Abstract
The contribution of bone marrow cells (BMC) in lung repair is controversial. We previously reported a subpopulation of BMC that express Clara cell secretory protein (CCSP). To determine the contribution of endogenous CCSP+ BMC to airway regeneration, we performed bone marrow transplantation studies using the CCtk mouse, which expresses a thymidine kinase suicide gene under regulation of the CCSP promoter. Mice were transplanted with wild-type or CCtk BMC and treated with ganciclovir to eliminate CCSP+ cells. After airway injury using naphthalene, mice depleted of CCSP+ BMC had more inflammatory cells in lung and decreased levels of oxygen in arterial blood. They also had reduced expression of airway epithelial genes and less Clara cells compared to control mice that had intact CCSP+ BMC and bone marrow derived CCSP+ cells in the airways. After naphthalene injury, administration of CCSP reproduced the beneficial effect of CCSP+ BMC by improving recovery of airway epithelium, reducing lung inflammation and increasing oxygen in arterial blood from mice depleted of CCSP+ BMC. Our data demonstrate that ablation of CCSP+ BMC delays airway recovery and suggests the beneficial effect of CCSP+ BMC in lung recovery is in part due to production of CCSP itself.
Introduction
The homeostasis of the airway epithelium is maintained by the infrequent proliferation of Clara cells which are progenitor cells capable of producing both more Clara cells and ciliated cells.1,2 An important characteristic of Clara cells is their production of Clara cell secretory protein (CCSP), which has anti-inflammatory and immunomodulatory properties besides playing a role in host defense and control of oxidative stress.1,3,4,5,6,7,8
The remodeling of the airway epithelium is a key factor in the pathogenesis of chronic lung diseases.1,9,10,11,12 Several pathologic changes take place after chronic lung injury, including loss of surface epithelial integrity, partial shedding of the epithelium, and the denudation of the basement membrane.13 In patients with chronic airway injury, there is a decreased concentration of CCSP in bronchial epithelium, bronchioalveolar lavage (BAL) and serum.14,15,16,17,18 For example, in the lung trasnplantation field, some publications had demonstrated that patients with bronchiolitis obliterans syndrome (BOS) had lower levels of CCSP in BAL compared to those without BOS.19,20,21 In contrast, some data suggest that the CCSP levels in BAL among patients that were BOS-free, BOS-free with acute rejection or acute infection were not significantly different.21 These data points towards the inability of some studies to assess if CCSP changes are a cause or consequence of the events that lead to disease21 and demonstrates the necessity to study in more detail the relation of CCSP levels, CCSP-expressing cells ablation and lung disease.
The CCtk transgenic mouse which expresses the Herpes simplex thymidine kinase suicide gene under regulation of the mouse CCSP promoter has been used to induce ablation of CCSP-expressing cells (CCSP+). Treatment of CCtk mice with ganciclovir results in ablation of epithelial stem and progenitor cell pools and initiates a stress response by remaining lung cells,22,23,24 induces an excessive deposition of extracellular matrix,25 and leads to failure of airway regeneration that is associated with rapid mortality.24
The potential of bone marrow cells (BMC) to facilitate lung repair after injury has been suggested by several studies in human and animal models.26,27,28,29,30 However, the role that endogenous bone marrow plays is less certain. The existence of a population of cells that express CCSP in the bone marrow of human and mouse has been demonstrated by our group and others.31,32,33,34 Further characterization of the CCSP+ BMC by flow cytometry, FACS-sorting, real time PCR and immunofluorescence staining has demonstrated that these cells also express mesenchymal markers CD73, CD90, and CD105 but not CD106, collagen type I or collagen type IV. On the other hand, these cells also express CD45 and CD34, which suggest the CCSP+ BMC are a unique population that coexpresses hematopoietic and mesenchymal markers.33
The CCSP+ BMC cells are increased in peripheral blood and home to the lung in response to injury.31,33 When administered transtracheally they increased bronchial epithelial repair and animal survival while reducing lung inflammation in CCtk mice after ablation of CCSP+ cells.31
The goal of this study was to determine if endogenous CCSP+ BMC affect airway regeneration. Prior depletion of CCSP+ BMC in mice subsequently injured by naphthalene was associated with decreased number of airway Clara cells, reduced expression of airway epithelial markers, and increased inflammatory cells in BAL. These mice also had decreased levels of oxygen in blood compared to control mice that had intact CCSP+ BMC and bone marrow-derived CCSP+ cells in the airways. Intratracheal administration of CCSP protein reproduced the beneficial effects of CCSP+ BMC in lung recovery when given to mice depleted of CCSP+ BMC and injured with naphthalene. These mice had increased levels of oxygen in blood and increased expression of airway epithelial markers and Clara cells; they also had less macrophages and neutrophils in BAL. Our findings demonstrate that CCSP+ BMC accelerate airway recovery while decreasing inflammation and suggest the beneficial effect of CCSP+ BMC in lung recovery is in part due to production of CCSP itself.
