Abstract
Objective
To investigate the normal data of pain-related evoked potentials (PREP) elicited with a concentric surface electrode among normal, healthy adults and the relationship between PREP and pain intensity.
Methods
Sixty healthy volunteers (22 men and 38 women; aged 36.4±10.7 years; height, 165.4±7.8 cm) were enrolled. Routine nerve conduction study (NCS) was done to measure PREP following electrical stimulation of hands (C7 dermatome) and feet (L5 dermatome). Negative peak (N), positive peak (P) latencies, peak to peak (NP) amplitudes, conduction velocity (CV), and verbal rating scale (VRS) score were obtained. Linear regression analysis tested for significant relevance between variables of PREP and VRS score.
Results
Normal NCS results were obtained in all subjects. N latency of hand PREP was 163.8 ±40.0 ms (right) and 161.0±39.9 ms (left). N latency of foot PREP was 178.0±43.9 ms (right), 180.4±43.4 ms (left). NP amplitude of hands was 20.6±10.6 µV (right) and 21.9±11.6 µV (left). NP amplitude of feet was 18.8±8.3 µV (right) and 19.0±8.4 µV (left). The calculated CV was 13.2±4.7 m/s and VRS score was 3.8±1.0. A highly significant positive correlation was evident between VRS score and NP amplitude (y=0.1069x+1.781, r=0.877, n=60, p<0.0001).
Conclusion
PREP among normal, healthy adults revealed a statistically significant correlation between PREP amplitude and VRS score.
Keywords: Evoked potentials, Pain measurement, Nociceptive pain, Electrodes
INTRODUCTION
Recording of pain-related evoked potential (PREP) is a tool for evaluation of human nociceptive systems [1]. Different techniques can evoke brain response by stimulation of nociceptive fibers. It is difficult to separate the nociceptive inputs from non-nociceptive inputs, because of the simultaneous activation of both non-nociceptive Aβ and nociceptive Aδ and C fibers in the somatosensory evoked potentials [2].
Several attempts have sought to increase the nociceptive sensitivity of various electrophysiological techniques [3]. These techniques have been suggested for selective stimulation of nociceptive Aδ and C fibers by avoiding co-stimulation of non-nociceptive Aβ fibers [1]. Intracutaneous low current intensity stimulation has been reported [3]. Valeriani et al. [2] reported that the contact thermode activates the cutaneous nociceptors. But, the intracutaneous technique and contact heat method are invasive and uncomfortable. More recently, stimulation of pure pain using a short pulse CO2 laser was introduced in pain research. Laser thermal stimulation requires expensive equipment and is only available in a few specialized laboratories [4,5,6].
All these techniques have proven adequate sensitivity but most have at least one major disadvantage. So these methods are limited for wide clinical use.
A novel technique to elicit PREP using a custom-built concentric surface electrode is noninvasive, cheap, and easy to perform [1]. Due to unique the concentric geometry and smaller anode-cathode distance compared with a conventional electrode, a high current density can be achieved at relatively low current intensities. As a result, depolarization is limited to the superficial layer of the dermis containing nociceptive Aδ fibers, without recruitment of deeper lying non-nociceptive fibers [7]. The novel technique has enabled studies of nociceptive blink reflex, and trigeminal and peripheral pain related potentials [1,7,8]. Pain-related potentials reportedly reflect pain processing in a quantitative way [9,10,11]. One study examined the suitability of the technique is suitable for examination of sensory neuropathy due to infection with human immunodeficiency virus (HIV) [3]. Another study assessed the utility of the technique in early detection of diabetic small-fiber neuropathy [12].
However, there is a lack of data on PREP in Asians. This study measured the normal data of PREP elicited with concentric surface electrode among normal, healthy adults. The relationships between verbal rating scale (VRS) score and variables of PREP were explored.
MATERIALS AND METHODS
Subjects
The study subjects were 60 healthy volunteers. All subjects who consented to participate in this study were informed about the experimental protocol, which was approved by the Institutional Review Board of our hospital. Inclusion criteria were age >18 years and informed written consent. None reported any history of headache or pain, nor took any medication regularly. Exclusion criteria were any diagnosis of malignant disease, diagnosis of diabetes mellitus, alcohol or drug abuse, diseases causing peripheral neuropathy, and central nervous system disease.
