Key Points
Liver-restricted expression of FIX-Padua induces immune tolerance to the transgene in hemophilia B inhibitor dog models.
Long-term toxicity studies show no increased risk of thrombogenicity of FIX-Padua in mice and dogs.
Abstract
Emerging successful clinical data on gene therapy using adeno-associated viral (AAV) vector for hemophilia B (HB) showed that the risk of cellular immune response to vector capsid is clearly dose dependent. To decrease the vector dose, we explored AAV-8 (1-3 × 1012 vg/kg) encoding a hyperfunctional factor IX (FIX-Padua, arginine 338 to leucine) in FIX inhibitor-prone HB dogs. Two naïve HB dogs showed sustained expression of FIX-Padua with an 8- to 12-fold increased specific activity reaching 25% to 40% activity without antibody formation to FIX. A third dog with preexisting FIX inhibitors exhibited a transient anamnestic response (5 Bethesda units) at 2 weeks after vector delivery following by spontaneous eradication of the antibody to FIX by day 70. In this dog, sustained FIX expression reached ∼200% and 30% of activity and antigen levels, respectively. Immune tolerance was confirmed in all dogs after challenges with plasma-derived FIX concentrate. Shortening of the clotting times and lack of bleeding episodes support the phenotypic correction of the severe phenotype, with no clinical or laboratory evidence of risk of thrombosis. Provocative studies in mice showed that FIX-Padua exhibits similar immunogenicity and thrombogenicity compared with FIX wild type. Collectively, these data support the potential translation of gene-based strategies using FIX-Padua for HB.
Introduction
Hemophilia B (HB) is an X-linked inherited bleeding disease characterized by deficiency of factor IX (FIX) caused by F9 gene mutations. Patients with severe HB (residual FIX activity <1% normal) have recurrent bleeding episodes associated with increase morbidity and mortality compared with those with moderate or mild disease. Treatment of HB is based on protein replacement therapy, and prophylactic therapy is associated with clinically beneficial outcomes. One of the main complications of protein replacement therapy is the development of inhibitory alloantibodies to the infused protein, which occurs in 1.5% to 3% of severe HB patients.1-3 One of the main determinants of inhibitor formation is the underlying F9 mutation. HB patients with mutations such as missense mutations that lead to circulating but defective FIX antigen, termed cross-reacting material (CRM) positive, exhibit a lower risk of inhibitor formation compared with CRM-negative patients.1,3,4 In contrast, ∼50% of patients with large gene deletions develop FIX inhibitors, followed by patients with premature stop codon, frameshift, or splice site mutations (20-30%).4 Thus, null mutations in F9 significantly increase the risk of inhibitor formation.
Therapies for hemophilia based on protein, nucleic acid, or cell therapies are under development aimed at increasing factor levels to the range of moderate or mild disease.5-9 Gene therapy using adeno-associated viral (AAV) vectors for liver FIX gene transfer is emerging as a successful strategy as long-term expression of circulating FIX and improvement of disease phenotype.10,11 Data from early-phase AAV-FIX trials demonstrated that immune responses to vector capsid proteins are a main safety concern and are directly correlated to vector dose.10,11 Thus, strategies to reduce the vector dose are highly attractive to overcome these safety concerns.
We previously reported a case of thrombophilia associated with an arginine 338 to leucine (FIX-R338L, FIX-Padua) substitution in F9. The mutant FIX circulates at normal antigenic levels but exhibits eightfold increased clotting activity.12 Thus, the use of FIX-Padua offers an alternative strategy for treating HB. Preclinical studies in severe HB dogs provide a unique opportunity to address both efficacy and safety. There are 2 severe canine HB models available, both of which are CRM negative. However, the underlying F9 mutation, mRNA levels, and risk of inhibitor formation differ.13-15 The University of North Carolina-Chapel Hill (UNC-CH) model is due to a missense mutation, glutamic acid 379 to glycine, which leads to normal RNA levels but probable disruption of protein folding.13,14 The University of Alabama at Birmingham (UAB) model results from a frameshift mutation, premature stop codon at position 146 (null mutation), and undetectable mRNA likely due to transcript instability.14 Infusion of canine FIX concentrate in naïve HB dogs resulted in inhibitor formation in the UAB model but not the UNC-CH model.16-19 Preclinical studies using these models for AAV muscle gene therapy showed that the parameters of vector dose tested (per site and per body weight), which proved safe in the UNC-CH model, resulted in inhibitor formation in the UAB dogs.16-18 Therefore, in the skeletal muscle-directed AAV trial, only severe HB men with F9 missense mutations were enrolled.20 With liver gene therapy, both canine models showed long-term sustained expression of FIX-WT.16-19 Thus, patients with missense and null mutations were enrolled in AAV liver trials. Overall, none of the 15 patients enrolled in these early-phase studies developed FIX inhibitors.10,20,21 Thus, data on immune responses to the transgene in these canine models are likely to be predictive of human responses.