Results
Characterization of bone marrow CCSP+ cells
Previously, we described CCSP+ BMC in C57/Bl6 and FVBn mice.31,33 In this study, we made use of FVBn mice and CCtk transgenic mice in the same FVB/n background to determine if ablation of CCSP+ BMC affects airway regeneration. As in our initial observations, we were able to isolate CCSP+ BMC by FACS-sorting (Supplementary Figure S1a–c). We corroborated that freshly isolated CCSP+ BMC expressed CCSP by immunocytochemistry for CCSP (Supplementary Figure S1d) and CCSP mRNA expression by PCR, obtaining an amplification product of approximately 468 bp, which is the expected size for the CCSP amplicon (Supplementary Figure S1e). We confirmed the identity of the amplification product by sequencing; analysis by Basic Local Alignment Search Tool (BLAST) demonstrated the product had 99.7% identity with respect to mouse CCSP mRNA (Supplementary Figure S1f).
As our objective was to eliminate the CCSP+ BMC without eliminating CCSP+ cells in lung, we developed an animal model of female FVBn mice transplanted with male CCtk or FVBn bone marrow. We demonstrated that it is possible to eliminate specifically the CCSP+ BMC by administering ganciclovir to mice transplanted with CCtk marrow (Supplementary Figure S1j–l).
As a control for this experiment, we explored the possibility of an immune response due to gender differences between bone marrow donors and recipients. We compared the inflammatory cells in BAL from female FVBn mice transplanted with male marrow versus nontransplanted FVBn females. No differences in the inflammatory cells of the BAL between these groups were observed (Supplementary Figure S1h,i).
Bone marrow CCSP+ cells extend survival after ablation of Clara cells and lung stem cells
To study how endogenous CCSP+ BMC affect airway regeneration and survival in absence of Clara cells, we irradiated CCtk female mice and transplanted them with BMC from either FVBn (wild-type) or CCtk male mice. To eliminate CCSP+ cells in the CCtk background, we treated the mice with ganciclovir (Figure 1a). We corroborated the presence of male cells in the blood of female recipients 2 months after transplantation by measuring the levels of Sry, which is a gene found in the Y chromosome. All mice transplanted had male cells in the blood with no significant differences between the groups (Figure 1b). After treatment with ganciclovir, the CCtk mice transplanted with FVBn BMC had preservation of the bone marrow CCSP+ cells as shown by the percentage of CCSP+ cells in the bone marrow detected by flow cytometry (Supplementary Figure S2a). After 10 days of ganciclovir administration, only the mice transplanted with FVBn bone marrow had expression of CCSP in blood cells (Figure 1c). Survival after ganciclovir administration was longer for mice transplanted with FVBn BMC although ultimately there was no change in mortality by 15 days after ganciclovir treatment (Figure 1d).
Figure 1.

Bone marrow CCSP+ cells extend survival after ablation of CCSP+ cells in lung. (a) We transplanted female CCtk mice with male wild-type (FVBn; green) or CCtk (purple) bone marrow (BM) cells. Two months later, mice were treated with ganciclovir to eliminate CCSP+ cells in lung and bone marrow. (b) Quantitative RT-PCR for Sry gene to corroborate the presence of male cells in the blood of female recipients 60 days after transplantation. (c) Quantitative RT-PCR to analyze the expression of CCSP in blood cells at 0 and 10 days after ganciclovir treatment. (d) Survival curve after ganciclovir treatment; the control group (blue) consisted in FVBn mice transplanted with FVBn BM. After 12 days of ganciclovir, we analyzed the (e) total cell count and the differential cell count of inflammatory cells (f) in bronchoalveolar lavage (BAL). (g) Quantitative RT-PCR using lung samples to analyze the expression of markers for Clara cells CCSP, Cyp2f2, Pon1, and Aox3; the type II pneumocyte Sftpc and the ciliated cell Foxj1. Confocal microscopic analysis of CCSP (green) in the lung of mice transplanted with (h) CCtk or (i) FVBn BM and graph showing the number of (j) CCSP+ cells per airway in each group. Representative photomicrographs of hematoxylin and eosin stained sections of airways from mice transplanted with (k) CCtk or (l) FVBn bone marrow. Data shown are means ± SD; n = 8 mice per group. Insets are representative isotype staining controls. Scale bar represents 40 μm. *P < 0.05, ***P < 0.0005.
