ± 0.6 | 8.0 ± 0.5 | 8.9 ± 0.5 | 8.0 ± 0.6 | 0.43 | 0.27 |
Notes: TOSL, Test of Strategic Learning; WAIS, Wechsler Adult Intelligence Scale; CVLT, California Verbal Learning Test; DKEFS, Delis Kaplan Executive Function System; ss, standard score; rs, raw score.
CBF Correlates of Cognitive Changes
Mean changes between groups in TOSL (measure of ability to synthesize complex information) and WAIS-III Similarities (concept abstraction) were found to correspond to mean changes between groups in brain blood flow in particular brain regions. Table 5 summarizes the Pearson correlation tests of temporal changes between groups on TOSL and WAIS-III Similarities scores with corresponding contrasts of CBF regions reported in Table 2. The significantly improved cognitive changes corresponded with increased regional brain plasticity as measured by blood flow increases at rest in specific brain–behavior patterns.
Table 5.
Correspondence between mean change in cerebral blood flow and mean change in cognitive gains between the cognitive training and control groups
| Cognitive exam | CBF region | r | P-value |
|---|---|---|---|
| TOSL (Linear) | L. MTG (Linear) | 0.50 | 0.002 |
| TOSL (Linear) | L. ITG (Quad) | 0.47 | 0.004 |
| TOSL (Linear) | L. Precuneus (Quad) | 0.35 | 0.03 |
| TOSL (Linear) | L/R PCG (Quad) | 0.33 | 0.04 |
| TOSL (Quad) | L. MTG (Linear) | 0.39 | 0.02 |
| TOSL (Quad) | L/R ACG (Linear) | 0.32 | 0.04 |
| TOSL (Quad) | L. tIFG (Linear) | 0.30 | 0.05 |
| TOSL (Quad) | L. Precuneus (Quad) | 0.33 | 0.04 |
| WAIS-III (Linear) | L/R PCG (Quad) | 0.39 | 0.02 |
| WAIS-III (Linear) | L. MTG (Linear) | 0.33 | 0.04 |
| WAIS-III (Linear) | L. SMFG (Linear) | 0.31 | 0.05 |
Notes: MTG, middle temporal gyrus; ITG, inferior temporal gyrus; PCG, posterior cingulate gyrus; ACG, anterior cingulate gyrus; tIFG, triangular part of inferior frontal gyrus; SMFG, superior medial frontal gyrus; L/R, left/right.
Discussion
Our principal finding was that strategy-based cognitive training has the potential to reverse some of the negative consequences of age-related functional and structural brain losses. The goal of this study was to evaluate functional and structural mechanisms of brain change in response to a manualized cognitive training program in healthy older adults. The data serve to inform whether a specific training program could induce positive brain plasticity, to complement previously identified cognitive gains in aging adults across abstract thinking, concept formation, and other executive function measures (Anand et al. 2011; Vas et al. 2011). Specifically, we found that the training positively altered the intrinsic activity of the brain at rest as well as its structural connectivity. To our knowledge, this work provides the first convergent evidence of significant positive neurophysiological and neuroanatomical changes across 3 brain measures at rest, namely: CBF, functional, and structural connectivity.
Training-Induced Brain Plasticity
The findings of regained global/regional CBF, increased functional interdependence within brain networks, and improved white matter integrity are important given the evidence that significant negative plasticity occurs with aging even in the absence of disease pathology (Rypma and D'Esposito 2000; Raz et al. 2005; Lu et al. 2011). Negative plasticity is a term used to refer to the age-related cognitive decline and degradation in brain function that results from decreased brain use and weakened function of top-down neuromodulatory systems that underlie efficient learning and memory (Moller et al. 2006). The training-induced gains in the present study were identified in relevant brain measures/regions that heretofore have shown decline in older adults. These significant increases support reversals in age-related declines as reflected by good agreement between increases in both CBF and greater functional connectivity in both the default mode and the central executive networks, and improvement in white matter integrity (Rypma and D'Esposito 2000; Lu et al. 2011).
The present convergent findings provide insight into modifiable brain plasticity mechanisms in healthy older adults given strategy-based cognitive training. We propose that the cognitive training increased the overall cellular activity and metabolic rate in certain brain regions included in 2 networks, the DMN and CEN. These changes are manifested by the indirect markers of fcMRI and CBF presumably via the dogma of neurovascular coupling (i.e., higher metabolic demand, higher blood supply) since cellular activity and metabolic rate are difficult to measure in humans.