Equipments
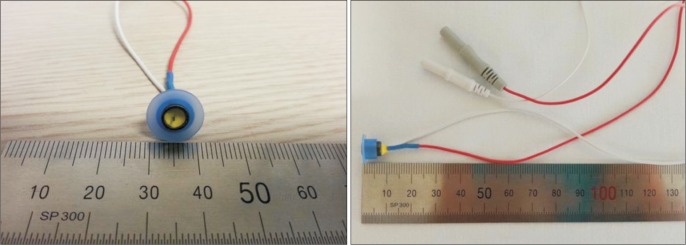
A Dantec Keypoint portable electromyography system (Alpine BioMed, Skovlunde, Denmark) was used for peripheral nerve conduction study (NCS) and recording of PREP. A custom-built concentric surface electrode was used to deliver electrical stimulation (Fig. 1).
Fig. 1. The custom-built concentric surface electrode. The design of this planar concentric electrode was previously described. Central cathode (diameter 0.5 mm) and external anode ring (diameter 6 mm) were assembled. A medical engineering company (Hurev Co. Ltd., Wonju, Korea) contractually produced the first sample electrode according. Then we tested and confirmed final production. This custom-built electrode use conventional carbon wire cable and socket that fits well with a portable electromyography system.
Study design
All subjects received routine NCS of the median, ulnar, tibial, peroneal, superficial peroneal and sural nerves, bilaterally.
PREP was recorded in 60 healthy volunteers 22-62 years of age. Individual perception and pain thresholds were determined by ascending and descending sequences with succeeding current intensities. In each subject, PREP was elicited bilaterally from upper and lower limbs. For upper limb stimulation, the stimulating electrode was placed on the middle phalanx of the second digit of the hand (C7 dermatome). For lower limb stimulation, the stimulating electrode placed on the middle phalanx of the second toe of the foot (L5 dermatome) (Fig. 2). Previously described stimulation parameters were used [1]. The parameters were application of 22 double pulses (monopolar square wave: intensity, 1.5-fold the individual pain threshold; double pulse interval, 5 ms; duration, 0.5 ms; interstimulus interval, 16-17 seconds). A recording electrode placed at Cz referred to linked earlobes of the international 10-20 system (bandwidth, 1 Hz-1 kHz; sweep length, 500 ms).
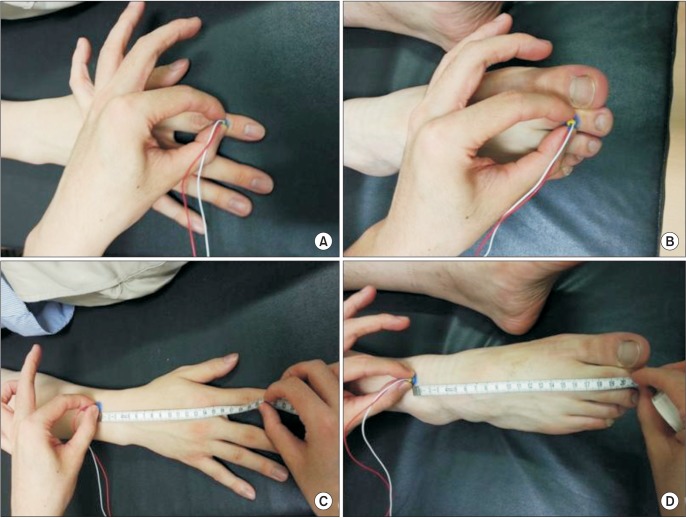
Fig. 2. Stimulation site of pain-related evoked potential. (A) In upper limb stimulation, the stimulating electrode was placed on the middle phalanx of the second digit of the hand (C7 dermatome). (B) In lower limb stimulation, the stimulating electrode was placed on the middle phalanx of the second toe of the foot (L5 dermatome). (C) For calculating upper extremity conduction velocity, distal N latency was measured by stimulating the middle phalanx of the second digit. Proximal N latency was measured by stimulating the point where it measured from 20 cm proximal from the middle phalanx of the second digit. The 20-cm distance between distal and proximal stimulation sites was divided by the difference between the distal N latency and the proximal N latency. (D) The same method was used for calculating lower extremity conduction velocity.