Twelve days after ganciclovir treatment, the mice transplanted with FVBn bone marrow had less inflammatory cells in the BAL with reduced numbers of neutrophils (Figure 1e,f). Analysis of the lungs of mice transplanted with FVBn bone marrow demonstrated higher expression of epithelial cell markers for Clara cells, type II pneumocytes and ciliated cells (Figure 1g) with no differences in the gene expression for Aquaporin 5 and T1-α (markers of type I pneumocytes), keratin 14, keratin 18, and keratin 5 (data not shown). Mice transplanted with FVBn BMC also had higher number of CCSP+ cells in the airways compared to mice transplanted with CCtk BMC as demonstrated by immunohistochemistry for CCSP protein (Figure 1h–j). Analysis of the lung sections stained with hematoxylin and eosin demonstrated less inflammation in mice transplanted with FVBn BMC compared with mice transplanted with CCtk BMC (Figure 1k,l).
As donors and recipients were sex-mismatched, it was also possible to track the FVBn BMC by confocal microscopy analysis of lung sections using fluorescence in situ hybridization (FISH) for Y-chromosome and immunohistochemistry for CCSP (Supplementary Figure S2b).
Taken together, these data demonstrate that CCSP+ BMC extended survival, reduced lung inflammatory cells, and increased expression of lung epithelial cell markers after ablation of Clara cells with ganciclovir.
Ablation of bone marrow CCSP+ cells delays lung recovery after naphthalene injury
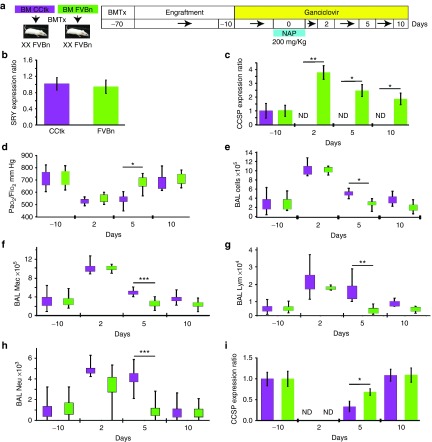
To study how endogenous CCSP+ BMC affect airway regeneration in a nonlethal model of Clara cell injury, we irradiated wild-type FVBn female mice and transplanted them with bone marrow from either FVBn or CCtk male mice. To eliminate CCSP+ cells in the bone marrow, we treated the mice with ganciclovir. We then examined the effect of subsequent airway epithelial injury using naphthalene (Figure 2a). We corroborated the presence of male cells in the blood of female recipients after 2 months of transplantation by measuring the levels of Sry. All the mice transplanted had male cells in the blood with no significant differences between the groups (Figure 2b). After ganciclovir administration, the mice transplanted with CCtk BMC had no expression of CCSP in blood in contrast to mice transplanted with FVBn BMC that had an increase in CCSP expression, maximal 2 days after lung injury with naphthalene (Figure 1c).
Figure 2.
Ablation of bone marrow CCSP+ cells delays lung recovery after naphthalene injury. (a) Female FVBn mice were transplanted with male wild-type (FVBn; green) or CCtk (purple) bone marrow (BM) cells. Sixty days later, mice were treated with ganciclovir to eliminate CCSP+ cells in the bone marrow. Ten days after ganciclovir exposure, mice were injected with naphthalene (NAP) to induce lung injury. (b) Quantitative RT-PCR for Sry gene to corroborate the presence of male cells in the blood of female recipients 60 days after transplantation. (c) Quantitative RT-PCR to analyze the expression of CCSP in blood cells at different time points showing a transient increase in CCSP expression peaking at 2 days of naphthalene injury in the FVBn group, while mice transplanted with CCtk BM didn't have CCSP+ cells in blood after ganciclovir treatment. Five days after naphthalene injury, the CCtk group had lower levels of oxygen in the arterial blood (d) (PaO2/FiO2 ratio), more inflammatory cells in (e) BAL, with increased numbers of (f) macrophages, (g) lymphocytes, and (h) neutrophils. These mice also showed (i) lower expression of CCSP in the lung, compared to mice transplanted with FVBn BM. Data shown are means ± SD, n = 8 mice per group. *P < 0.05, **P < 0.005, ***P < 0.0005.
