Abstract
Current hypotheses suggest that aberrant wound healing has a critical role in the pathogenesis of idiopathic pulmonary fibrosis (IPF). In these hypotheses, continuous TGF-β1 secretion by alveolar epithelial cells (AECs) in abnormal wound healing has a critical role in promoting fibroblast differentiation into myofibroblasts. Mesenchymal stem cells (MSCs) home to the injury site and reduce fibrosis by secreting multifunctional antifibrotic humoral factors in IPF. In this study, we show that MSCs can correct the inadequate-communication between epithelial and mesenchymal cells through STC1 (Stanniocalcin-1) secretion in a bleomycin-induced IPF model. Inhalation of recombinant STC1 shows the same effects as the injection of MSCs. Using STC1 plasmid, it was possible to enhance the ability of MSCs to ameliorate the fibrosis. MSCs secrete large amounts of STC1 in response to TGF-β1 in comparison to AECs and fibroblasts. The antifibrotic effects of STC1 include reducing oxidative stress, endoplasmic reticulum (ER) stress, and TGF-β1 production in AECs. The STC1 effects can be controlled by blocking uncoupling protein 2 (UCP2) and the secretion is affected by the PI3/AKT/mTORC1 inhibitors. Our findings suggest that STC1 tends to correct the inappropriate epithelial–mesenchymal relationships and that STC1 plasmid transfected to MSCs or STC1 inhalation could become promising treatments for IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a specific form of idiopathic interstitial pneumonia (IIPs) with the histological appearance of usual interstitial pneumonia (UIP). The prognosis is poor because of the lack of effective therapies.1 Current prevailing hypotheses suggest aberrant wound healing, rather than inflammation, is one of the major problems in the pathogenesis because inflammation is minimal in UIP tissues and immunosuppressive drugs do not alter the natural history of IPF.2,3 Endoplasmic reticulum (ER) stress is caused by burdens that perturb the processing and folding of proteins, resulting in the accumulation of misfolded proteins in the ER and the activation of the unfolded protein response (UPR). The UPR in alveolar epithelial cells (AECs) is a key component in the pathogenesis of IPF because UPR causes continuous stress in AECs.4,5 In the microenvironment of IPF, various insults increase ER-stress in AECs.6 Continuous UPR alters the secretion status of AECs by activating several receptor tyrosine kinase pathways.7,8 Subsequently, AECs increase the secretion of profibrotic growth factors, such as TGF-β1, FGF-2, and PDGF-BB.9 TGF-β1 promotes the differentiation of fibroblasts into myofibroblasts, which have a central role in the pathogenesis of IPF. In normal wound healing, TGF-β1 secretion from AECs is downregulated at the appropriate time during tissue repair. However, continuous TGF-β1 secretion from AECs is observed in the abnormal wound healing of IPF. To treat IPF, this miscommunication between epithelial and mesenchymal cells must be blocked.3
Mesenchymal stem cells (MSCs) have been proved to ameliorate lung remodeling in animal models through differentiating into specific cells, but differentiation into specific cells is relatively rare.10,11,12 Recent studies suggest humoral factors secreted by MSCs play more important roles in ameliorating IPF.13,14,15 In previous studies, we showed that MSCs secrete mitochondria-related hormone Stanniocalcin-1 (STC1) in a paracrine fashion under stress conditions, which improves the cell survival through the upregulation of uncoupling protein 2 (UCP2).16,17,18 In addition, recent evidence suggests the involvement of STC1 and STC2 in the subcellular function of ER, particularly through responding to UPR.17,18,19,20 In this study, we show that MSCs enhance STC1 secretion under the control of the mTORC1 pathway, which is related to aerobic glycolysis and ER-stress, in the presence of profibrotic growth factors.21 Sufficient quantities of STC1 derived from MSCs can diminish oxidative and ER-stress and the production of profibrotic growth factors in AECs. The ability of MSCs to ameliorate fibrosis depended on the secretion of STC1 and these functions of STC1 were blocked by the inhibition of UCP2 gene, which is downstream of STC1.17,19 Our findings about MSCs and STC1 in the pathology of the disease may be helpful in developing promising treatments from IPF.
Results
Growth factors promote human MSCs to secrete STC1 under the control of the PI3/AKT/mTOR pathway
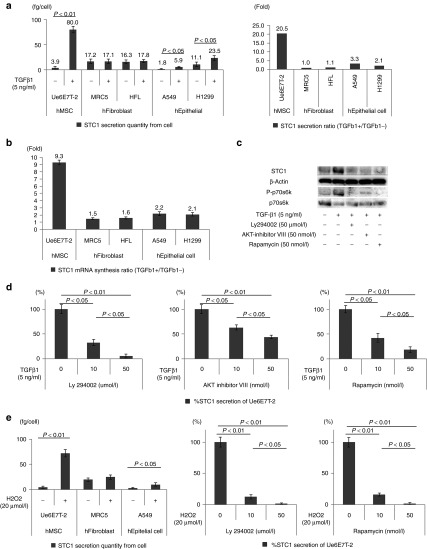
Our hypothesis is that MSCs secrete STC1 to maintain homeostasis when the microenvironment is disturbed by profibrotic factors. Therefore, the responses of MSCs exposed to a typical profibrotic factor, human TGF-β1, were evaluated. In the preceding experiment, we determined the suitable concentration (5 ng/ml) of and incubation time (24 hours) for TGF-β1 in the experiments using a human MSC cell line (hMSC: Ue6E7T-2) and naive human MSCs (MSC240L and MSC5062L) of healthy volunteers (Supplementary Figure S1). Previous studies showed that Ue6E7T-2 maintained the characteristics and functions of naive MSCs.22,23 TGF-β1 more strongly stimulated Ue6E7T-2 cells to synthesize and release STC1 into the culture medium compared to fibroblasts and epithelial cells (Figure 1a–c). Other profibrotic growth factors (human FGF-2, human PDGF-BB) and oxidative stress (hydrogen peroxide; H2O2) also more strongly stimulated Ue6E7T-2 cells to release STC1 compared to human epithelial and fibroblast cells (Figure 1e and Supplementary Figure S2a,b). Especially, FGF-2 was a stronger inducer of STC1 in hMSC compared to TGF-β1 and PDGF-BB (Supplementary Figure S2c). Next, we focused on PI3/AKT/mTOR among the pathways stimulated by growth factors because this pathway is closely involved in cell metabolism.24,25 Specific inhibitors of PI3 (Ly294002), AKT (AKT specific inhibitors VIII), and mTORC1 (rapamycin) inhibited STC1 secretion from Ue6E7T-2 under the control of other human profibrotic growth factors (PDGF-BB, FGF-2) and H2O2 (Figure 1c–e and Supplementary Figure S2d). We confirmed that these inhibitors also inhibited STC1 secretion from MSC240L and MSC5062L (Supplementary Figure S3).
Figure 1.