Increases in Global CBF
This study provides preliminary evidence that complex cognitive training may serve to increase whole-brain blood flow in healthy older adults. As a tightly regulated system, resting CBF is remarkably consistent (Raichle and Gusnard 2002). In contrast, regional CBF may increase or decrease during mental activation tasks due to redistribution of blood. The regional activation effect is transient and the local CBF elevation typically is restored to baseline level when the brain returns to resting state (Raichle and Gusnard 2002). The present results, showing a maintained increase in global CBF from T2 to T3, support the possibility of increasing resting whole-brain blood flow and achieving a new homeostasis level in the aging brain. Furthermore, the capacity to increase resting whole-brain blood flow as an outcome of complex mental training may have clinical implications in light of evidence that resting blood flow shows an age-related decline beginning in early adulthood (Lu et al. 2011). With further validation, CBF measures could potentially become useful markers of treatment effects at an individual level, indexing key physiologic brain changes to detect reversal of losses, preserved brain function, or continuing brain decline. We propose that greater connectivity and neural activity result in higher CBF as well as the reverse pattern where lower connectivity would be indicative of lower CBF demands.
Convergence in Functional Connectivity and Resting CBF
The current investigation identified increases in 2 relevant networks: default mode and central executive networks. Despite diminishing DMN's connectivity with age (Hafkemeijer et al. 2012), we have shown that cognitive training has the potential to reverse this age-related loss which is in agreement with prior investigations in younger adults (Takeuchi et al. 2012). We found monotonic increases from T1 to T3 in functional connectivity that was mirrored in resting CBF of DMN's regions, posterior cingulate, and medial prefrontal cortices. This increasing pattern in the DMN suggests that the potential benefit of training did not plateau within the time period of our study training. These results are consistent with recent work showing training-induced changes in both resting functional connectivity and resting CBF in the DMN following working memory training in young adults (Takeuchi et al. 2012).
The improvement of CEN's functional connectivity in the training group compared with the control group is a novel finding. Similar to DMN, the CEN's connectivity change was mirrored in its regional CBF, dorsolateral prefrontal (BA 9 and 46) and inferior parietal cortices. However, rather than a monotonic increase as identified in DMN, we found a peak increase at T2. It is important to note that the increases in connectivity and CBF at T2 do not return to baseline by T3. We interpret this quadratic pattern in CEN to mean that the training had a large onset effect, which then reached plateau or may even have slightly settled down, which happens when the neuronal changes are consolidated (e.g., forming new synapses rather than firing at higher frequency using existing synapses).This pattern of increased connectivity in the CEN was not found in young adults following working memory training by Takeuchi et al. (2012). The divergent patterns between studies in the CEN's intrinsic activity may be due to differences in the nature of the cognitive training, delineation of network nodes since these are currently undergoing refinement (Bressler and Menon 2010), or differences related to age effects. In sum, cognitive training induced changes in the DMN and CEN connectivity, accompanied by a similar temporal pattern of change in CBF.
Convergence of Regional CBF and Structural Connectivity
Another noteworthy pattern was reflected in the concomitant improvements in the left middle temporal and left superior medial frontal resting CBF and FA of left UF, which connects these 2 regions. Higher FA has been associated with higher white matter integrity (i.e., myelination) as water molecules diffuse more anisotropically along the axonal fibers (Teipel et al. 2010). It has been shown that healthy older adults have reduced FA in the intracortical projecting fiber tracts such as UF (Teipel et al. 2010), and it is associated with age-related cognitive decline (Charlton et al. 2006). The present evidence offers promise that some of the losses at the level of white matter tracts may be reversible with strategy-based cognitive training.
Whereas the functional changes in CBF and functional connectivity were apparent earlier in the time course at T2, the structural changes on DTI emerged later at T3. This time course is concordant with accumulating evidence that neurophysiologic plasticity is followed by structural plasticity occurring concomitantly with acquisition of new skills within a few weeks of cognitive training (Kennedy et al. 2009). The present findings confirm prior evidence that functional brain changes are more frequent and rapid than structural plasticity (Bruel-Jungerman et al. 2007). A similar sequence in brain markers of decline was characterized in individuals showing memory loss manifested first by decline in functional neuroimaging (PET-CBF) followed by structural brain changes (MRI) then cognitive decline (Clark et al. 2012).
