Abstract
Recent evidence has shown that Ras homolog enriched in brain (Rheb) is dysregulated in Alzheimer's disease (AD) brains. However, it is still unclear whether Rheb activation contributes to the survival and protection of hippocampal neurons in the adult brain. To assess the effects of active Rheb in hippocampal neurons in vivo, we transfected neurons in the cornu ammonis 1 (CA1) region in normal adult rats with an adeno-associated virus containing the constitutively active human Rheb (hRheb(S16H)) and evaluated the effects on thrombin-induced neurotoxicity. Transduction with hRheb(S16H) significantly induced neurotrophic effects in hippocampal neurons through activation of mammalian target of rapamycin complex 1 (mTORC1) without side effects such as long-term potentiation impairment and seizures from the alteration of cytoarchitecture, and the expression of hRheb(S16H) prevented thrombin-induced neurodegeneration in vivo, an effect that was diminished by treatment with specific neutralizing antibodies against brain-derived neurotrophic factor (BDNF). In addition, our results showed that the basal mTORC1 activity might be insufficient to mediate the level of BDNF expression, but hRheb(S16H)-activated mTORC1 stimulated BDNF production in hippocampal neurons. These results suggest that viral vector transduction with hRheb(S16H) may have therapeutic value in the treatment of neurodegenerative diseases such as AD.
Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disorder and is characterized by impaired cognitive function, particularly for memory.1 There is no cure for the disease, which worsens as it progresses, and eventually causes death. In contrast to known pathological hallmarks including neuronal degeneration, extracellular neuritic plaques, and intracellular neurofibrillary tangles, the pathogenetic mechanisms underlying neurodegeneration remain largely unknown, and treatments that are able to interfere with the progression of the disease are limited.2 However, despite the lack of complete understanding of the etiology of AD for guiding the development of knowledge-based targeted therapeutics, which includes increased levels of mammalian target of rapamycin complex 1 (mTORC1) in the brains of patients with AD,2,3,4 a number of studies suggest that the control of mTORC1 signaling pathway could be a beneficial strategy for the protection and functional maintenance of hippocampal system in the brain.5,6,7,8
Ras homolog enriched in brain (Rheb) is a member of the Ras family of small GTP-binding proteins. It mediates the activation of mTORC1, which enhances the activity of intracellular cell survival pathways.9,10,11,12,13 The serine at position 16 of Rheb has sensitivity to tuberous sclerosis complex GTPase activation, and Rheb(S16H), a mutation of the serine to histidine, results in persistence of the GTP-bound Rheb as an activated state owing to the resistance to tuberous sclerosis complex activation.12,13,14 In nigral dopaminergic neurons in vivo, adeno-associated virus 1 (AAV1) transduction with a gene encoding human Rheb(S16H) (hRheb(S16H)) induces trophic effects resulting in the protection of the dopaminergic system in a neurotoxin model of Parkinson's disease.12,13
In the hippocampus, Rheb mRNA is rapidly and transiently induced in hippocampal granule cells by seizures and by N-methyl-aspartate-dependent synaptic activity in a long-term potentiation (LTP) paradigm.15 However, recent results have shown that Rheb is dysregulated in the brains of patients with AD, and its overexpression promotes the degradation of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in a model of AD.16 These findings suggest that Rheb may be an important regulator of the survival of hippocampal neurons in the adult brain. Although the effects of Rheb on the level of BACE1 are mTORC1 independent,16 it is largely unknown whether the activation of Rheb/mTORC1 signaling pathways induces neurotrophic effects in the hippocampus and protects hippocampal neurons against neurotoxicity. In this study, we therefore investigated the effects of mTORC1 activation through the transduction of hippocampal neurons with hRheb(S16H) in the normal rat brain and the neuroprotective mechanisms of hRheb(S16H) against thrombin-induced neurodegeneration in the hippocampus.
Results
Neurotrophic effects of hRheb(S16H) in hippocampal neurons in normal adult rat brains
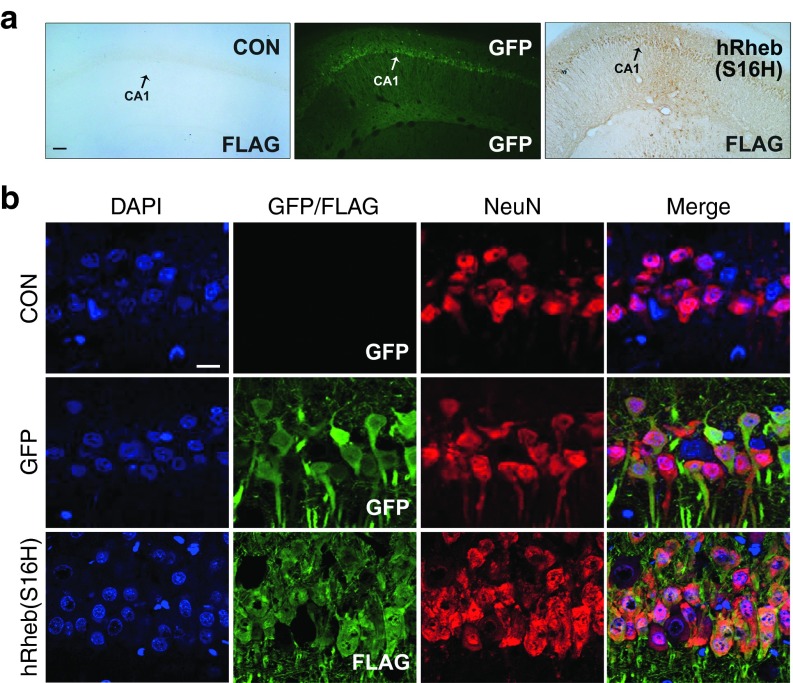
AAV-hRheb(S16H) or AAV-green fluorescent protein (GFP) as a control vector was unilaterally injected into the hippocampus by targeting the cornu ammonis 1 (CA1) region of normal adult rats. Four weeks later, the brains were removed, and sections were processed to determine the transduction of hippocampal neurons by the viral injection. The transduction of hippocampal neurons with GFP and hRheb(S16H) was demonstrated by the immediate observation of GFP expression and immunoperoxidase staining for the FLAG epitope (Figure 1a), respectively, and by triple immunofluorescence labeling for 4',6-diamidino-2-phenylindole, GFP, and neuronal nuclei (NeuN) or 4',6-diamidino-2-phenylindole, FLAG, and NeuN (Figure 1b), respectively. Immunofluorescence labeling of microglia (OX-42 and ionized calcium-binding adapter molecule 1 (Iba1)-immunopositive cells) and astrocytes (glial fibrillary acidic protein (GFAP)-immunopositive cells) indicated no transduction in each cell type, indicating the specific transduction of AAV-hRheb(S16H) and AAV-GFP in the hippocampal neurons (Supplementary Figure S1).
Figure 1.
Transduction of hippocampal neurons with AAV-GFP and AAV-hRheb(S16H) in normal adult SD rats. (a) Animals received a unilateral injection of AAV-GFP or AAV-hRheb(S16) in the hippocampus and were sacrificed 4 weeks later. AAV-hRheb(S16H)-injected brain sections (30 μm) were processed for immunostaining with antibodies against FLAG. The expression of GFP and FLAG (brown reaction product) is observed in the hippocampus for each viral injection, but no expression of FLAG is seen in the noninjected control side (CON). Bar = 100 μm. (b) Immunofluorescence triple labeling for DAPI (blue), GFP (green), and NeuN (red), or DAPI, FLAG (green), and NeuN shows that transgene expression is identifiable within neurons in the CA1 of hippocampus. Bar = 20μm. All of the pictures show the representative coronal sections following each immunostaining (n = 3, each group).