TGF-β1 and oxidative stress strongly stimulated the capacity of human mesenchymal stem cells (MSC) (Ue6E7T-2) to synthesize and release Stanniocalcin-1 (STC1) in comparison with human fibroblasts and lung epithelial cells. The secretion of STC1 by MSC is controlled by the PI3/AKT/mTOR pathway. (a) The quantities of secreted STC1 from human MSC (Ue6E7T-2), human fibroblasts (MRC5, HFL), and human lung epithelial cells (A549, H1299), with or without TGF-β1 (5 ng/ml) stimulation, were measured by enzyme-linked immunosorbent assay (ELISA) (left). STC1 secretion ratios (TGFβ1+/TGFβ1−) were calculated based on the STC1 protein quantities measured by ELISA as shown in Figure 1a left (right). (b) In addition, the STC1 mRNA synthesis ratios (TGFβ1+/TGFβ1−) were measured using RT-PCR. (c) Western blotting of Ue6E7T-2. STC1 production and the phosphorylation of p70s6k (P-p70s6k), as an indicator of mTOR activation, were activated by TGF-β1. Specific PI3 (Ly294002), AKT (AKT inhibitor VIII), and mTOR (Rapamycin) inhibitors reduce STC1 production and P-p70s6k expression in Ue6E7T-2. (d) Specific PI3 (Ly294002), AKT (AKT inhibitor VIII), and mTOR (rapamycin) inhibitors reduced STC1 secretion of human MSCs in the presence of TGF-β1. The values of %STC1 secretion of Ue6E7T-2 were calculated with the quantities of STC1 measured by ELISA. (e) Oxidative stress (hydrogen peroxide; H2O2 20 µmol/l) also strongly induced human MSCs to secrete STC1 in comparison with human fibroblasts and epithelial cells. Ly294002 and rapamycin also inhibited the secretion of STC1 by human MSCs. In all experiments, 5 × 105 cells were seeded in each well of 12-well plates for 48 hours before the addition of the reagents. All evaluations were done 48 hours after the addition of the reagents. Data are presented as mean ± SD of three separate experiments, each performed in triplicate. RT-PCR, real-time PCR.
STC1 diminished the oxidative stress induced by bleomycin (BLM) exposure in AECs
BLM increased intracellular reactive oxygen species (ROS) in human AECs and A549, and rSTC1 reduced the expression of ROS in A549 (Supplementary Figure S4). The experiments with H1299 showed similar results (data not shown). These results suggest that STC1 can ameliorate oxidative stress in BLM-induced pulmonary fibrosis.
hMSC and recombinant STC1 (rSTC1) ameliorated tissue damage by reducing collagen-synthesis and the accumulation of ROS in the BLM-induced pulmonary fibrosis model
First, C57BL/6J mice were injected with BLM via the trachea with a needle. Successively, rSTC1 injection via the trachea or Ue6E7T-2 injection via the tail vein was conducted. The lungs were evaluated at day 14 after the first injection of BLM (Supplementary Figure S5a and Supplementary Material and Methods). Staining with HE, EM, and 8-OHdG was used to evaluate tissue destruction, fibrotic changes (collagen deposition), and oxidative stress (ROS deposition), respectively. Remarkable pathological improvements were observed in the rSTC1- and hMSC-treated groups compared with the control group (Figure 2a). Quantification of the collagen deposition and expression intensity of 8-OHdG showed significant decreases of collagen deposition and intracellular ROS in the lungs of these groups (Figure 2b,c). Although N-acetylcystein (NAC) also had antioxidative and antifibrotic effects, rSTC1 inhalation via the trachea or injection of human MSCs via the tail vein was more strongly effective than NAC in the same model (Figure 2a–c).
Figure 2.
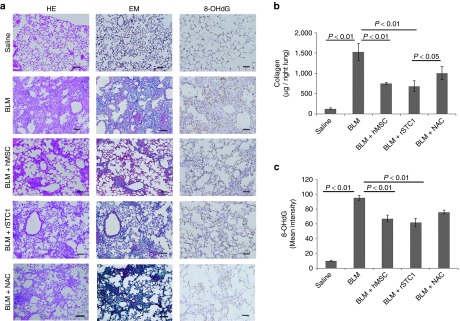
The administration of human mesenchymal stem cells (MSCs) (Ue6E7T-2) or recombinant Stanniocalcin-1 (STC1) (rSTC1) ameliorated the tissue damage in bleomycin (BLM)-induced pulmonary fibrosis. C57BL/6J mice were intratracheally injected with BLM (1 mg/kg; 20 µg/mice) in 200 µl of saline at day 0. These mice were then injected with rSTC1 (100, 250 µg/kg) or N-acetylcystein (150 mg/kg) via the trachea at day 0 or human MSCs (5 × 105 cells in 200 µl of PBS) via the tail vein at day 1. Control mice were injected with an equal volume of saline. Mice were sacrificed on day 14 and the evaluation was then conducted (see Supplementary Figure S5a). (a) Histologic examination in the left lung was performed by hematoxyline-eosin-staining (HE) and Elastica-Masson-staining (EM), and 8-hydroxy-2′-deoxyguanosine-staining (8-OHdG) in the control (saline and BLM), human MSC, rSTC1, and NAC-treated groups for the evaluation of destructive changes in the tissue, collagen-deposition and oxidative stresses. (b) Collagen deposition in the whole right lung was measured using a Sircol collagen kit in the same groups. (c) Quantification of the mean intensity of 8-OHdG in lungs was measured by HistoQUEST (Tissue Gnostics) expression analysis in the same groups. Data are presented as mean ± SD (n = 6). The bar in the photographs represents 200 µm for each section.
The ability of human MSCs to ameliorate lung injuries was affected by STC1. The enhancement of STC1 secretion by gene manipulation in human MSCs increased their capacity to ameliorate lung injury.
First, C57BL/6J mice were injected BLM via the trachea with a needle. Ue6E7T-2 transfected with/without sh-STC1, sh-CTRL, CMV-STC1, and CMV-CTRL plasmids was injected via the tail vein on the next day. The lungs of each group were evaluated at day 14 after BLM injection (Supplementary Figure S5a and Supplementary Material and Methods).
The amounts of STC1 secreted from hMSCs transfected with plasmids were measured (Supplementary Figure S5b). Exacerbated tissue destruction, increased collagen accumulation, and aggravated oxidative stress were observed in the group treated with MSCs transfected with sh-STC1 plasmid. On the other hand, amelioration of the tissue destruction, decreased collagen accumulation, and amelioration of the oxidative stress were observed in the group treated with MSCs transfected with CMV-STC1 plasmid in comparison with the control (Figure 3a). The quantification of collagen deposition and the intensity of 8-OHdG staining are shown in Figure 3b,c. A mouse MSC line, KUM10, was transfected with these plasmids and checked for their therapeutic effects in BLM-induced pulmonary fibrosis. KUM10 also ameliorated the destruction, collagen-deposition, and oxidative stress of BLM-induced pulmonary fibrosis in a STC1-dependent manner (Supplementary Figure S6). These findings suggest that STC1 is an essential factor for MSCs in the protection against lung injuries and fibrosis from excessive collagen deposition and oxidative stress in mammals.