Cognitive Plasticity and CBF Correlates
The present results support positive cognitive plasticity from the complex cognitive training—that is, synthesizing global meanings and concept abstraction. Previous literature revealed that synthesized thinking is related to cognitive control measures purported to activate the prefrontal networks included within the CEN and DMN; further complex strategy-based training generalizes to untrained cognitive control (Mahncke et al. 2006; Anand et al. 2011; Vas et al. 2011). There is growing motivation to investigate congruent evidence in both brain and cognitive mechanisms to more objectively assess training-induced gains (Takeuchi et al. 2012). The present study provides promising evidence that complex mental training may facilitate parallel gains in regional blood flow and cognition in older adults. The significant relationships between gains in complex synthesizing and increased CBF in both the left inferior frontal and left middle temporal gyri represent brain-cognition relationships that have been previously implicated (Seghier et al. 2010). Synthesizing abstracted and original meanings and conceptual integration have been previously linked to nodes of the CEN, but heretofore training-induced changes have not been examined according to parallel changes in both CBF and cognition. We propose that cognitively challenging application of strategies to improve information processing is supported by intrinsic brain mechanisms, but perhaps more importantly, mental exercise can bring about significant positive changes that modify brain systems (Bressler and Menon 2010).
Plausible Mechanisms of Brain Plasticity
To date, little is known about the temporal pattern of training-induced changes to intrinsic brain activity in 2 or more brain networks as measured by resting CBF as well as functional connectivity in aging. This pilot study represents a first step to investigate cognitive training changes in older adults over 3 time periods in 2 distinct brain networks, central executive network (CEN) and the default mode network (DMN) in 2 imaging methods measuring resting metabolic brain activity. We demonstrated that functional connectivity/CBF changes in CEN occurred more rapidly emerging at T2 and DMN increased continuously over time with greatest increases observed at T3 in the cognitive training group compared with controls, respectively. Comparing across imaging modalities, the present data suggest that CBF may be a more sensitive marker as the changes are detected globally whereas diffusion and functional connectivity measures only showed regional effects.
The cognitive training targeted the CEN, but may also have impacted the DMN. The gist reasoning training involved top-down cognitive control of complex information that is maintained, manipulated, and synthesized into abstracted meanings (Anand et al. 2011). Cognitive control processes have been associated with both CEN and DMN networks/nodes. The increases in connectivity and CBF in CEN did not return to baseline level instead remaining elevated above baseline at T3 suggesting that the gains were relatively stable. The nature of the cognitive gist training and its prior link to frontal brain networks within the CEN perhaps account for why increases in both connectivity and CBF appeared more rapidly in CEN at T2 (i.e., Week 6). The DMN did not plateau in the short training period and continued to increase from T1 to T3, raising the possibility that the DMN is also integrally involved and facilitates consolidation and maintenance of complex cognitive performance. The significant relation between improved brain metabolism and higher cognitive performance suggest that the increases in both CEN and DMN were positive as suggested by Hampson et al. (2006).
We propose a coherent mechanism by which complex mental exercise promotes brain plasticity and improves cognitive brain health. Specifically, we speculate that the cognitive training regime would leave a “footprint” on the resting brain such that greater resting-state spontaneous neural activity occurs in DMN and CEN regions. This increased activity is manifested as greater functional connectivity. The reason that the footprint is still present even though the training has ended could be because of aggregations of neurotransmitter receptors as a consequence of previous activations, which essentially prepare the brain to react “better” for future stimulus of similar type, even at resting state. Protein and lipid synthesis in the neuron may also be enhanced, which may serve to form/strengthen new synapses if the trained habits are maintained for a substantial period of time. All of these will cost energy, manifested as greater blood supply to these brain regions. The increased activities in dendrites/synapses/somas are likely to be accompanied by white matter changes such as increased myelin thickness, which may be the reason for a greater FA in DTI scans.
In sum, we propose that the multidimensional increases in brain function and structure are driven by task-dependent brain activation during training that increases resting brain network synchrony. Evidence reveals tight coupling of brain activity during active thinking that is mirrored to a large degree in intrinsic resting brain activity during internally driven cognitive thought processes (Smallwood et al. 2012). A precedent for a link between active cognitive training and increases in intrinsic resting activity in brain networks has been previously established (Takeuchi et al. 2012).
