SUMMARY
Our understanding of the mechanisms that establish wiring specificity of complex neural circuits is far from complete. During Drosophila olfactory circuit assembly, axons of 50 olfactory receptor neuron (ORN) classes and dendrites of 50 projection neuron (PN) classes precisely target to 50 discrete glomeruli, forming parallel information-processing pathways. Here we show that Toll-6 and Toll-7, members of the Toll receptor family best known for functions in innate immunity and embryonic patterning, cell-autonomously instruct the targeting of specific classes of PN dendrites and ORN axons, respectively. The canonical ligands and downstream partners of Toll receptors in embryonic patterning and innate immunity are not required for the function of Toll-6/Toll-7 in wiring specificity, nor are their cytoplasmic domains. Interestingly, both Toll-6 and Toll-7 participate in synaptic partner matching between ORN axons and PN dendrites. Our investigations reveal that olfactory circuit assembly involves dynamic and long-range interactions between PN dendrites and ORN axons.
INTRODUCTION
Neuronal circuit assembly involves a coordinated sequence of developmental steps that culminates in the formation of precise connections between highly specific, often anatomically distant, groups of neurons. This necessitates mechanisms that 1) guide axons of presynaptic neurons near their targets, 2) direct the elaboration of dendrites of postsynaptic neurons within a target zone, 3) act locally to determine specificity of connections between axons and dendrites, and 4) recruit protein complexes required for synapse formation. While great progress has been made in our understanding of the mechanisms governing long-range axon guidance and synapse formation (Chia et al., 2013; Kolodkin and Tessier-Lavigne, 2011), the intermediate steps in which cognate pre- and post-synaptic partners identify one another locally amongst a multitude of incorrect choices remains less understood (Zipursky and Sanes, 2010).
From insects to mammals, the sensory and 2nd order neurons in the olfactory system form parallel, discrete information processing channels, making them useful models for investigating the general mechanisms by which wiring specificity is established during development (Luo and Flanagan, 2007). In Drosophila, most of the 50 classes of olfactory receptor neurons (ORNs) each express a single olfactory receptor (OR) gene and target axons to a single invariant position in the antennal lobe termed a glomerulus (Couto et al., 2005; Fishilevich and Vosshall, 2005; Silbering et al., 2011). Projection neurons (PNs), which form the output of the antennal lobe, each arborize dendrites within a single glomerulus and receive direct inputs from axons of the corresponding ORN class (Jefferis et al., 2001; Stocker et al., 1990). Thus, the olfactory circuit exhibits highly specific one-to-one pairings between 50 ORN classes and 50 PN classes, presumably via cell surface recognition molecules. Previous work suggests that PN dendrites pre-pattern the developing antennal lobe. By 18 hours after pupae formation (APF), when pioneering ORN axons just contact the periphery of the developing antennal lobe, PNs have already elaborated dendrites within the antennal lobe, and occupy areas corresponding to their future glomerular positions (Jefferis et al., 2004). The transmembrane semaphorin Sema-1a instructs coarse PN dendrite targeting along the dorsolateral–ventromedial axis in response to a gradient of secreted Sema-2a/Sema-2b (Komiyama et al., 2007; Sweeney et al., 2011). Locally acting determinants such as Capricious segregate PN dendrites into discrete glomeruli through a binary choice (Hong et al., 2009). Several distinct mechanisms of ORN axon targeting have also been identified. In addition to its role in PNs, Sema-1a mediates repulsive axon-axon interactions to segregate ORN axons originating from different sensory organs (Lattemann et al., 2007; Sweeney et al., 2007). Sema-2b and its receptor PlexB link Notch-dependent ORN cell-fate decisions with axon trajectory choice and target selection (Joo et al., 2013). Hedgehog signaling coordinates peripheral ORN cell body position with antennal lobe glomerular targeting (Chou et al., 2010). Finally, recent work identified a matching mechanism that pairs pre-synaptic ORN axons with their cognate PN dendrite partners via Teneurin-mediated homophilic attraction (Hong et al., 2012).
Given the inherent complexity of wiring 50 ORN and PN classes, we anticipate that additional molecules and mechanisms regulate olfactory circuit assembly (Hong and Luo, 2014). Here we carried out a high-resolution RNAi screen to identify cell surface molecules required for olfactory circuit wiring specificity. We found that Toll-6 and Toll-7, two members of the Toll-family receptors best known for their conserved role as innate immunity receptors (Imler and Zheng, 2004; Takeda et al., 2003), instruct PN dendrite and ORN axon targeting, respectively. Toll-6 and Toll-7 also participate in partner matching of cognate pairs of PN dendrites and ORN axons, similar to our previous findings with the Teneurins (Hong et al., 2012), suggesting that multiple partner matching mechanisms are required for ensuring a robust wiring process. Analyses of the functions of these Toll receptors implied non-canonical signaling mechanisms and revealed that olfactory circuit assembly involves dynamic, long-range interactions between PN dendrites and ORN axons.
RESULTS
An RNAi Screen for Wiring Specificity Molecules in the Olfactory System Identifies the Toll-Family Receptors Toll-6 and Toll-7
To further elucidate the mechanisms that instruct PN dendrite and ORN axon targeting specificity in the Drosophila antennal lobe, we designed a high-resolution confocal-based RNAi screen of candidate transmembrane and secreted molecules (Figure 1A). To visualize PN dendrites, we utilized the Q-system driver Mz19-QF, which specifically labels two classes of PNs (DA1 and VA1d) that arborize dendrites at the anterior surface of the antennal lobe (Hong et al., 2012). Simultaneously, ORNs that express the Or88a odorant receptor and whose axons target to the VA1d glomerulus (hereafter VA1d ORNs) were labeled by the expression of myristolated tdTomato from the Or88a-myr-tdT transgene. Likewise, ORNs that express Or47b, and whose axons target to the adjacent VA1v glomerulus (also known as VA1lm), were labeled by the expression of rat CD2 from Or47b-rCD2 (Figure 1A and 1B). In addition to these markers, we visualized the entire antennal lobe neuropil with an antibody against N-Cadherin. Four-color confocal imaging enabled us to observe both matching and neighboring classes of PNs and ORNs, and to identify defects in PN dendrite targeting, ORN axon targeting, axon segregation between neighboring ORN classes, and ORN axon–PN dendrite matching. The C155-GAL4 driver line was used for pan-neuronal RNAi knockdown of predicted transmembrane and secreted proteins (Kurusu et al., 2008, Figure 1A).
Figure 1. Identification of Toll-6 and Toll-7 as Wiring Specificity Molecules in an RNAi Screen.
All images are single confocal sections of adult antennal lobes, with magenta showing neuropil staining and other colors showing axons of specific ORN classes and dendrites of specific PN classes as indicated. N is number of antennal lobes tested.
(A) Schematic of RNAi screen. A pan-neuronal C155-GAL4 drives UAS-RNAi of predicted transmembrane and secreted molecules. Dendrites of two PN classes, DA1 and VA1d, are labeled by Mz19QF > QUAS-mCD8GFP. Axons of two ORN classes, VA1d and VA1v, are labeled by two different markers driven directly from odorant receptor promoters. This four-color screen can in principle identify phenotypes in four processes listed on the right.
(B) In wild type, dendrites of Mz19-QF+ PNs and axons of VA1d and VA1v ORNs target to their glomeruli in stereotyped positions in the lateral antennal lobe. D, dorsal; L, lateral (scale bar is 10μm).
(C) Pan-neuronal RNAi knockdown of toll-6 causes dorsomedial (shown here) or dorsolateral (not shown) mistargeting of VA1d ORN axons and PN dendrites (arrow).
(D) Pan-neuronal RNAi knockdown of toll-7 causes VA1d ORN axons and PN dendrites to mistarget to a medial position.
(E) Wild-type VA1d ORNs target axons to the VA1d glomerulus ventral to the DA1 glomerulus (dashed circle).
(F) In toll-6 mutants, VA1d ORN axons mistarget either dorsomedially (shown here) or dorsolaterally (see Figure 4I) in 50% of cases (N=20).
(G) In toll-7 mutants, VA1d ORN axons partially mistarget to a medial position. Mistargeting was observed in all antennal lobes examined (N=24).
(H) Quantification of VA1d ORN axon mistargeting in toll-6 mutants, shown as percent dorsomedial (grey) and dorsolateral (black) mistargeting.
(I) Quantification of VA1d ORN axon mistargeting in toll-7 mutants. Top, normalized fluorescence intensity was binned along the lateral (bin 1) to medial (bin 10) axis of the antennal lobe and averaged across all animals (top graph). Bottom, mean intensity of VA1d ORN axons (each dot represents one fly; t-test, P< 0.0001) is shifted medially in toll-7 mutants (bottom graph).