Figure 3.
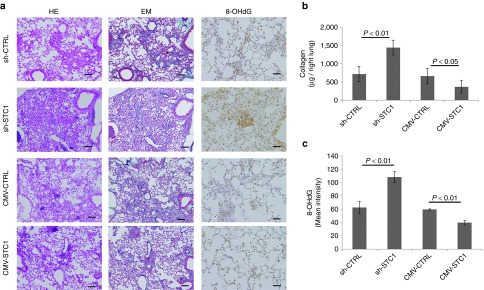
Inhibition or promotion of Stanniocalcin-1 (STC1) secretion in human mesenchymal stem cells (MSCs) (Ue6E7T-2) using plasmids affected the capacity of human MSCs to reduce collagen synthesis and oxidative stress. C57BL/6J mice were injected with bleomycin (BLM) (1 mg/kg; 20 µg/mice) in 200 µl of saline via the trachea at day 0. These mice were then injected with human MSCs (5 × 105 cells in 200 µl of phosphate-buffered saline) transfected with sh-CTRL, sh-STC1, CMV-CTRL, and CMV-STC1 plasmid via the tail vein at day 1. Mice were sacrificed on day 14 and the evaluation was then conducted (see Supplementary Figure S5a). (a) Histologic examination in the left lung was performed using HE, EM, and 8-OHdG staining in the sh-CTRL, sh-STC1, CMV-CTRL, and CMV-STC1 treated groups. (b) Collagen deposition in the whole right lung was measured using a Sircol collagen kit. (c) Quantification of the mean intensity of 8-OHdG in lungs was done by HistoQUEST expression analysis. The bar in the photographs represents a length of 200 µm. Data are presented as mean ± SD (n = 6).
ER stress causes upregulation of TGF-β1 in AECs in BLM-induced pulmonary fibrosis
Next, we investigated whether STC1 reduces ER-stress and depresses TGF-β1-synthesis continuously through relieving ROS in AECs in the animal model. In the unstressed state, UPR pathway-related proteins (PERK, ATF6, and IRE1) are bound by immunoglobulin heavy-chain-binding protein (BiP), also known as glucose regulated protein-78 (GRP78). With misfolded protein accumulation in the ER, BiP is sequestered away from these proteins; therefore, BiP expression in cells is considered to be a sensitive marker of ER stress in cells.4,26 We used GRP78/BiP antibody as the ER-stress marker and performed coimmuno-staining for it together with TGF-β1 and surfactant protein C (Sftpc). The fluorescence intensities of TGF-β1 and BiP in AECs were increased in the BLM-treated mice in comparison with the saline control, and the expressions of TGF-β1, BiP, and Sftpc were observed in the same location in the majority of AECs exposed to BLM (Figure 4a). The expression levels of TGF-β1 and BiP protein derived from whole lung homogenates were increased in the BLM-treated group. On the other hand, the administration of human MSCs and STC1 decreased the TGF-β1 and BiP expression (Figure 4b,c). The experiments with human MSCs transfected by sh-STC1, sh-CTRL, CMV-STC1, and CMV-CTRL revealed that the promotion or inhibition of STC1 synthesis in human MSCs resulted in a decrease or increase of the TGF-β1 and BiP protein expression levels in whole lung, respectively (Figure 4d,e). These data suggest that ER-stress can induce the upregulation of TGF-β1 in AECs and that the ability of human MSCs to inhibit ER-stress and TGF-β1 synthesis in AECs is dependent on STC1.
Figure 4.
Endoplasmic reticulum (ER) stress caused upregulation of TGF-β1 in alveolar epithelial cells (AECs) in bleomycin (BLM)-induced pulmonary fibrosis. C57BL/6J mice were injected with saline (200 µl) with or without BLM (1 mg/kg; 20 µg/mice) via the trachea at day 0. Mice were sacrificed on day 14. Immunofluorescence staining was performed with TGF-β1 (red), the ER stress marker BiP (green), the alveolar-epithelial-cell marker Sftpc (orange) antibodies, and DAPI (blue). (a) Immunofluorescence staining of phosphate-buffered saline (PBS)- (left) and BLM-treated groups (central and right; enlarged). (b) Western blotting analysis for TGF-β1 and BiP in whole lung in the CTRL, BLM, rSTC1, N-acetylcystein (NAC) groups. (c) Relative expressions of TGF-β1 and BiP proteins to β-actin were measured by densitometric analysis in each group, as shown in Figure 4b. (d) Western blotting analysis for TGF-β1 and BiP in whole lung in the sh-CTRL, sh-STC1, CMV CTRL, and CMV STC1 treated groups. (e) Relative expressions of TGF-β1 and BiP proteins to β-actin were measured by densitometric analysis in each group, as shown in Figure 4d. Data are presented as mean ± SD (n = 6). Scale bars: 200 µm (a, left and middle panel), 50 µm (a, right panel). DAPI, 4′,6-diamidino-2-phenylindole.