For comparing the values of nerve conduction velocities related to the nerve fibers stimulated by the concentric electrode with established data for conduction velocities of Aδ fibers, conduction velocity (CV) was calculated as previously described [1]. Briefly, to calculate upper extremity CV, distal N latency and proximal N latency was measured by stimulating the middle phalanx of the second digit and stimulating the point measured from 7 cm proximal from the head of the ulna, respectively. The distance (about 20 cm) between a distal and proximal stimulation site was divided by the difference between the distal N latency and the proximal N latency. For calculating lower extremity CV, distal N latency and proximal N latency were measured by stimulating the middle phalanx of the second toe and by stimulating the point measured from 4 cm proximal from the middle of medial and lateral malleolus, respectively. Lower extremity CV was similarly calculated (Fig. 2).
Signal analysis was performed by an investigator blinded to the study design. For preventing bias by initial startle responses, the first sweep was eliminated [1]. Therefore, in total 21 sweeps were averaged. Latencies, peak-to-peak amplitudes of nociceptive fibers, stimulation intensities and subjective pain perception with a VRS score ranging for 0 (no pain) to 10 (worst possible pain) were recorded. All statistical analyses were performed using IBM SPSS Statistics ver. 20 (IBM Korea, Seoul, Korea).
RESULTS
Demographic data
Sixty subjects (22 men and 38 women) were enrolled. Age ranged from 22-62 years, with an average age of 36.33±10.63 years. Height ranged from 153-186 cm, with an average height of 165.4±7.8 cm. Weight ranged from 46-85 kg, with an average of subjects 61.5±10.3 kg (Table 1).
Table 1. Participants' demographics and descriptive statistics of PCOQ (n=50).
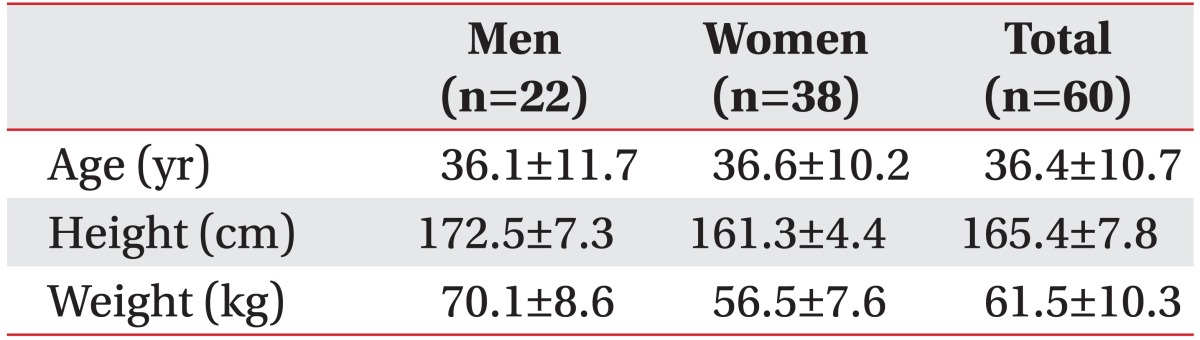
Values are presented as mean±standard deviation.
Peripheral NCS
Motor and sensory nerve conduction data of all subjects were normal (Table 2).
Table 2. Peripheral nerve conduction study data.
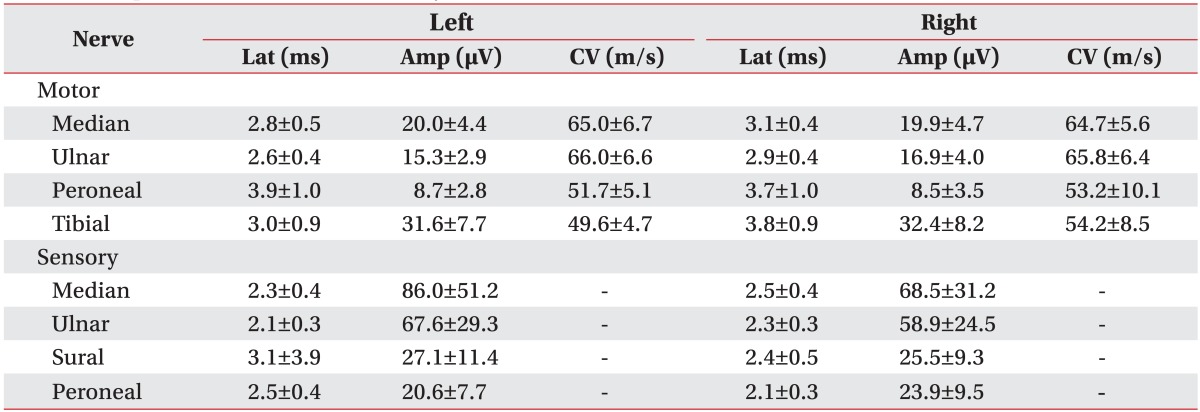
Values are presented as mean±standard deviation.