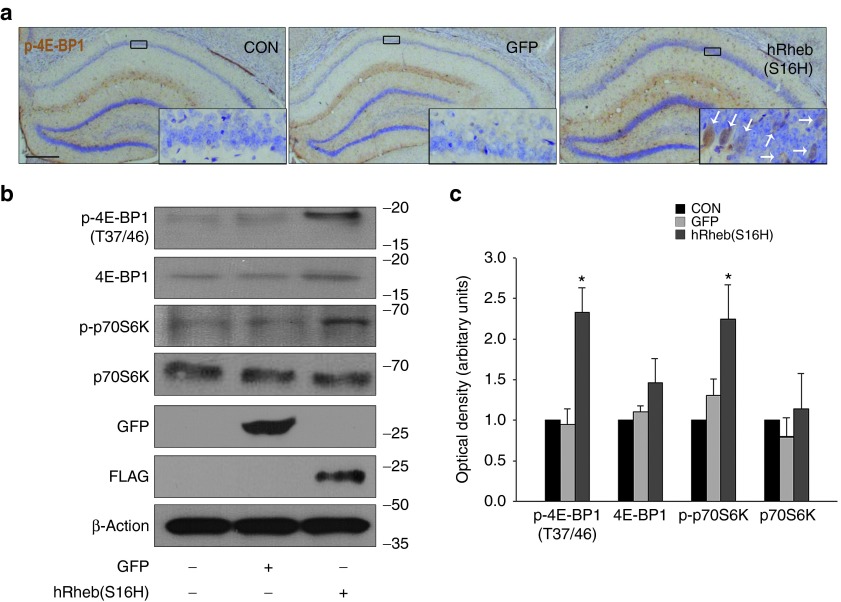
We next examined whether the hRheb(S16H) transduction of hippocampal neurons induced an increase in phosphorylation of the mTORC1 substrates 4E-BP1 and p70S6K, which indicates mTORC1 activation, and assessed the neurotrophic effects in hippocampal neurons. Similar to our recent results on mTORC1 activation by hRheb(S16H) in adult dopaminergic neurons,13 the levels of phospho-4E-BP1 (p-4E-BP1) and phospho-p70S6K (p-p70S6K) were significantly increased in rats transduced with hRheb(S16H) compared to intact controls and GFP-treated rats, indicating mTORC1 activation in hippocampal neurons, as demonstrated by immunohistochemical (Figure 2a) and western blot analysis (P < 0.01 versus controls; Figure 2b,c). GFP and FLAG were strongly detected in samples subjected to each viral injection, indicating the successful transduction of AAV-hRheb(S16H) and AAV-GFP, respectively (Figure 2b), and hRheb(S16H) expression increased the hippocampal levels of total choline with a modest alteration in level of acetylcholine, which are important for cognitive function, learning, and memory performance17,18,19 (P = 0.005 and P = 0.117, respectively, versus controls; Supplementary Figure S2).
Figure 2.
hRheb(S16H) expression activates mTORC1 in the hippocampus. (a) Brain sections were stained with anti-phospho-4E-BP1, a mTORC1 substrate, at 4 weeks postinjection of viral vectors. Immunoperoxidase staining for p-4E-BP1 (with thionin counterstain) shows that brown reaction products are clearly observed in the neurons of the hRheb(S16H)-treated group, compared to a modest level in the vehicle-treated group. Bar = 500 μm. Insets show magnified photomicrographs of the area in the CA1 layer. An example of neuronal p-4E-BP1 staining (white arrows) is shown in the inset. All pictures show the representative coronal section of each group (n = 3, each group). (b) Western blot analysis of p-4E-BP1, 4E-BP1, p-p70S6K, and p70S6K expression at 4 weeks after intrahippocampal injection of AAV-GFP and AAV-hRheb(S16H). Successful transduction of the hippocampus was confirmed in each case by western blot analysis of GFP and FLAG expressions. (c) The histogram results show the results of a quantitative analysis based on the density of the p-4E-BP1, 4E-BP1, p-p70S6K, and p70S6K bands normalized with the β-actin band for each sample. All values represent the mean ± SEM of four pooled samples for each group. *P < 0.01, significantly different from contralateral control side (CON) and AAV-GFP (one-way analysis of variance and Student–Newman–Keuls analysis).
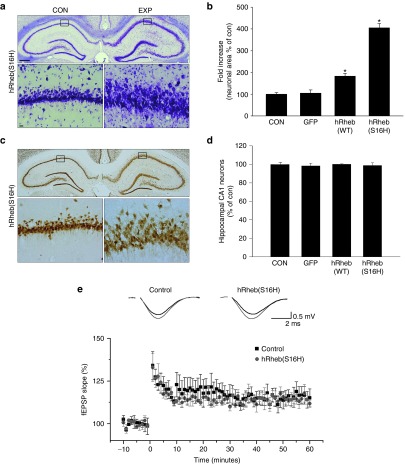
In addition to the activation of mTORC1 and the increase in total choline, hRheb(S16H) induced morphological changes to hippocampal neurons, as demonstrated by Nissl staining (Figure 3a) and NeuN immunostaining (Figure 3c), indicating the increased area of neurons with hRheb(S16H) expression compared to the intact controls and GFP-expressed controls (P < 0.01 versus controls; Figure 3b). The cytoarchitectural abnormalities of hippocampal neurons could be involved in neuronal circuitry impairment20 or abnormal behavioral changes.21 To ascertain if there were side effects such as impaired LTP and abnormal behaviors from the morphological changes in the hippocampal neurons, we additionally investigated the effects of hRheb(S16H) on changes in LTP in the hippocampus and on abnormal behavior, such as seizures. Our results showed that the hRheb(S16H)-induced morphological changes of hippocampal neurons did not affect basal LTP in the hippocampus (Figure 3e) and did not cause behavioral disorders, such as seizures, compared to kainic acid-induced behavioral abnormalities (Supplementary Figure S3), suggesting that the hRheb(S16H) transduction of hippocampal neurons induces cellular morphologic changes without side effects such as neuronal circuitry impairment or seizures in the hippocampus. Similar to the effects in the substantia nigra of adult mice brains,13 the number of rat hippocampal neurons was not influenced by the viral injection (Figure 3d).
Figure 3.
hRheb(S16H) induces a hypertrophic effect without LTP impairment from the cytoarchitectural changes in the hippocampus. (a) Morphologic analysis of hippocampal neurons at 4 weeks after intrahippocampal injection of AAV-hRheb(S16H). The upper panel shows a representative coronal section of the hippocampus following Nissl staining by cresyl violet. The experimental side (EXP) injected with AAV-hRheb(S16H) shows an increase in the area of Nissl-positive neurons, compared to the contralateral control side (CON), as shown in representative micrographs at higher power in the lower panels. All pictures show the representative coronal section of each group (n = 5, each group). Bar = 500 μm (upper panel) and 20 μm (lower panel). (b) The area of Nissl-positive neurons in the CA1 layer. The area of neurons was expressed quantitatively as a percentage relative to the contralateral control. *P < 0.01, significantly different from CON and AAV-GFP (one-way analysis of variance and Student–Newman–Keuls analysis; n = 5, each group). WT, wild-type. (c) NeuN-positive neurons also show morphologic changes in the hippocampus at 4 weeks after injection of hRheb(S16H). (d) The number of the NeuN-positive neurons in the CA1 layer injected with viral vectors. Note that there is no difference between groups (one-way analysis of variance and Student–Newman–Keuls analysis; n = 5, each group). (e) Effect of hRheb(S16H) on the induction of LTP in the hippocampus. Hippocampal slices were prepared from the rats 3 weeks (n = 6) after injection of hRheb(S16H), and noninjected slices were used as controls (n = 6). The slices were perfused with artificial cerebrospinal fluid (ACSF) for 60 minutes. For the induction of LTP, the slices were tetanized with a 100 Hz for 1 second stimulation at time 0 minute. Note that there is no difference between the groups. Each point and line represents the mean ± SEM. Representative field excitatory postsynaptic potential (fEPSP) recordings at time −5 and 30 minutes are shown as insets (upper side).