(J) Schematic of the domain organization of Toll-6 and Toll-7 proteins. Toll-6 and Toll-7 both have extracellular leucine-rich repeat (LRR) domains and intracellular conserved Toll-interleukin receptor (TIR) domains. CF and NF, C-terminal and N-terminal LRR cysteine cluster motif. TM, transmembrane domain.
We screened a total of 768 lines representing 278 genes containing the following domain types: immunoglobulin (Ig), leucine-rich repeat (LRR), cadherin, fibronectin (FN), and epidermal growth factor (EGF) repeats. We identified two related LRR proteins, Toll-6 and Toll-7, which showed specific targeting defects of VA1d ORN axons and PN dendrites following RNAi knockdown of their respective genes. The VA1d glomerulus is located at the anterior surface of the lateral antennal lobe, ventral to the DA1 glomerulus and dorsal to the VA1v glomerulus (Figure 1B). toll-6 RNAi knockdown caused a pronounced dorsal shift of VA1d PN dendrites and ORN axons, either medial (Figure 1C) or lateral (data not shown) to the DA1 glomerulus. On the other hand, toll-7 RNAi knockdown consistently caused medial mistargeting of VA1d PN dendrites and ORN axons (Figure 1D).
Toll-family receptors are single-pass transmembrane proteins with extensive LRRs in their extracellular domain and a conserved Toll/interleukin-1 receptor (TIR) domain in the cytoplasmic region (Figure 1J). Drosophila toll-6 and toll-7 are expressed in the peripheral and central nervous system during development, and are implicated in motor axon targeting and neuronal survival in embryos (McIlroy et al., 2013). To validate the RNAi phenotypes, we tested VA1d ORN axon targeting in toll-6 and toll-7 null mutants (see Experimental Procedures), both of which are viable. toll-6 mutants exhibited dorsal mistargeting of VA1d ORN axons (Figure 1F, compared to Figure 1E, quantified in 1H) similar to that caused by RNAi-mediated knockdown (Figure 1C). Likewise, toll-7 mutants recapitulated the medial mistargeting of VA1d ORN axons (Figure 1G) observed in RNAi experiments (Figure 1D). We quantified VA1d ORN axon mistargeting by analyzing the distribution of fluorescence intensity across the antennal lobe (Komiyama et al., 2007). VA1d ORN axons displayed a significant medial shift in toll-7 mutants compared with wild-type animals (Figure 1I).
In summary, the RNAi-based screen followed by loss-of-function mutant analysis identified two Toll-family receptors, Toll-6 and Toll-7, to be required for targeting of olfactory neuron processes to the VA1d glomerulus. We next explore the developmental mechanisms by which these Toll receptors regulate wiring specificity.
Toll-7 Is Expressed in ORN Axons Targeting Anterolateral Glomeruli
To determine the spatial distribution of Toll-7 during development, we generated an antibody against Toll-7 and used it to stain developing antennal lobe. At 48 APF, dendrites of individual PNs and axons of individual ORNs have just coalesced into specific glomeruli, and begin to be identifiable from neuropil staining (Jefferis et al., 2004). During this period of development, Toll-7 protein was enriched in a cluster of glomeruli in the anterior and lateral regions of the antennal lobe, which includes the DA1, VA1d, and VA1v glomeruli (Figure 2A-C). Toll-7 signal was absent in toll-7 mutants (Figure 2D), confirming antibody specificity.
Figure 2. Toll-7 Is Expressed in ORN Axons Targeting Anterolateral Glomeruli and is required in ORNs.
Panels A-E show Toll-7 antibody staining (green) and a merged image with neuropil staining (magenta) in the same single confocal section of antennal lobes at 48 hours after pupae formation (APF). Panels F-K show labeling of specific ORN and PN classes, and a merged image with neuropil labeling of the same single confocal section of adult antennal lobes.
(A-C) In anterior (A), middle (B), and posterior (C) sections of the antennal lobe, Toll-7 protein localizes to lateral glomeruli in the anterior and middle sections, including DA1, VA1d, and VA1v glomeruli, but is largely absent in the posterior section. Because of the limited strength of Toll-7 antibody staining, we were not able to determine the identity of all glomeruli that are Toll-7+ unambiguously.
(D) Toll-7 antibody is specific as signal is absent in toll-7 mutants.
(E) Toll-7 antibody signal is abrogated following RNAi knockdown of toll-7 in ORNs using Pebbled-GAL4.
(F) In wild-type animals, VA1d ORN axons and Mz19-QF-labeled PN dendrites target the lateral antennal lobe.
(G) ORN RNAi knockdown of toll-7 by Pebbled-GAL4 causes VA1d ORN axons to mistarget medially.
(H) Occasionally Mz19-QF+ (likely VA1d) PN dendrites mistarget medially following ORN-specific RNAi knockdown of toll-7. (N=3/9 mistarget, VA1d PN dendrites were labeled in only 9 of the 16 antennal lobes in Figure 2G due to inconsistent Mz19-QF labeling).
(I-K) VA1d ORN axon targeting in wild type (K), toll-7 mutant (L), and in toll-7 mutant in which a Toll-7 transgene is expressed in all ORNs with Pebbled-GAL4 (K).
(L) Quantification of VA1d ORN axon mistargeting comparing toll-7 mutants with tissue specific RNAi and rescue.
To identify which cells produce Toll-7, we used a cell-type-specific RNAi knockdown approach. Pebbled-GAL4 is broadly expressed in all ORNs but not in central neurons (Sweeney et al., 2007). Toll-7 staining was abrogated following Pebbled-GAL4-based RNAi knockdown of toll-7 (Figure 2E), suggesting that Toll-7 is predominantly produced by ORNs. Indeed, we detected Toll-7 in the antennal commissure of wild-type pupae consisting mostly of commissural ORN axons (data not shown). Thus, Toll-7 is differentially expressed in ORN axons that innervate a cluster of anterolateral glomeruli, including VA1d ORN axons that display targeting defects in toll-7 mutants.
Toll-7 Acts Cell-autonomously in VA1d and DA1 ORNs to Control Axon Targeting
To identify which neurons require toll-7 for VA1d ORN axon targeting, we first used cell type-specific RNAi knockdown. Knockdown of toll-7 with the PN specific driver Mz19-GAL4 (expressed in VA1d, DC3, and DA1 PNs) had no effect on targeting of PN dendrites and ORN axons to VA1d (Figure S1A). Likewise, toll-7–/– VA1d PNs in Mz19-GAL4-based MARCM clones targeted their dendrites normally (Figure S1B). However, toll-7 knockdown in ORNs using Pebbled-GAL4 recapitulated the VA1d targeting defects observed with pan-neuronal RNAi (Figure 2F-H, L). Expression of UAS-toll-7 in ORNs using Pebbled-GAL4 rescued VA1d ORN axon targeting defects observed in toll-7 mutants (Figure 2I-K, L), indicating that Toll-7 in ORNs is necessary and sufficient for proper VA1d wiring. ORN knockdown of toll-7 also resulted in occasional PN dendrite mistargeting events (Figure 2H), indicating that mistargeted ORN axons can affect targeting of partner PN dendrites during development. This is surprising in light of our previous work showing that PN dendrites pre-pattern the antennal lobe to instruct target selection of later-arriving ORN axons (Jefferis et al., 2004). Our toll-7 ORN-specific RNAi findings suggest that target selection of PN dendrites is more flexible than was previously appreciated, and that the interactions of PN dendrites and ORN axons can mutually affect each other's final target selection.
In support of this, we simultaneously examined PN dendrites and ORN axons during antennal lobe development. We labeled DA1/VA1d PN dendrites using Mz19-QF, and DM6/DL4 ORN axons using the enhancer trap Am29-GAL4, which is expressed early in pupae development (Endo et al., 2007; Joo et al., 2013). At 24-36 hours APF, both AM29+ ORNs and Mz19+ PNs extended fine branches to relatively broad areas in the antennal lobe, and these processes partially overlapped at 36 hours APF even though these ORNs and PNs are not synaptic partners (Figure S2A-C). This suggests that targeting of these PN dendrites and ORN axons is dynamic before their eventual coalescence into specific glomeruli between 42-48 hours APF (Figure S2D-E).
Having established that toll-7 is required in ORNs alone for targeting to the VA1d glomerulus, we next tested whether toll-7 functions cell-autonomously in ORNs. We first employed MARCM to label VA1d ORN clones mutant for toll-7 using eyFLP, which produces mutant clones in ORNs but not in their central targets (Hummel et al., 2003). eyFLP-based MARCM generated mosaic toll-7–/– clones in 30-50% of ORNs from all classes, but only toll-7–/– VA1d ORN axons were visualized. toll-7–/– VA1d ORN axons mistargeted medially (Figure 3B, I), similar to phenotypes in toll-7 RNAi and whole-animal mutants. Using hsFLP-based MARCM, we generated sparser mosaic toll-7–/– clones and again observed qualitatively similar mistargeting (Figure 3C, I). We also performed eyFLP-based reverse MARCM (Zhu and Luo, 2004), in which wild-type VA1d ORN axons are visualized in the background of large (30-50% of all ORNs) mutant ORN clones. Despite the presence of these mutant ORNs, targeting of the labeled wild-type VA1d ORN axons was normal (Figure 3D, I). Together, these experiments indicate that Toll-7 functions cell-autonomously for VA1d ORN axon targeting.