Lat, N latency; Amp, peak-to-peak amplitude; CV, conduction velocity.
PREP
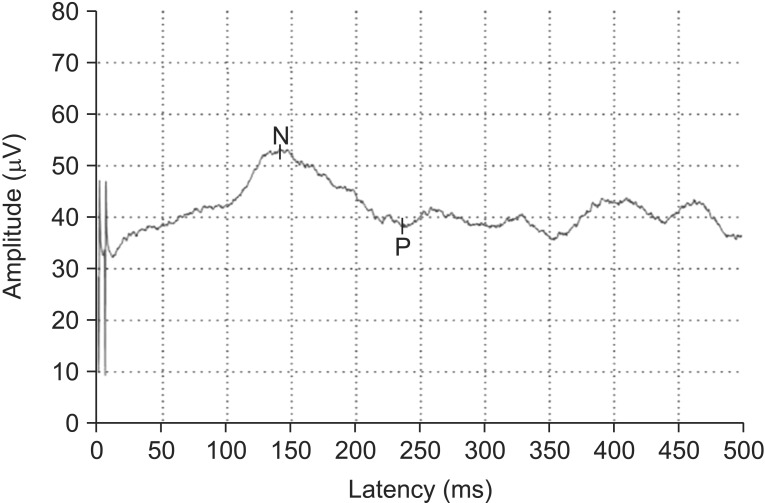
Stimulation with the concentric surface electrode produced a pinprick-like painful sensation (VRS 2-3 and average pinprick pain threshold of 0.8±3.2 mA). A negative peak (N) and subsequently positive peak (P) were identified in the averaged PREP waveforms (Fig. 3). Negative-positive peaks (NP) were clearly recognized in four different stimulation sites. N latency of hand PREP was 163.8±40.0 ms (right) and 161.0±39.9 ms (left). N latency of foot PREP was 178.0±43.9 ms (right) and 180.4±43.4 ms (left), NP amplitude of hand was 20.6±10.6 µV (right) and 21.9±11.6 µV (left), and NP amplitude of foot was 18.8±8.3 µV (right) and 19.0±8.4 µV (left) (Table 3). The calculated CV was 13.2±4.7 m/s and VRS score was 3.8±1.0. The data of PREP were divided into groups according to age and height (Tables 4 and 5). An independent t-test was used to assess the statistical significance between the right and left PREP data with p-values <0.05 considered statistically significant. There were no significant differences.
Fig. 3. Representative acquired graph of C7 dermatomal stimulation of pain-related evoked potential (PREP). Initially negative peak (N) and subsequently positive peak (P) were identified in the averaged waveforms of PREP. Negative-positive peaks were clearly recognized in four different stimulation sites.
Table 3. Pain-related evoked potential data.
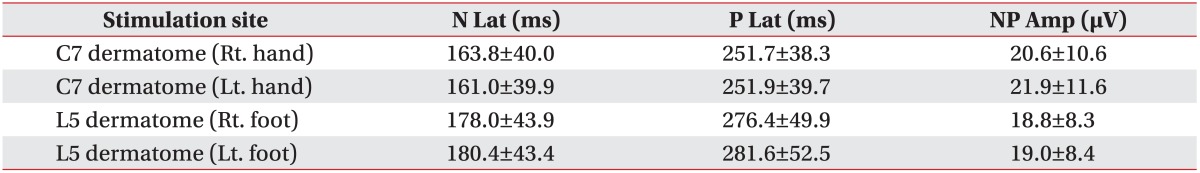
Values are presented as mean±standard deviation.