Induction of brain-derived neurotrophic factor by hRheb(S16H) in adult hippocampal neurons in vivo
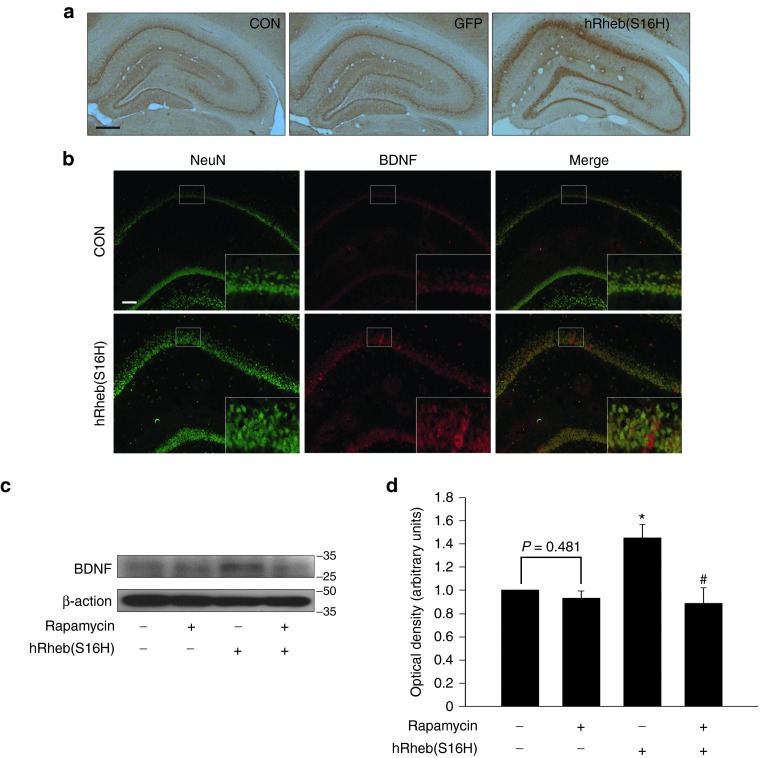
A number of studies have shown a reduction in brain-derived neurotrophic factor (BDNF) expression in the brains of patients with AD.22,23,24 Moreover, BDNF delivery has neuroprotective effects in animal models of AD,25,26 suggesting that the sustained support of BDNF can be useful for the protection of hippocampal neurons in the adult brain. The cellular effects of BDNF can be mediated by the Akt/mTOR signaling pathway in neurons.27,28 However, it is largely unknown whether the increase in mTORC1 activity by specific gene delivery can produce BDNF in hippocampal neurons in the adult brain, suggesting the role of mTORC1 as an activator of BDNF production, in addition to its role as a downstream molecule that is mediated by BDNF. We therefore examined whether the continual stimulation of mTORC1 by transduction with hRheb(S16H) induced BDNF expression in hippocampal neurons in vivo. As demonstrated by the immunohistochemical analysis conducted 4 weeks postinjection, hRheb(S16H) induced an increase in the hippocampal expression of BDNF but not in GFP-expressed hippocampus compared to the intact controls (Figure 4a), and the increased BDNF expression was apparently observed in NeuN-positive neurons (Figure 4b), indicating BDNF induction by hRheb(S16H) in hippocampal neurons. Consistent with the BDNF staining, western blot analysis showed that hRheb(S16H)-transduced hippocampal side had significantly increased levels of BDNF compared to the contralateral controls (P = 0.002; Figure 4c,d).
Figure 4.
hRheb(S16H) increases BDNF expression in hippocampal neurons. (a) Immunoperoxidase staining for BDNF was performed at 4 weeks postinjection of AAV-hRheb(S16H). Both the contralateral control side (CON) and the AAV-GFP-injected side (GFP) show similar levels of BDNF in the hippocampus. However, the AAV-hRheb(S16H)-injected side (hRheb(S16H)) shows an increase in the level of BDNF in the hippocampus. Bar = 500 μm. (b) Immunofluorescence double staining for NeuN (green) and BDNF (red) shows that the hRheb(S16H)-increased expression of BDNF is identifiable within hippocampal neurons. Bar = 100 μm. Insets are higher magnifications of each CA1 layer. All pictures in a and b show representative coronal sections following each immunostaining (n = 3, each group). (c) Western blot analysis of BDNF in the hippocampus. Rats were intraperitoneally injected with rapamycin (5 mg/kg), starting 3 weeks after AAV-hRheb(S16H) injection and continued daily injection until 4 weeks postinjection, and then they were sacrificed for western blot analysis. The results show that treatment with 5 mg/kg rapamycin alone causes no alteration to the basic level of BDNF compared to the intact control (first band). However, its treatment attenuated the level of hRheb(S16H)-increased BDNF. (d) All values of the optical density of each band represent the mean ± SEM of six pooled samples in each group. *P = 0.002 and #P < 0.001, significantly different from CON and hRheb(S16H) alone, respectively (one-way analysis of variance and Student–Newman–Keuls analysis).
To ascertain whether the induction of BDNF by hRheb(S16H) was mTORC1 dependent, we further examined the effects of rapamycin, which is a specific inhibitor of mTORC1,21 on the levels of BDNF expression and mTORC1 activity. Rats received an intraperitoneal injection of rapamycin (5 mg/kg) 3 weeks after the viral injection, and the treatment was continued until 4 weeks postinjection. Our results showed that treatment with 5 mg/kg of rapamycin significantly attenuated the expression of p-4E-BP1 and p-p70S6K in the hippocampus compared to that in intact controls (Supplementary Figure S4a,c), but its treatment did not alter the basal levels of BDNF in the hippocampus (P = 0.481 versus controls; Figure 4c,d). However, it significantly attenuated the hRheb(S16H)-induced increase in BDNF expression (P < 0.001 versus hRheb(S16H) alone; Figure 4c,d). To clarify the involvement of mTORC1 in the basal levels of BDNF, we further investigated whether treatment with 10 mg/kg of rapamycin attenuated the basal levels of BDNF in the hippocampus. Although 10 mg/kg of rapamycin also showed a significant reduction in mTORC1 activity compared to the intact controls, it did not show any significant reductions in basal BDNF expression (Supplementary Figure S4b,d). Taken together, these results suggest that hRheb(S16H)-activated mTORC1 may induce the production of BDNF in the hippocampal neurons, even though the basal levels of BDNF are not affected by mTORC1.
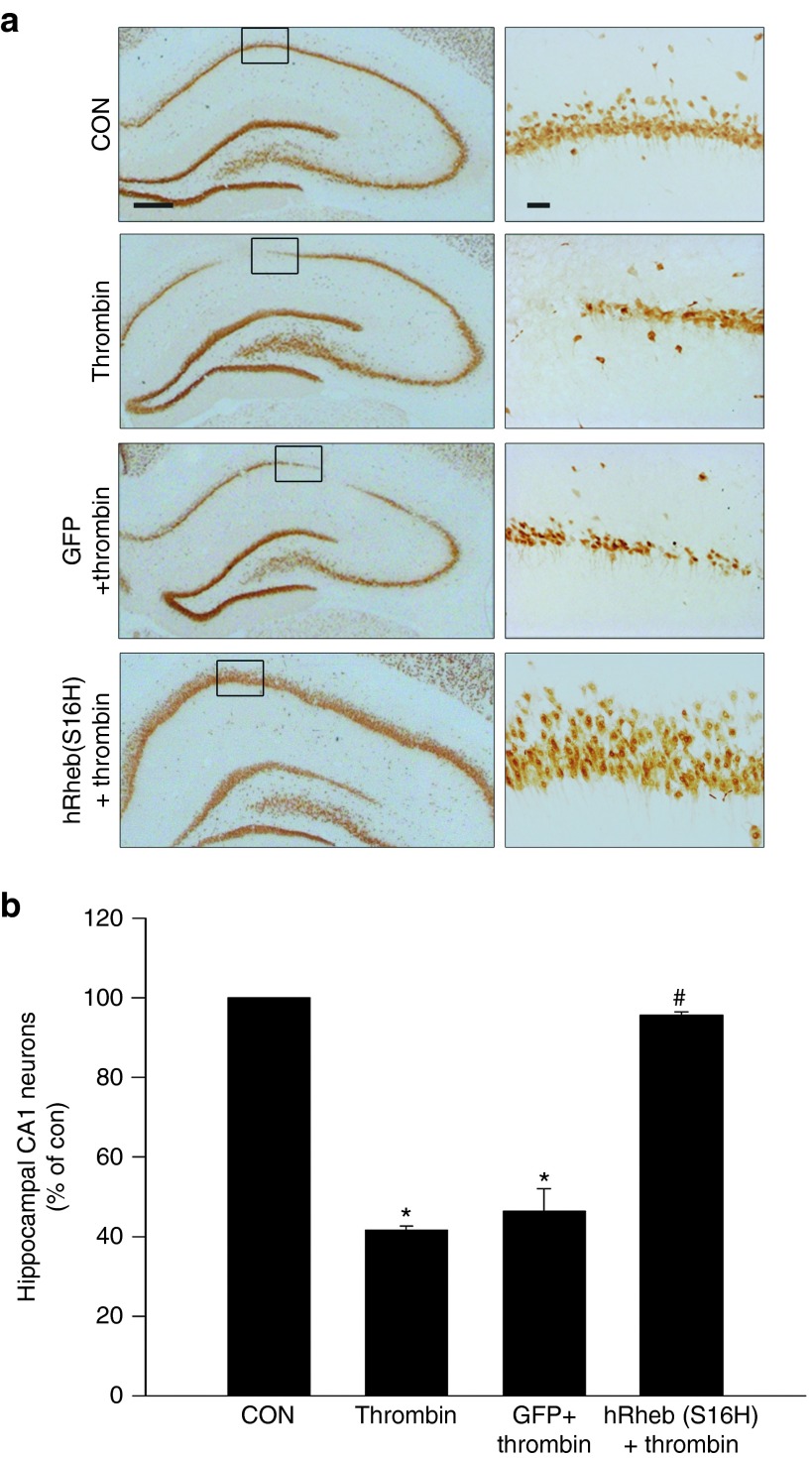
Neuroprotective effects of hRheb(S16H) against thrombin-induced neuronal cell death in the CA1 of rat hippocampus
Increased levels of thrombin, which result in the death of hippocampal neurons and AD-like cognitive impairment, have been observed in AD brains.29,30,31,32 This suggests that thrombin may be an important endogenous pathogen for AD. Given the pronounced trophic effects of hRheb(S16H) in normal rats, we next investigated whether hRheb(S16H) had neuroprotective effects in a thrombin-treated rat model of AD.29 Thrombin (20 U) or phosphate-buffered saline (PBS), which was used as a control (data not shown), was unilaterally injected into the hippocampus. Seven days later, the brains were removed, and the sections were processed for anti-NeuN immunostaining (Figure 5a and Supplementary Figure S5). As previously reported,29 there was a significant loss of NeuN-positive neurons in the thrombin-treated CA1, compared to the control CA1 (P < 0.001 versus controls; Figure 5a,b and Supplementary Figure S5). However, the AAV transduction of hippocampal neurons with hRheb(S16H) 3 weeks before the intrahippocampal injection of thrombin provided significant neuroprotection as demonstrated by the immunohistochemical analysis (Figure 5a,b and Supplementary Figure S5). When quantified and expressed as a percentage of the CA1 neurons in the counting area of the ipsilateral hippocampus relative to the contralateral control, only 42% of the hippocampal neurons were preserved in the hippocampal CA1 region of the thrombin-treated rats, whereas 95% of the hippocampal neurons were preserved in the presence of hRheb(S16H) (P < 0.001 versus thrombin; Figure 5b). Moreover, the levels of total choline and acetylcholine increased by hRheb(S16H) transduction were preserved in the thrombin-treated hippocampus (Supplementary Figure S6). Although thrombin alone shows no reduction in both levels of total choline and acetylcholine compared with intact controls (Supplementary Figure S6), these results suggest that hRheb(S16H) is capable of inducing neuroprotection against the thrombin-induced death of hippocampal neurons.