Figure 3. Toll-7 Acts Cell-autonomously in VA1d and DA1 ORNs to Control Their Axon Targeting.
All images are confocal projections of adult antennal lobe showing MARCM labeling of VA1d (A-D) or DA1 (E-H) ORN axons in green and neuropil staining in magenta. The right panel shows the MARCM experimental design for each pair of images on the left; green rectangles indicate labeled cells.
(A, E) Wild-type VA1d (A) or DA1 (E) eyFLP MARCM labeled ORN clones target axons to the VA1d or DA1 glomerulus in the anterolateral antennal lobe.
(B, F) toll-7–/– VA1d (B) or DA1 (F) ORN axons in eyFLP MARCM clones partially mistarget to the medial antennal lobe. About 30-50% of all ORN axons are toll-7–/– but only VA1d or DA1 ORNs are labeled.
(C, G) Smaller toll-7–/– VA1d (C) or DA1 (G) clones produced by hsFLP MARCM also display partial axon mistargeting to the medial antennal lobe.
(D, H) In eyFLP reverse MARCM clones, toll-7–/– VA1d (D) or DA1 (H) ORN axons target normally to the VA1d or DA1 glomerulus despite the presence of 30-50% unlabeled toll-7–/– ORN axons.
(I-J) Quantification of VA1d (I) and DA1 (J) ORN axon mistargeting in toll-6 whole animal mutants, eyFLP MARCM clones, hsFLP MARCM clones, and reverse MARCM clones.
To determine whether toll-7 is broadly required for ORN axon targeting, or instead plays a specific role in only a subset of ORN classes, we performed whole-animal toll-7 mutant analysis in different ORN classes using Or-GAL4 driver lines. Interestingly, eight different ORN classes displayed significant targeting defects (Figure S3). Of these, DA1 is clearly Toll-7+, and VA3 and VA7l may also be Toll-7+ based on proximity to the Toll-7 enriched signal (Figure 2A-C). To investigate whether toll-7 acts cell autonomously in these ORN classes, we conducted eyFLP MARCM experiments using the same Or-GAL4 driver lines. To our surprise, among the eight ORN classes examined, only DA1 ORNs displayed defects in axon targeting, whereas ORN axon targeting for the other classes was normal (data not shown). DA1 ORN axons mistargeted to a medial position of the antennal lobe (Figure 3F, J), similar to the phenotype exhibited by VA1d ORN axons. Medial DA1 ORN axon targeting defects were also observed with toll-7–/– hsFLP MARCM (Figure 3G, J), and targeting was normal when we visualized wild-type DA1 axons in eyFLP-based reverse MARCM experiments (Figure 3H, J). Therefore, toll-7 acts cell-autonomously in VA1d and DA1 ORNs, while the axon targeting defects in ORN classes VA7l, DM3, DA2, VM7, VA3, VC1, and VM5v were likely due to cell-non-autonomous effects. These cell-non-autonomous effects could result from ORN axon–axon interactions (Sweeney et al., 2007), or from disrupted matching of ORN axons–PN dendrites (see below), which could cause unmatched PN dendrites to influence targeting/matching of other ORN axons.
Expression of Toll-6 in Small Subsets of PNs Is Sufficient to Rescue VA1d ORN Axon Mistargeting
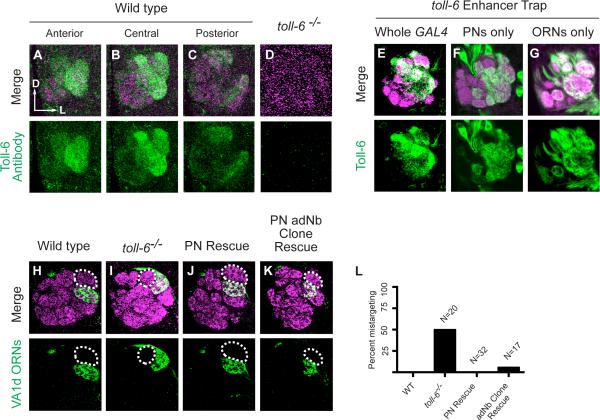
To determine the expression pattern of the other Toll-receptor identified in our screen, Toll-6, we generated an antibody and stained the developing antennal lobe. Interestingly, Toll-6 was enriched in the lateral antennal lobe in many of the same glomeruli as seen with the Toll-7 antibody, including DA1, VA1d, and VA1v (Figure 4A-C). Toll-6 is also found in DL3, DC3, DC1, DA4l, and DA4m glomeruli, which are all innervated by trichoid ORNs (Couto et al., 2005). These staining patterns were absent in toll-6 mutant animals (Figure 4D), confirming antibody specificity.
Figure 4. Expression of Toll-6 in Small Subsets of PNs Is Sufficient to Rescue VA1d ORN Mistargeting.
(A-C) Single confocal section of anterior (A), middle (B), and posterior (C) antennal lobe at 48 hours APF stained with anti-Toll-6 antibody, revealing that Toll-6 is enriched in lateral glomeruli in the anterior and middle sections, including DA1, VA1d and VA1v.
(D) Toll-6 antibody is specific as signal is absent in toll-6 mutants.
(E) D42-GAL4 staining recapitulates endogenous Toll-6 antibody staining in 48 hours APF antennal lobes.
(F-G) Intersection of GH146-FLP (F) or eyFLP (G) and D42-GAL4 labels PN dendrites or ORN axons, respectively, targeting the glomeruli observed with endogenous D42-GAL4.
(H) Wild-type VA1d ORNs labeled by Or88a-myr-tdTomato (pseudocolored in green) target axons ventral to the DA1 glomerulus (dashed circle).
(I) In toll-6 mutants, VA1d ORN axons mistarget dorsally, lateral to the DA1 glomerulus in this example (see also Figure 1H).
(J) Mz19-GAL4 driven toll-6 expression in DA1, VA1d, and DC3 PNs fully rescues VA1d ORN axon mistargeting in toll-6 mutants.
(K) Expression of Toll-6 in Mz19-GAL4+ MARCM adNb clones, which include VA1d and DC3 PNs, rescues VA1d ORN axon mistargeting (labeled by Or88a-rCD2 in green) in toll-6 mutants.
(L) Quantification of VA1d ORN axon mistargeting in toll-6 whole animal mutants compared to rescue by expression of toll-6 in Mz19+ PNs or in adNb MARCM clones.
We also examined the Toll-6 expression pattern using a putative toll-6 enhancer trap line, D42, which has a GAL4-containing P-element inserted approximately 1.5 kilobases upstream of the toll-6 transcriptional start site. D42-GAL4 strongly labeled the same 8 trichoid glomeruli observed with the Toll-6 antibody, and weakly labeled a few additional glomeruli. Further, D42-GAL4 labeled PN cell bodies as well as the antennal commissure and fasciculating ORN axons entering the antennal lobe (data not shown), suggesting that Toll-6 is expressed in both PNs and ORNs. To more precisely determine the cellular source of Toll-6, we employed an intersectional strategy using a FLP- and GAL4-dependent reporter. Intersections between GH146-FLP (expressed in PNs) and D42-GAL4 recapitulated the original D42-GAL4 expression pattern (Figure 4F). Intersection between eyFLP (expressed in ORNs) and D42-GAL4 also resulted in strong expression in anterolateral glomeruli, consistent with the original D42 expression pattern (Figure 4G). Taken together, these experiments indicate Toll-6 is expressed in ORN axons and PN dendrites that target to largely overlapping glomeruli.
Since Toll-6 is expressed in both ORNs and PNs, we next asked whether Toll-6 acts in ORNs, PNs, or both for targeting to the VA1d glomerulus. VA1d ORN axon targeting was normal following Pebbled-GAL4 driven RNAi knockdown (Figure S1C) or eyFLP MARCM removal of toll-6 from ORNs (Figure S1D), suggesting that Toll-6 is not required in ORNs. On the other hand, misexpression of toll-6 in PNs with Mz19-GAL4 fully rescued dorsal mistargeting of VA1d ORN axons (Figure 4J compared to 4I, 4L). Using hsFLP and heatshocking in newly hatched larvae to generate MARCM PN neuroblast clones (Jefferis et al., 2001), we further limited Mz19-GAL4 misexpression of toll-6 to VA1d and DC3 PNs in the anterodorsal neuroblast (adNb) lineage. Expression of toll-6 in VA1d and DC3 PNs alone was sufficient to rescue VA1d ORN axon targeting defects (Figure 4K-L). Consistently, RNAi removal of toll-6 from PNs caused a low penetrance VA1d ORN axon mistargeting phenotype in 2 of 15 antennal lobes analyzed (data not shown). The low penetrance is possibly due to weak GH146-GAL4 expression or perdurance of early expressed Toll-6 protein, yet supports the notion that Toll-6 acts primarily in PNs for VA1d ORN axon targeting.
