Abstract
Striking parallels exist between the neurochemical and toxic effects of stress and methamphetamine. Despite these similarities, no studies have examined how stress may promote the toxic effects of methamphetamine (METH). The current study tested the hypothesis that chronic stress enhances METH toxicity by augmenting glutamate (GLU) release and excitotoxicity in response to METH administration. Adult male Sprague-Dawley rats were exposed to 10 days of unpredictable stress and then received either saline or METH (7.5 mg/kg, i.p., once every 2 h × four injections). Prior exposure to unpredictable stress acutely enhanced the striatal extracellular GLU concentrations in response to METH, and eventually caused proteolysis of the cytoskeleton protein spectrin. Administration of the corticosterone synthesis inhibitor, metyrapone (25 mg/kg, i.p., prior to each stressor), during unpredictable stress attenuated the enhanced striatal GLU release in response to METH, blocked spectrin proteolysis, and attenuated METH-associated toxicity measured by long-term depletions in the dopamine and serotonin tissue content as well as depletions in dopamine and serotonin transporter immunoreactivity of the striatum. In summary, prior exposure to unpredictable stress enhances METH-induced elevations of GLU in the striatum, resulting in long-term excitotoxic damage and an augmentation of damage to dopamine and serotonin terminals. These studies provide a neurochemical basis for how stress contributes to the deleterious effects of METH abuse.
Keywords: spectrin proteolysis, glucocorticoids, metyrapone, dopamine transporter, serotonin transporter
INTRODUCTION
Methamphetamine (METH) is an addictive psychostimulant with neurotoxic effects. The neurotoxic effects are evidenced by long-term depletions in striatal dopamine (DA) and serotonin (5-HT) concentrations, and their uptake sites (Friedman et al., 1998; Ricaurte et al., 1982; Wagner et al., 1980), decreases in tryptophan and tyrosine hydroxylase activity (Bakhit et al., 1981), and cell loss (Deng et al., 2007; Sonsalla et al., 1996; Thiriet et al., 2005; Yu et al., 2004; Zhu et al., 2006). In addition to the hypotheses of oxidative stress and apoptosis as mechanisms of METH-induced neurotoxicity (for review, see Davidson et al., 2001; Kita et al., 2003; Quinton and Yamamoto, 2006), increases in the extracellular concentrations of glutamate (GLU) have been also suggested to play an important role (Abekawa et al., 1994; Nash and Yamamoto, 1992; Sonsalla et al., 1989; Stephans and Yamamoto, 1994). Recently, it has been demonstrated that spectrin proteolysis resulting from the activation of ionotropic GLU receptors, influx of Ca2+, and the subsequent activation of the calcium-dependent protease, calpain (Siman et al., 1989), is increased after METH administration (Staszewski and Yamamoto, 2006). Furthermore, evidence suggests that administration of METH causes the activation of calpain and various caspases including -3, -9, and -12, both in vivo (Jayanthi et al., 2004; Warren et al., 2005) and in vitro (Deng et al., 2002; Samantaray et al., 2006).
Elevations in GLU have also been associated with psychological stress. Increases in GLU after stress produces structural damage to the hippocampus, whereas antagonism of NMDA receptors or blockade of excitatory amino acid release protects against this damage (Armanini et al., 1990; Magarinos and McEwen, 1995). Furthermore, the increases in extracellular GLU in various brain areas after exposure to acute or chronic stressors (Broom and Yamamoto, 2005; Lowy et al., 1995; Moghaddam, 1993) are attenuated by adrenalectomy (Lowy et al., 1993; Moghaddam et al., 1994). Moreover, elevations in glucocorticoids or psychological stress exacerbate the increase in extracellular GLU, neuron loss, and spectrin proteolysis in the hippocampus produced by kainic acid (Stein-Behrens et al., 1992, 1994b).
Despite the similarities between METH and stress on GLUergic transmission, there is a paucity of data regarding the interactions between GLU-mediated excitotoxicity produced by METH and its relationship to chronic stress. This is particularly surprising given the close association between stress and the vulnerability to substance abuse (Sinha, 2001; Soderpalm et al., 2003). Recent findings indicate that prior exposure to chronic unpredictable stress (CUS) enhances METH-associated toxicity to striatal DA terminals (Matuszewich and Yamamoto, 2004), but the underlying mechanisms of this enhancement are unknown.
Exposure to elevated glucocorticoids or stress has been shown to exacerbate kainic acid-induced GLU accumulation and spectrin proteolysis (Stein-Behrens et al., 1992, 1994a). Therefore, the current study examined how chronic stress enhances METH toxicity, and tested the hypothesis that prior exposure to stress will augment the acute METH-induced increases in GLU and the production of spectrin proteolysis. A CUS paradigm was employed because, in contrast to repeated predictable stress (e.g., chronic restraint stress), it is not confounded with adaptation and causes significantly higher basal corticosterone levels (Araujo et al., 2003; Bielajew et al., 2002). We also investigated the effects of the inhibition of endogenous adrenal corticosteroid synthesis by metyrapone. Metyrapone inhibits corticosteroid biosynthesis by binding to 11β-hydroxylase, the enzyme that converts 11-deoxycorticosterone to corticosterone in the adrenal glands (Sonino, 1982). Previous studies have shown that metyrapone administration blocks stress-induced increases in corticosterone (Calvo et al., 1998; Cordero et al., 2002; Dal-Zotto et al., 2003; Der-Avakian et al., 2006; Marinelli et al., 1996; Piazza et al., 1994). Therefore, we examined whether metyrapone during unpredictable stress will attenuate the acute METH-induced increases in GLU concentrations and the longer-term changes in spectrin proteolysis and markers of toxicity to DA terminals.
MATERIALS AND METHODS
Subjects
Adults Sprague-Dawley rats (175–200 g; Harlan, Indianapolis, IN) were used in the experiments. All rats were acclimated to the colony for 4 days before experimentation and housed in pairs in a temperature- (21–23°C) and humidity (30–40%)-controlled room with a 12-h light–dark cycle (lights on 7.00 a.m.–off 7.00 p.m.). Food and water were available ad libitum.