Human MSCs attenuate ER stress in a STC1-dependent manner in BLM-induced pulmonary fibrosis
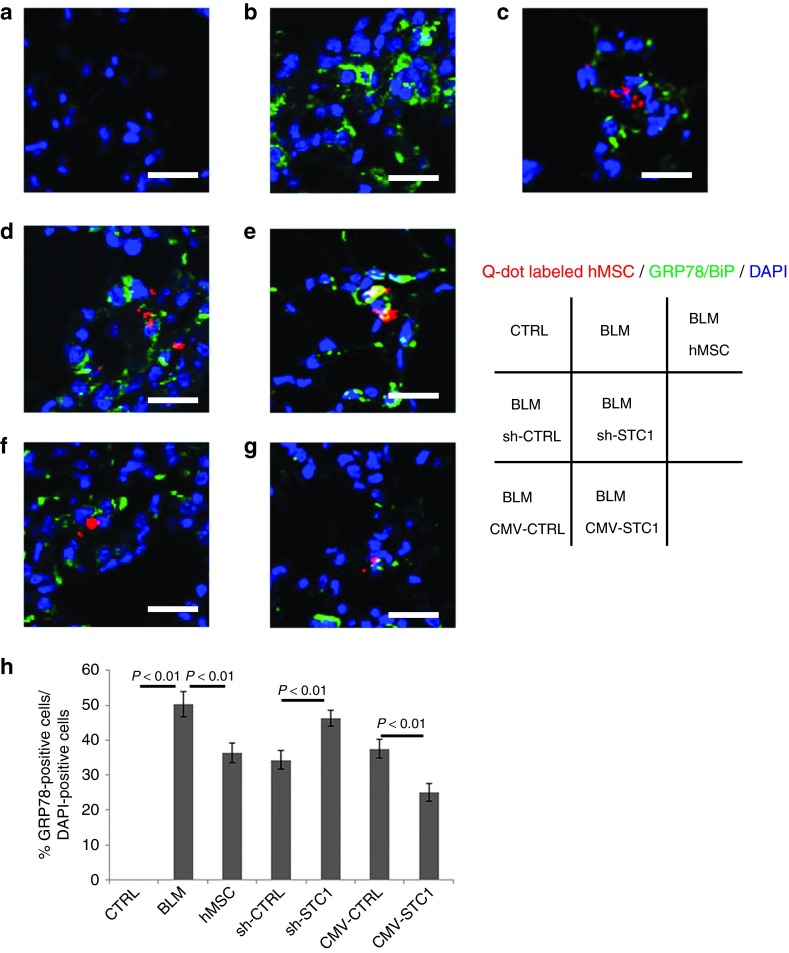
To investigate the effect of STC1 on BiP expression in AECs, we evaluated lung sections immunostained for BiP and 4′,6-diamidino-2-phenylindole (DAPI) antibodies in BLM-treated mice at day 3 after intratracheal injection of Q-dot-labeled human MSCs via the tail vein. The expression of BiP increased in the AECs of BLM-treated mice in comparison with the saline-treated group (Figure 5a,b,h). The administered human MSCs were engrafted in damaged AECs. Q-dot-labeled human MSCs transfected with sh-CTRL and CMV-STC1 attenuated the BiP expression in the surrounding tissues in comparison with BLM-treatment without MSC, shSTC1, and CMV-CTRL, respectively (Figure 5b–h). The expression of CD45 (antileucocyte common antigen) with these Q-dots cells was negative (Supplementary Figure S7). In the case of human MSCs transfected with sh-STC1 plasmid, the immune fluorescence of labeled MSCs merged with that of BiP (Figure 5e). Human MSCs transfected with CMV-STC1 plasmid showed strongly reduced BiP expression compared to the other MSCs (Figure 5g). These findings suggest that MSCs reduced the ER-stress in the lung microenvironment through the paracrine secretion of STC1.
Figure 5.
Human mesenchymal stem cells (Ue6E7T-2) ameliorated ER stress in bleomycin (BLM)-induced pulmonary fibrosis in a Stanniocalcin-1 (STC1)-dependent manner. C57BL/6J mice were injected with PBS 200 µl (a) or BLM (1 mg/kg; 20 µg/mice) in 200 µl PBS (b–g) into the trachea on day 0. PBS 200 µl (b), 1 × 105 Ue6E7T-2 in PBS 200 µl with Q-dot-label (red) (c), Q-dot-labeled (red) transfected with sh-CTRL (d) sh-STC1 (e), CMV-CTRL (f), or CMV-STC1 plasmid (g) was injected into the tail vein on day 1. Mice were sacrificed on day 3. Immunofluorescence staining was performed with the ER stress marker BiP (green) and DAPI (blue) antibodies. Bar represents 100 µm in each photograph. (h) Proportion of BiP-positive cells to DAPI-positive cells (%). Data are presented as mean ± SD (n = 6). DAPI, 4′,6-diamidino-2-phenylindole.
STC1 reduction in ER stress and TGF-β1 production in BLM-treated AECs was dependent on UCP2
Firstly, we examined whether oxidative stress could induce ER-stress in AECs because H2O2 induces BiP in AECs (Supplementary Figure S8a). Next, we confirmed that BLM also induced BiP and determined that the appropriate concentration of BLM for the evaluation of ER-stress is 10 or 50 µg/ml because this concentration induced BiP but not CHOP expression in AECs (Supplementary Figure S8b,c). Prolonged or severe ER stress can result in cellular apoptosis through transcription factor CHOP, sequential caspase-12, and JNK pathway activation, which is activated by all three UPR pathways. Therefore, CHOP expression in cells indicates that the stimulation for inducing ER stress was too severe to benefit from the protective functions of these factors.4,27 More than 100 µg/ml of BLM induced CHOP expression and decreased the cell viability (Supplementary Figure S8d).
BLM 50 µg/ml upregulated the mRNA of fibrosis-related growth factors TGF-β1, FGF2, and PDGF-B in AECs and the addition of rSTC1 50 ng/ml downregulated the mRNA expression of these factors. UCP2 inhibition using specific siRNA in AECs abolished the ability of STC1 to diminish these growth factors (Figure 6a). The immunoblotting experiment for TGF-β1 in AECs showed similar results to those of real-time PCR, as shown in Figure 6a left (Figure 6b). These findings suggest UCP2 is an essential molecule for the function of STC1 in diminishing TGF-β1, as is STC1 in diminishing oxidative stress.17
Figure 6.
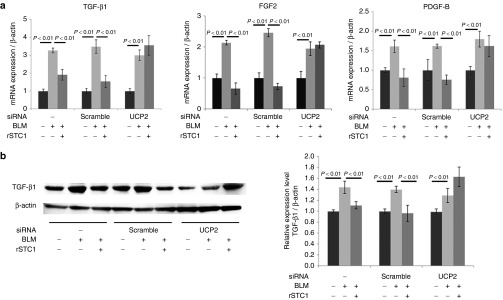
Stanniocalcin-1 (STC1) reduced TGF-β1 production in lung epithelial A549 cells exposed to bleomycin (BLM). The function depended on uncoupling protein 2 (UCP2). STC1 also reduced other fibrosis-related growth factors, FGF2 and PDGF-B, in A549. A549 cells were exposed to culture medium, BLM (50 µg/ml) and BLM with rSTC1 (50 ng/ml) for 24 hours. (a) TGF-β1, FGF2, and PDGF-B mRNA expressions were measured by real-time PCR. Specific siRNA to UCP2 blocked the ability of mesenchymal stem cells (MSCs) to reduce the mRNA level of these growth factors in A549. (b) The level of TGF-β1 protein in A549 cells was determined by western blotting (left). Relative protein levels of TGF-β1 (normalized by β-actin) in A549 cells were measured by densitometric analysis (right). Specific siRNA to UCP2 blocked the ability of MSCs to reduce the relative expression of TGF-β1 protein to β-actin in A549. Data are presented as mean ± SD of three separate experiments, each performed in triplicate.