Here we sought to determine the immunogenicity of FIX-Padua following AAV8 liver gene transfer in HB dogs with a null mutation and perform comprehensive safety studies in mice. If the expression of FIX-Padua is safe, these data will further enhance the potential of clinical translation of FIX-Padua to HB patients, including those with underlying F9-null mutations.
Materials and methods
Recombinant AAV vector
Production of AAV8 vectors was carried out as previously described.22 The expression cassette contained canine FIX-R338L (cFIX-Padua), human FIX-R338L (hFIX-Padua), or human FIX wild-type (hFIX-WT) behind a liver-specific promoter.23
Canine studies
The local and UAB Institutional Animal Care and Use Committee approved all experiments. Adult male HB dogs received AAV at doses of 1 × 1012 or 3 × 1012 vector genomes per kilogram body weight (vg/kg) via the saphenous vein diluted in PBS over a 30-minute period.
Systemic and local toxicity
Hematologic and biochemical analyses of blood and serum samples for liver and kidney functions and pathologic activation of coagulation was monitored by d-dimer and thrombin-antithrombin (TAT) complexes.24
Canine FIX antigen, activity, and antibody assays
Whole blood clotting time (WBCT), cFIX antigen and activity, and thromboelastography (TEG) were assayed as previously described.25,26 Neutralizing antibodies to cFIX were determined by the Bethesda assay. Antibodies against cFIX were measured by enzyme-linked immunosorbent assay (ELISA) against immunoglobulin (Ig)G1, IgG2, and total IgG (Bethyl Laboratories, Montgomery, TX).24
Immune responses to AAV8 capsid proteins
To detect antibody responses against AAV8 capsid proteins, plates were coated with AAV8 empty capsids (1 µg/mL) as previously described.22 Coating with serially diluted dog reference serum with known quantities of IgG1, IgG2, and total IgG (Bethyl Laboratories, Montgomery, TX) served as a standard.27
Immunological challenges with canine FIX-WT protein concentrate
Dogs were challenged with 0.5 mg of pooled plasma-derived, purified cFIX concentrate (Enzyme Research Laboratory, South Bend, IN) by intravenous injection. Humoral responses to cFIX-WT were monitored using assays for inhibitory and noninhibitory antibodies to cFIX as described above.24
Canine multiplex cytokine array
Cytokines were assayed using a Milliplex canine cytokine magnetic (MAG)MAG Panel kit (EMD Millipore, Billerica, MA) 8-plex containing murine interleukin (IL) 15-MAG, canine (c) granulocytes macrophage-colony-stimulating factor (GM-CSF)-MAG, canine interferon (cIFN)-γ-MAG, cIL2-MAG, cIL6-MAG, cIL10-MAG, cIL18-MAG, and canine tumor necrosis factor (TNF)-α-MAG. Standards, quality controls, and samples were assayed in duplicate. The bead counts and fluorescence intensity of the beads were measured using a Luminex 100/200 analyzer. Samples were measured once, and blank values were subtracted from all readings. Standard curves were produced and sample values determined using xPONENT software.
Murine studies
Assessing immunogenicity of hFIX-Padua
HB male mice on a C57Bl/6 background (n = 7-9 mice per group) were injected with AAV-hFIX-WT or AAV-hFIX-Padua at 4 × 1010 vg/kg. Mice then received 2 μg/mouse of hFIX-WT or hFIX-Padua subcutaneously every week for 4 weeks starting at week 10 after vector. Ten weeks after the last protein injection, mice received hFIX-WT or hFIX-Padua (2 μg/mouse) in complete Freund’s adjuvant (CFA) and then in incomplete Freund’s adjuvant (IFA; Sigma-Aldrich, St. Louis, MO) 8 weeks later. The control group consisted of adult naïve HB mice (n = 4-5 mice per group) injected with hFIX-WT or hFIX-Padua protein alone in a similar fashion. We monitored FIX antigen levels, inhibitory antibodies, and murine anti-hFIX total IgG at baseline, week 4 after AAV injection, and 4 weeks after the last of each protein challenge (protein alone, protein in CFA, and protein in IFA).