All treatment and testing protocols were approved by the Boston University Institutional Animal Care and conformed to the National Institutes of Health guidelines.
Experimental design
Rats were either stressed for 10 days or not stressed and received saline (Nostress/Saline, Stress/Saline) or METH (Nostress/METH, Stress/METH) injections on Day 11. In separate experiments, the effects of the administration of the corticosterone synthesis inhibitor, metyrapone, during the CUS period was examined on METH-induced GLU release and METH-associated striatal toxicity. Rats that received metyrapone (MetStress/METH) during the CUS period as well as controls that were not stressed and received vehicle for 10 days prior to METH (VehNostress/METH) were also included in the study. All animals underwent intracranial surgery on Day 7 of the 10-day period and were killed by rapid decapitation 7 days after METH or saline treatment for the measurement of DA and 5-HT tissue content (Expt. 1). In another set of experiments (Expts. 2a and 2b), spectrin proteolysis was examined 5 days after METH administration in rats preexposed to unpredictable stress. Stressed rats and rats that were not stressed and received saline or METH were tested (Nostress/Saline, Stress/Saline, Nostress/METH, Stress/METH) (Expt. 2a). In addition, we investigated whether spectrin proteolysis will be blocked by metyrapone treatment during CUS (Expt. 2b). Rats received either metyrapone or vehicle 15 min prior to each stressor during the 10 days of CUS exposure. METH or saline was injected on Day 11 (VehStress/METH-MetStress/METH). In Experiment 2, striatal DA transporter (DAT) and serotonin transporter (SERT) immunoreactivity were examined as markers of METH toxicity.
CUS paradigm
The CUS paradigm was employed in this study. According to the CUS model, both type and time of stressor exposure vary. Therefore, this paradigm is not confounded with adaptation and produces higher baseline corticosterone levels compared to models of predictable stress (Araujo et al., 2003; Bielajew et al., 2002; Herman et al., 1995). Rats were exposed to stressors for 10 days (Matuszewich and Yamamoto, 2004). The stressors were varied by day and time and were administered according to the following schedule: 1st day: 10.00 h 60 min cage rotation, 14.00 h 4 min swim stress (22–23°C); 2nd day: 15.00 h 60 min restraint stress, 19.00 h isolation housing overnight; 3rd day: 11.00 h 3 h lights off, 19.00 h food/water deprivation; 4th day: 14.00 h 50 min cage agitation, 19.00 h lights on overnight; 5th day: 11.00 h 60 min restraint stress, 16.00 h 15 min cold room isolation (4°C); 6th day: 12.00 h 4 min swim stress (22–23°C), 16.00 h isolation housing overnight; 7th day: surgery; 8th day: 10.00 h 20 min cage rotation, 15.00 h 2 h lights off; 9th day: 11.00 h 50 min cold room (4°C), 19.00 h lights on overnight; 10th day: 9.00 h 3 h lights off, 15.00 h 30 min rotation. All animals were transported daily to the area where stressors were administered, in order to control for possible effects of transport.
In the experiments in which intracranial surgery was not employed (Expt. 2), the schedule was modified slightly as follows: 1st day:10.00 h 50 min cold room (4°C), 13.00 h 60 min cage rotation; 2nd day: 14.00 h 4 min swim stress (22–23°C), 19.00 lights on overnight; 3rd day: 10.00 h 3 h lights off, 16.00 h 60 min restraint stress; 4th day: 12.00 h 50 min cage rotation, 19.00 h food and water deprivation overnight; 5th day: 15.00 h 15 min cold room isolation, 19.00 isolation housing overnight; 6th day: 9.00 h 3 h lights off, 16.00 h swim stress (22–23°C); 7th day: 10.00 h 30 min restraint 19.00 h food and water deprivation overnight; 8th day: 11.00 h 20 min cage rotation, 19.00 h lights overnight; 9th day: 13.00 h 3 min swim stress, 19.00 h food and water deprivation; 10th day: 10.00 h 60 min restraint stress, 14.00 h 2 h lights off.
Drugs and drug administration
d-METH hydrochloride, 2-methyl-1,2-di-3-pyridyl-1-propanone (metyrapone), and Dulbecco’s powdered medium were purchased from Sigma-Aldrich (St. Louis, MO). Fifteen minutes prior to each stressor during the 10-day CUS period, metyrapone (25 mg/kg), dissolved in 10% ethanol was administered i.p. (i.p.). This dose has been previously shown to block the enhancement of plasma corticosterone levels produced by psychological stress (Liu et al., 1999). One day after the last stressor (11th day), 7.5 mg/kg d-METH, dissolved in 0.9% NaCl (1 ml/kg), or an equivalent volume of 0.9% NaCl was injected (i.p.) once every 2 h over an 8-h period, for a total of four injections (7.5 mg/kg; q 2 h × 4).
On the day of the experiments, body temperatures were measured using a rectal probe digital thermometer (Thermalert TH-8 monitor, Physitemp Instruments, Clinton, NJ). The probe was inserted into the rectum of each rat, where it remained until a stable temperature was obtained. Rectal measurements were recorded 1 h prior to the first METH or saline injection and 1 h following each injection. In case rectal temperatures were higher than 41.0°C, rats were cooled by placing wet ice in a tray underneath their cage for a period of ~30 min. During that period, temperature was taken every 15 min to prevent overexposure to the ice and, consequently, hypothermia. Rats were allowed to acclimate to the treatment room for ~18 h before the start of the injections.
Intracranial surgery
On the 7th day of the stress regimen, rats in the microdialysis experiment (Expt. 1) were anesthetized with a combination of xylazine (6 mg/kg, i.p.) and ketamine hydrochloride (70 mg/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The skull was exposed, and a 1-mm hole was drilled over the striatum (+2.00 mm anterior and +3.2 mm medial to bregma) (Paxinos and Watson, 1982). A 21-gauge stainless steel guide cannula was stereotaxically inserted into the hole and onto the cortex above the striatum. The guide cannula with a stylet obturator was secured to the skull with cranioplastic cement. After the surgery, all rats were housed individually.