Figure 5.
hRheb(S16H) protects hippocampal neurons from thrombin-induced neurotoxicity in vivo. (a) Rats received an intrahippocampal injection of thrombin (20 U) at 3 weeks postinjection of viral vectors, and NeuN immunostaining was assessed at 1 week following thrombin treatment. Bar = 500 μm. The right panels show higher magnifications of each CA1 layer. Bar = 20 μm. All pictures show the representative coronal section of each group (n = 5, each group). (b) The histogram results show the number of hippocampal neurons in the target area of the CA1 layer, which is expressed quantitatively as a percentage compared to the contralateral control. The results show that hRheb(S16H) has protective effects against thrombin-induced neurotoxicity. *P < 0.001 and #P < 0.001, significantly different compared to the contralateral control (CON) and thrombin alone, respectively (one-way analysis of variance and Student–Newman–Keuls analysis; n = 5, each group).
However, it was still unclear whether the increased levels of thrombin substantially reduced the expression of p-4E-BP1 in the hippocampal neurons that may be involved in the neuroprotection from hRheb(S16H) transduction against thrombin-induced neurotoxicity in the hippocampus. As demonstrated by western blot analysis (Supplementary Figure S7a,b), our results unexpectedly showed that thrombin treatment significantly increased the levels of p-4E-BP1 compared to the intact controls. However, immunohistochemical staining for p-4E-BP1 showed that its increased expression by thrombin was localized within nonneuronal cells, and, on the contrary, its expression was decreased in hippocampal neurons (Supplementary Figure S7c,d). These results suggest that neuronal mTORC1 activity mediated by hRheb(S16H) may be important for the neuronal survival in the thrombin-treated hippocampus.
BDNF contributed to the neuroprotection induced by hRheb(S16H) in the thrombin-treated hippocampus
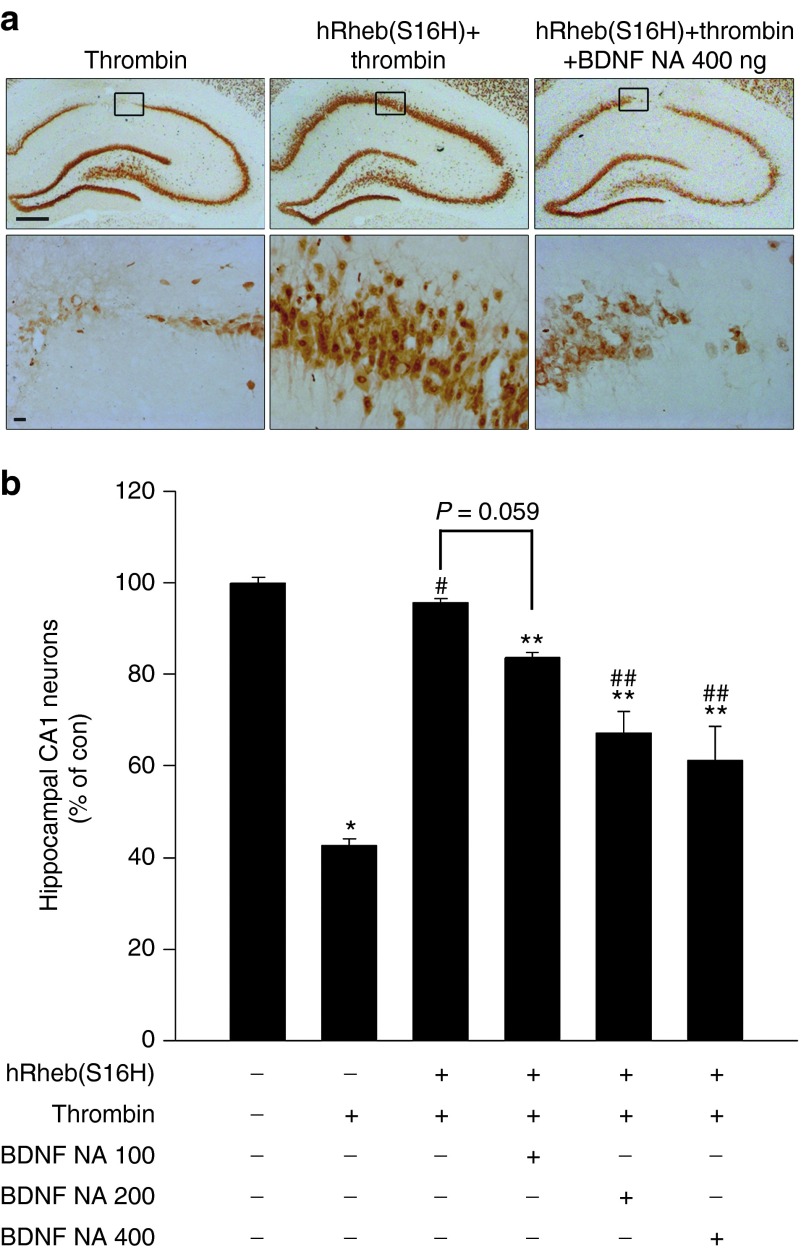
To evaluate whether the induction of BDNF correlated with hRheb(S16H)-induced neuroprotection in the thrombin-treated rat model of AD, rats received a unilateral injection of neutralizing antibodies against BDNF (100, 200, or 400 ng) with thrombin (20 U) into the hippocampal CA1 region 3 weeks after hRheb(S16H) treatment, and the brains were analyzed by immunohistochemical staining 1 week after injection of neutralizing antibodies (Figure 6a,b). The results showed that the protection by hRheb(S16H) against thrombin-induced neurotoxicity that was described in Figure 5 was attenuated by treatment with neutralizing antibodies against BDNF in a dose-dependent manner (Figure 6b). When quantified and expressed as a percentage of CA1 neurons in the counting area of the ipsilateral hippocampus compared to the contralateral controls, the administration of 100 ng BDNF neutralizing antibodies preserved the number of NeuN-positive neurons by 83%, indicating a modest inhibitory effect on the hRheb(S16H)-induced protection of hippocampal neurons (P = 0.059 versus hRheb(S16H)+thrombin). However, treatment with 200 and 400 ng of neutralizing antibodies significantly attenuated the preservation of NeuN-positive neurons by 67 and 61%, respectively (P < 0.01 versus hRheb(S16H)+thrombin). Rats treated with neutralizing antibodies alone in the absence of hRheb(S16H) showed no alterations in the number of NeuN-positive neurons (Supplementary Figure S8).
Figure 6.