Toll-6 Also Functions Cell-autonomously for PN Dendrite Targeting
In addition to being required for VA1d ORN axon targeting (Figure 4I-K), we found that Toll-6 was also required in PNs for their dendrite targeting. In these experiments, we used Mz19-GAL4-based hsFLP MARCM to label toll-6–/– PN neuroblast clones, which normally target to the VA1d and DC3 glomeruli in the dorsolateral antennal lobe (Figure 5A). In toll-6–/– adNb clones, however, the VA1d and DC3 PN dendrites mistargeted extensively to dorsomedial and ventral positions in regions that normally did not express Toll-6 (Figure 5B, F). We further quantified fluorescence intensity of PN dendrites along the medial–lateral axis and found that removal of toll-6 caused a significant medial PN dendritic shift compared with wild type (Figure 5E). We also observed mistargeting of PN dendrites in single-cell MARCM clones of VA1d or DC3 PNs (Figure 5C, F). PN dendrite targeting defects were rescued when we expressed a UAS-toll-6 transgene in MARCM adNb clones (VA1d and DC3 PNs) that were toll-6–/– (Figure 5D, G). Together, these experiments indicate that Toll-6 is cell-autonomously required in VA1d and DC3 PNs for dendrite targeting.
Figure 5. Toll-6 Functions Cell-autonomouly for PN Dendrite Targeting.
(A) Mz19-GAL4 labeled wild-type VA1d and DC3 PNs in adNb clones target dendrites to the VA1d and DC3 glomeruli in the lateral antennal lobe.
(B) Mz19-GAL4 labeled toll-6–/– VA1d and DC3 PNs in adNb clones mistarget dendrites to the medial antennal lobe.
(C) An Mz19-GAL4 labeled toll-6–/– VA1d (or DC3) single cell PN clone mistargets to the medial antennal lobe.
(D-E) PN dendrite targeting in Mz19-GAL4 labeled toll-6–/–adNb clones is rescued by restricted misexpression of Toll-6 (D) or Toll-7 (E) only in these clones.
(F) Quantification of VA1d and DC3 PN dendrite mistargeting in toll-6–/– adNb clones, as in Figure 1I. VA1d and DC3 PN dendrite distribution (top) and mean intensity (bottom) are shifted medially in toll-6 mutants.
(G) Quantification of VA1d and DC3 PN dendrite mistargeting in wild-type Mz19+ adNb clones, toll-6–/– Mz19-GAL4 adNb clones, toll-6–/– Mz19-GAL4 single cell clones, and intoll-6–/– adNb clones in which UAS-toll-6 or UAS-toll-7 is misexpressed in the clones.
To explore the role of toll-6 in dendrite targeting of other PN classes, we conducted hsFLP-based MARCM experiments and labeled with available PN-specific driver lines. We examined DA1 and VA1v PNs, which normally express Toll-6, as well as DM6 PNs, which do not express Toll-6. toll-6–/– DA1 PNs exhibited a low-penetrance localized medial dendrite mistargeting phenotype (Figure S4A), and toll-6–/– VA1v PNs displayed medial mistargeting of dendrites similar to the VA1d/DC3 defects (Figure S4B). Conversely, toll-6–/– DM6 PNs did not exhibit dendrite targeting defects (Figure S4C). Thus, Toll-6 is required for dendrite targeting in 4 classes of PNs that express Toll-6, but is not required for dendrite targeting in one PN class that does not express Toll-6.
Toll-6 and Toll-7 Play Instructive Roles in Target Selection
So far, we have shown that Toll-7 is cell-autonomously required for axon targeting of specific ORN classes (Figure 3) and Toll-6 is cell-autonomously required for dendrite targeting of specific PN classes (Figure 5). These ORN axons and PN dendrites target to Toll-7+ (Figure 2A-C) and Toll-6+ (Figure 4A-C) antennal lobe regions. These data suggest that Toll-7 and Toll-6 play instructive roles in ORN axon and PN dendrite targeting, respectively. To further test this idea, we performed the following gain-of-function experiments.
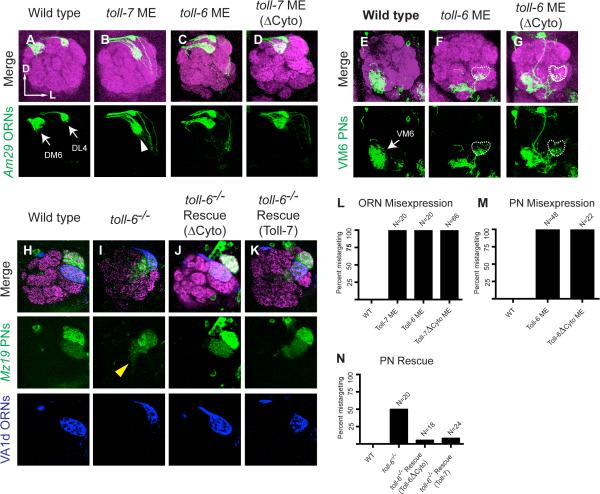
We first misexpressed UAS-toll-7 in two classes of ORNs, DM6 and DL4, during development using Am29-GAL4 (Figure 6A). DM6 is normally located at the dorsomedial edge of the antennal lobe, outside the Toll-7+ region (Figure 7A), but misexpression of toll-7 caused a severe lateral mistargeting of DM6 ORN axons to the Toll-7+ region (Figure 6B, L). DL4 ORN axons were unaffected, which may be explained by their proximity to, or inclusion within, the Toll-7+ region. Thus, misexpression of Toll-7 caused the axons of a Toll-7-negative ORN class to mistarget to the Toll-7+ region, supporting an instructive role of Toll-7 in ORN axon targeting.
Figure 6. Toll-6 and Toll-7 Play Instructive Roles in Target Selection, and Their Cytoplasmic Domains Are Dispensable.
All panels show labeling of specific PN and ORN classes and a merged image with neuropil labeling of the same single confocal section.
(A) In wild-type, Am29-GAL4 labels ORN axons targeting to the DM6 and DL4 glomeruli (arrows) in the dorsomedial and dorsolateral antennal lobe, respectively.
(B) Am29-GAL4 misexpression of Toll-7 causes lateral mistargeting of DM6 ORN axons to the Toll-7+ region (white arrowhead). DL4 ORNs appear unperturbed.
(C) Am29-GAL4 misexpression of Toll-6 also causes DM6 ORN axon mistargeting.
(D) Misexpression of a truncated Toll-7 in which the cytoplasmic domain is replaced with GFP recapitulates wild-type Toll-7 misexpression.
(E) In wild type, 71D09-GAL4 labels dendrites of VM6 PNs (arrow) targeting the posterior ventromedial antennal lobe.
(F) 71D09-GAL4 misexpression of Toll-6 causes lateral mistargeting of VM6 PN dendrites to the Toll-6+ region including the VA1v glomerulus (dashed line).
(G) Misexpression of a truncated Toll-6 transgene in which the cytoplasmic domain is replaced with GFP recapitulates wild-type Toll-6 misexpression.
(H) In wild-type control, dendrites of DA1 and VA1d PNs visualized by Mz19-GAL4 (green) and VA1d ORNs labeled with Or88a-myr-tdT (pseudocolored blue) target to the lateral antennal lobe. Mz19-GAL4 also labels dendrites of DC3 PNs, which are not in this confocal section.
(I) In toll-6 mutants, VA1d ORN axons mistarget dorsally to a lateral position with respect to DA1. Mz19-GAL4 PN dendrites mistarget (yellow arrowhead) diffusely in the antennal lobe, and partially colocalize with mistargeted VA1d ORNs.
(J) Misexpression of a cytoplasmic domain truncated Toll-6 in PNs by Mz19-GAL4 rescues VA1d ORN axon and PN dendrite targeting defects.
(K) Misexpression of Toll-7 in PNs by Mz19-GAL4 also rescues VA1d ORN axon and PN dendrite targeting defects.
(L) Quantification of DM6 ORN axon targeting in wild type and following Am29-GAL4 misexpression of UAS-toll-7, UAS-toll-6, or UAS-toll-7Δcyto.
(M) Quantification of VM6 PN dendrite mistargeting in wild type and following 71D09-GAL4 misexpression of UAS-toll-6 or UAS-toll-6Δcyto.