In vivo microdialysis
Four days after surgery and 15 h prior to the start of the experiment, the obturator was removed and replaced with a microdialysis probe. A 26-gauge stainless steel needle with a beveled tip extending ~0.5 mm beyond the end of the guide cannula was used to puncture the dura prior to the insertion of the probe. The microdialysis probes were constructed as described previously (Yamamoto and Pehek, 1990). The length of the membrane (Spectrum Laboratories, Rancho Dominguez, CA; 13,000 molecular weight cutoff; 210 μm o.d.) was 4 mm. Fifteen hours after the insertion of the probes, Dulbecco’s phosphate-buffered saline (138 mM NaCl, 2.1 mM KCl, 0.5 mM MgCl2, 1.5 mM KH2PO4, 8.1 mm NaHPO4, 1.2 mm CaCl2, and 0.5 mM D-glucose, pH 7.4) was perfused at a flow rate of 2.0 μl/min using a model 22 syringe perfusion pump (Harvard Apparatus, Holliston, MA), and dialysis samples were collected every 30 min. After a 2-h equilibration period, three baseline samples were collected followed by 16 samples during systemic saline or METH injections over the 8-h period of treatment. Rats were killed by decapitation 7 days after injections. Their brains were removed and frozen in dry ice. Coronal slices (40-μm-thick) were taken to verify probe placement.
High-performance liquid chromatography
Biochemical measurements of extracellular GLU
Extracellular concentrations of striatal GLU were determined by high-performance liquid chromatography (HPLC) with fluorescence detection. An aliquot (20 μl) of the dialysis sample was assayed for GLU after precolumn derivatization with o-pthaldialdehyde (10 μl). o-Pthaldialdehyde was automatically added to the dialysate sample by an ESA model 542 autosampler (ESA, Chelmsford, MA), mixed, and allowed to react with the sample for exactly 90 s before injection onto the column (3 μm C18 column). The mobile phase used for GLU detection consisted of 0.1 M sodium phosphate, 0.1 mM EDTA, and 10% methanol (pH 6.65). Fluorescence detection was performed with a Waters 474 Fluorescence Detector (Waters Corp, Milford, MA) and data was collected using EZChrom® software (Scientific Software, Pleasanton, CA).
Tissue DA and 5-HT content
Seven days after the saline or METH injections, rats were killed by rapid decapitation. In the microdialysis experiment (Expt. 1), whole brains were frozen in dry ice and coronal slices were subsequently sectioned on a cryostat in 40-μm increments. Once the probe placement was identified, a coronal 400-μm-thick section was taken and striatal tissue on the side contralateral to the probe placement was microdissected for tissue content analysis. The tissue was sonicated in 300 μl of cold 0.25 M perchloric acid and centrifuged at 15,000 rpm for 10 min. The supernatant (20 μl) was analyzed with HPLC-EC, and the protein was determined using a Bradford assay (Bio-Rad, Hercules, CA) after resuspending the pellet in 1 N NaOH.
Western blot analysis of striatal spectrin breakdown products, and DAT and SERT immunoreactivity
Rats were killed by rapid decapitation 5 days after the last METH or saline injection. This time point was chosen based on previous findings, which indicate that calpain-mediated spectrin proteolysis reaches a maximum 5 days after METH administration (10 mg/kg; q 2 h × 4) (Staszewski and Yamamoto, 2006). Brains were removed, whole striata were dissected, frozen in dry ice, and then stored at −80°C until homogenization.
Striata were homogenized in 20:1 vol/wt buffer containing 10 mM Tris (pH 7.4), 10 mM EGTA, 250 mM sucrose, 2 μg/ml aprotinin, 10 μg/ml leupeptin, 2 μg/ml pepstatin A, and 1 mM phenylmethylsulfonyl fluoride. After estimation of protein by the Bradford method, samples were diluted with 2× sodium dodecyl sulfate loading buffer (Invitrogen, Carlsbad, CA) and heated to 85°C for 5 min.
Electrophoresis was performed in a XCell SureLock mini-cell apparatus (Invitrogen). For spectrin breakdown products (SBPs) analysis, 10 μg of protein were loaded in a 6% Tris-glycine gels (Invitrogen). For DAT or SERT immunoreactivity, 5 μg or 20 μg of protein, respectively, were loaded in 10% Tris-glycine gels. DAT and SERT immunoreactivity was used as an indication of toxicity to DA and 5-HT terminals. Following electrophoresis, samples were transferred to poly-vinylidene difluoride membrane. Membranes for SBPs and SERT immunoreactivity were rinsed in Tris-buffered saline containing Tween 20 (TBS-T; 20 mM Tris, 137 mM NaCl, pH 7.6, 0.1% Tween 20) and blocked in TBS-T containing 5% milk for 1 h at room temperature (RT). Membranes for DAT protein immunoreactivity were rinsed in Tris-buffered saline (TBS), and then blocked for 1 h in TBS containing 0.5% Tween 20 and 5% nonfat dry milk at RT. Membranes were then incubated with the primary antibody for the nonerythroid α-spectrin MAB 1622 (1:5000, Chemicon International, Temecula, CA), DAT (1:2500, s.c.-1433; Santa Cruz Biotechnology, Santa Cruz, CA), or SERT (1:2500, s.c.-1458; Santa Cruz Biotechnology) overnight at 4°C. The following day, membranes were rinsed in TBS-T (SBPs, SERT) or TBS (DAT) and incubated with an antimouse (1:2500, noradrenaline (NA) 931, GE Healthcare, Piscataway, NJ), antirabbit (1:2500, NA 934, GE Healthcare, Piscataway, NJ), or antigoat (1:2500, s.c. 2768, Santa Cruz) horseradish peroxidase-linked IgG antibody, respectively, for 1 h (RT). Following rinses in TBS-T or TBS, the membranes were visualized using chemiluminescence reagents and Hyperfilm (GE Healthcare). The densities of SBPs, DAT, and SERT protein were analyzed using a Kodak Gel logic imaging system (Rochester, NY).