The induction of BDNF by hRheb(S16H) contributes to the protection of hippocampal neurons. (a) Rats received an intrahippocampal injection of a mixture of BDNF neutralizing antibodies (100, 200 or 400 ng) and thrombin (20 U) at 3 weeks after AAV-hRheb(S16H) injection. Brains were removed and processed for NeuN immunostaining at 1 week following injection of the mixture. As described in Figure 5, hRheb(S16H) protects hippocampal neurons from thrombin-induced neurotoxicity. However, treatment with BDNF neutralizing antibodies attenuates hRheb(S16H)-induced neuroprotection. All pictures show the representative coronal section of each group (n = 5, each group). Bars = 500 and 20 μm, respectively. (b) The number of NeuN-positive hippocampal neurons in the target area of the CA1 layer was expressed quantitatively as a percentage compared to the contralateral control. The results show that hRheb(S16H)-induced BDNF contributes to neuroprotection in the thrombin-treated hippocampus. *P < 0.001, #P < 0.001, **P < 0.05, and ##P < 0.01, significantly different compared with contralateral control side (CON), thrombin alone, CON, and hRheb(S16H)+thrombin, respectively (one-way analysis of variance and Student–Newman–Keulsan analysis; n = 5, each group).
To ascertain the effects of BDNF neutralization on mTORC1 activity, we further examined the changes in 4E-BP1, p70S6K, p-4E-BP1, and p-p70S6K expression induced by BDNF neutralizing antibodies in the absence or presence of hRheb(S16H). Rats received a unilateral injection of 300 ng neutralizing antibodies against BDNF into the hippocampal CA1 region 3 weeks after hRheb(S16H), and the brains were analyzed by western blotting 2 days after the injection of neutralizing antibodies. Although the treatment with neutralizing antibodies resulted in no alterations in basic mTORC1 activity in the hippocampus, it significantly attenuated the levels of p-4E-BP1 and p-p70S6K that were increased by hRheb(S16H) compared to hRheb(S16H) alone (Supplementary Figure S9), suggesting that BDNF neutralization inhibited the activation of mTORC1 induced by hRheb(S16H) transduction in the hippocampus. Thus, all of these results show that the activation of the hRheb(S16H)/mTORC1 signaling pathway can protect hippocampal neurons through the production of BDNF.
Discussion
It is controversial whether the activation of mTORC1 has neuroprotective effects in the hippocampus of adult brains because there is no evidence for the direct mTORC1-mediated protection of hippocampal neurons, the increased levels of mTORC1 in AD brains,3,4 and the correlation between mTORC1 activation and cognitive severity of AD patients.2 However, there are a number of studies describing the putative role of mTORC1 in protecting hippocampal neurons and the functional maintenance of hippocampal system.5,6,7,8 In addition to the previous results, Chen et al.28 have reported that amyloid-β, which plays a central role in AD, interrupts the mTOR signaling pathway in rat cortical neurons in vitro. Furthermore, kainic acid treatment, which is a well-established model of hippocampal neuron death, induces the activation of Akt/mTOR signaling pathways, which correlates with the control of autophagic stress.33 Taken together, these results suggest that increased levels of mTORC1 in the brains of patients with AD may trigger a potential negative feedback loop to inhibit further neurotoxicity, and the stimulation of the mTORC1 signaling pathway could be an important strategy for the control of neurodegeneration in the hippocampus.
GTP-bound active Rheb stimulates the activation of mTORC1,14 and a constitutively active form of Rheb such as hRheb(S16H) apparently activates mTORC1 in adult neurons in vivo.12,13 Recently, Shahani et al.16 reported that Rheb is dysregulated in AD brains, and Rheb overexpression promotes the degradation of BACE1, which is involved in amyloid-β generation, through a mTORC1-independent pathway in a model of AD.16 This indicates that Rheb may be an important regulator of the survival of hippocampal neurons in the adult brain. However, it is still unclear whether the induction of active Rheb in hippocampal neurons can contribute to neuroprotection in vivo and whether the Rheb-induced effects are mTORC1 dependent or independent.
In this study, we first investigated whether AAV1 transduction with a gene encoding hRheb(S16H) induced trophic effects through mTORC1 activation in the hippocampal neurons in vivo. 4E-BP1, which is a representative substrate of mTORC1, has seven phosphorylation sites.34,35 Its phosphorylation proceeds in a sequential manner,34 and the Thr37/46 sites are directly phosphorylated by the activation of mTORC1.35 Here, we demonstrated that hRheb(S16H) transduction significantly increased 4E-BP1 phosphorylation at Thr37/46 in the hippocampus of normal adult rats compared to the intact controls and GFP controls (Figure 2). In addition to an increase in p-4E-BP1 level, phosphorylation of p70S6K, which is another substrate of mTORC1, was also increased by hRheb(S16H) transduction (Figure 2). These results suggest that the transduction of hippocampal neurons with hRheb(S16H) can apparently increase mTORC1 activity in the hippocampus in vivo.
In normal adult rat brains, the levels of acetylcholine and total choline, which are important for cognitive function, learning, and memory performance,17,18,19 were increased in the hRheb(S16H)-expressed hippocampus, even though acetylcholine level showed a modest alteration with no significant change compared to that in the intact hippocampus (Supplementary Figure S2). hRheb(S16H) expression led to increases in the area of neurons in the hippocampus (Figure 3) due to a hypertrophic effect in neurons13,36 and in the levels of neuronal BDNF, which is a well-recognized neurotrophic factor against AD28,37,38 (Figure 4). The cytoarchitectural abnormalities in hippocampal neurons could be involved in neuronal circuitry impairments20 or abnormal behavioral changes.21 However, our investigations to clarify these side effects from the changes in cytoarchitecture showed that the hRheb(S16H) transduction of hippocampal neurons had no effect on changes in LTP (Figure 3) and abnormal behaviors, such as seizures (Supplementary Figure S3), suggesting that hRheb(S16H) expression was not involved in the alterations in the synaptic structure in the hippocampus.39 We therefore conclude that transduction of hippocampal neurons with hRheb(S16H) can induce neurotrophic effects without side effects such as neuronal circuitry impairment and seizures in the hippocampus of normal adult rats, even though there were cellular morphologic changes in the hippocampal neurons.
In addition to the hRheb(S16H)-induced trophic effects in the hippocampus of normal rats, we evaluated the ability of hRheb(S16H) to protect hippocampal neurons from the thrombin-induced neurotoxicity.29 Thrombin, which is a serine protease of the trypsin family, is a key enzyme of the blood coagulation system and is generated from prothrombin by cleavage following prothrombinase activation.31 Its expression is increased in the brains of patients with AD. It accumulates in senile plaques, reactive microglial cells, and neurofibrillary tangles in AD brains and microvessels,31,32 whereas the activity of the thrombin inhibitor, protease nexin I, is sharply reduced in AD brains.40 Moreover, a number of studies have shown that thrombin can act as a neurotoxin, leading to the death of hippocampal neurons and AD-like cognitive impairment,29,30,32 suggesting that alterations in thrombin levels may be an important endogenous pathogen for AD. In the current study, our observations showed that thrombin treatment decreased the expression of p-4E-BP1 in hippocampal neurons (Supplementary Figure S7), suggesting that the maintenance of mTORC1 activity in hippocampal neurons may be crucial for neuronal survival against thrombin-induced neurotoxicity. Furthermore, our results showed that hRheb(S16H) had a robust ability to protect hippocampal neurons from thrombin-induced neurotoxicity (Figure 5) with an increase in the levels of total choline and acetylcholine in the hippocampus (Supplementary Figure S6).
BDNF is a neurotrophin that mediates neuronal survival and differentiation.41 A number of studies have shown that BDNF expression is decreased in AD brains22,23,24 and that BDNF delivery has neuroprotective effects in animal models of AD.25,26 The cellular effects of BDNF are initiated by its binding to the specific receptor, tropomyosin receptor kinase B,42 and BDNF treatment activates the Akt/mTOR signaling pathways in neurons.27 However, it is largely unknown whether the activation of Rheb/mTORC1 signaling pathways gives hippocampal neurons the ability to produce BDNF, thereby contributing to neuroprotection in the hippocampus of adult brains. To ascertain whether the activation of mTORC1 by hRheb(S16H) mediated the induction of BDNF production, we examined the effects of rapamycin, which is a specific inhibitor of mTORC1 (ref. 21), on the levels of BDNF in the hippocampus. Although rapamycin did not alter the basic levels of BDNF in the hippocampus (P = 0.481 versus normal controls), it significantly inhibited an increase in BDNF in the hRheb(S16H)-treated hippocampus, suggesting that the increased BDNF expression may be mTORC1 dependent (Figure 4). Moreover, our observations regarding the effects of neutralizing antibodies against BDNF on hRheb(S16H)-induced neuroprotection indicated that BDNF neutralization could significantly reduce the hRheb(S16H)-increased mTORC1 activity although treatment with neutralizing antibodies showed no alterations in basic mTORC1 activity in the hippocampus of normal rat brains (Supplementary Figure S9), suggesting that hRheb(S16H)-increased BDNF, at least in part, might be involved in the activation of mTORC1 with direct stimulation by hRheb(S16H) and that the induction of BDNF contributed to the protection of hippocampal neurons from thrombin-induced neurotoxicity (Figure 6). Taken together, these results suggest that the synergetic effects of hRheb(S16H) and hRheb(S16H)-induced BDNF on the activation of mTORC1 may contribute to neuroprotection in the thrombin-treated hippocampus (Figure 7).