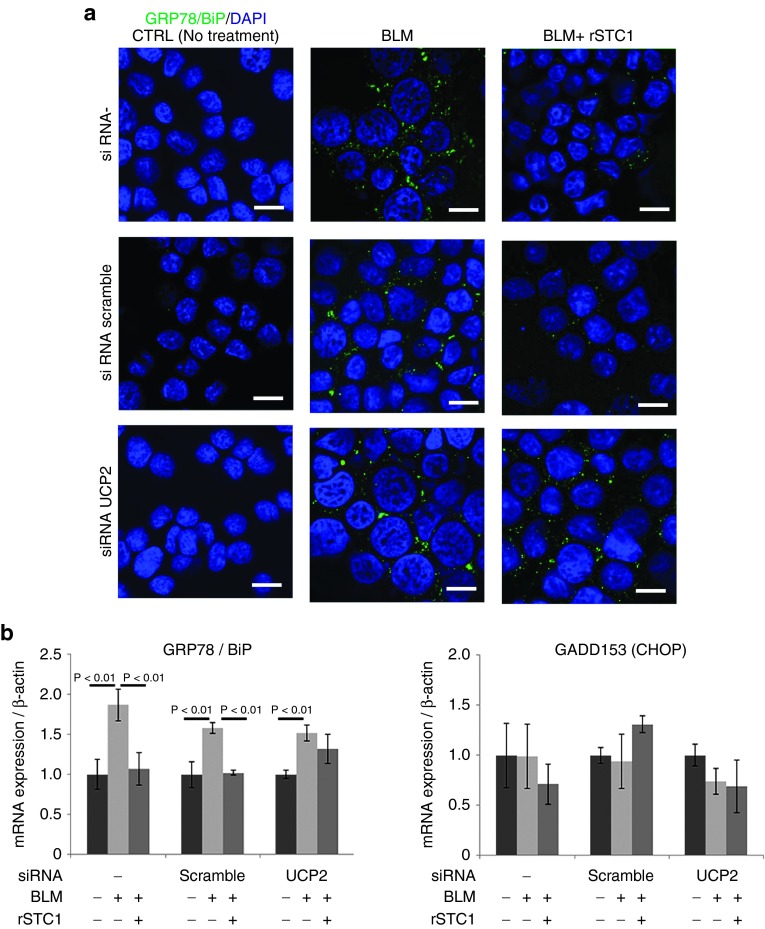
Decrease of ER stress by STC1 in BLM-treated AECs is dependent on UCP2
To clarify the effect of STC1 on ER-stress, we evaluated the BiP expression in BLM-exposed (50 µg/ml) AECs by immunofluorescence staining and real-time PCR. The addition of rSTC1 (50 ng/ml) to A549 cells cultured with BLM reduced the BiP expression to the same level as that of CTRL in the immunofluorescence staining of DAPI and BiP (Figure 7a). The addition of rSTC1 to A549 cells cultured with BLM decreased the expression of BiP and mRNA expression. However, knock down of UCP2 with specific siRNA diminished the ability of STC1 to reduce the expression of BiP and mRNA expression (Figure 7a,b). The expression level of CHOP mRNA in A549 cells was not affected by the addition of BLM (50 µg/ml), rSTC1 (50 ng/ml), or UCP2 blocking with specific siRNA (Figure 7b). These data suggest that the ability of STC1 to reduce ER-stress (BiP) depends on UCP2, which induces uncoupling respiration in mitochondria. Uncoupling respiration is synonymous with aerobic glycolysis, known as the “Warburg Effect” in cancer. In other words, noncancerous cells use the Warburg effect to reduce oxidative stress, ER stress and TGF-β1 production for cell protection, and antifibrosis through STC1 secretion. Although the quantity of STC1 from AECs and fibroblasts may be insufficient to reduce fibrosis, that from MSCs may be enough to cure fibrosis.
Figure 7.
Stanniocalcin-1 (STC1) reduced the expression level of BiP in A549 cells exposed to BLM. The ability of STC1 depended on UCP2. On the other hand, bleomycin (BLM) and rSTC1 did not affect the expression level of CHOP. A549 cells were exposed to culture medium, BLM (50 µg/ml) and BLM with rSTC1 (50 ng/ml) for 24 hours. (a) Immunofluorescence staining was performed with the ER stress marker BiP (green) and DAPI (blue) antibodies. (b) BiP and CHOP mRNA expressions in A549 cells exposed to BLM were measured by real-time PCR. The ability of STC1 to reduce BiP was inhibited by UCP2 knock down using siRNA in A549 (left). On one side, BLM and rSTC1 did not affect the CHOP expression level in A549 (right). The bar in the photographs represents a length of 20 µm. Data are presented as mean ± SD of three separate experiments, each performed in triplicate. DAPI, 4′,6-diamidino-2-phenylindole.
The oxidative stress scavenger N-acetylcysteine (NAC) also decreases TGF-β1 production and ER stress in BLM-treated AECs, but NAC does not depend on the function of UCP2.
NAC is a strong scavenger of products in cells derived from oxidative stress and is used for the treatment of IPF. We attempted to confirm whether NAC also decreases the mRNA expressions of TGF-β1, FGF2, PDGF-B, and ER-stress in AECs. NAC (5 mmol/l = 1.63 mg/ml) can decrease these growth factors and ER-stress (GRP78/BiP) induced by BLM (50 µg/ml) in AECs. UCP2 knock-down with specific siRNA in AECs did not affect the NAC functions that reduce growth factors and ER-stress (BiP) (Supplementary Figure S9a,b). These findings suggest that the mechanism by which STC1 reduces fibrosis is different from that of NAC. STC1 (50 ng/ml) can reduce fibrosis at an extremely low concentration compared with NAC (1.63 mg/ml).
The mTORC1 function induced by TGF-β1 is essential for the protection against ER stress in human MSCs
To investigate the significance of mTOR in ameliorating the ER-stress induced by TGF-β1 in human MSCs, we made an experiment with a specific mTORC1 inhibitor, rapamycin. Rapamycin increased the ER-stress of human MSCs in both a normal culture condition and one with TGF-β1 (Supplementary Figure S10). This result suggests that the mTORC1 function is an essential factor for reducing ER-stress and maintaining homeostasis in MSCs.
STC1 reduced ER-stress and TGF-β1 synthesis in alveolar macrophages in bleomycin-induced pulmonary fibrosis
Macrophages are also considered one of the important sources of TGF-β1 in the pathogenesis of IPF.2 The relationship between ER-stress and TGF-β1 production in macrophages is still obscure. We confirmed that STC1 also reduces ER-stress and TGF-β1 synthesis in macrophages separated from BAL fluid of C57BL/6J mice (Supplementary Figure S11a,b) and THP-1 cell differentiated into macrophage exposed by bleomycin (Supplementary Figure S11c). This result suggests that STC1 can reduce ER-stress and TGF-β1 synthesis in macrophages in bleomycin-induced pulmonary fibrosis.