N Lat, negative peak latency; P Lat, positive peak latency; NP Amp, peak-to-peak amplitude.
Table 4. Pain-related evoked potential data by age group.
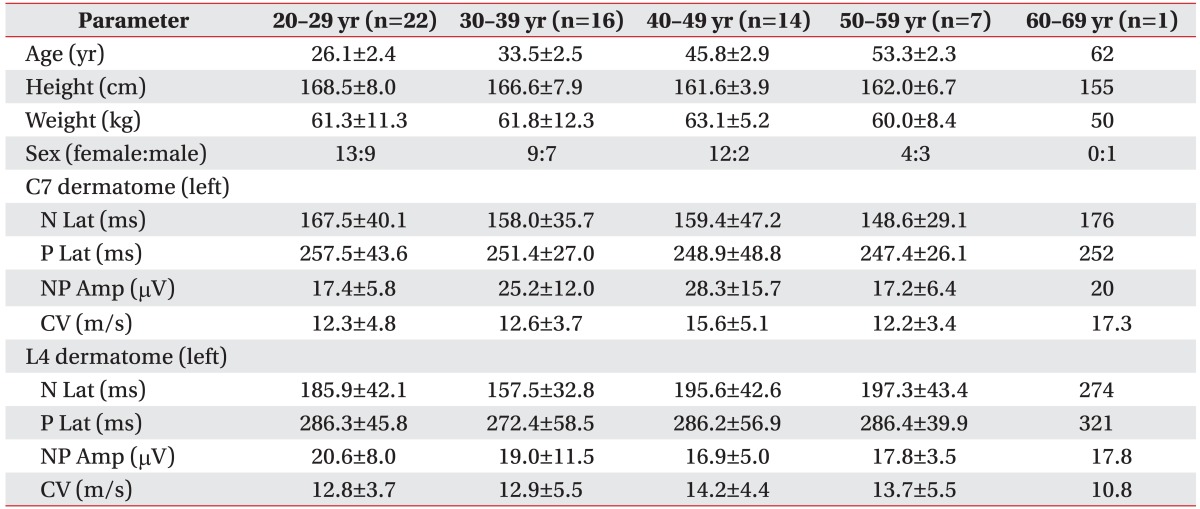
Values are presented as mean±standard deviation.
N Lat, negative peak latency; P Lat, positive peak latency; NP Amp, peak-to-peak amplitude; CV, conduction velocity.
Table 5. Pain-related evoked potential by height group.
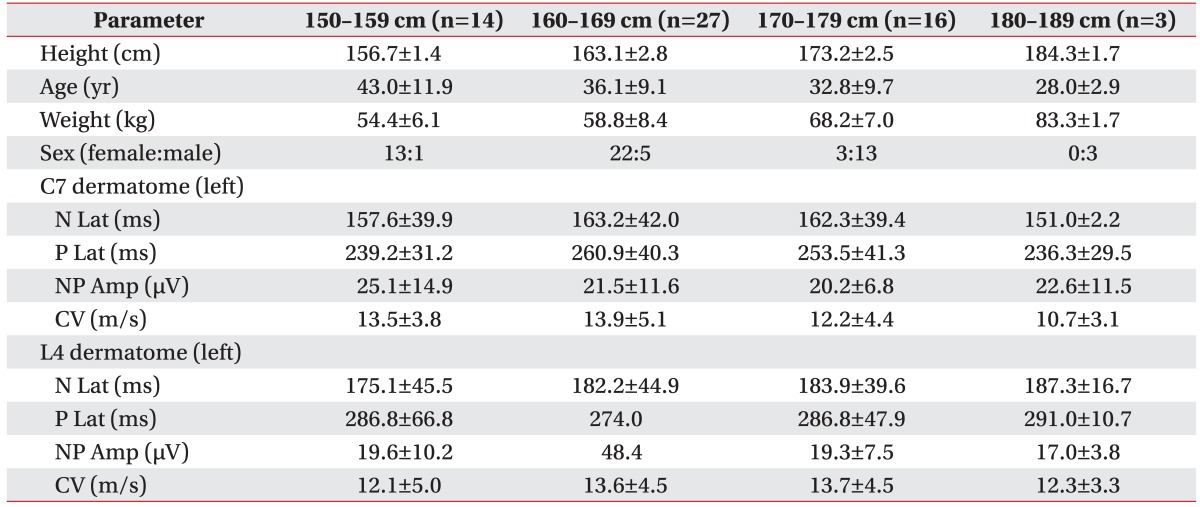
Values are presented as mean±standard deviation.