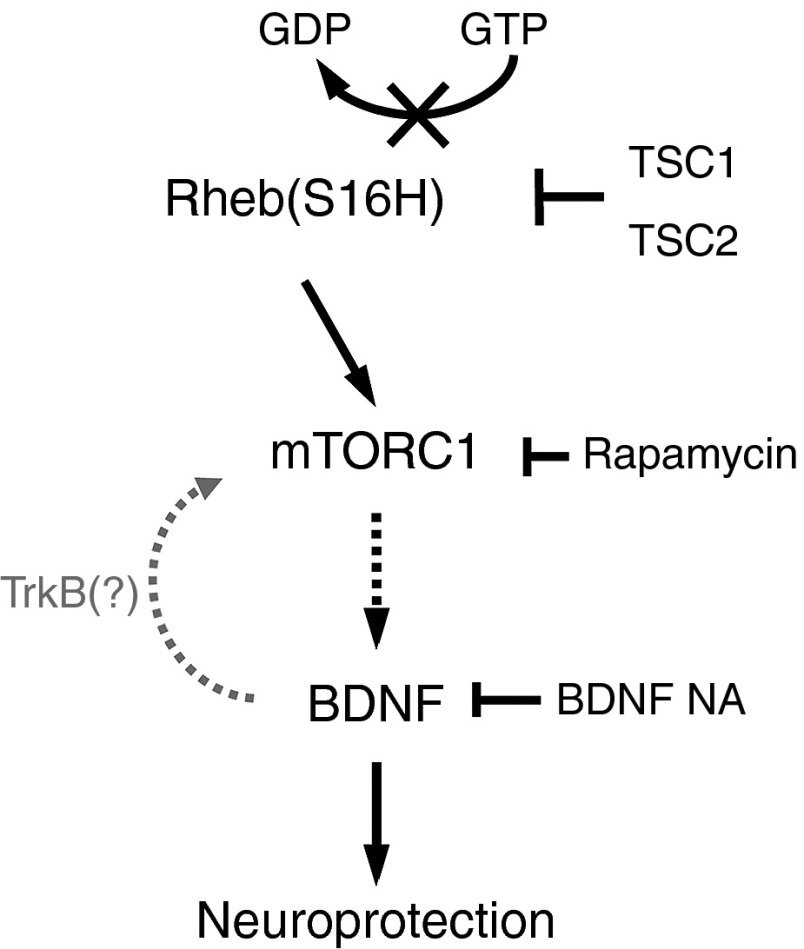
Figure 7.
Schematic representation of hRheb(S16H)-induced neuroprotection in the hippocampus. The serine at position 16 of hRheb has sensitivity to tuberous sclerosis complex (TSC) GTPase activation, and hRheb(S16H) mutation of the serine to histidine results in resistance of hRheb to this activation and consequently a persistence of the GTP-bound, activated state.13 The accumulation of hRheb(S16H) stimulates mTORC1, and the activation of mTORC1 induces BDNF production in hippocampal neurons. Moreover, hRheb(S16H)-induced BDNF contributes to the activation of mTORC1, even though it is unclear whether the induction of BDNF activates its specific receptor, tropomyosin receptor kinase B (TrkB), which is expressed in hippocampal neurons.48 Taken together, the synergetic effects of hRheb(S16H) and hRheb(S16H)-induced BDNF on the activation of mTORC1 may contribute to neuroprotection in the hippocampus.
In conclusion, we found that hRheb(S16H) expression had robust trophic and protective effects in adult hippocampal neurons. Moreover, we demonstrated that the hRheb(S16H) transduction of hippocampal neurons induced the sustained production of BDNF via mTORC1-dependent pathway, contributing to neuroprotection in the adult brain (Figure 7). Although we cannot exclude the possibility that another mechanism may be involved in the production of BDNF by hRheb(S16H) due to the potential possibility of an interaction between hRheb(S16H) and rapamycin, the present observations suggest that mTORC1 activation by specific gene delivery to hippocampal neurons may be a useful strategy for protecting neurons in the hippocampus of adult brains.
Materials and Methods
Production of AAV viral vectors. All vectors used for these studies were AAV1 serotype as previously described.12,13 A plasmid carrying the hRheb was purchased from OriGene Technologies (Rockville, MD). hRheb DNA was amplified and modified to incorporate a FLAG-encoding sequence at the 3′-end by expanded long-template PCR (Roche, Indianapolis, IN). Constitutively active hRheb (hRheb(S16H)) was generated by use of the Phusion Site-directed Mutagenesis Kit of New England Biolabs (Ipswich, MA) in the pGEM-T vector (Promega, San Luis Obispo, CA) and then cloned into an AAV packaging construct that utilizes the chicken β-actin promoter and contains a 3′ WPRE (pBL). AAVs were produced by the University of North Carolina Vector Core, and the genomic titer of hRheb(WT) and hRheb(S16H) were 3 × 1012 and 2 × 1012 viral genomes/ml, respectively. Enhanced green fluorescent protein (GFP), used as a control, was subcloned into the same viral backbone, and viral stocks were produced at a titers of 1 × 1012 viral genomes/ml.
Institutional review of animal protocols. Sprague Dawley (SD) rats (10-week-old, 220–240 g) were obtained from Daehan Biolink (Eumseong, Korea). All surgical experiments were performed in accordance with approved animal protocols and guidelines established by the Animal Care Committee of Kyungpook National University (no. KNU 2012–37).
Intrahippocampal AAV injection. SD rats were anesthetized by intraperitoneal injection of chloral hydrate (360 mg/kg; Sigma, St Louis, MO) and placed in a stereotaxic frame (David Kopf Instrument, Tujunga, CA). Each rat received a unilateral injection of AAV- GFP as a control vector or AAV-hRheb using a 10 μl Hamilton syringe (30 S needle) attached to a syringe pump (KD Scientific, New Hope, PA) into the right CA1 of the hippocampus (anteroposterior (AP): −3.8 mm; mediolateral (ML): −2.4 mm; dorsoventral (DV): −3.0 mm, relative to the bregma), according to the atlas of Paxinos and Watson.43 After the injection, the needle was left in place for an additional 5 minutes before being slowly retracted. A viral vector suspension in a volume of 2.0 μl was injected at 0.1 μl/minute over 20 minutes.
Intrahippocampal BDNF neutralizing antibody injection and thrombin injection. BDNF neutralizing antibodies (100, 200, or 400 ng in 4 μl PBS as modified doses of murine BDNF neutralizing antibodies44; Santa Cruz, Santa Cruz, CA), thrombin (20 U in 4 μl PBS; Sigma), and mixture of BDNF neutralizing antibodies and thrombin were injected at a rate of 0.5 μl/minute into the right CA1 of the hippocampus (AP: −3.8 mm; ML: −2.4 mm; DV: −3.0 mm). Rats injected with neutralizing antibodies alone were used as a control (Supplementary Figure S8). For western blot analysis, rats received a unilateral injection of 300 ng neutralizing antibodies against BDNF into the hippocampal CA1 region at 3 weeks after hRheb(S16H), and the brains were harvested 2 days after treatment (Supplementary Figure S9). All injections were made using a Hamilton syringe (30 S needle) attached to a syringe pump (KD Scientific).
Rapamycin administration. To investigate whether the production of BDNF after hRheb(S16H) transduction of hippocampal neurons is dependent on the activation of mTORC1, animals were posttreated with rapamycin (5 mg/kg, intraperitoneal injection; LC Laboratory, Woburn, MA), starting 3 weeks after hRheb(S16H) injection and continued daily until 4 weeks postinjection. The brain tissues treated with rapamycin or vehicle (4% ethanol, 5% Tween 80, and 5% PEG400)21 in the absence of hRheb(S16H) were used as controls. To clarify the effects of rapamycin on the basal level of BDNF expression and mTORC1 activity in the hippocampus, additional rats were treated with 10 mg/kg rapamycin for a week and then sacrificed for western blot analysis (Supplementary Figure S4).