Discussion
Traditional therapies that are not based on sufficient knowledge of molecular mechanisms have failed to achieve satisfactory results in IPF. New drugs including Pirfenidone and the multiple RTKs inhibitor BIBF1120 were suggested to reduce the annual decline of the vital capacity and improve the prognosis, but the improvement in the prognosis was inadequate.1,28,29 Therefore, additional new therapies for IPF are needed.3 MSCs hold promise as a novel treatment in multiple diseases including intractable pulmonary diseases.13,30
Intratracheal or intravenous administration of MSCs was efficacious in a rodent model of IPF.14,31,32 Phase 1 clinical trials for IPF have been started and intravenous infusions of autologous or allogeneic MSCs have been shown to be well tolerated.33 However, the precise mechanisms by which MSCs ameliorate fibrosis remain elusive. Perhaps, multiple functions of MSCs, including cell fusion, cell–cell interactions, immunomodulation, wound healing, and cell metabolism, are involved in these effects. Paracrine factors from MSCs have also been proposed as an important mechanism of action in an animal model and in several clinical trials.34 When MSCs meet the new microenvironment at the sites of damaged tissue, they release large amounts of paracrine factors (“first encounter effects”).35 These paracrine factors have myriad functions such as anti-inflammatory and antibacterial functions and by enhancing phagocytosis while decreasing metabolic stress.36,37,38 MSCs also exert an effect on mitochondria for cell protection.39,40 Interestingly, UCP2 upregulation induced by STC1 could increase the uncoupling respiration of mitochondria, decrease oxidative stress, and promote the survival of AECs under harmful microenvironments.17,19,20
Recent evidence suggests that STCs exert cell protection in a manner dependent on UPR.18,41 Therefore, we hypothesized that STC1 has essential roles in the amelioration of fibrosis in lung by MSCs through regulating oxidative and ER-stress and by reducing TGF-β1 production in the microenvironment of pulmonary fibrosis.
In this study, we confirmed that human and mouse MSCs reduced collagen deposition and oxidative stress in the BLM-induced pulmonary fibrosis model (Figure 2 and Supplementary Figure S6). These effects depended on STC1 secreted from MSCs, and recombinant STC1 reproduced the same results (Figures 2 and 3 and Supplementary Figures S5 and S6).
The side effects of STC1 are still unknown. STC1 expression is upregulated in several types of cancer.42,43 Perhaps, intracellular, upregulated STC1 protects cells in tissues from harmful situations including infarction, infection, and inflammation. Though STC1 transgenic mice do not appear susceptible to carcinogenesis, MSCs transfected with STC1 plasmid for homing to the injury site may have the advantage of requiring less STC1 compared with rSTC1 inhalation to the whole bronchus in lung.44 It is noteworthy that even rSTC1 inhalation requires 600–1,500 times less STC1 (100, 250 µg/kg) than NAC (150 mg/kg) (Figure 4 and Supplementary Figure S9). Such a reduction in the quantity may have the advantage of increasing the safety and applicability of inhalation therapy, as with corticosteroid for bronchial asthma.
The first encounter effects of MSCs were evaluated by culturing MSCs with TGF-β1, PDGF, FGF2 or hydrogen peroxidase, representing the IPF microenvironment.9,45 As expected, these substances induced the MSCs to secrete STC1 in a robust manner. The synthesis or secretion ratio of the STC1 of MSCs was considerably larger than those of AECs and fibroblasts in vitro (Figure 1 and Supplementary Figure S2).
The pathway shared by these growth factors and oxidative stress in MSCs in the secretion of STC1 was investigated. We focused on the PI3/AKT/mTOR pathway because of its relationship with glycolysis and ER-stress.21,24,25 As expected, rapamycin, a specific inhibitor of mTORC1, which belongs to the PI3/AKT/mTOR pathway, can reduce STC1 secretion from MSCs. Rapamycin also induced ER-stress in MSCs with or without TGF-β1 (Figure 1 and Supplementary Figures S2 and S10). These results suggest that mTORC1 in MSCs is a central factor in the control of STC1 synthesis and secretion for protecting MSCs and the surrounding tissue. Our findings suggest that mTORC1 is a sensor of extracellular stress that prompts cells to adapt to harmful conditions through the secretion of STC1. FGF2 was a strong inducer of STC1 in comparison with PDGF-BB and TGF-β1 in epithelial and mesenchymal cells, but the reason is still unknown. FGF2 has various functions in MSCs including the promotion of wound healing, maintenance of stemness and differentiation into specific cells.45,46 FGF2 may act as a speedy initiator of the early stage of wound healing by activating STC1 secretion by MSCs. Interestingly, STC1 has a significant role in sensing calcium and ATP waves through P2X and P2γ receptors as the first signal of injury in cells.7 FGF2 and STC1 may collaborate in early wound healing.
Finally, we revealed that rSTC1 or STC1 released from MSCs decreased oxidative stress, ER-stress and TGF-β1 synthesis in AECs with BLM-induced pulmonary fibrosis and AEC cell lines (A549, H1299) exposed to BLM (Figures 4–7 and Supplementary Figures S8 and S9). ER-stress is a strong inducer of TGF-β1 in AECs.4,5,47 STC1 reduced TGF-β1 production and ER-stress (GRP78/BiP) in AECs through the UCP2 function, as demonstrated by the fact that UCP2 knock down with specific siRNA abolished these effects (Figures 6 and 7). These results suggest that the decrease of oxidative stress in AECs via UCP2 upregulation by STC1 results in ameliorating ER-stress, and the decrease of ER-stress causes a reduction of TGF-β1 synthesis in AECs.
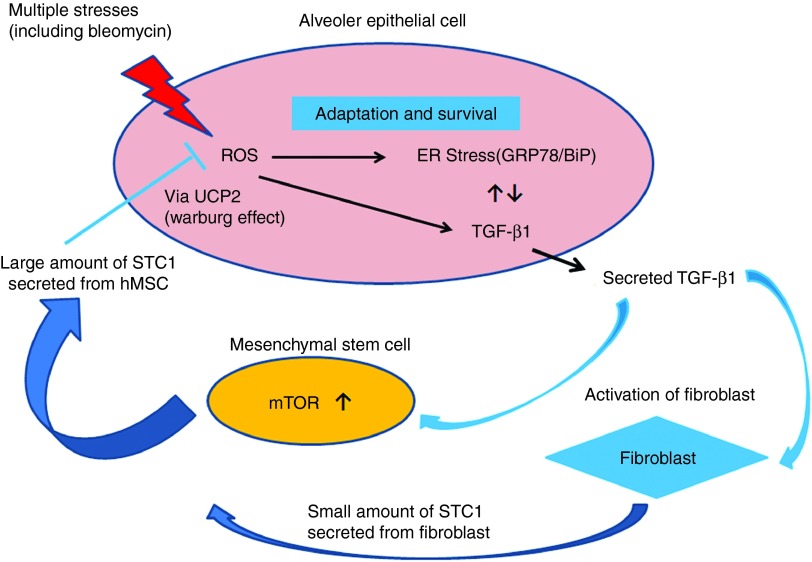
The relationship among these factors is summarized in Figure 8. MSCs form a kind of negative feedback loop and cancel the inappropriate epithelial–mesenchyme relationship in order to sustain homeostasis in the lung microenvironment. The utilization of MSCs transfected with STC1 plasmid and/or direct rSTC1 inhalation could lead to promising new treatments for IPF.