N Lat, negative peak latency; P Lat, positive peak latency; NP Amp, peak-to-peak amplitude; CV, conduction velocity.
Correlation between VRS scores and PREP
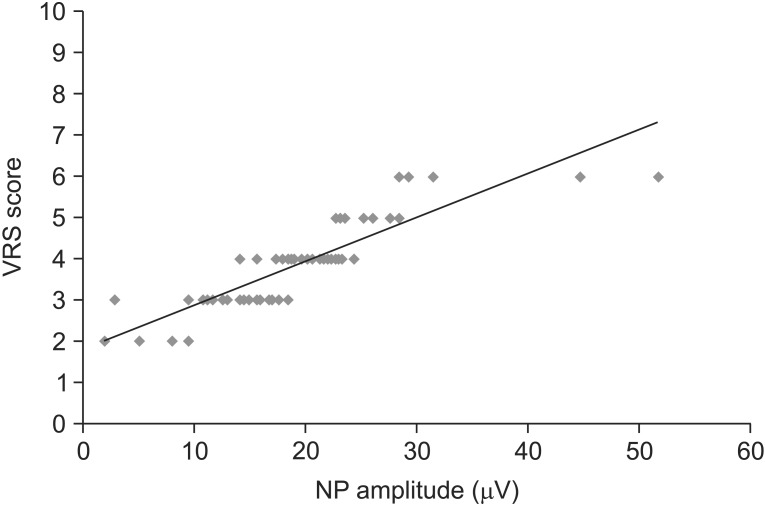
Linear regression analysis revealed a highly significant positive correlation between VRS scores (y-axis) and amplitude of PREP (x-axis) (y=0.1069x+1.781, r=0.877, n=60, p<0.0001) (Fig. 4). N latency, P latency, and CV showed no statistically significant correlations.
Fig. 4. Correlation between verbal rating scale (VRS) scores and pain-related evoked potential (PREP). VRS score for each of the subject was plotted against the amplitude of PREP. Linear regression analysis was used to determine the relation between VRS scores (y-axis) and amplitude of PREP (x-axis), which revealed a highly significant positive correlation (y=0.1069x+1.781, r=0.877, n=60, p<0.0001).
DISCUSSION
The current study was conducted to elicit evoked potentials among healthy adults by stimulation with a concentric electrode. The elicited sensation was a pinpricklike pain that is typical for Aδ fiber mediated pain. Reproducible potentials were observed using stimulation intensities 1.5-fold over the individual pain threshold. The calculated CV was 13.2±4.7 m/s. Previously reported CVs of peripheral nerves for PREP were approximately 10-15 m/s, in a range of Aδ fibers [1,13,14,15].
This study is the first among Asians. The 60 subjects were healthy and none reported any history of pain or took any medication. PREP was bilaterally measured from both upper and lower extremities. To the best of our knowledge, this is only the second study to adopt this measurement approach. Mueller et al. [12] studied 60 healthy subjects and 57 diabetic patients. The healthy subjects were classified into two groups according to age. In our study, subjects were classified into five groups according to age and four groups according to height (Tables 4 and 5). The subjects were not evenly distributed with respect to age, particularly in the 50s and 60s. Also, only three subjects were 180-189 cm in height. These uneven distributions are an acknowledged limitation.
CV of those aged 40-49 years was faster than other age groups except for the 60-69 years old group (Table 4). Comparing those 40-49 years of age concerning mean height with other group's mean height revealed a faster CV. There was no reference group because this is the first study to include data of age, height, weight, and parameters of PREP. Anthropometric factors of consequence include circumference of the stimulation site (subcutaneous tissue depth), body mass index, and body surface area. Attention and distraction effects during stimulation are also influential. More investigations on healthy subjects are needed incorporating more of these factors.