Immunohistochemical staining procedures. Animals were transcardially perfused and fixed, and brain sections (30 μm thick) were processed for immunohistochemical staining as previously described29 with some modifications. Briefly, brain sections (30 μm thick) were rinsed in PBS and then incubated at 4 °C with primary antibody for 48 hours, and then brain sections were rinsed with PBS–0.5% bovine serum albumin, incubated at room temperature with the appropriate biotinylated secondary antibody, and processed with an avidin–biotin complex kit (Vector Laboratories, Burlingame, CA). The signal was detected by incubating sections in 0.5 mg/ml 3,3'-diaminobenzidine (Sigma) in 0.1 mol/l PB containing 0.003% H2O2. The stained samples were analyzed under a bright-field microscope (Axio Imager, Carl Zeiss, Germany). The primary antibodies were rabbit anti-FLAG (1:3,000; Sigma), rabbit anti-phospho-4E-BP1 (1:1,000; Cell Signaling, Beverly, MA), mouse anti-NeuN (1:500; Millipore, Temecula, CA), and rabbit anti-BDNF (1:200; Santa Cruz). For Nissl staining, brain sections were mounted on gelatin-coated slides and stained in 0.5% cresyl violet (Sigma), and then analyzed with a bright-field microscope (Axio Imager).
For immunofluorescence labeling, brain sections were rinsed and incubated for 48 hour with one of the following pairs: rabbit anti-FLAG (1:3,000; Sigma) and mouse anti-NeuN (1:500; Millipore) or mouse anti-NeuN (1:500; Millipore) and rabbit anti-BDNF (1:200; Santa Cruz). Sections were then rinsed and incubated with Cy3-conjugated antimouse IgG or anti-rabbit IgG (1:200; Millipore), and FITC-conjugated anti-rabbit IgG or antimouse IgG (1:200; Millipore) for 1 hour, and then washed and mounted with Vectashield mounting medium (Vector Labs, Burlingame, CA). The stained sections were viewed using a confocal microscopy (LSM700, Carl Zeiss).
Immunofluorescence staining for glia and FLAG epitope. Brain tissues were prepared for immunohistochemical staining as previously described.29 For immunofluorescence labeling, while AAV-GFP-treated tissues (30 μm thick) were incubated with mouse anti-OX-42 (1:400; Serotec, Oxford, UK) or mouse anti-GFAP (1:500; Sigma), AAV-hRheb(S16H)-treated sections were incubated with one of the following pairs for 48 hours: mouse anti-FLAG (1:2,000; Sigma) and rabbit anti-Iba1 (1:2,000; Wako Pure Chemical Industries, Osaka, Japan) or rabbit anti-FLAG (1:3,000; Sigma) and mouse anti-GFAP (1:500; Sigma). And then, sections were rinsed and incubated with Cy3-conjugated antimouse IgG (1:200; Millipore) and FITC-conjugated antirabbit IgG (1:200; Millipore), or with Cy3-conjugated antirabbit IgG (1:200; Millipore) and FITC-conjugated antimouse IgG (1:200; Millipore) for 1hour. The stained sections were viewed using a confocal microscopy (LSM700; Carl Zeiss).
Western blot analysis. Hippocampal tissues were prepared from the animals at 4 weeks after injection of viral vectors as previously described with some modifications.29 The tissues were homogenized and centrifuged at 4 °C for 15 minutes at 14,000g. The supernatant was transferred to a fresh tube, and the concentration was determined using a BCA kit (Bio-Rad Laboratories, Hercules, CA). The samples were boiled at 100 °C for 5 minutes before gel loading, and equal amounts of protein (50 μg) were loaded into each lane with the loading buffer. Proteins analyzed by gel electrophoresis were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) using an electrophoretic transfer system (Bio-Rad Laboratories), and then, the membranes were incubated overnight at 4 °C with specific primary antibodies. The following primary antibodies were used: mouse anti-β-actin (1:5,000; Abcam, Cambridge, UK), rabbit anti-phospho-4E-BP1 (1:1,000; Cell Signaling), rabbit anti-4E-BP1 (1:1,000; cell Signaling), rabbit anti-phospho-p70S6K (1:1,000; Cell Signaling), rabbit anti-p70S6K (1:1,000; Cell Signaling), rabbit anti-GFP (1:2,000; Millipore), mouse anti-FLAG (1:4,000; Sigma), and rabbit anti-BDNF (1:500; Santa Cruz). After washing, the membranes were incubated with secondary antibodies (Amersham Biosciences, Piscataway, NJ) for 1 hour at room temperature, and the blots were finally developed with the ECL western-blotting detection reagents (Amersham Biosciences). For semi-quantitative analyses, the density of the immunoblot bands was measured with a Computer Imaging Device and accompanying software (Fuji Film, Tokyo, Japan).
Measurement of total choline and acetylcholine in the rat hippocampus. The quantitative levels for total choline and acetylcholine were measured by commercial colorimetric/fluorimetric kit according to the manufacturer's instructions (Abcam, Cambridge, UK; Supplementary Figure S2 and S6). Briefly, the hippocampal tissues were prepared from the animals at 4 weeks after intrahippocampal injection of AAV-GFP or AAV-hRheb(S16H), and the tissues were homogenized and centrifuged. The supernatant was transferred to a fresh tube, and 50 μl of the each sample was mixed with 50 μl of reaction solution including choline assay buffer, choline probe, enzyme mix, and acetylcholinesterase. The standard curve, according to the colorimetric procedure as indicated by manufacturer's instructions, was obtained by diluting the choline standard. The measurement was obtained by SoftMax software (Molecular Devices, Sunnyvale, CA) at 570 nm.
Counting of hippocampal CA1 neurons. As previously described,29 the number of CA1 neurons was counted in the rat hippocampus. Briefly, alternate sections were obtained at 3.3, 3.6, 4.16, and 4.3 mm posterior to the bregma, and two regions from each level (eight regions for each animal) were used to count cells in the CA1 region. The number of neurons within the CA1 layer was counted using a light microscope (Carl Zeiss) at a magnification of ×400 and expressed as the number of CA1 neurons per millimeter of linear length as described previously with some modifications.45 To maintain consistency across animals, a rectangular box (1 × 0.05 mm) was centered over the CA1 cell layer beginning 1.5 mm lateral to the midline, and only neurons with normal visible nuclei were counted. For quantification of p-4E-BP1-positive neurons, cells with p-4E-BP1 immunoreactivity (brown color) in Nissl-positive cells, which have a size of over 10 μm, were counted. The number of neurons in the ipsilateral hippocampus was quantitatively expressed as a percentage compared to the contralateral control.
Cell area analysis. The area of Nissl stained neurons in the CA1 of hippocampus was determined by use of the DP2-BSW program (Olympus, Tokyo, Japan) on a computer attached to a light microscope (BX51; Olympus), interfaced with a charge-coupled device video camera (DP20-5; Olympus). Alternate sections were obtained at 3.3, 3.6, 4.16, and 4.3 mm posterior to the bregma, and a rectangular box (0.2 × 0.2 mm) was centered over the CA1 cell layer in each section, and five neurons in each section were randomly selected to provide 20 neurons per rat brain. The mean area of neurons in the ipsilateral CA1 was expressed as a percentage compared to the contralateral control.
Hippocampal slice preparation. Rats were anesthetized with ether and decapitated. All animal experiments were performed according to the policies and guidelines of the Institutional University, Seoul, ROK, study protocol #KHUASP(SE)-13-002. The brains were removed at 4 weeks after the injection of AAV-hRheb(S16H) and placed in ice-cold artificial cerebrospinal fluid (ACSF) that was composed of the following (in mmol/l): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 dextrose, and 2 CaCl2 and bubbled with 95% O2–5% CO2. Transverse hippocampal slices (350 μm) were prepared with a vibrating microtome (VT-1000S; Leica Biosystems Nussloch, Nussloch, Germany) in ice-cold ACSF. Slices were then maintained in a recording chamber at room temperature for at least 1 hour. All solutions were continuously bubbled with 95% O2 and 5% CO2.
LTP recording. Hippocampal slices were transferred to a recording chamber and continuously perfused with ACSF (21–23 °C). The Schaffer collateral/commissural-CA1 pyramidal neuron responses were induced by stimulation of the Schaffer collateral/commissural pathway with a concentric bipolar electrode (200 μm diameter; FHC, Bowdoin, ME). Extracellular recordings were obtained with a glass micropipette that was filled with 3 mol/l NaCl (2–3 MΩ). The recording electrode was placed along the trajectory of the Schaffer collateral/commissural pathway. The stimulus intensity was set to produce ~30% (150–200 μV) of the maximum field excitatory postsynaptic potential (fEPSP). CA1 LTP was induced by tetanic stimuli at 100 Hz for 1 second. Field EPSP amplitudes were averaged over 10-second intervals and expressed as percentages of the mean fEPSP amplitude that was measured during the 30-minute baseline period perfused with ACSF, which was expressed as 100%.