Statistical analyses
In experiment 1, extracellular GLU concentrations and rectal temperature measurements of all six groups were analyzed with a two-way repeated-measures analysis of variance (ANOVA), with treatment (NoStress/Saline, Stress/Saline, NoStress/METH, Stress/METH, VehNoStress/METH, and MetStress/METH) as the between-subjects factor and time as the repeated-measures factor. A one-way ANOVA was used for the analysis of DA and 5-HT tissue content (Expt. 1). In Experiment 2a, SBPs, DAT, and SERT immunoreactivity were analyzed with a two-way ANOVA (drug × stress; NoStress/Saline, Stress/Saline, NoStress/METH, Stress/METH), while in the Experiment 2b, a one-way ANOVA was performed (Nostress/Saline, VehStress/METH, MetStress/METH).
Significant interactions were further analyzed with Newman’s Keuls post hoc comparisons. All statistics were determined using Sigma Stat 3.0. Statistical significance was set at P < 0.05 for all tests.
RESULTS
Experiment 1: Effects of unpredictable stress and metyrapone on METH-associated hyperthermia, extracellular GLU concentrations, and DA and 5-HT content in the striatum
Body temperature
The rectal temperatures of stressed or rats that were not stressed and received saline or METH as well as Stress/METH rats that received metyrapone are illustrated in Figure 1. Analysis of rectal temperatures prior to and 1 h after each injection showed a significant treatment [F(5, 311) = 25.57, P < 0.001], time [F(4, 311) = 56.46, P < 0.001] and treatment × time interaction [F(20, 311) = 4.85, P < 0.001]. The baseline values of rectal temperatures did not significantly differ among groups (P > 0.05). METH injections significantly increased body temperatures over time (Nostress/Saline vs. Nostress/METH, P < 0.001), and prior exposure to stress enhanced the METH-associated hyperthermia (Nostress/METH vs. Stress/METH, P < 0.01). Administration of metyrapone (Met) to stressed rats that were treated with METH (MetStress/METH) did not attenuate the stress-related enhanced hyperthermia (Stress/METH vs. MetStress/METH, P > 0.05).
Fig. 1.
Body temperatures were measured 1 h prior and 1 h after each saline (0.9% NaCl, 1 ml/kg) or METH (7.5 mg/kg, i.p.; q 2 h × 4) injections. METH caused hyperthermia compared with no-stressed saline-treated rats (#P < 0.05). Preexposure to unpredictable stress significantly enhanced the hyperthermic response (*P < 0.05). Administration of metyrapone during the CUS period did not attenuate the enhanced hyperthermia in response to METH. Data are expressed as mean temperature (°C) ± SEM. Arrows indicate the time of saline or METH injections; n = 5–8 per treatment group.
Extracellular GLU levels
Figure 2 illustrates the extracellular GLU concentrations prior to and after saline or METH (7.5 mg/kg; q 2 h × 4) injections in no-stressed and stressed rats. Analysis revealed a significant treatment [F(5, 588) = 23.47 P < 0.001], time [F(18, 588) = 19.70 P < 0.001], and treatment × time interaction [F(90, 588) = 2.46, P < 0.001]. No significant differences were found between any of the groups with regard to the average baseline values of extracellular GLU [F(5, 31) = 0.89, P > 0.05]. METH administration produced a gradual and significantly sustained increase in GLU concentrations relative to saline controls (Nostress/Saline vs. Nostress/METH, P < 0.01). Prior exposure to CUS further increased the METH-induced extracellular GLU levels when compared with no-stressed rats treated with METH (Nostress/METH vs. Stress/METH, P < 0.001). No differences were detected in the extracellular GLU concentrations between the two saline-treated groups (Nostress/Saline vs. Stress/Saline, P > 0.05). Metyrapone administration during CUS significantly attenuated the METH-induced GLU levels in response to METH to the levels of no-stressed rats (MetStress/METH vs. VehNostressMETH, P > 0.05, MetStress/METH vs. Stress/METH, P < 0.001).
Fig. 2.
In vivo microdialysis of extracellular glutamate concentrations in the striatum prior to and during injections of saline (0.9% NaCl, 1 ml/kg) or METH (7.5 mg/kg, i.p.; q 2 h × 4). Dialysate samples were collected in the striatum every 30 min during a 1.5-h baseline period and following each saline or METH injection over a 8-h period (total of 19 samples). METH increased glutamate levels compared with saline controls (#P < 0.05). Rats previously stressed exhibited greater increases of extracellular glutamate compared with no-stressed METH rats (*P < 0.05). Pretreatment of Stress/METH rats with metyrapone significantly attenuated the enhanced glutamatergic response to levels similar to VehNostress/METH rats (P > 0.05) Values are expressed as means ± SEM percentage of baseline values. Arrows indicate the time of METH or saline injections; n = 5–8 per treatment group.
DA and 5-HT tissue concentrations
Figure 3 illustrates the DA and 5-HT tissue concentrations in the striatum 7 days after saline or METH (7.5 mg/kg; q 2 h × 4) in stressed and no-stressed rats as well as in stressed/METH rats that received metyrapone. The DA tissue content was significantly depleted after METH treatment, and prior exposure to CUS further enhanced this effect [F(5, 64) = 18.78, P < 0.001; Nostress/Saline vs. Nostress/METH, P < 0.001, Nostress/METH vs. Stress/METH, P < 0.001; Fig. 3A]. Metyrapone administration during CUS attenuated the augmented DA depletions following METH treatment to the levels of VehNostress/METH rats (VehNostress/METH vs. MetStress/METH, P > 0.05; Stress/METH vs. MetStress/METH, P < 0.01).
Fig. 3.
Striatal DA (A) and 5-HT (B) tissue concentrations 7 days after injections of saline (0.9%NaCl, 1 ml/kg) or METH (7.5 mg/kg, i.p.; q 2 h × 4) in stressed and no-stressed rats, as well as in Stress/METH rats that received metyrapone during CUS. Metyrapone treatment of METH-treated rats with prior exposure to CUS attenuated the enhanced DA and blocked the 5-HT depletions in the striatum. # indicates a significant difference from the Nostress/Saline and Stress/Saline groups (P < 0.05). * indicates a significant difference from the Nostress/METH group (P < 0.05). ¥ indicates a significant difference from the Stress/METH group. Values are expressed as means ± SEM picogram per microgram of protein; n = 6–8 per treatment group.