(N) Quantification of VA1d ORN axon mistargeting in wild type, toll-6 mutants (same as Figure 1H), and in rescue experiments in which UAS-toll-6Δcyto or UAS-toll-7 is misexpressed in Mz19-GAL4 labeled PNs in toll-6 mutants.
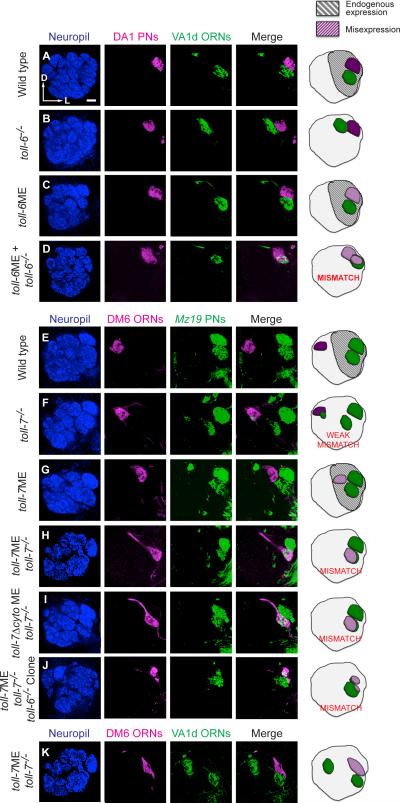
Figure 7. Toll-6 and Toll-7 Mediate Synaptic Partner Matching.
Left panels are single-section confocal images stained with neuropil marker, PN dendrites, ORN axons, and their merge as indicated. The right panel shows the schematic interpretation. Panels A-D test the role of Toll-6, and panels E-K test the role of Toll-7, in synaptic partner matching.
(A) In wild type, axons of VA1d ORNs labeled with Or88a-myr-tdT (pseudocolored green) target to the VA1d glomerulus ventral to DA1. The DA1 glomerulus is labeled here by dendrites of DA1 PNs (magenta) visualized by Mz19-GAL4-based MARCM lateral neuroblast clones. No intermingling of VA1d ORN axons and DA1 PN dendrites results (scale bar is 10μm).
(B) In toll-6–/– whole-animal mutants, VA1d ORN axons mistarget dorsally (see also Figure 1F). No intermingling of VA1d ORN axons and DA1 PN dendrites results (0/10 mismatch).
(C) When Toll-6 is misexpressed in DA1 PNs in Mz19-GAL4-based MARCM lateral neuroblast clones in an otherwise wild-type background, no intermingling of VA1d ORN axons and DA1 PN dendrites results (0/10 mismatch).
(D) When Toll-6 is misexpressed in DA1 PNs in Mz19-GAL4-based MARCM lateral neuroblast clones in toll-6–/– whole-animal mutants, extensive intermingling of VA1d ORN axons and DA1 PN dendrites occurs (13/15 mismatch).
(E) In wild type, Am29-GAL4 labels axons of DM6 ORN axons targeting the dorsomedial antennal lobe (it also labels ORN axons targeting the DL4 glomerulus, which is in a more posterior confocal plane and thus is not visible). Mz19-QF labels dendrites of DA1 and VA1d PNs in the lateral antennal lobe, far away from DM6 ORN axons.
(F) In toll-7 whole-animal mutants, a subset of VA1d PN dendrites mistarget medially, following the mistargeted VA1d ORNs, some of which mismatch with DM6 ORNs in the vicinity (3/10 antennal lobes exhibit mismatching of comparable severity to this example).
(G) Misexpressing Toll-7 with AM29-GAL4 in an otherwise wild-type background causes DM6 ORN axons to mistarget laterally (similar to Figure 6B) to a Toll-7+ area, adjacent to Mz-19+ dendrites, but does not result in intermingling with Mz-19+ PN dendrites (0/8 mismatch).
(H) When Toll-7 is misexpressed with AM29-GAL4 in a toll-7 whole-animal mutant background, laterally mistargeted DM6 ORN axons extensively intermingle with Mz19+ PN dendrites (31/38 antennal lobes exhibit mismatching of comparable severity to this example).
(I) Misexpressing a truncated Toll-7 lacking its cytoplasmic domain (Toll-7Δcyto) with AM29-GAL4 in a toll-7 whole-animal mutant background gives a similar mismatching phenotype as misexpressing wild-type Toll-7 in panel H (6/6 antennal lobes mismatch with a comparable severity to this example).
(J) The DM6 ORN axon and VA1d PN dendrite still mismatch when Toll-7 is misexpressed with AM29-GAL4 in a toll-7 whole-animal mutant background following MZ19 Q-MARCM removal of toll-6 from adNb clones (4/7 antennal lobes mismatch with a comparable severity to this example).
(K) When Toll-7 is misexpressed by AM29-GAL4 in a toll-7 whole-animal mutant background, laterally mistargeted DM6 ORN axons do not comingle with VA1d ORN axons (0/30 antennal lobes display DM6 and VA1d ORN axon comingling).
In an analogous experiment, we misexpressed UAS-toll-6 in VM6 PNs during development using the Janelia GAL4 line 71D09 (Jenett et al., 2012; B. Wu & L. L., unpublished observation). VM6 is normally located at the posterior ventromedial edge of the antennal lobe (Figure 6E), outside the Toll-6+ region. However, misexpression of Toll-6 in VM6 PNs caused an anterolateral shift in PN dendrite targeting, such that some VM6 PN dendrites mistargeted within the VA1v glomerulus (Figure 6F, M), which normally expresses Toll-6 (Figure 4A). Together, these data indicate that toll-6 and toll-7 act instructively in PNs and ORNs, respectively, for targeting their dendrites and axons to the Toll-6/Toll-7 enriched region.
Misexpressed Toll-6 and Toll-7 Appear to Function Equivalently
Given the different phenotypes and cell-type specificities of these two highly related Toll receptors in olfactory circuit wiring, we next examined their molecular mechanisms. Blast comparison revealed that Toll-6 and Toll-7 proteins share 40% sequence identity, with similar domain organization (Figure 1J). Given these gross structural similarities, we asked whether Toll-6 and Toll-7 could replace each other's function. Using the Am29-GAL4 misexpression assay, we found that Toll-6 misexpression in DM6 ORNs caused severe lateral mistargeting of ORN axons (Figure 6C, L), similar to Toll-7 misexpression (Figure 6B). Toll-2, Toll-6, Toll-7, and Toll-8 are closest among the 9 Drosophila Toll receptors and represent a subfamily based on alignment of the Toll-family cytosolic TIR-containing domains (Leulier and Lemaitre, 2008). Interestingly, Am29-GAL4 misexpression of Toll-2 and Toll-8 also resulted in lateral targeting defects of DM6 ORN axons (data not shown) similar to Toll-6 and Toll-7 misexpression. By contrast, targeting of DM6 ORN axons was normal following Am29-GAL4 misexpression of the other Toll-family members, including Toll-1, -3, -4, -5, and -9 (data not shown). Thus, only the Toll-2/6/7/8 subfamily members exhibited similar activity in this misexpression assay.
We also examined whether Toll-7 is capable of rescuing toll-6 mutant ORN axon and PN dendrite targeting defects. Expression of UAS-toll-7 using Mz19-GAL4 rescued the VA1d ORN axon targeting defects seen in mutants (Figure 6K, N). Further, expression of toll-7 in toll-6–/– Mz19+ MARCM adNb PN clones (Figure 5E, G) rescued the severe PN dendrite targeting defects observed in toll-6–/– clones (Figure 5B). Thus, in addition to the equivalence in misexpression assays (Figure 6A-C), Toll-7 can replace the olfactory wiring functions of Toll-6 in PNs during development.
The Canonical Toll Signaling Pathway Is Not Required for VA1d ORN Axon Targeting
In the context of embryonic dorsal-ventral pattern formation and innate immune response, Toll-1 and Toll-like receptors (TLRs) function upstream of a conserved, canonical Toll-signaling cascade to activate NF-κB factors and regulate transcription (Imler and Zheng, 2004). To investigate whether Toll-6 and Toll-7 utilize canonical Toll signaling for olfactory circuit wiring, we examined the requirement of critical Toll-pathway components, including the TIR adaptor protein dMyD88, and the two NF-κB factors Dorsal and Dif, using VA1d ORN axon targeting as an assay. VA1d ORN axon targeting was normal in two different dMyD88 alleles, dMyD88EP2133 and dMyD88C03881, as well as in dl1 and Dif1 (Figure S6A-D). Drosophila Toll-1 is activated by its ligand Spätzle (Spz) (Imler and Zheng, 2004), and evidence suggests the Spz homologs DNT1 and DNT2 (also named Spz-2 and Spz-5, respectively) are ligands for Toll-6 and Toll-7 (McIlroy et al., 2013). VA1d ORN axon targeting was normal in two different Spz alleles (spz2 and spz3; data not shown). We also tested whether DNT1 and DNT2 are involved in olfactory circuit wiring specificity. Mz19+ PN dendrites and VA1d ORN axons targeted normally in dNT2, dNT1 double null mutant flies (Figure S6E). These findings indicate the canonical Toll-signaling cascade, from ligands to downstream signaling partners, is not required for VA1d ORN axon targeting.