When comparing the two groups of mice at the peak of lung damage (2 days after naphthalene injury), during the healing phase (5 days after injury), and at the resolution phase (10 days after injury), we only found significant differences during the healing phase. Five days after naphthalene injury, mice transplanted with CCtk BMC had lower levels of oxygen in arterial blood (Figure 2d). These mice also had more inflammatory cells in the BAL with increased numbers of macrophages, lymphocytes, and neutrophils (Figure 2e–h). We analyzed recovery of airway epithelium by RT-PCR and demonstrated that mice transplanted with CCtk BMC had lower expression of CCSP (Figure 2i) and other Clara cell markers (Cyp2f2, Pon1, Aox3, and FMO3; Supplementary Figure S3b) compared to mice transplanted with FVBn BMC. CCSP immunofluorescence at the same time point demonstrated that mice transplanted with CCtk BMC had lower number of CCSP+ cells in the airways compared to mice transplanted with FVBn BMC (Figure 3a–c).
Figure 3.

Bone marrow CCSP+ cells express epithelial markers and localize in the airways. Confocal microscopic analysis of Clara cell marker CCSP (green) 5 days after lung injury in mice transplanted with (a) CCtk or (b) FVBn bone marrow and (c) graph showing the number of CCSP+ cells per airway in each group. Lung sections from female mice transplanted with male FVBn bone marrow after naphthalene injury. The sections were stained for Y chromosome (red) using FISH and then subjected to immunohistochemistry for (d) CCSP (green) and (e) the pan-cytokeratin epithelial cell marker (yellow). Arrows point to male donor cells. Insets are representative isotype staining controls. Scale bar represents 40 and 10 μm. Data shown are means ± SD; n = 6 mice per group; *P < 0.05.
As BMC donors and recipients were sex-mismatched, we tracked FVBn BMC by fluorescence in situ hybridization (FISH) for Y-chromosome and immunohistochemistry for CCSP or pan-cytokeratin, an epithelial cell marker. We demonstrated the presence of donor male cells in the airways expressing CCSP (Figure 3d) and pan-cytokeratin (Figure 3e).
As a control for this experiment, we analyzed if bone marrow transplantation, ganciclovir administration and naphthalene injury could alter bone marrow homeostasis, especially after the ablation of CCSP+ BMC in mice transplanted with CCtk marrow. We compared the percentages of different cell types in bone marrow from mice transplanted with FVBn or CCtk marrow. No significant differences in the percentages of BMC were seen between the groups (Supplementary Figure S3c).
In sum, these data demonstrate that ablation of CCSP+ cells in the bone marrow specifically delays the regeneration of airway epithelium, decreases oxygen in arterial blood, and increases lung inflammation in a model of airway injury. We also demonstrated that CCSP+ cells increase in the blood and can localize in the airways where they express CCSP and cytokeratin.
Administration of CCSP protein improves airway regeneration after naphthalene damage
Considering that CCSP has anti-inflammatory, immunomodulatory, and antioxidative properties1,3,4,5,6,7,8 along with our findings that CCSP+ BMC can be localized to the injured lung where they express CCSP, we hypothesized that the beneficial effect of the CCSP+ BMC could be due, in part, to their production of CCSP. To further explore if CCSP reproduced the beneficial effect of CCSP+ BMC, we administered CCSP intratracheally in naphthalene-injured mice that had ablation of CCSP+ BMC by ganciclovir.
We used the same model of bone marrow transplantation from either FVBn or CCtk male marrow into wild-type female mice and eliminated the CCSP+ BMC in the CCtk background using ganciclovir. Airway epithelial damage was initiated using naphthalene and 24 hours later we administered CCSP or vehicle (Figure 4a). Five days after naphthalene injury, the mice transplanted with CCtk bone marrow and treated with CCSP protein had higher levels of oxygen in the arterial blood (Figure 4b), less inflammatory cells in BAL (Figure 4c), with reduced numbers of macrophages (Figure 4d) and neutrophils (Figure 4e). Analysis of airway recovery by RT-PCR in lung samples from these mice demonstrated increased expression of CCSP and other Clara cell markers in the lung (Figure 4f) compared to mice transplanted with CCtk BM and administered with saline.
Figure 4.
Administration of CCSP protein improves lung recovery after naphthalene injury. (a) Female FVBn mice were transplanted with male wild-type (FVBn; green) or CCtk (purple) bone marrow cells. Sixty days later, mice were treated with ganciclovir to eliminate CCSP+ cells in the bone marrow. Ten days after ganciclovir exposure, mice were injected with naphthalene (NAP) to induce lung injury and 1 day lather mice were administered CCSP protein or saline transtracheally. Five days after naphthalene injury, mice transplanted with CCtk bone marrow and treated with CCSP protein had a higher level of oxygen in the arterial blood (b) (PaO2/FiO2 ratio) less inflammatory cells in (c) BAL, with reduced numbers of (d) macrophages and (e) neutrophils. These mice also showed (f) increased expression of CCSP, Cyp2f2, Pon1, Aox, and Fmo in the lung, compared to mice transplanted with CCtk BM and administered with saline. Data shown are means ± SD; n = 8 mice per group. *P < 0.05, **P < 0.005, ***P < 0.0005.