Presently, the correlation between VRS score and PREP variables were analyzed. There was a significant positive correlation between amplitude of PREP and VRS score. But, the limited range of the VRS score is a weakness of the study (mean VRS score for all subjects 3.8±1.0, ranging from 2-6). Confidence about this correlation is likely to rise further by adding the data for various amplitude and VRS score by adjusting the stimulation intensity. Two previous studies also showed a correlation between pain perception intensity and PREP variable. Using laser stimulation, Kanda et al. [13] compared visual analogue scale (VAS) score and three different stimulation durations, and reported a significant positive correlation between VAS score and stimulation duration. Using a concentric electrode, Katsarava et al. [1] observed a good linear correlation between amplitude of PREP and VRS scores.
There have been several studies about this technique of eliciting PREP. One study addressed trigeminal and peripheral pain-related potentials [1]. The authors reported a correlation of NP amplitude and subjective pain perception of somatic and trigeminal PREP, and an effect of selective loss of cutaneous pain sensitivity. After application of local anaesthesia, the pinprick sensation and PREP disappeared and a light touch sensation persisted, indicating blockade of Aδ and C fibers. These findings suggest that the Aδ and C fibers are responsible for PREP induction. Also, the CV of PREP and very low stimulation intensities, without recruitment of unmyelinated fibers, indicates that stimulation with a concentric electrode mainly involves Aδ fibers.
Few studies have evaluated of small fiber neuropathy using this novel technique. Methods for evaluation of small fiber neuropathy exist [16]. They can be time consuming. Laser-evoked potential is expensive and skip biopsy is invasive, rendering them unsuitable for routine clinical use. Standard NCS are typically unremarkable and not helpful. Three studies have investigated noninvasive detection of HIV associated sensory fiber neuropathy using this technique [17,18,19]. Electrophysiological abnormality of PREP increased with duration of HIV infection and with advanced disease stage. PREP had a greater sensitivity in detecting impairment of small fiber function in patients with HIV sensory neuropathy [3]. Another study showed the utility of this technique in the early detection of diabetic small-fiber neuropathy [12]. Patients with neuropathic symptoms showed prolonged latencies and decreased amplitude compared to healthy controls and patients without neuropathic symptoms, demonstrating that PREP elicited by a nociceptive electrical stimulation can be useful in detecting early diabetic sensory neuropathy. Skin biopsy can reveal the correlation of PREP with intraepidermal nerve fiber density [19]. In this study, a negative correlation was evident between latencies and intraepidermal nerve fiber density, and a positive correlation was evident between peak-to-peak amplitude and intraepidermal nerve fiber density. Strong correlation of intraepidermal nerve fiber density with both PREP latencies and amplitudes were found.
In our study, subject groups were not gender matched. However, in previous two studies, there no gender specific differences were apparent [1,12]. Larger scale studies are needed to establish reliable normative data controlling for age, gender, and height. Skin biopsy was done for verification of electrophysiological findings in a previous study [19], so it can be considered in further clinical studies.
In conclusion, PREP of normal, healthy adults demonstrated a statistically significant correlation between amplitude of PREP and VRS score in Asians. PREP could be useful to test peripheral nociceptive functions. Theses normative adult data will be helpful for the detection of impairment in peripheral nociceptive fibers.