The Cytosolic Domains of Toll-6 and Toll-7 Are Dispensable for Olfactory Circuit Wiring
To further examine the signaling mechanisms of Toll-6 and Toll-7 in olfactory circuit wiring, in particular whether signaling through the conserved Toll-6 and Toll-7 TIR domains is required, we made UAS-transgenes in which two critical TIR domain residues (Ohnishi et al., 2009; Xu et al., 2000) were mutated (TIR-dead). We also replaced the entire cytosolic domain by GFP (ΔCyto). Surprisingly, Am29-GAL driven misexpression of UAS-toll-7TIR-dead (Figure S5A) and UAS-toll-7ΔCyto (Figure 6D, L) resulted in lateral mistargeting of DM6 ORN axons, a phenotype indistinguishable from misexpressing the wild-type UAS-toll-7 transgene (Figure 6B). In addition, 71D09-GAL4-driven misexpression of UAS-toll-6ΔCyto caused lateral mistargeting of VM6 PN dendrites (Figure 6G, M). Thus, the cytosolic domains of Toll-6 and Toll-7 are dispensable for their gain-of-function effects on PN dendrite and ORN axon targeting, respectively.
To test whether the TIR/cytosolic domains are required for rescue of VA1d PN and ORN phenotypes observed in toll-6 mutants, we used Mz19-GAL4 to express the mutant transgenes in DA1, VA1d, and DC3 PNs. We found that UAS-toll-6TIR-dead (Figure S5B) and UAS-toll-6ΔCyto (Figure 6H-J, N) rescued both ORN axon and PN dendrite targeting phenotypes, indicating that the functions of Toll-6 and Toll-7 in olfactory wiring do not depend on the intracellular signaling capacities of these proteins.
Toll-6 and Toll-7 Mediate Synaptic Partner Matching Using Heterophilic Molecular Partners
Lastly, we describe a set of experiments that shed light on the cellular mechanisms by which Toll receptors regulate olfactory system wiring specificity. VA1d ORN axons and DA1 PN dendrites normally targeted to adjacent glomeruli and never mismatched in wild-type animals (Figure 7A). However, under the condition where all cells lacked endogenous Toll-6 and DA1 PNs expressed Toll-6 from a transgene (in Mz19-GAL4-based MARCM lateral neuroblast clones), there was a strong mismatch between VA1d ORN axons and DA1 PN dendrites (Figure 7D). This mismatching did not occur either when all cells lacked Toll-6 alone (Figure 7B), or when DA1 PNs expressed Toll-6 from a transgene in an otherwise wild-type background (Figure 7C).
Given the cell-autonomous function of Toll-6 in PN dendrite targeting (Figure 5), and that expressing Toll-6 in PNs can rescue ORN axon mistargeting phenotypes in toll-6 whole-animal mutants (Figure 4), the simplest model that can explain the mismatching phenotypes is that, in addition to regulating PN dendrite targeting, Toll-6 in PNs also regulates ORN–PN partner matching with its cognate ORN partners. Adding Toll-6 back only in DA1 PNs in a whole-animal mutant background makes DA1 PN dendrites the preferred PN partner for VA1d ORN axons. We have previously identified two Teneurins, Ten-m and Ten-a, as homophilic partner matching molecules that differ in their expression between VA1d and DA1 and contribute to the ORN–PN partner matching of these glomeruli (Hong et al., 2012). The fact that manipulation of toll-6 alone is capable of causing mismatching of DA1 PN dendrites and VA1d ORN axons suggests Toll-6 is a potent partner-matching factor that can override other matching mechanisms. That mismatching occurred between Toll-6-expressing DA1 PN dendrites and Toll-6-lacking VA1d ORN axons suggests that Toll-6 interacts with a heterophilic ligand for partner matching.
Next, we designed a similar set of experiments to explore whether Toll-7 could have an analogous role in synaptic partner matching, this time between Toll-7-expressing ORN axons and their cognate PN dendrites. As shown earlier, misexpression of Toll-7 in DM6 ORNs using Am29-GAL4 caused a lateral mistargeting of DM6 ORN axons (Figure 6B). When we co-labeled Mz19+ PNs in the same animal, we found that Toll-7-misexpressing DM6 ORN axons mistargeted to an antennal lobe position just medial to the VA1d glomerulus, but did not intermingle with Mz19+ PN dendrites (Figure 7G, compared to control in Figure 7E). However, when we performed the same misexpression experiments in the toll-7 whole-animal mutant background, we observed extensive mismatching between DM6 ORN axons and Mz19+ PN dendrites—most likely VA1d dendrites as judged from the location (Figure 7H). To test whether this Toll-7-mediated mismatching phenotype is dependent on the activity of Toll-6 in PNs, we used Q-MARCM (Potter et al., 2010) to remove toll-6 from Mz19+ MARCM adNb clones in a toll-7 mutant background while simultaneously misexpressing Toll-7 in DM6 ORNs (Figure 7J). We still observed mismatching between DM6 ORNs and the toll-6−/− clones, suggesting Toll-6 is not a ligand for Toll-7 in this matching experiment.
The mismatching between DM6 ORNs and Mz19+ PN dendrites could be due to (1) direct mismatching between ORNs and PNs or (2) failed segregation between DM6 and VA1d ORN axons, and as a secondary consequence DM6 ORNs mismatch with VA1d PNs. To distinguish between these two possibilities, we differentially labeled DM6 and VA1d ORNs in the above mismatching experiment, and observed that DM6 and VA1d ORN axons remained segregated (Figure 7K). This strongly suggests that the overlap between DM6 ORN axons with Mz19+ PN dendrites in experiments shown in Figure 7H resulted from direct mismatching between ORN axons and PN dendrites, as opposed to failed segregation between DM6 and VA1d ORN axons. Moreover, in toll-7 mutants we occasionally observed minor mismatching phenotypes between DM6 ORN axons and mistargeted VA1d PN dendrites (Figure 7F), indicating that these classes are partially competent to match. As shown previously, PNs labeled by Mz19-GAL4 elaborate dendrites that partially overlap with ORN axons of non-matching classes during early stages of olfactory circuit development (Figure S2). Thus, olfactory wiring appears to be a dynamic process subject to global mismatching events, such as in cases when the partner matching cell-surface code is altered (Figure 7H).
Finally, we tested whether Toll-7 signaling through its cytoplasmic domain is required for these mismatching phenotypes. We found that AM29-GAL4 driven misexpression of UAS-toll-7ΔCyto in the toll-7 mutant background recapitulated the mismatching phenotypes (Figure 7I), indicating that signaling through the cytoplasmic domain of Toll-7 is not required for its synaptic partner matching function.
DISCUSSION
Our high-resolution confocal-based screen of transmembrane and secreted proteins identified two Toll-family receptors that regulate different aspects of olfactory circuit wiring (Figure 1). Toll-7 is expressed in ORN axons that target to a cluster of anterolateral glomeruli (Figure 2), and functions cell-autonomously in VA1d and DA1 ORNs for their axon targeting (Figure 3). Toll-6 is localized to a similar cluster of anterolateral glomeruli, but is expressed from both ORNs and PNs (Figure 4). Toll-6 cell-autonomously regulates PN dendrite targeting (Figure 5), and Toll-6 in PNs is sufficient to regulate ORN axon targeting (Figure 4). Thus, Toll-6 and Toll-7 instruct PN dendrite and ORN axon targeting, respectively. Both Toll-6 and Toll-7 also participate in partner matching of the VA1d glomerulus (Figure 7), with Toll-6 functioning in PNs and Toll-7 in ORNs. This raises the intriguing idea that partner matching may be part of a larger dynamic strategy of PN dendrite and ORN axon target selection. The activities of Toll-6 and Toll-7 in olfactory circuit wiring do not require their cytoplasmic domains or their canonical signaling pathway members (Figure 6), suggesting the involvement of signaling pathways with novel ligand(s) and co-receptor(s).
Toll-6 and Toll-7 Instruct Olfactory Circuit Assembly via a Non-canonical Mechanism
Toll-family receptors are best known for their functions in innate immunity and also have important roles in development, including embryonic patterning and in tissue morphogenesis (Imler and Zheng, 2004; Pare et al., 2014). In addition, Toll-1, Toll-6, Toll-7 and Toll-8 have been implicated in regulating embryonic motor axon targeting (McIlroy et al., 2013; Rose et al., 1997) and synaptic growth at the neuromuscular junction (Ballard et al., 2014). Vertebrate Toll-like receptors (TLRs) regulate hippocampal neurogenesis in adult mice (Rolls et al., 2007) and promote dendrite outgrowth in vitro (Liu et al., 2013; Ma et al., 2006). However, neurodevelopmental defects have so far not been observed in mouse TLR knockouts, which could be due to redundancy in the TLR family.