CCSP immunofluorescence staining also demonstrated that mice transplanted with CCtk bone marrow and treated with saline had less CCSP+ cells in the airways compared to mice transplanted with CCtk BM and administered with CCSP protein (Figure 5a–c). In the mice transplanted with FVBn bone marrow, there were no significant differences between groups treated with saline or CCSP (Figure 5d–f).
Figure 5.

Administration of CCSP protein increased the recovery of the airway epithelium after injury. Confocal microscopic analysis of Clara cell marker CCSP (green) 5 days after lung injury in FVBn mice transplanted with CCtk bone marrow and administered with (a) saline or (b) CCSP and (c) graph showing the number of CCSP+ cells per airway in each group. Lung samples from mice transplanted with FVBn bone marrow and administered with (d) saline or (e) CCSP and (f) graph showing the number of CCSP+ cells per airway in each group. Insets are representative isotype staining controls. Scale bar represents 40 μm. Data shown are means ± SD; n = 6 mice per group; **P < 0.005.
Overall, these data demonstrate that administration of CCSP reproduced the beneficial effect of CCSP+ BMC in airway regeneration and control of lung inflammation in a model of airway injury.
Discussion
The contribution of BMC in lung repair has been studied in several publications and is still controversial. The expression of CCSP by a population of BMC in human and mice has been demonstrated by our group using flow cytometry, FACS-sorting, immunocytochemistry, western blot, and RT-PCR.31,33,34 Independently of our research, Londhe et al.32 confirmed that CCSP+ cells are found in bone marrow from wild-type mice treated with saline or ganciclovir, and, more importantly, they found that CCtk mice harbor thymidine kinase/CCSP dual-positive cells in the bone marrow. In accordance with these publications, we corroborated that it is possible to isolate CCSP+ BMC from CCtk and FVBn mice by FACS-sorting. We also demonstrated that sorted CCSP+ BMC expressed protein and mRNA for CCSP. In agreement with Londhe et al. findings of a thymidine kinase/CCSP dual-positive BMC in CCtk mice, our results shown that it is possible to specifically eliminate the CCSP+ BMC by ganciclovir administration to wild-type mice transplanted with CCtk bone marrow.
As a control for our bone marrow transplantation studies, we analyzed inflammatory cells in BAL comparing transplanted females versus nontransplanted females. These results suggest that gender differences between donors and recipients do not cause a pre-existing inflammatory condition in the lung after bone marrow transplantation.
We analyzed the beneficial effects of CCSP+ BMC in a lethal model of lung injury by administering ganciclovir to CCtk transgenic mice. We found that CCtk mice transplanted with FVBn BMC had preservation of bone marrow CCSP+ cells after ganciclovir administration. These mice had reduced lung inflammatory cells in BAL and increased expression of lung epithelial cell markers for Clara cells, type II pneumocytes, and ciliated cells. In contrast to the findings from Londhe et al.32 that demonstrated a reduction in alveolar type I cells after the ablation of CCSP+ cells, we didn't find significant differences in the expression of epithelial markers for alveolar type I cells when eliminating specifically the CCSP+ BMC population. These differences may be due to the fact that Londhe et al. based their results in the ablation of CCSP+ cells in both the lung and bone marrow while in our approach, we specifically eliminated the CCSP+ cells in the bone marrow, which allowed us to differentiate their effects from the effect that other CCSP-expressing cells in the lung have in lung recovery.
This model of lung injury by administration of ganciclovir to CCtk mice is characterized by the loss of epithelial stem and progenitor cells in the lung that leads to a failure of airway regeneration and is associated with rapid mortality.24 After ganciclovir administration to the CCtk mice, we found an increased survival time in mice with preserved CCSP+ cells in the bone marrow, although there were no long-term differences in mortality 15 days after ganciclovir exposure. This suggests that either the beneficial effects of CCSP+ BMC are not mediated by direct regeneration of the epithelial stem and progenitor cells in the lung or any direct regeneration is quantitatively insufficient in this acute injury model of massive stem cell loss.
Using naphthalene to induce a nonlethal airway injury, we demonstrated that CCSP+ BMC increase in peripheral blood and localize in the lung in response to injury where they express CCSP and cytokeratin. These cells had a beneficial effect by promoting airway regeneration, reducing pulmonary inflammation, and increasing oxygen in arterial blood. Using our bone marrow transplantation approach, we demonstrated that it is possible to eliminate specifically the CCSP+ cells in blood and bone marrow which results in the loss of these beneficial effects observed in the lung after naphthalene injury.