ACKNOWLEDGMENTS
This study was supported by the Korea Health Technology R&D project, Ministry of Health & Welfare (No. A100054).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Katsarava Z, Ayzenberg I, Sack F, Limmroth V, Diener HC, Kaube H. A novel method of eliciting pain-related potentials by transcutaneous electrical stimulation. Headache. 2006;46:1511–1517. doi: 10.1111/j.1526-4610.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 2.Valeriani M, Le Pera D, Niddam D, Chen AC, Arendt-Nielsen L. Dipolar modelling of the scalp evoked potentials to painful contact heat stimulation of the human skin. Neurosci Lett. 2002;318:44–48. doi: 10.1016/s0304-3940(01)02466-1. [DOI] [PubMed] [Google Scholar]
- 3.Katsarava Z, Yaldizli O, Voulkoudis C, Diener HC, Kaube H, Maschke M. Pain related potentials by electrical stimulation of skin for detection of small-fiber neuropathy in HIV. J Neurol. 2006;253:1581–1584. doi: 10.1007/s00415-006-0262-4. [DOI] [PubMed] [Google Scholar]
- 4.Beydoun A, Morrow TJ, Shen JF, Casey KL. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr Clin Neurophysiol. 1993;88:173–181. doi: 10.1016/0168-5597(93)90002-7. [DOI] [PubMed] [Google Scholar]
- 5.Bromm B, Jahnke MT, Treede RD. Responses of human cutaneous afferents to CO2 laser stimuli causing pain. Exp Brain Res. 1984;55:158–166. doi: 10.1007/BF00240510. [DOI] [PubMed] [Google Scholar]
- 6.Kakigi R, Shibasaki H, Ikeda A. Pain-related somatosensory evoked potentials following CO2 laser stimulation in man. Electroencephalogr Clin Neurophysiol. 1989;74:139–146. doi: 10.1016/0168-5597(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 7.Kaube H, Katsarava Z, Kaufer T, Diener H, Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol. 2000;111:413–416. doi: 10.1016/s1388-2457(99)00295-3. [DOI] [PubMed] [Google Scholar]
- 8.Katsarava Z, Ellrich J, Diener HC, Kaube H. Optimized stimulation and recording parameters of human 'nociception specific' blink reflex recordings. Clin Neurophysiol. 2002;113:1932–1936. doi: 10.1016/s1388-2457(02)00307-3. [DOI] [PubMed] [Google Scholar]
- 9.Ayzenberg I, Obermann M, Nyhuis P, Gastpar M, Limmroth V, Diener HC, et al. Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia. 2006;26:1106–1114. doi: 10.1111/j.1468-2982.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 10.Katsarava Z, Lehnerdt G, Duda B, Ellrich J, Diener HC, Kaube H. Sensitization of trigeminal nociception specific for migraine but not pain of sinusitis. Neurology. 2002;59:1450–1453. doi: 10.1212/wnl.59.9.1450. [DOI] [PubMed] [Google Scholar]
- 11.Kaube H, Katsarava Z, Przywara S, Drepper J, Ellrich J, Diener HC. Acute migraine headache: possible sensitization of neurons in the spinal trigeminal nucleus? Neurology. 2002;58:1234–1238. doi: 10.1212/wnl.58.8.1234. [DOI] [PubMed] [Google Scholar]
- 12.Mueller D, Obermann M, Koeppen S, Kavuk I, Yoon MS, Sack F, et al. Electrically evoked nociceptive potentials for early detection of diabetic small-fiber neuropathy. Eur J Neurol. 2010;17(6):834–841. doi: 10.1111/j.1468-1331.2009.02938.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanda M, Matsuhashi M, Sawamoto N, Oga T, Mima T, Nagamine T, et al. Cortical potentials related to assessment of pain intensity with visual analogue scale (VAS) Clin Neurophysiol. 2002;113:1013–1024. doi: 10.1016/s1388-2457(02)00125-6. [DOI] [PubMed] [Google Scholar]
- 14.Tran TD, Lam K, Hoshiyama M, Kakigi R. A new method for measuring the conduction velocities of Abeta-, Adelta- and C-fibers following electric and CO(2) laser stimulation in humans. Neurosci Lett. 2001;301:187–190. doi: 10.1016/s0304-3940(01)01639-1. [DOI] [PubMed] [Google Scholar]
- 15.Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of Adelta fibers by intra-epidermal needle electrode in humans. Pain. 2002;96:247–252. doi: 10.1016/S0304-3959(01)00453-5. [DOI] [PubMed] [Google Scholar]
- 16.Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002;26:173–188. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- 17.Polydefkis M, Yiannoutsos CT, Cohen BA, Hollander H, Schifitto G, Clifford DB, et al. Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–119. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- 18.Tagliati M, Grinnell J, Godbold J, Simpson DM. Peripheral nerve function in HIV infection: clinical, electrophysiologic, and laboratory findings. Arch Neurol. 1999;56:84–89. doi: 10.1001/archneur.56.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Obermann M, Katsarava Z, Esser S, Sommer C, He L, Selter L, et al. Correlation of epidermal nerve fiber density with pain-related evoked potentials in HIV neuropathy. Pain. 2008;138:79–86. doi: 10.1016/j.pain.2007.11.009. [DOI] [PubMed] [Google Scholar]