There was a significant difference among the groups with regard to 5-HT content, [F(5, 64) = 5.20, P < 0.001; Fig. 3B]. Although METH alone did not cause a significant depletion on 5-HT content compared with saline rats (Nostress/Saline vs. Nostress/METH, P > 0.05), METH treatment did significantly deplete 5-HT in previously stressed rats (Nostress/METH vs. Stress/METH, P < 0.01). Similar to DA tissue content, metyrapone administration blocked this effect (MetStress/METH vs. VehNostress/METH, P > 0.05; MetStress/METH vs. Stress/METH, P < 0.05). The two groups of saline-treated animals did not significantly differ from each other with regard to DA and 5-HT tissue content (Nostress/Saline vs. Stress/Saline, P > 0.05).
Experiment 2: Effects of unpredictable stress and metyrapone on spectrin proteolysis and DAT and SERT immunoreactivity in the striatum of METH-treated rats with prior exposure to unpredictable stress
Spectrin proteolysis
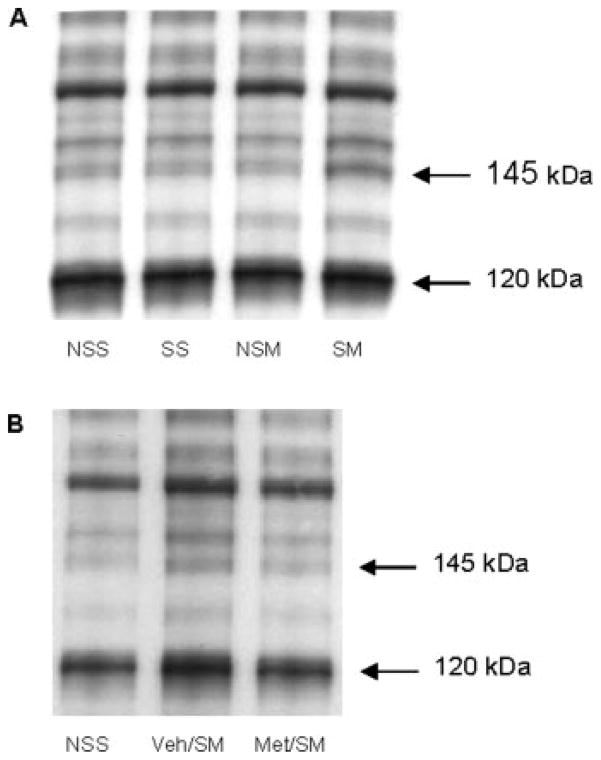
As illustrated in Figure 4A, analysis of 145 kDa SBPs of stressed or no-stressed rats 5 days after saline or METH (7.5 mg/kg; q 2 h × 4) injections revealed that there was a differential effect of METH on calpain-mediated SBPs [stress × drug: F(1, 27) = 6.41, P < 0.05]. METH alone did not affect spectrin proteolysis compared with no-stressed controls (Nostress/Saline vs. Nostress/METH, P > 0.05), but exposure to stress prior to METH administration caused calpain-mediated spectrin proteolysis compared with METH controls 5 days after the last injection (Nostress/METH vs. stress/METH, P < 0.05; Figs. 4A and 5A). There was no difference between the two saline-treated groups (Nostress/Saline vs. Stress/Saline, P > 0.05). Interestingly, the increased immunoreactivity of the 145-kDa band from rats exposed to both unpredictable stress and METH was blocked by metyrapone administration during CUS [F(2, 14) = 5.71, P < 0.05; Figs. 4B and 5B].
Fig. 4.
Effects of unpredictable stress on METH-induced changes in 145-kDa SBP (A, B) and 120-kDa SBP (C, D) in the striatum as well as in stress/METH rats that received metyrapone injections prior to each stressor. Preexposure to stress caused a significant increase in the calpain-mediated spectrin proteolysis (*P < 0.05), while METH alone did not have an effect. Metyrapone administration blocked increases in striatal calpain-mediated spectrin proteolysis in METH-treated rats preexposed to unpredictable stress. ¥ indicates a significant difference from the Stress/METH group. There was no difference among the groups in caspase-3 dependent 120-kDa SBP; Values are expressed as means ± SEM percent of saline relative optical densities n = 4–8 per treatment group.
Fig. 5.
Representative Western blots of spectrin proteolysis after METH or saline treatment. (A) The intensity of the bands indicates that METH administration in rats with prior exposure to CUS increased 145-kDa SBPs compared with the saline controls, while there is no difference in the intensity of the 120-kDa SBP among the groups. (B) Administration of metyrapone during CUS blocked the increases in 145-kDa SBPs. NSS, NoStress/Saline; SS, Stress/Saline; NSM, NoStress/METH; SM, Stress/METH; Veh/SM, Veh/StressMETH; Met/SM, Met/StressMETH.
Caspase-3 dependent spectrin proteolysis, as indicated by the 120-kDa band, did not change among the groups [Expt. 2A: F(1, 27) = 0.03, P > 0.05; Figs. 4C and 5A; Expt. 2B: F(2, 14) = 0.84, P > 0.05; Figs. 4D and 5B].
DA and SERT depletions
Figures 6 and 7 illustrate the DAT and SERT immunoreactivity in the striatum 5 days after saline or METH (7.5 mg/kg; q 2 h × 4) in stressed and no-stressed rats as well as in stressed/METH rats that received metyrapone during CUS. Analysis of the DAT immunoreactivity revealed that METH caused a significant depletion compared with saline controls, as noted by a significant overall drug effect [F(1, 29) = 40.72 P < 0.001]. As illustrated in Figure 6A, METH alone produced a significant 20% decrease in DAT immunoreactivity relative to saline controls (Nostress/Saline vs. Nostress/METH: P < 0.05) and previous exposure to CUS significantly enhanced this effect [drug × stress: F(1, 29) = 11.24, P < 0.01; Nostress/METH vs. Stress/METH, P < 0.001]. There was no significant difference in DAT expression between the two saline-treated groups (Nostress/Saline vs. Stress/Saline, P > 0.05). Metyrapone administration during the CUS period attenuated DAT depletions in the Stress/METH rats (VehStress/METH vs. Nostress/Saline, P < 0.05; MetStress/METH vs. VehStress/METH, P < 0.01; Fig. 6B).