We had a particular interest in the possibility that the CCSP+ BMC may exert their beneficial effects through paracrine-mediated activity, specifically by their production of CCSP, given previous publications that suggest protective roles of CCSP in oxidative stress and inflammation due to its immunoregulatory characteristics.4,14,22,35,36,37,38 This hypothesis is further supported by our data that showed that ablation of CCSP+ BMC increased lung inflammation and delayed airway regeneration. Conversely, treatment with CCSP reduced neutrophils in the lung while increasing oxygen in arterial blood and the number of CCSP+ cells in the airways. We cannot rule out the possibility that increased numbers of CCSP+ cells in the airways is due to the uptake of CCSP by other epithelial cells. Our findings of increased mRNA expression of CCSP and other Clara cell markers in the same lung samples favors the idea that CCSP administration increases Clara cell numbers by inducing their proliferation. In accordance with this idea, our previous publication demonstrated in an in vitro assay that administration of CCSP protein produced a dose-dependent increase in the rate of epithelial proliferation while protecting against cell death by oxidative stress.31 CCSP+ BMC may exert their beneficial effect in lung regeneration by the production of multiple factors, our data demonstrate that administration of CCSP reproduced much of the beneficial effects of CCSP+ BMC themselves.
Our findings clearly suggest that intratracheal administration of CCSP may be a potential therapeutic strategy for the treatment of lung diseases where re-epithelialization of the airways is compromised and inflammation is one of the main characteristics of these diseases. In accordance with this idea, intratracheal administration of CCSP to preterm infants with or at risk of respiratory distress syndrome demonstrated a significant reduction in neutrophils and total cell count of BAL and lung protein concentration.39 Additional studies will be required in order to determine the doses and number of treatments necessary to achieve a therapeutic effect in patients with different types of lung diseases.
In summary, this study proposes a novel concept that a unique population of CCSP+ BMC participates in airway repair in part through secretion of CCSP and suggests the intratracheal administration of CCSP as a potential therapeutic strategy for the treatment of lung diseases.
Materials and Methods
Animal procedures. Mice received care in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Experimental Animals formulated by the Canadian Council on Animal Care. FVB/n and CCtk mice on an FVB/n background were kindly provided by Dr Barry Stripp (Duke University, Durham, NC). We identified the transgenic CCtk mice and their wild-type littermates by genotyping as described previously.22 Bone marrow from wild-type or CCtk male littermates was harvested and transplanted into irradiated female littermates as described previously.33 For flow cytometry and sorting, BMC were blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA), stained with rabbit antimouse CCSP (1:200; Upstate, Tremecula, CA) followed by AlexaFluor 488 secondary IgG (1:200; Invitrogen, Eugene, OR). Two months after bone marrow transplantation female CCtk mice received 4.5 mg/day of ganciclovir (Hoffmann-La Roche, Mississauga, Ontario, Canada) by subcutaneous osmotic pump (ALZET, Cupertino, CA).
For lung injury studies, mice were given intraperitoneal injections of naphthalene (>99% pure; Sigma-Aldrich, St Louis, MO), 200 mg per kg of body weight. CCSP recombinant mouse protein (Life technologies, Grand Island, NY) was administered transtracheally (30 µg/day) for 5 days. Control animals were treated with saline solution. Arterial blood gases (PaO2/FiO2 ratio) were analyzed at different times after lung injury. Blood was sampled under direct vision by means of a heparinized needle inserted into the femoral artery and the sample was measured in a blood gas analyzer (Harvard Apparatus, Holliston, MA).
The left and right femurs of each animal were exposed and the marrow sample was flushed with saline using a needle. The cells were re-suspended by pipetting the sample and the bone marrow smears were stained with Hemacolor kit (EMD Millipore, Billerica, MA). One observer blinded with respect to the group evaluated the percentage of different cell types in bone marrow smears for eight mice in each group.
Lung assessment. Animals were anesthetized with 5% isoflurane and sacrificed by cervical dislocation. Lungs were removed en bloc together with the heart. After ligation of the left bronchus, bronchoalveolar lavage (BAL) was performed twice on the right side using 0.5 ml saline through an endotracheal tube. Total and differential cell count in the BAL was perform as described.40 Left lungs were fixed by intratracheal instillation of 10% formalin (EMD Chemicals, Gibbstown, NJ) introduced in the bronchial tree by gravity. The lungs were immersed in formalin for further fixation for 10 minutes prior to paraffin embedding. Lung sections were stained with hematoxylin-eosin (Sigma-Aldrich). An average of CCSP+ cells in the lungs was calculated for each group based on the number of CCSP+ cells lining the airway in sections stained with antibodies against CCSP (Upstate). For each group, 16 fields were examined in each sample using a 40× objective. Two observers blinded with respect to the group evaluated the images for four mice in each group.