Our study represents the first example of a role for Toll-family receptors in wiring specificity of neural circuits in the brain. Surprisingly, the activation and signaling mechanisms of Toll-6 and Toll-7 are non-canonical, in contrast to a previous report based on in vitro assays (McIlroy et al., 2013). In Drosophila, the embryonic patterning and immune functions of Toll-1 are activated in response to its ligand Spz, resulting in signaling through NF-κB members Dorsal and Dif, respectively. At the neuromuscular junction, the Spz homologs DNT2 and DNT1 have been proposed to serve as ligands for Toll-6 and Toll-7, and evidence suggests that Toll-6 and Toll-7 signal through a canonical NF-κB pathway (McIlroy et al., 2013). We found that targeting of VA1d PN dendrites and ORN axons was normal in dNT2, dNT1 double null mutants (Figure S6E), indicating that Toll-6 and Toll-7 do not use the DNT1/2 ligands in the context of olfactory circuit wiring. Previous work has also shown that Toll receptors promote cell aggregation in in vitro S2 cell assays (Keith and Gay, 1990; Pare et al., 2014). We did not observe homophilic or heterophilic interaction of Toll-6 and Toll-7 in S2 cell aggregation assays (data not shown). Thus, Toll-6 and Toll-7 do not appear to function as homophilic or heterophilic ligand-receptor pairs. Indeed, our findings suggest Toll-6 and Toll-7 interact with heterophilic partner(s) in synaptic partner matching functions (Figure 7). The distinct toll-6 and toll-7 mutant phenotypes (Figure 1) and the Toll-7-mediated mismatching observed with toll-6−/− PN clones (Figure 7J) argue against the possibility that they are a receptor-ligand pair. Lastly, we found the cytoplasmic domains of Toll-6 and Toll-7 to be dispensable for their activities in PNs and ORNs, respectively, suggesting that these receptors signal through a non-canonical co-receptor mechanism.
In summary, our studies identified essential functions of Toll receptors in regulating wiring specificity of the olfactory circuit. Our in vivo analyses suggest that Toll receptor signaling in olfactory circuit assembly differs from early development or innate immunity. These data imply the existence of novel Toll-6/7 ligands and co-receptors that mediate their function in wiring specificity. Future identification of these proteins will broaden our knowledge of Toll receptor function, and shed further light on the molecular mechanisms that establish wiring specificity of neural circuits.
Families of Wiring Specificity Molecules in Neural Circuit Assembly
Toll-6 and Toll-7 regulate reciprocal biological functions in PNs and ORNs, respectively. While Toll-6 functions in PNs and Toll-7 in ORNs, they each regulate targeting and matching of neuronal processes to the VA1d glomerulus. Interestingly, we found that all four members of the subfamily to which Toll-6 and Toll-7 belong have similar misexpression phenotypes in ORNs, and that Toll-6 and Toll-7 are functionally equivalent for rescuing toll-6 mutant VA1d PN dendrite and ORN axon targeting defects. Thus, the Toll-2/6/7/8 subfamily members exhibit similar functional properties in olfactory neuron wiring in the context of misexpression.
Drosophila Robo receptors are a well-characterized family of receptors that regulate different wiring decisions in longitudinal axon pathway choice in the embryonic CNS (Rajagopalan et al., 2000; Simpson et al., 2000). Robo1, Robo2, and Robo3 establish a combinatorial code for axon pathway selection in response to the midline repellent ligand Slit. The Robo swap experiments showed that pathway choice specificity was not dependent on the functional differences of Robo receptor proteins, but on their different spatiotemporal expression patterns (Spitzweck et al., 2010). Our finding of the functional equivalence of Toll-6 and Toll-7 in olfactory wiring when misexpressed resembles the Robo code: the distinct spatial expression pattern of a particular Toll receptor, rather than its unique structural facets, can determine its biological function. The fact that Toll-2 and Toll-8 have activities similar to Toll-6 and Toll-7 when misexpressed suggests that Toll-2 and Toll-8 could play analogous functions in wiring specificity in other parts of the nervous system, including part of the olfactory system not examined in this study. Toll-6 and Toll-7 receptors, and possibly other Toll receptors, may share a common ligand, co-receptor, or both, making them wiring specificity molecules whose spatiotemporal expression patterns can evolve to play distinct roles in wiring of the complex CNS circuits.
Our data also suggest that Toll-6 and Toll-7 do not play completely identical, redundant functions. For instance, our expression study indicates that VA1d ORNs express both Toll-6 and Toll-7 (Figure 2, Figure 4). Yet whole animal or ORN-specific loss-of-function of Toll-7 is sufficient to produce VA1d ORN axon targeting defects (Figures 1, Figure 2). This may be accounted for by differences in Toll-6 and Toll-7 temporal patterns of expression, different levels of expression, or their differential affinity for the ligand(s) or co-receptor(s).
In the Drosophila antennal lobe, thousands of neurons belonging to over 100 different classes extend axons and dendrites within a small, compact space, making specific choices during targeting to specific stereotyped positions. This process requires a large number of cell-surface recognition molecules to bring axons and dendrites of the same neuronal class together and separate axons and dendrites from different classes. A certain level of redundancy could make the wiring process more robust (i.e., more resistant to perturbations of individual molecules), thus requiring a greater number of identity recognition molecules than the theoretical minimum (Hong and Luo, 2014). In principle, expressing multiple molecules such as Toll receptors in distinct spatiotemporal patterns and levels (or having distinct ligand-receptor affinities) can increase the capacity for a limited number of molecules to encode different neuronal identities, providing each class of olfactory neuron with a unique, partially redundant combination of recognition molecules.
Long-range Mismatching Phenotypes Suggest a Dynamic Wiring Process
Teneurins were previously identified to be essential for synaptic partner matching between PN dendrites and ORN axons, wherein loss-of-function and misexpression perturbations cause neighboring classes of PN dendrites and ORN axons to form ectopic connections (Hong et al., 2012). This mismatching appears local, as the mismatched glomeruli are located adjacently within the same region of the antennal lobe. Given that there are 50 pairs of PNs and ORNs, two Teneurins are clearly not the only molecules involved in synaptic partner matching. Indeed, we observed ectopic connections between DA1 PN dendrites with VA1d ORN axons following misexpression of toll-6 in DA1 PNs in the whole-animal toll-6 mutant background (Figure 7D). This mismatching also occurs locally, qualitatively similar to what we observed with Teneurin perturbations. Surprisingly, we also observed mismatching between DM6 ORN axons and VA1d PN dendrites when misexpressing Toll-7 in DM6 ORNs in the whole-animal toll-7 mutant background (Figure 7H). This is unexpected considering that DM6 ORN axons and VA1d PN dendrites normally target to the medial and lateral edge of the antennal lobe, respectively; in this complex genetic manipulation, they mismatch across the entire antennal lobe, which we term “global mismatching.”
Previous work showed that PNs target their dendrites to approximate regions of the antennal lobe that correspond to their final glomerular position, before the arrival of pioneering ORN axons (Jefferis et al., 2004; Komiyama et al., 2007). This suggests a model whereby PN dendrites pre-pattern the antennal lobe, serving as a relatively fixed target for projecting ORN axons to partner with, and therefore matching would be a local mechanism segregating neighboring glomeruli. Here, the Toll-7 findings serve as the first example of a global mismatching between glomeruli that are at much greater distance. This suggests PN dendrites are more dynamic, and are capable of sampling a relatively large portion of the antennal lobe during ORN axon invasion. Additionally, synaptic partner matching between PN dendrites and ORN axons may begin at an early stage before confinement of PN dendrites to their final target area (Figure S2). Together, these data suggest that PN dendrite and ORN axon targeting is more dynamic than previously appreciated. As a result, synaptic partner sampling would occur on a larger, more global scale, and may serve as an additional strategy for axon and dendrite targeting. In this view, the dynamic interplay of both PN dendrites and ORN axons is pivotal for final target selection, emphasizing the need for multiple partner matching molecules, such as Tolls and Teneurins, to prevent not only local, but also global, mismatching.
EXPERIMENTAL PROCEDURES
See Supplemental Experimental Procedures for detailed descriptions of mutants and transgenes used in this study.