Fig. 6.
DAT immunoreactivity in the striatum 5 days after injections of saline (0.9% NaCl, 1 ml/kg) or METH (7.5 mg/kg, i.p.; q 2 h × 4). (A) METH alone depleted DAT levels relative to saline-treated rats (#P < 0.05). Previously stressed rats showed greater decreases in DAT immunoreactivity in response to METH compared with no-stressed controls (*P < 0.05). (B) Metyrapone attenuated the enhanced DAT depletions in Stress/METH group relative to VehStress/METH rats (¥P < 0.05). Representative Western blots are shown below the graphs, representing the 78-kDa DAT band. Values are expressed as means ± SEM percent of saline relative optical densities; n = 4–8 per treatment group. NSS, NoStress/Saline; SS, Stress/Saline; NSM, NoStress/METH; SM, Stress/METH; Veh/SM, Veh/StressMETH; Met/SM, Met/StressMETH.
Fig. 7.
SERT immunoreactivity in the striatum 5 days after injections of saline (0.9% NaCl, 1 ml/kg) or METH (7.5 mg/kg, i.p.; q 2 h × 4). (A) METH depleted SERT levels in the striatum of stressed rats relative to METH controls (*P < 0.05), but METH alone had no effect. (B) Metyrapone blocked the SERT depletions in Stress/METH group relative to VehStress/METH rats (¥P < 0.05). Representative Western blots are shown below the graphs, representing the 63- to 68-kDa SERT band. Values are expressed as means ± SEM percent of saline relative optical densities; n = 4–8 per treatment group. NSS, NoStress/Saline; SS, Stress/Saline; NSM, NoStress/METH; SM, Stress/METH; Veh/SM, Veh/StressMETH; Met/SM, Met/StressMETH.
Analysis of SERT immunoreactivity indicated that the significant depletion of SERT by METH [drug: F(1, 28) = 16.47, P < 0.001] was dependent upon prior CUS exposure [drug × stress: F(1, 28) = 5.36, P < 0.05]. Post hoc comparisons to further analyze the significant interaction revealed that METH alone did not affect SERT (Nostress/Saline vs. Nostress/METH, P > 0.05), but preexposure to stress resulted in a significant depletion compared with no-stressed METH-treated rats (Nostress/METH vs. Stress/METH, P < 0.01; Fig. 7A). Similar to DAT, the two saline-treated animals did not significantly differ from each other (Nostress/Saline vs. Stress/Saline, P > 0.05). Administration of metyrapone during the CUS period blocked the CUS-associated SERT depletions [F(2, 13) = 4.47, P < 0.05, Nostress/Saline vs. Vehstress/METH, P < 0.05; MetStress/METH vs. VehStress/METH, P > 0.05; Fig. 7B].
DISCUSSION
Preexposure to 10 days of unpredictable stress enhanced the striatal extracellular concentrations of the excitatory amino acid, GLU, in response to METH. Chronic stress and METH synergized to cause calpain-mediated proteolysis of the cytoskeletal protein, spectrin. Interestingly, attenuation of the augmented striatal GLU levels in rats treated with metyrapone during CUS was associated with a blockade of spectrin proteolysis and an attenuation of METH-induced depletions of DA and 5-HT tissue concentrations as well as DAT and SERT immunoreactivities. These findings suggest that the enhanced toxicity in stressed rats in response to METH is mediated by the augmented GLU release in response to METH, and that stress-related elevations in glucocorticoids during CUS play an important role in the METH-induced GLUergic response and long-term striatal toxicity.
Although there was no change in basal concentrations of extracellular GLU 1 day after the last stressor, a METH challenge significantly increased GLU concentrations in the striatum, without altering the time course of these increases (Fig. 2). Extracellular concentrations of GLU in response to METH were similar in duration and magnitude to those reported previously (Abekawa et al., 1994; Nash and Yamamoto, 1992). The current study did not examine the mechanism of the METH-induced increases in striatal GLU, but previous studies showed a D1 receptor-dependent activation of the striatonigral GABAergic pathway (Mark et al., 2004), resulting in a disinhibition of corticostriatal GLUergic projections (Burrows and Meshul, 1999).
The current study is the first to report that prior exposure to stress exacerbates METH-induced GLU release in the striatum. This finding extends previous reports of an augmentation of kainic acid-induced increases in extracellular GLU by glucocorticoids in the rat hippocampus (Stein-Behrens et al., 1992). The mechanism by which CUS enhances GLU levels in the striatum during METH administration is unknown, but it is likely mediated by stress-associated elevations in glucocorticoids. Based on the findings that extracellular hippocampal GLU levels increase after corticosterone administration (Venero and Borrell, 1999) and adrenalectomy attenuates the stress-induced GLU elevations in the same brain area (Lowy et al., 1993; Moghaddam et al., 1994), our data suggest that the enhanced GLU response in METH-treated rats preexposed to stress may be paralleled by enhanced plasma corticosterone levels. In fact, data from our laboratory indicate higher corticosterone levels in stressed rats 1 h after METH administration compared with no-stressed controls (unpublished observations).
Our finding that metyrapone administration during CUS attenuated the enhanced extracellular GLU in response to METH further supports the role of glucocorticoids in the enhanced GLUergic response. Existing evidence indicates that adrenalectomy or administration of metyrapone decreases D1 binding or mRNA in various brain regions (striatum, substantia nigra, nucleus accumbens; Biron et al., 1992; Czyrak et al., 1997). Based on the involvement of D1 receptors in METH-associated increases in corticostriatal GLU (Mark et al., 2004), it is possible that elevated glucocorticoids during CUS may cause an upregulation of the striatonigral D1 receptor system, which, in turn, contributes to the augmented GLU release during METH administration. However, no change was observed in D1 mRNA in either striatum or substantia nigra after 5 weeks of chronic mild stress (Dziedzicka-Wasylewska et al., 1997). Further investigation into the mechanism through which glucocorticoids elevations lead to the augmented GLU rise is warranted.