Genomic DNA and total RNA were prepared from the right lung, blood, BM, and sorted CCSP+ cells using the DNeasy and RNeasy kits, respectively (Qiagen, Valencia, CA), and quality assessed by spectrophotometry. The cDNA was used to amplify the whole mRNA sequence of mouse CCSP using the following primers: forward 5'CCCCACATCTACAGACACCA3' and reverse 5'GCTCGCAGTTTATTGCAAAG3'. The 468 bp amplification product was analyzed by agarose gel electrophoresis, cut from the gel under ultraviolet light, and recovered using a DNA gel extraction kit (Millipore, Bedford, MA). The product was sequenced as previously described41 and the sequence results were analyzed by Basic Local Alignment Search Tool (BLAST; Bethesda, MD).
Quantitative RT-PCR analysis. Differential gene expression (SYBR green detection method; Applied Biosystems, Carlsbad, CA) was determined for CCSP, Cyp2f2, Pon1, Fmo3, Aox3, Cldn10, Sftpc, and Foxj1. Quantification of bone marrow donor cells retained in the lung was performed using Sex determining region Y (Sry) primers (for details see Supplementary Table S1). Normalized mRNA or gDNA levels are expressed as relative to the control samples. 18S was used to normalize gene expression levels using the REST-384 program.42
Immunocytochemistry, immunohistochemistry, and FISH. For immunocytochemistry, sorted CCSP+ BMC were fixed in 4% paraformaldehyde, washed in PBS and blocked with 5% normal serum. Slides were then incubated at 4 °C overnight with primary antibody against CCSP (CC10 T-18; Santa Cruz, Dallas, TX). After washing, samples were incubated with secondary AlexaFluor-546 antibody and nuclei were counterstained with Vectashield mounting media with DAPI (Vector Laboratories). For immunohistochemistry, lung sections underwent deparaffinization and heat-antigen retrieval and the samples were blocked with 5% normal serum for 1 hour. Slides were then incubated at 4 °C overnight with primary antibodies against CCSP (Upstate) and pancytokeratin (eBioscience, San Diego, CA). After washing, samples were incubated for 1 hour with the appropriate secondary AlexaFluor-conjugated antibody and nuclei were counterstained with Vectashield mounting media with DAPI (Vector Laboratories). Dual Y-chromosome fluorescent in situ hybridization (FISH) and immunohistochemical detection was performed as described.43 For negative controls, primary antibodies were replaced with isotype-specific IgG (see details in Supplementary Table S2).
Statistical analysis. Data are presented as mean ± SD as stated in each figure legend. Statistical analysis was performed using GraphPad Prism Software (GraphPad Software, La Jolla, CA). Survival curves were generated by the Kaplan–Meier method and groups compared with the log-rank test. Continuous variables were compared among different groups using the Student's t-test or one-way analysis of variance followed by Tukey's test. Significance was defined as P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Characterization and ablation of bone marrow CCSP+ cells. Figure S2. Percentage of CCSP+ BMC and localization in the airways of CCtk mice. Figure S3. Ablation of bone marrow CCSP+ cells delays airway recovery. Table S1. Primers for RT-PCR. Table S2. Antibodies for Immunohistochemistry.
Acknowledgments
The authors thank Mingyao Liu and Masashi Gotoh for experimental advice; and the staff of the TGRI Animal Care Facility for animal manipulation advice. Funded by Canadian Institutes of Health Research (MOP 86760), Consejo Nacional de Ciencia y Tecnologia CONACyT, and the Kinnear Foundation. The authors declare no conflict of interest.
Supplementary Material
Characterization and ablation of bone marrow CCSP+ cells.
Percentage of CCSP+ BMC and localization in the airways of CCtk mice.
Ablation of bone marrow CCSP+ cells delays airway recovery.
Primers for RT-PCR.
Antibodies for Immunohistochemistry.