Figure 8.
A proposed model of our study is shown. Normalization of the inappropriate relation between epithelium and mesenchyme is achieved by Stanniocalcin-1 (STC1) secretion from mesenchymal stem cells (MSCs) stimulated by TGF-β1 in the microenvironment of pulmonary fibrosis. ER stress and oxidative stress generated by various types of stress led to excessive TGF-β1 synthesis in AECs. TGF-β1 promoted the secretion of large amounts of STC1 by MSCs via PI3/AKT/mTOR pathway. STC1 derived from MSCs reduced oxidative stress, ER stress and TGF-β1 synthesis in AECs under the function of UCP2, which shifts mitochondrial respiration into aerobic glycolysis. This is one of the negative feedback pathways for the protection against fibrosis in cooperation with AECs and MSCs.
Various cells beside AECs, such as macrophages, lymphocytes, vascular endothelial and pericyte cells etc., have multiple roles in the pathogenesis of IPF. Some papers mention that STC1 affects the cell function of macrophages and endothelial cells.48 STC1 reduces the macrophage immunological function. STC1 production by macrophages is very low.48,49,50 Besides AECs, macrophages are an important source of TGF-β1 in the pathogenesis of IPF.2 Therefore, we evaluated the effects of STC1 on macrophages and confirmed STC1 also reduce ER-stress and TGF-β1 expression in macrophage (Supplementary Figure S11). Whether our hypothesis is applicable to other cells involved in the pathogenesis of IPF and related to ER-stress, oxidative stress and immunological function etc. should be investigated in the future.
Materials and Methods
See the detailed explanation of the materials and methods in the Supplementary Information.
Cell lines. Ue6E7T-2, a human mesenchymal stem cell line and KUM-10, a C57BL/6J mouse mesenchymal stem cell line were kindly provided by RIKEN (Tsukuba, Japan). The human naive MSCs, MSC240L and MSC5062L, were kindly provided by Dr Prockop of Texas A&M University (Austin, TX).
Plasmid vector construction. CMV-STC1 and CMV-CTRL plasmids were based on pBluescript II KS(+) vectors (Agilent Technologies, Santa Clara, CA). sh-STC1 and sh-CTRL plasmids included short-hairpin RNA (sh-RNA) for inhibiting STC1 and the negative control, respectively. These plasmids were constructed using pBAsi hU6 Pur DNA (TaKaRa, Ohtsu, Japan).
Animal and experimental protocol. All procedures were performed according to protocols approved by the Institutional Committee for the Use and Care of Laboratory Animals of Tohoku University. Female C57BL/6J mice, 8 weeks old, were purchased from Japan Charles River (Yokohama, Japan).
Small interfering RNA trasfections. All siRNAs were purchased from Ambion (Ambion, Austin, TX). SiRNAs were transfected to cells using siPORT NeoFX.
Real-time PCR analysis. TGF-β1, FGF-2, PDGF-B, BiP, CHOP, STC1, UCP2, and β-actin expressions were determined at the mRNA level by real-time PCR analysis using SYBR Select master mix (Life-technologies, Tokyo, Japan).
Western blotting. To detect the expression levels of STC1, TGF-β1, and BiP protein, protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane and incubated with anti-STC1 (#AF2958;R&D), anti-TGF-β1 (#9016;R&D), anti-GRP78/BiP (#ab21685; Abcam, Tokyo, Japan) antibodies.
Enzyme-linked immunosorbent assay (ELISA). The concentrations of STC1 in culture media were analyzed using an ELISA kit (DY2958; R&D, Minneapolis, MN).
Measurement of ROS. ROS were measured using acetoxymethyl ester dye, 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (Life-technologies).
Labeling of hMSCs with Q-tracker. Ue6E7T-2 was labeled using a Qtracker 655 Cell Labeling Kit (Life-technologies).
Immunohistochemistry. The staining intensity of 8-OHdG antibodies (Nichirei, Tokyo, Japan) in lungs was quantified by tissue cytometry using HistoQUEST analysis software (Tissue Gnostics, Vienna, Austria). The immunohistochemistry of anti-TGF-β1 (R&D) and Anti-GRP78 BiP (Abcam) antibodies and anti-surfactant protein C (#SC-7706; Santa Cruz Biotechnology, Dallas, TX) was performed, followed by incubation with Alexa Fluor 633 anti-mouse IgG for TGF-β1 and Alexa Fluor 488 anti-rabbit IgG (Life-technologies) for BiP and Alexa Fluor 555 anti-goat IgG (Life technologies) for surfactant protein C.
Quantification of collagen in lung. Total lung collagen was determined using the Sircol Collagen Assay kit (Biocolor, Carrickfergus, UK).
Statistics. Data are presented as mean ± SD for statistical analysis; the data were analyzed by one-way analysis of variance with either Tukey–Kramer or Student's t-test (JMP software, SAS Institute, Cary, NC). The differences were considered statistically significant when the P value was less than 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Determining suitable concentration and culture time of MSCs with TGF-β1. Figure S2. PDGF-BB and FGF2 more strongly stimulated the capacity of human MSCs (Ue6E7T-2) to release STC1 compared with human fibroblasts and lung epithelial cells. Figure S3. Specific inhibitors against PI3, AKT, mTORC1 decrease STC1 production induced by TGF-β1 in MSC240L and MSC5062L. Figure S4. STC1 decreased ROS in BLM-exposed AECs. Figure S5. Animal experiment methods and STC1 secretion of human MSCs transfected with plasmids. Figure S6. The administration of recombinant STC1 or mouse MSCs (KUM10) ameliorated the tissue damage caused by the BLM-induced pulmonary fibrosis. Figure S7. CD45 positive cells did not take in Q-dots. Figure S8. The concentration of BLM (10 or 50 µg/ml) used in this study induced GRP78/BiP but not GADD153/CHOP expression in AECs. Figure S9. NAC (N-acetylcystein) reduced TGF-β1 (FGF-2, PDGF-B) and GRP78/BiP expression induced by bleomycin (BLM) in AECs. Figure S10. Rapamycin increased ER-stress (GRP78/BiP) in human MSCs with or without the presence of TGF-β1. Figure S11. The administration of recombinant STC1 or human MSCs (Ue6E7F-2) reduced the expression of TGF-β1 and ER stress in macrophages from BAL fluid of bleomycin-treated mice. Materials and Methods
Acknowledgments
This work was supported by grants from the Japan society for the Promotion of Science (JSPS-24591149, 24592080) and Japan Science and Technology Agency (JST-AS242Z01715P). Thanks to RIKEN BioResource Center (Tsukuba, Japan) and Prockop at TEXAS A&M University for kindly providing the MSCs cell lines and human naïve MSCs and for supporting our facilities damaged by the East-Japan Earthquake in 2011. We also thank Brent Bell for reading the manuscript. All authors have no conflict of interest to commercial companies concerning this work. Conception and design: M.O., S.O.; analysis and interpretation: M.O., S.O., M.K., N.T., M.K., M.E., T.K., T.I., H.O., Y.O.; drafting the manuscript for important intellectual content: S.O., H.K., T.A., M.I.