The results illustrate that the stress-induced enhancement of GLU release after METH is excitotoxic and corticosterone-dependent, because the increases in proteolytic cleavage of spectrin into 145-kDa fragments were blocked by the administration of the corticosterone synthesis inhibitor, metyrapone (Figs. 4A, 4B, and 5B). The cleavage of spectrin is most likely the result of increased extracellular concentrations of GLU and overactivation of GLU receptors to increase intracellular Ca2+ and activate a number of Ca2+-dependent proteases, such as calpain and caspase-3 (Siman et al., 1989; Wang, 2000). Consequently, calpain specifically degrades the scaffolding protein spectrin into 145-kDa SBPs under both necrotic and apoptotic conditions. In contrast, caspase-3 exclusively lyses spectrin into a 120-kDa SBPs and is involved primarily in apoptosis (Wang, 2000; Wang et al., 1998). Because METH administration to rats preexposed to CUS increased the 145-kDa SBP without a change in 120-kDa SBP, these findings indicate that the synergistic interaction between stress and METH on spectrin proteolysis is selective to calpain activation and is similar to the excitotoxic cell death that occurs in response to traumatic brain injury (Pike et al., 2001) and ischemia (Neumar et al., 2001). This finding is in agreement with previous reports that exposure to high glucocorticoids levels or psychological stress exacerbate neuron loss and cytoskeletal pathology caused by the excitotoxin, kainic acid (Stein-Behrens et al., 1994a).
Although METH administration to rats that were not exposed to CUS (NoStress/METH group) increased striatal extracellular GLU levels, these elevations were not associated with spectrin proteolysis (Figs. 4A and 5A). However, higher doses of METH (10 mg/kg, q 2 h × 4) that have been shown to cause a maximal 7.5-fold increase in extracellular GLU (Stephans and Yamamoto, 1994) did result in calpain-mediated spectrin proteolysis (Staszewski and Yamamoto, 2006). The differences between this past study and the current study illustrating an approximate threefold increase in extracellular GLU and no change in the 145-kDa SBPs after METH administration to rats not exposed to CUS could be explained by the lower dose of METH used and the smaller increase in extracellular GLU levels. Regardless, the lower dose of METH in the current study, combined with the preexposure to chronic stress, produced similar increases in extracellular GLU and spectrin proteolysis observed with the higher dose of METH alone (Stephans and Yamamoto, 1994).
In contrast to previously published observations using the acute toxic dosing model (i.e., four injections of METH every 2 h; Daberkow et al., 2005; Eyerman and Yamamoto, 2005; Friedman et al., 1998; Ricaurte et al. 1982; Straiko et al., 2007; Wallace et al., 2001), METH administration to rats not exposed to CUS did not produce depletions in 5-HT tissue content (Fig. 3B). Higher doses of METH used in previous studies and/or differences in rat strain (e.g., Clemens et al., 2005) may explain this discrepancy. In contrast, prior exposure to CUS resulted in METH-induced 5-HT and SERT depletions, and enhanced DA and DAT depletions in the striatum compared with rats treated with METH but not exposed to CUS as reported previously (Matuszewich and Yamamoto, 2004; Tata and Yamamoto, 2005). CUS rats that were treated with metyrapone during CUS and subsequently exposed to METH had DA and 5-HT content levels that approximated those of rats not exposed to CUS but treated with METH. Importantly, this protective effect of metyrapone was independent of changes in body temperature. As indicated by our data, preexposure to stress enhanced hyperthermia in response to METH, but metyrapone administration did not attenuate this effect (Fig. 1). Thus, an attenuation of hyperthermia does not appear to account for the protective effects of metyrapone.
In conclusion, these new findings demonstrate an interdependent relationship between unpredictable stress, METH administration, and GLU-mediated excitotoxicity in the rat striatum. The results demonstrate that the enhanced GLU response to METH observed in stressed rats is associated with excitotoxic damage. The findings that the CUS-induced augmentation of extracellular GLU concentrations, calpain-mediated spectrin proteolysis, and the striatal toxicity after METH are attenuated by metyrapone administration during stress exposure support the conclusion that CUS augments METH-induced excitotoxicity in a corticosterone-dependent manner. Based on the increase in drug craving under stress conditions in humans (Soderpalm et al., 2003) and the high comorbidity associated with posttraumatic stress disorder and substance abuse (Brown et al., 1998), these current findings provide a neurochemical basis for how stress can contribute to the deleterious effects of METH abuse.
Acknowledgments
This work was supported by the National Institutes of Health grants DA07606 and DA16866 and a gift from Hitachi America, Inc.