Behavioral test for seizures. To examine whether the cellular morphologic changes induced by hRheb(S16H) in the dentate granular neurons caused behavioral changes, such as seizures, the animals were recorded by video monitoring as previously described46 with some modifications. Rats were anesthetized with chloral hydrate (360 mg/kg; Sigma), and they received a unilateral intrahippocampal injection of kainic acid (0.2 μg in 4 μl PBS; Sigma) using a 10 μl Hamilton syringe (30 S needle) attached to a syringe pump (KD Scientific) into the right CA1 of the hippocampus (AP: −3.8 mm; ML: −2.4 mm; DV: −3.0 mm, relative to the bregma). After the injection, the needle was left in place for an additional 5 minutes before being slowly retracted. Recordings of spontaneous seizures were started 3 weeks after hRheb(S16H) or kainic acid treatment, and continued for 4 days. The rats were monitored daily for 8 hours in order to evaluate the behavioral seizures. The seizure stage was determined as previously described47: stage 1, facial movement; stage 2, wet dog shake; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling; and stage 6, jumping. The number of seizures was counted in the rats showing recurrent seizures.
Statistical analysis. Differences between the two groups were analyzed by the Student's t-test. Multiple comparisons between groups were performed by one-way analysis of variance and Student–Newman–Keuls analysis. All statistical analyses were performed using Sigma Stat Software (Systat Software, San Leandro, CA).
SUPPLEMENTARY MATERIAL Figure S1. Nontransduction of hippocampal microglia and astrocytes by AAV-GFP or AAV-hRheb(S16H) in normal adult SD rats. Figure S2. The levels of total choline (tCh) and acetylcholine (ACh) in the rat hippocampus. Figure S3. Cytoarchitectural changes in the granule cell layers by hRheb(S16H) transduction and behavioral tests for seizures. Figure S4. Effects of rapamycin on the hRheb(S16H)-increased mTORC1 activity. Figure S5. Thrombin causes neuronal cell death in the hippocampus. Figure S6. hRheb(S16H)-increased tCh and ACh are preserved in the thrombin-treated hippocampus. Figure S7. Thrombin reduces mTORC1 activity in the hippocampal neurons. Figure S8. Treatment with BDNF neutralizing antibodies (BDNF NA) has no neurotoxicity. Figure S9. BDNF neutralization attenuates the hRheb(S16H)-increased mTORC1 activity in the hippocampus.
Acknowledgments
This research was supported by the National Research Foundation of Korea grant funded by the Korean government (no. 2012R1A1A1039140 and 2008-0061888).
The authors declared no conflict of interest.
M.-T.J., J.H.N., W.-H.S., B.K.J., and S.R.K. planned and carried out the experiments, analyzed the data, and generated the figures. S.R.K., N.K., and R.E.B. designed the viral vectors. M.-T.J., J.H.N., E.L., and U.J.J. carried out immunohistochemical staining. M.-T.J., J.H.N., and S.-G.L. carried out western blot analysis. S.R.K., K.H.J., and Y.-S.B. analyzed behavioral data. Y.-H.J. measured the change of long-term potentiation. S.R.K. and B.K.J. supervised the whole project and wrote paper. All authors contributed to data analysis and preparation of the manuscript.
Supplementary Material
References
- Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- Sun YX, Ji X, Mao X, Xie L, Jia J, Galvan V.et al. (2014Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer's disease J Alzheimers Dis 38437–444. [DOI] [PubMed] [Google Scholar]
- Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X.et al. (2013Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: implication for Alzheimer disease J Biol Chem 28815556–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R.et al. (2005mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer's disease J Neurochem 94215–225. [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT.et al. (2010Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease PLoS One 5e12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwarha G, Dasari B, Prabhakara JP, Schommer J, Ghribi O. β-Amyloid regulates leptin expression and tau phosphorylation through the mTORC1 signaling pathway. J Neurochem. 2010;115:373–384. doi: 10.1111/j.1471-4159.2010.06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2000;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Chang N, El-Hayek YH, Gomez E, Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–586. doi: 10.1016/j.tins.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C.et al. (2011Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration Ann Neurol 70110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Kareva T, Yarygina O, Kholodilov N, Burke RE. AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol Ther. 2012;20:275–286. doi: 10.1038/mt.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Findlay GM, Jones R, Procter J, Cao Y, Lamb RF. Hyperactivation of mammalian target of rapamycin (mTOR) signaling by a gain-of-function mutant of the Rheb GTPase. J Biol Chem. 2006;281:19793–19797. doi: 10.1074/jbc.C600028200. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D.et al. (1994rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein J Biol Chem 26916333–16339. [PubMed] [Google Scholar]
- Shahani N, Pryor W, Swarnkar S, Kholodilov N, Thinakaran G, Burke RE.et al. (2014Rheb GTPase regulates β-secretase levels and amyloid β generation J Biol Chem 2895799–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Lenz B, Rotter A, Biermann T, Peters O, Ramirez A.et al. (2013Neurokinin3 receptor as a target to predict and improve learning and memory in the aged organism Proc Natl Acad Sci USA 11015097–15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ. Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience. 2008;151:476–488. doi: 10.1016/j.neuroscience.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C.et al. (2009Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice J Neurosci 291773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Lee J, Fukumoto H, Orne J, Klucken J, Raju S, Vanderburg CR.et al. (2005Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms Exp Neurol 19491–96. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Arancibia S, Silhol M, Moulière F, Meffre J, Höllinger I, Maurice T.et al. (2008Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats Neurobiol Dis 31316–326. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM.et al. (2009Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease Nat Med 15331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SV, Ghoda L, Hoffman PL. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: signal transduction cascades and site of ethanol action. J Neurosci. 1999;19:3277–3286. doi: 10.1523/JNEUROSCI.19-09-03277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TJ, Wang DC, Chen SS. Amyloid-beta interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor-induced Arc expression in rat cortical neurons. J Neurosci Res. 2009;87:2297–2307. doi: 10.1002/jnr.22057. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 2008;100:576–581. [PubMed] [Google Scholar]
- Yin X, Wright J, Wall T, Grammas P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer's disease. Am J Pathol. 2010;176:1600–1606. doi: 10.2353/ajpath.2010.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacka JJ, Lu J, Xie ZL, Uchiyama Y, Roth KA, Zhang J. Kainic acid induces early and transient autophagic stress in mouse hippocampus. Neurosci Lett. 2007;414:57–60. doi: 10.1016/j.neulet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK.et al. (2001Hierarchical phosphorylation of the translation inhibitor 4E-BP1 Genes Dev 152852–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol. 2005;25:2558–2572. doi: 10.1128/MCB.25.7.2558-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustilo MC, Markowska AL, Breckler SJ, Fleischman CA, Price DL, Koliatsos VE. Evidence that nerve growth factor influences recent memory through structural changes in septohippocampal cholinergic neurons. J Comp Neurol. 1999;405:491–507. doi: 10.1002/(sici)1096-9861(19990322)405:4<491::aid-cne4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Wang M, Pham S, Sze CI, Eckman CB. Downregulation of CREB expression in Alzheimer's brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener. 2011;6:60. doi: 10.1186/1750-1326-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Studer D, Chai X, Graber W, Brose N, Nestel S.et al. (2012Structural plasticity of spines at giant mossy fiber synapses Front Neural Circuits 6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan PJ, Su J, Cotman CW, Cunningham DD. Protease nexin-1, a potent thrombin inhibitor, is reduced around cerebral blood vessels in Alzheimer's disease. Brain Res. 1994;668:160–170. doi: 10.1016/0006-8993(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN.et al. (2010Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents J Clin Invest 1201774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, Okun MS, Mandel RJ. Gene therapy for neurological disorders: challenges and future prospects for the use of growth factors for the treatment of Parkinson's disease. Curr Gene Ther. 2009;9:375–388. doi: 10.2174/156652309789753400. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th edn. Academic Press; San Diego, CA; 1998. [Google Scholar]
- Kurauchi Y, Hisatsune A, Isohama Y, Mishima S, Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br J Pharmacol. 2012;166:1151–1168. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, González-Falcón A, García-Cabrera M, Alvarez D, Al-Dalain S, Martínez G.et al. (2003Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia J Neurochem 86545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröticke I, Hoffmann K, Löscher W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol. 2008;213:71–83. doi: 10.1016/j.expneurol.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gelowitz DL, Lai CT, Boulton AA, Yu PH. Gradation of kainic acid-induced rat limbic seizures and expression of hippocampal heat shock protein-70. Eur J Neurosci. 1997;9:760–769. doi: 10.1111/j.1460-9568.1997.tb01424.x. [DOI] [PubMed] [Google Scholar]
- Lambert WM, Xu CF, Neubert TA, Chao MV, Garabedian MJ, Jeanneteau FD. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol Cell Biol. 2013;33:3700–3714. doi: 10.1128/MCB.00150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.