Limitations to Current Findings
The present findings need to be interpreted cautiously in light of a few key limitations. The first is small sample size. We propose that the convergent findings in 2 major networks—CEN and DMN identified in 2 brain mechanisms—both CBF and functional connectivity of intrinsic brain activity at rest, combined with enhanced white matter connectivity support the potential for a strategy-based cognitive training program to significantly enhance brain integrity in cognitive healthy seniors even given this small sample size. Moreover, the current cognitive gains replicate previous findings of generalized improvement in cognitive skills in healthy older adults (Anand et al. 2011).
Another limitation in our study was the potential for nonresponse bias due to missing data from those participants whose MRI data had to be excluded due to missing MRI scans or excessive movement. We investigated this possibility by comparing all of our neuropsychological contrasts of interest between the subjects with and without MRI scans in the cognitive training group. We found that the contrast estimates themselves exhibited evidence of a “missing-at-random” mechanism of data loss, wherein the actual signs of the estimates were evenly distributed, a finding that would not be expected from nonresponse bias. Interestingly, a significant linear interaction contrast was identified from the TOSL, exhibiting a mean increase in those without MRI scans relative to those with MRI scans in the cognitive training group (P = 0.048.) If a nonresponse bias exists, therefore, the bias may be reducing the magnitude of our reported findings, rather than inflating them.
Lack of an active control group may be a third limitation. However, we do not believe that the significant increases in intrinsic brain activity across large-scale brain networks could be accounted for simply by active stimulation alone. This assumption is based on a randomized trial study whereby a strategy-trained group was compared with an active-stimulation control group, with the latter not manifesting significant cognitive gains (Vas et al. 2011).
We were not able to address whether the increases in functional and structural brain connectivity or the cognitive gains were maintained at a distant time point (beyond 12 weeks) from the immediate training period. We expect that the training gains may persist at least for individuals who are continual strategy-adopters, because the training was embedded in real-life activities and prior work has shown maintained and even improved performance after training ceased (Anand et al. 2011; Vas et al. 2011).
Finally, our post labeling delay time may not be sufficient for individuals with hemodynamic delays related to cerebrovascular diseases (Macintosh et al. 2012). A longer post labeling delay time may be useful in alleviating this possible limitation, although at a potential cost of sensitivity.
Conclusions and Future Directions
The potential for individuals to improve their own cognitive brain health by habitually exercising high-order mental strategies is intriguing and is just beginning to be more fully exploited. Our work is in accord with prior claims that engaging in complex mental activity may offer promising ways to enhance brain integrity to promote successful cognitive aging. Valenzuela and colleagues claim that complex mental activity induces broad-based changes in brain function and structure (Valenzuela et al. 2007). In the present study, the training-dependent brain changes were likely achieved by top-down (i.e., high-level) strategy-based stimulation that are believed to activate large-scale brain circuitry that work interdependently within widely distributed brain regions (Bressler and Menon 2010). Specifically, the cognitive training involved considerable usage of lengthy language-based materials as well as rich visual stimuli where participants were required to construct novel and abstract interpretations. This processing required complex top-down information processing, implicating multiple brain regions within the DMN and CEN. Not only were functional connectivity and CBF increased to these regions but the specific white matter tract connecting certain regions was enhanced. Animal models of complex cognitive stimulation have shown to be protective against cognitive decline, diminishing brain amyloid burden, and increasing hippocampal synaptic immunoreactivity (Cracchiolo et al. 2007). The present findings offer a promising complement to large-scale randomized trials where smaller trials may be informative and cost effective, particularly as a first run trial, when they incorporate multiple levels of the neurobiological mechanisms of brain and cognitive plasticity in well-defined populations. Clarifying both the brain and cognitive plasticity changes in response to strategy-based mental training will elucidate the neurogenerative potential in the cognitively healthy aging brain.
Funding
This work was supported by a grant from the National Institute of Health (RC1-AG035954, R01-NS067015, R01-AG033106) and by grants from the T. Boone Pickens Foundation, the Lyda Hill Foundation, and Dee Wyly Distinguished University Endowment. Funding to pay the Open Access publication charges for this article was provided by funds from the Dee Wyly Distinguished University Endowed Chair held by Sandra Bond Chapman.
Notes
Conflict of Interest: None declared.