Abbreviations
- CUS
chronic unpredictable stress
- DA
dopamine
- DAT
DA transporter
- GLU
glutamate
- HPLC
high performance liquid chromatography
- 5-HT
serotonin
- METH
methamphetamine
- SBPs
spectrin breakdown products
- SERT
serotonin transporter
References
- Abekawa T, Ohmori T, Koyama T. Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res. 1994;643:276–281. doi: 10.1016/0006-8993(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Araujo AP, DeLucia R, Scavone C, Planeta CS. Repeated predictable or unpredictable stress: Effects on cocaine-induced locomotion and cyclic AMP-dependent protein kinase activity. Behav Brain Res. 2003;139:75–81. doi: 10.1016/s0166-4328(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Armanini MP, Hutchins C, Stein BA, Sapolsky RM. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- Bakhit C, Morgan ME, Gibb JW. Propranolol differentially blocks the methamphetamine-induced depression of tryptophan hydroxylase in various rat brain regions. Neurosci Lett. 1981;23:99–103. doi: 10.1016/0304-3940(81)90194-4. [DOI] [PubMed] [Google Scholar]
- Bielajew C, Konkle AT, Merali Z. The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats. I. Biochemical and physiological analyses. Behav Brain Res. 2002;136:583–592. doi: 10.1016/s0166-4328(02)00222-x. [DOI] [PubMed] [Google Scholar]
- Biron D, Dauphin C, Di Paolo T. Effects of adrenalectomy and glucocorticoids on rat brain dopamine receptors. Neuroendocrinology. 1992;55:468–476. doi: 10.1159/000126158. [DOI] [PubMed] [Google Scholar]
- Broom SL, Yamamoto BK. Effects of subchronic methamphetamine exposure on basal dopamine and stress-induced dopamine release in the nucleus accumbens shell of rats. Psychopharmacology (Berl) 2005;181:467–476. doi: 10.1007/s00213-005-0007-6. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stout RL, Gannon-Rowley J. Substance use disorder-PTSD comorbidity. Patients’ perceptions of symptom interplay and treatment issues. J Subst Abuse Treat. 1998;15:445–448. doi: 10.1016/s0740-5472(97)00286-9. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Meshul CK. High-dose methamphetamine treatment alters presynaptic GABA and glutamate immunoreactivity. Neuroscience. 1999;90:833–850. doi: 10.1016/s0306-4522(98)00506-5. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res. 1998;800:227–235. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Li KM, Hunt GE, McGregor IS. MDMA (‘ecstasy’) and methamphetamine combined: Order of administration influences hyperthermic and long-term adverse effects in female rats. Neuropharmacology. 2005:195–207. doi: 10.1016/j.neuropharm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5:73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- Czyrak A, Wedzony K, Michalska B, Fijal K, Dziedzicka-Wasylewska M, Mackowiak M. The corticosterone synthesis inhibitor metyrapone decreases dopamine D1 receptors in the rat brain. Neuroscience. 1997;79:489–495. doi: 10.1016/s0306-4522(96)00649-5. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S, Marti O, Armario A. Glucocorticoids are involved in the long-term effects of a single immobilization stress on the hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology. 2003;28:992–1009. doi: 10.1016/s0306-4530(02)00120-8. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL. Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology. 2002;42:837–845. doi: 10.1016/s0028-3908(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;1:1235–1243. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Bland ST, Schmid MJ, Watkins LR, Spencer RL, Maier SF. The role of glucocorticoids in the uncontrollable stress-induced potentiation of nucleus accumbens shell dopamine and conditioned place preference responses to morphine. Psychoneuroendocrinology. 2006;31:653–663. doi: 10.1016/j.psyneuen.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Willner P, Papp M. Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol. 1997;8:607–618. doi: 10.1097/00008877-199711000-00017. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Phamacol Exp Ther. 2005;312:160–169. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: Animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- Liu L, Tsuji M, Takeda H, Takada K, Matsumiya T. Adrenocortical suppression blocks the enhancement of memory storage produced by exposure to psychological stress in rats. Brain Res. 1999;821:134–140. doi: 10.1016/s0006-8993(99)01085-9. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Wittenberg L, Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res. 1996;724:251–255. doi: 10.1016/0006-8993(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience. 2004;124:637–646. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: Comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: Comparison to 3,4-methylenedioxyme-thamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Neumar RW, Meng FH, Mills AM, Xu YA, Zhang C, Welsh FA, Siman R. Calpain activity in the rat brain after transient forebrain ischemia. Exp Neurol. 2001;170:27–35. doi: 10.1006/exnr.2001.7708. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson D. The rat brain in stereotaxic coordinates. New York: Academic Press; 1982. [Google Scholar]
- Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Le Moal M, Simon H. Inhibition of corticosterone synthesis by metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL. Accumulation of non-erythroid α II-spectrin and calpain-cleaved α II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Ray SK, Ali SF, Banik NL. Calpain activation in apoptosis of motoneurons in cell culture models of experimental Parkinsonism. Ann NY Acad Sci. 2006;74:349–356. doi: 10.1196/annals.1369.034. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9:1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Soderpalm A, Nikolayev L, de Wit H. Effects of stress on responses to methamphetamine in humans. Psychopharmacology (Berl) 2003;170:188–199. doi: 10.1007/s00213-003-1536-5. [DOI] [PubMed] [Google Scholar]
- Sonino N. Inhibition of adrenal steroid biosynthesis by metyrapone. In: Agarwal MK, editor. Hormone antagonists. Berlin: Walter de Gruyter & Co; 1982. pp. 419–429. [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED. Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res. 1996;738:172–175. doi: 10.1016/0006-8993(96)00995-x. [DOI] [PubMed] [Google Scholar]
- Staszewski RD, Yamamoto BK. Methamphetamine-induced spectrin proteolysis in the rat striatum. J Neurochem. 2006;96:1267–1276. doi: 10.1111/j.1471-4159.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Elliott EM, Miller CA, Schilling JW, Newcombe R, Sapolsky RM. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58:1730–1735. doi: 10.1111/j.1471-4159.1992.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994a;14:5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Behrens BA, Lin WJ, Sapolsky RM. Physiological elevations of glucocorticoids potentiate glutamate accumulation in the hippocampus. J Neurochem. 1994b;63:596–602. doi: 10.1046/j.1471-4159.1994.63020596.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: Roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Straiko MM, Coolen LM, Zemlan FP, Gudelsky GA. The effect of amphetamine analogs on cleaved microtubule-associated protein-tau formation in the rat brain. Neuroscience. 2007;144:223–231. doi: 10.1016/j.neuroscience.2006.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata DA, Yamamoto BK. Chronic stress enhances extracellular glutamate concentrations in the striatum after methamphetamine. Washington, DC: Society for Neuroscience; 2005. Program No. 341.5. 2005 Abstract viewer/itinerary planner. Available at http://sfn.scholarone.com/itin2005/ [Google Scholar]
- Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, Palmiter RD, Cadet JL. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J Neurosci. 2005;25:5273–5279. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: A microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: Can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB, Morrow JS. Simultaneous degradation of αII- and βII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Neurotoxic regimen of methamphetamine produces evidence of behavioral sensitization in the rat. Synapse. 2001;39:1–7. doi: 10.1002/1098-2396(20010101)39:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Warren MW, Kobeissy FH, Liu MC, Hayes RL, Gold MS, Wang KK. Concurrent calpain and caspase-3 mediated proteolysis of alpha II-spectrin and tau in rat brain after methamphetamine exposure: A similar profile to traumatic brain injury. Life Sci. 2005;78:301–309. doi: 10.1016/j.lfs.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Pehek EA. A neurochemical heterogeneity of the rat striatum as measured by in vivo electrochemistry and microdialysis. Brain Res. 1990;506:236–242. doi: 10.1016/0006-8993(90)91256-g. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–131. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: Selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]