RNAi screening
The RNAi screen fly was generated as follows: C155-GAL4 was recombined with UAS-dcr2 on the X chromosome. Mz19-QF was recombined with QUAS-mCD8GFP, Or88a-Myr-tdTomato and Or47b-ratCD2 on the 2nd chromosome. These flies were crossed together to make a stable RNAi screen stock. Virgin females were collected from the RNAi screen stock and crossed to UAS-RNAi males. Females were allowed to lay eggs for two days at 25°C then shifted to 29°C to enhance the GAL4/UAS expression system. Progeny were collected within two days of eclosion and the brains were dissected and fixed. We tested between two to three independent RNAi lines (different hairpins) per gene. For each RNAi line tested, 12 brains were dissected, fixed and immunostained, and of these, at least three were imaged by confocal microscopy.
Mosaic Analysis
hsFlp MARCM analyses were performed as previously described (Jefferis et al., 2001; Komiyama et al., 2004; Lee and Luo, 1999). In summary, for adNb and lNb clones, flies were heat shocked for 1 hr at 37°C between 2 and 26 hr after larval hatching. To analyze ORN axon projections in adult brains using hsFlp MARCM, animals between 0-24h APF were heat shocked for 1 hour at 37°C. eyFLP MARCM and reverse MARCM was performed as described previously (Zhu and Luo, 2004).
Immunostaining
Tissue dissection and immunostaining were performed according to previously described methods (Sweeney et al., 2007). Rat anti-Toll-6 antibody was custom produced by Thermo Scientific Pierce against a peptide epitope containing Toll-6 residues 62 to 81 (RPLTAGAGGDPSLYDAPDDC). Rabbit anti-Toll-7 antibody was custom produced by YenZym against Toll-7 residues 1425 to 1442 (QQPNPTAVSGQQQGPHVQ). Commercially available antibodies used in these studies include mouse nc82 [1:35; Developmental Studies Hybridoma Bank, (DSHB)], rat anti-DNcad (DN-Ex #8; 1:40; DSHB), chicken anti-GFP (1:1000; Aves Labs), rabbit anti-DsRed (1:250; Clontech) and mouse anti-ratCD2 (OX-34; 1:200; AbD Serotec). Secondary antibodies were raised in goat or donkey against rabbit, mouse, rat and chicken antisera (Jackson Immunoresearch), conjugated to Alexa 405, 488, FITC, Cy3, Cy5, or Alexa 647.
Imaging and Quantification Procedure
Confocal images were collected with a Zeiss LSM 510 or LSM 780 and processed with ImageJ and Adobe Photoshop. For assessment of toll-7 mutant ORN axon and toll-6 mutant PN dendrite targeting defects the relative fluorescence intensity was quantified by binning along the lateral-medial axis using MATLAB as described previously (Komiyama et al., 2007). Quantification of the toll-6 mutant ORN axon targeting phenotype was scored by the experimenter according to position of VA1d ORN axons relative to the DA1 glomerulus (i.e. ventral, dorsomedial, or dorsolateral) per antennal lobe. Mistargeting frequency was calculated as (hemispheres with mistargeting)/(total brain hemispheres). Graphs were generated using the GraphPad Prism software.
Supplementary Material
Highlights.
RNAi screen uncovers the Toll-Family Receptors Toll-6 and Toll-7 in neuronal wiring
Toll-6 and Toll-7 instruct targeting of specific dendrites and axons, respectively
Toll-6/7 mediate synaptic partner matching through heterophilic molecular partners
Toll-6/7 function in neuronal wiring does not require canonical signaling pathway
Acknowledgement
We thank D. Luginbuhl for technical assistance, B. Wu and H. Zhu for sharing unpublished results, the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) and the VDRC for providing transgenic RNAi fly stocks, the Bloomington Stock Center for mutant alleles and transgenic flies, the Kyoto DGRC for NP lines, S. Cherry and J.M. Reichhart for dMyD88 alleles, A. Hidalgo for the dNT1 allele, T. Lee and G. Rubin for 71D09-GAL4, and T. Clandinin for 853-GAL4. We thanks K. Beier, L. DeNardo, X. Gao, C. Guenthner, T. Mosca, B. Wu, Xin Wang, and Xiang Wang for discussion and critiques on the manuscript. A.W. is a Damon Runyon HHMI fellow supported by the Damon Runyon Cancer Research Foundation (DRG-2098-11). L.L. is an investigator of the Howard Hughes Medical Institute. This work was supported by an NIH grant (R01-DC005982 to L.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: A.W., H.W., and L.L. designed the study. A.W. and H.W. performed the RNAi screen and initial phenotypic analysis. A.W. completed the rest of the experiments with contribution from V.F. A.W. wrote the manuscript with substantial contribution from H.W. and L.L. L.L. supervised the project.
References
- Ballard SL, Miller DL, Ganetzky B. Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J Cell Biol. 2014;204:1157–1172. doi: 10.1083/jcb.201308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PH, Li P, Shen K. Cell biology in neuroscience: cellular and molecular mechanisms underlying presynapse formation. J Cell Biol. 2013;203:11–22. doi: 10.1083/jcb.201307020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Zheng X, Beachy PA, Luo L. Patterning axon targeting of olfactory receptor neurons by coupled hedgehog signaling at two distinct steps. Cell. 2010;142:954–966. doi: 10.1016/j.cell.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Endo K, Aoki T, Yoda Y, Kimura K, Hama C. Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat Neurosci. 2007;10:153–160. doi: 10.1038/nn1832. [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Hong W, Luo L. Genetic control of wiring specificity in the fly olfactory system. Genetics. 2014;196:17–29. doi: 10.1534/genetics.113.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Mosca TJ, Luo L. Teneurins instruct synaptic partner matching in an olfactory map. Nature. 2012;484:201–207. doi: 10.1038/nature10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Zhu H, Potter CJ, Barsh G, Kurusu M, Zinn K, Luo L. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat Neurosci. 2009;12:1542–1550. doi: 10.1038/nn.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Vasconcelos ML, Clemens JC, Fishilevich Y, Vosshall LB, Zipursky SL. Axonal targeting of olfactory receptor neurons in Drosophila is controlled by Dscam. Neuron. 2003;37:221–231. doi: 10.1016/s0896-6273(02)01183-2. [DOI] [PubMed] [Google Scholar]
- Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. Journal of leukocyte biology. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, Luo L. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell reports. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo WJ, Sweeney LB, Liang L, Luo L. Linking cell fate, trajectory choice, and target selection: genetic analysis of Sema-2b in olfactory axon targeting. Neuron. 2013;78:673–686. doi: 10.1016/j.neuron.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith FJ, Gay NJ. The Drosophila membrane receptor Toll can function to promote cellular adhesion. EMBO J. 1990;9:4299–4306. doi: 10.1002/j.1460-2075.1990.tb07878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Carlson JR, Luo L. Olfactory receptor neuron axon targeting: intrinsic transcriptional control and hierarchical interactions. Nat Neurosci. 2004;7:819–825. doi: 10.1038/nn1284. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;59:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattemann M, Zierau A, Schulte C, Seidl S, Kuhlmann B, Hummel T. Semaphorin-1a controls receptor neuron-specific axonal convergence in the primary olfactory center of Drosophila. Neuron. 2007;53:169–184. doi: 10.1016/j.neuron.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Leulier F, Lemaitre B. Toll-like receptors--taking an evolutionary approach. Nature reviews Genetics. 2008;9:165–178. doi: 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- Liu HY, Hong YF, Huang CM, Chen CY, Huang TN, Hsueh YP. TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 pathway. J Neurosci. 2013;33:11479–11493. doi: 10.1523/JNEUROSCI.5566-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy G, Foldi I, Aurikko J, Wentzell JS, Lim MA, Fenton JC, Gay NJ, Hidalgo A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat Neurosci. 2013;16:1248–1256. doi: 10.1038/nn.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Tochio H, Kato Z, Orii KE, Li A, Kimura T, Hiroaki H, Kondo N, Shirakawa M. Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc Natl Acad Sci U S A. 2009;106:10260–10265. doi: 10.1073/pnas.0812956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA. A positional Toll receptor code directs convergent extension in Drosophila. Nature. 2014;515:523–527. doi: 10.1038/nature13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rose D, Zhu X, Kose H, Hoang B, Cho J, Chiba A. Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron growth cone in Drosophila. Development. 1997;124:1561–1571. doi: 10.1242/dev.124.8.1561. [DOI] [PubMed] [Google Scholar]
- Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, Benton R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Spitzweck B, Brankatschk M, Dickson BJ. Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell. 2010;140:409–420. doi: 10.1016/j.cell.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- Sweeney LB, Chou YH, Wu Z, Joo W, Komiyama T, Potter CJ, Kolodkin AL, Garcia KC, Luo L. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron. 2011;72:734–747. doi: 10.1016/j.neuron.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney LB, Couto A, Chou YH, Berdnik D, Dickson BJ, Luo L, Komiyama T. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron. 2007;53:185–200. doi: 10.1016/j.neuron.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, Tong L. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- Zhu H, Luo L. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron. 2004;42:63–75. doi: 10.1016/s0896-6273(04)00142-4. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.