References
- Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. 2010;42:1–4. doi: 10.1016/j.biocel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu HJ, Wang N, Zhang YN, Huang SK, Cui YH.et al. (2013Clara cell 10-kDa protein inhibits T(H)17 responses through modulating dendritic cells in the setting of allergic rhinitis J Allergy Clin Immunol 131387–94.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CH, Chen LC, Zhang Z, Chowdhury B, Lee WL, Plunkett B.et al. (2004Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein J Allergy Clin Immunol 114664–670. [DOI] [PubMed] [Google Scholar]
- Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol. 2001;167:3025–3028. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Sajjan US. Repair and remodeling of airway epithelium after injury in chronic obstructive pulmonary disease. Curr Respir Care Rep. 2013;2:145–154. doi: 10.1007/s13665-013-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–1032. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013;140:290–305. doi: 10.1016/j.pharmthera.2013.07.008. [DOI] [PubMed] [Google Scholar]
- MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45:291–300. doi: 10.3109/07853890.2012.732703. [DOI] [PubMed] [Google Scholar]
- Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol. 2007;293:L114–L123. doi: 10.1152/ajplung.00014.2007. [DOI] [PubMed] [Google Scholar]
- Snyder JC, Teisanu RM, Stripp BR. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217:254–264. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1999;159:646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C.et al. (2001Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease Am J Respir Crit Care Med 163185–194. [DOI] [PubMed] [Google Scholar]
- Robin M, Dong P, Hermans C, Bernard A, Bersten AD, Doyle IR. Serum levels of CC16, SP-A and SP-B reflect tobacco-smoke exposure in asymptomatic subjects. Eur Respir J. 2002;20:1152–1161. doi: 10.1183/09031936.02.02042001. [DOI] [PubMed] [Google Scholar]
- Nord M, Schubert K, Cassel TN, Andersson O, Riise GC. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation. 2002;73:1264–1269. doi: 10.1097/00007890-200204270-00013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics. 2006;6:1001–1010. doi: 10.1002/pmic.200500105. [DOI] [PubMed] [Google Scholar]
- Kelly FL, Kennedy VE, Jain R, Sindhwani NS, Finlen Copeland CA, Snyder LD.et al. (2012Epithelial clara cell injury occurs in bronchiolitis obliterans syndrome after human lung transplantation Am J Transplant 123076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y.et al. (2000Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells Am J Physiol Lung Cell Mol Physiol 278L1256–L1263. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1256–L1265. doi: 10.1152/ajplung.00203.2004. [DOI] [PubMed] [Google Scholar]
- Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40:633–642. doi: 10.1165/rcmb.2008-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R.et al. (2001Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell Cell 105369–377. [DOI] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N.et al. (2003Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects Proc Natl Acad Sci USA 1008407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J.et al. (2005Bone marrow-derived mesenchymal stem cells in repair of the injured lung Am J Respir Cell Mol Biol 33145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng Part A. 2014;20:1735–1746. doi: 10.1089/ten.tea.2013.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos ML, Mura M, Marcus P, Hwang D, Ludkovski O, Wong AP.et al. (2013Bone marrow cells expressing clara cell secretory protein increase epithelial repair after ablation of pulmonary clara cells Mol Ther 211251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londhe VA, Maisonet TM, Lopez B, Jeng JM, Li C, Minoo P. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am J Respir Cell Mol Biol. 2011;44:804–812. doi: 10.1165/rcmb.2009-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A.et al. (2009Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium J Clin Invest 119336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin SE, Lung K, de Couto GT, Cypel M, Sato M, Singer LG.et al. (2013Bone marrow-derived progenitor cells in end-stage lung disease patients BMC Pulm Med 1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1523–L1530. doi: 10.1152/ajplung.2001.281.6.L1523. [DOI] [PubMed] [Google Scholar]
- Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275 5 Pt 1:L924–L930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N.et al. (2004Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions J Exp Med 1991317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shijubo N, Kawabata I, Sato N, Itoh Y. Clinical aspects of Clara cell 10-kDa protein/ uteroglobin (secretoglobin 1A1) Curr Pharm Des. 2003;9:1139–1149. doi: 10.2174/1381612033455026. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif ME, Osborn DA. Intratracheal Clara cell secretory protein (CCSP) administration in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2011;2011:CD008308. doi: 10.1002/14651858.CD008308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH.et al. (2007Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs Shock 28227–238. [DOI] [PubMed] [Google Scholar]
- Casas L, Galvan SC, Ordoñez RM, Lopez N, Guido M, Berumen J. Asian-american variants of human papillomavirus type 16 have extensive mutations in the E2 gene and are highly amplified in cervical carcinomas. Int J Cancer. 1999;83:449–455. doi: 10.1002/(sici)1097-0215(19991112)83:4<449::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J, Colombo C, Conese M. Engraftment of bone marrow-derived stem cells to the lung in a model of acute respiratory infection by Pseudomonas aeruginosa. Mol Ther. 2009;17:1257–1265. doi: 10.1038/mt.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization and ablation of bone marrow CCSP+ cells.
Percentage of CCSP+ BMC and localization in the airways of CCtk mice.
Ablation of bone marrow CCSP+ cells delays airway recovery.
Primers for RT-PCR.
Antibodies for Immunohistochemistry.