References
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Anand R, Chapman SB, Rackley A, Keebler M, Zientz J, Hart J., Jr Gist reasoning training in cognitively normal seniors. Int J Geriatr Psychiatry. 2011;26:961–968. doi: 10.1002/gps.2633. [DOI] [PubMed] [Google Scholar]
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63:765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, et al. Advanced Cognitive Training for I, Vital Elderly Study G. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y, Rieckmann A, Bellander M, Westerberg H, Fischer H, Backman L. Neural correlates of training-related working-memory gains in old age. Neuroimage. 2011;58:1110–1120. doi: 10.1016/j.neuroimage.2011.06.079. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. Neuroscientist. 2007;13:492–505. doi: 10.1177/1073858407302725. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- Chapman SB, Bonte FJ, Wong SB, Zientz JN, Hynan LS, Harris TS, Gorman AR, Roney CA, Lipton AM. Convergence of connected language and SPECT in variants of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2005;19:202–213. doi: 10.1097/01.wad.0000189050.41064.03. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Chen AJ, Abrams GM, D'Esposito M. Functional reintegration of prefrontal neural networks for enhancing recovery after brain injury. J Head Trauma Rehabil. 2006;21:107–118. doi: 10.1097/00001199-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Clark VH, Resnick SM, Doshi J, Beason-Held LL, Zhou Y, Ferrucci L, Wong DF, Kraut MA, Davatzikos C. Longitudinal imaging pattern analysis (SPARE-CD index) detects early structural and functional changes before cognitive decline in healthy older adults. Neurobiol Aging. 2012;33:2733–2745. doi: 10.1016/j.neurobiolaging.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracchiolo JR, Mori T, Nazian SJ, Tan J, Potter H, Arendash GW. Enhanced cognitive activity—over and above social or physical activity—is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem. 2007;88:277–294. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, Rombouts SA. Imaging the default mode network in aging and dementia. Biochim Biophys Acta. 2012;1822:431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychol Sci Publ Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O'Neil JP, Wilson RS, Jagust WJ. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol. 2012;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Liu P, Uh J, Lu H. Determination of spin compartment in arterial spin labeling MRI. Magn Reson Med. 2011;65:120–127. doi: 10.1002/mrm.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh BJ, Marquardt L, Schulz UG, Jezzard P, Rothwell PM. Hemodynamic alterations in vertebrobasilar large artery disease assessed by arterial spin-labeling MR imaging. Am J Neuroradiol. 2012;33:1939–1944. doi: 10.3174/ajnr.A3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci USA. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Moller A, Chapman S, Lomber S. Reprogramming the brain. Amsterdam: Elsevier; 2006. [Google Scholar]
- Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. Anat Rec. 1999;257:102–109. doi: 10.1002/(SICI)1097-0185(19990615)257:3<102::AID-AR7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Front Hum Neurosci. 2010;4:16. doi: 10.3389/neuro.09.016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R. Where the brain appreciates the moral of a story. Neuroreport. 1995;6:2309–2313. doi: 10.1097/00001756-199511270-00010. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, Backman L. Neural correlates of training-related memory improvement in adulthood and aging. Proc Natl Acad Sci USA. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci USA. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Schlee W, Leirer V, Kolassa IT, Weisz N, Elbert T. Age-related changes in neural functional connectivity and its behavioral relevance. BMC Neurosci. 2012;13:16. doi: 10.1186/1471-2202-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci. 2010;30:16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Hashizume H, Sekiguchi A, Kotozaki Y, Nakagawa S, Miyauchi CM, Sassa Y, Kawashima R. Effects of working memory training on functional connectivity and cerebral blood flow during rest. Cortex. 2012 doi: 10.1016/j.cortex.2012.09.007. pii: S0010-9452(12)00291-2. doi: 10.1016/j.cortex.2012.09.007. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Breakspear M, Sachdev P. Complex mental activity and the aging brain: molecular, cellular and cortical network mechanisms. Brain Res Rev. 2007;56:198–213. doi: 10.1016/j.brainresrev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Vas AK, Chapman SB, Cook LG, Elliott AC, Keebler M. Higher-order reasoning training years after traumatic brain injury in adults. J Head Trauma Rehabil. 2011;26:224–239. doi: 10.1097/HTR.0b013e318218dd3d. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, Chen Z, Zhu C, He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. Cognitive training and plasticity: theoretical perspective and methodological consequences. Restor Neurol Neurosci. 2009;27:375–389. doi: 10.3233/RNN-2009-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart J, Jr, Yezhuvath US, Gu H, Yang Y, Lu H. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Antuono PG, Jones J, Xu Y, Wu G, Ward D, Li SJ. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007;69:1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]