Supplementary Material
References
- Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M.et al. (2008Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis Am J Respir Crit Care Med 178838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer RJ, Elias JA, Lee CG, Herzog E. Modern concepts on the role of inflammation in pulmonary fibrosis. Arch Pathol Lab Med. 2011;135:780–788. doi: 10.5858/2010-0296-RA.1. [DOI] [PubMed] [Google Scholar]
- Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011;163:141–172. doi: 10.1111/j.1476-5381.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon JA, Nagy LH, Llamas CB, Tucker HA, Lee RH, Prockop DJ. Integrin expression and integrin-mediated adhesion in vitro of human multipotent stromal cells (MSCs) to endothelial cells from various blood vessels. Cell Tissue Res. 2010;341:147–158. doi: 10.1007/s00441-010-0994-4. [DOI] [PubMed] [Google Scholar]
- Jenkins G, Blanchard A, Borok Z, Bradding P, Ehrhardt C, Fisher A.et al.; ECIPF workshop 2012In search of the fibrotic epithelial cell: opportunities for a collaborative network Thorax 67179–182. [DOI] [PubMed] [Google Scholar]
- Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol. 2013;14:249–262. doi: 10.1038/nrm3541. [DOI] [PubMed] [Google Scholar]
- Maitra M, Cano CA, Garcia CK. Mutant surfactant A2 proteins associated with familial pulmonary fibrosis and lung cancer induce TGF-β1 secretion. Proc Natl Acad Sci U S A. 2012;109:21064–21069. doi: 10.1073/pnas.1217069110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nishioka Y, Azuma M, Kishi M, Aono Y. Targeting platelet-derived growth factor as a therapeutic approach in pulmonary fibrosis. J Med Invest. 2013;60:175–183. doi: 10.2152/jmi.60.175. [DOI] [PubMed] [Google Scholar]
- Sharma A, Janocha AJ, Hill BT, Smith MR, Erzurum SC, Almasan A. Targeting mTORC1-mediated metabolic addiction overcomes fludarabine resistance in malignant B cells. Mol Cancer Res. 2014;12:1205–1215. doi: 10.1158/1541-7786.MCR-14-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M.et al. (2007Targeted cell replacement with bone marrow cells for airway epithelial regeneration Am J Physiol Lung Cell Mol Physiol 293L740–L752. [DOI] [PubMed] [Google Scholar]
- Toonkel RL, Hare JM, Matthay MA, Glassberg MK. Mesenchymal stem cells and idiopathic pulmonary fibrosis. Potential for clinical testing. Am J Respir Crit Care Med. 2013;188:133–140. doi: 10.1164/rccm.201207-1204PP. [DOI] [PubMed] [Google Scholar]
- Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T.et al. (2011Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model Mol Ther 19196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K.et al. (2007Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury Proc Natl Acad Sci USA 10411002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R.et al. (2009Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1 Stem Cells 27670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkouchi S, Block GJ, Katsha AM, Kanehira M, Ebina M, Kikuchi T.et al. (2012Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1 Mol Ther 20417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung BH, Law AY, Wong CK. Evolution and roles of stanniocalcin. Mol Cell Endocrinol. 2012;349:272–280. doi: 10.1016/j.mce.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Samudio I, Fiegl M, McQueen T, Clise-Dwyer K, Andreeff M. The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res. 2008;68:5198–5205. doi: 10.1158/0008-5472.CAN-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I, Fiegl M, Andreeff M. Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22:274–282. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Mori T, Kiyono T, Imabayashi H, Takeda Y, Tsuchiya K, Miyoshi S.et al. (2005Combination of hTERT and bmi-1, E6, or E7 induces prolongation of the life span of bone marrow stromal cells from an elderly donor without affecting their neurogenic potential Mol Cell Biol 255183–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Mori T, Imabayashi H, Kiyono T, Gojo S, Miyoshi S.et al. (2004Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation J Gene Med 6833–845. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C.et al. (2006An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis Chest 130227–237. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK.et al.; ASCEND Study Group 2014A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis N Engl J Med 3702083–2092. [DOI] [PubMed] [Google Scholar]
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U.et al.; INPULSIS Trial Investigators 2014Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis N Engl J Med 3702071–2082. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Thompson BT, Read EJ, McKenna DH, Jr, Liu KD, Calfee CS.et al. (2010Therapeutic potential of mesenchymal stem cells for severe acute lung injury Chest 138965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D.et al. (2009Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis Cell Transplant 18405–422. [DOI] [PubMed] [Google Scholar]
- Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S.et al. (2009Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury Am J Pathol 175303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A.et al. (2013A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis J Transl Med 11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty K, Janes SM. Stem cells and pulmonary fibrosis: cause or cure. Proc Am Thorac Soc. 2012;9:164–171. doi: 10.1513/pats.201201-010AW. [DOI] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW.et al. (2012Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes Am J Physiol Lung Cell Mol Physiol 302L1003–L1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V.et al. (2012Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia Thorax 67533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R.et al. (2001Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell Cell 105369–377. [DOI] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K.et al. (2012Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury Nat Med 18759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Walker JR, Thompson CS, Moroz I, Lin W, Veselits ML.et al. (2004Characterization of stanniocalcin 2, a novel target of the mammalian unfolded protein response with cytoprotective properties Mol Cell Biol 249456–9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S, Oshima T, Yoshihara K, Kanazawa A, Yamada T, Inagaki D.et al. (2011Clinical significance of STC1 gene expression in patients with colorectal cancer Anticancer Res 31325–329. [PubMed] [Google Scholar]
- Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J.et al. (2010Stanniocalcin 1 and ovarian tumorigenesis J Natl Cancer Inst 102812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese R, Gagliardi AD, Bialek PE, Yee SP, Wagner GF, Dimattia GE. Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology. 2002;143:868–876. doi: 10.1210/endo.143.3.8671. [DOI] [PubMed] [Google Scholar]
- Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U.et al. (2008PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages Blood 112295–307. [DOI] [PubMed] [Google Scholar]
- Wu J, Huang GT, He W, Wang P, Tong Z, Jia Q.et al. (2012Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla J Endod 38614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG.et al. (2010Defining the risks of mesenchymal stromal cell therapy Cytotherapy 12576–578. [DOI] [PubMed] [Google Scholar]
- Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010;298:F248–F254. doi: 10.1152/ajprenal.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Ko JH, Lee HJ, Yu JM, Choi H, Kim MK.et al. (2014Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species Stem Cells 321553–1563. [DOI] [PubMed] [Google Scholar]
- Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.