Long-term safety studies in mouse models
Adult hemostatically normal male C57Bl/6 mice received AAV encoding hFIX-Padua or hFIX-WT at 5 vector doses ranging from 1 × 1010 to 2 × 1012 vg/kg (n = 5 mice per dose per vector). Blood was collected following distal tail amputation into 3.8% sodium citrate. We determined hFIX expression levels by antigen and activity assays and TAT complex levels28 following kit instructions (Enzygnost TAT micro; Siemens, Munich, Germany). d-dimer levels (Asserachrom D-Dimer, Parsippany, NJ) were monitored following kit instructions only at 2 and 8 months after vector injection due to sample volume limitation.28 Kaplan-Meier analysis was used to determine the survival rates; the control group (n = 5) received 1 × 1011 vg/kg AAV empty capsid.
Recombinant hFIX-WT and hFIX-Padua protein production
We used an expression system consisting of pLenti6.3 (ViralPower HiPerform Expression System; Invitrogen, Carlsbad, CA) to generate a stable HEK293 cell line expressing hFIX-WT and hFIX-Padua. Protein purification consisted of ion exchange chromatography.
Results
Canine studies
Sustained expression of cFIX Padua in inhibitor-prone HB dogs is safe
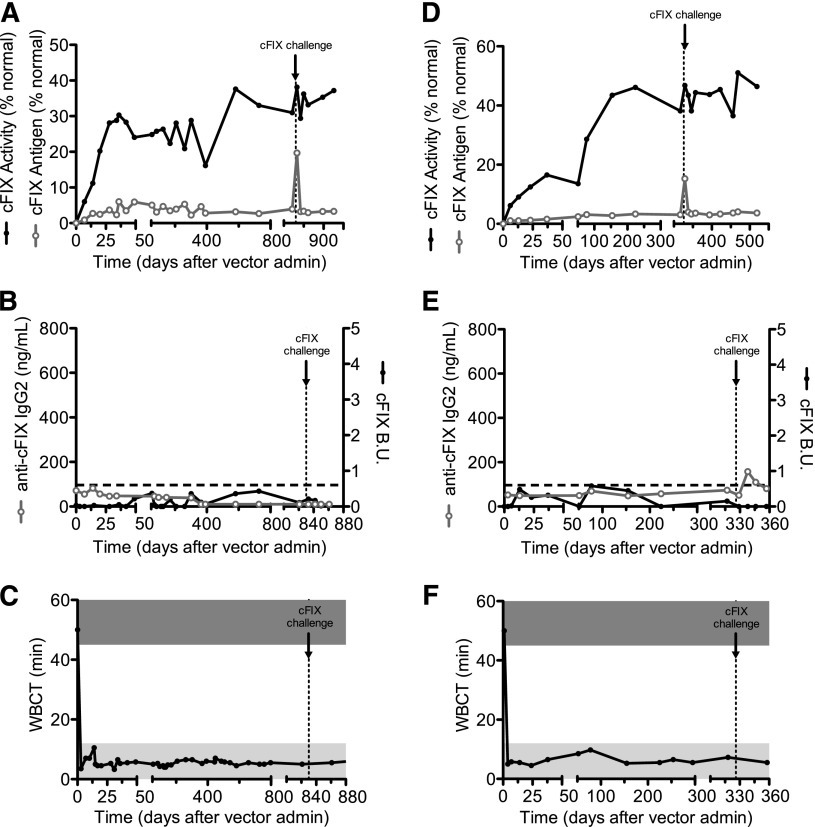
Dogs (Wick and Trex) received AAV8-cFIX-Padua at doses of 1 × 1012 or 3 × 1012 vg/kg, respectively, by intravenous injection (Figure 1; Table 1). At both doses the resulting FIX activity levels were 7 to 12 times higher than the antigen levels, and activity levels in the range of mild hemophilia. In these dogs, the WBCT (Figure 1C,F) and the TEG (supplemental Figure 1 and supplemental Table 1, available on the Blood Web site) normalized within a week following AAV delivery. This was accompanied by a complete lack of bleeding episodes, further supporting the correction of the disease phenotype (Table 1).
Figure 1.
cFIX expression and anti-cFIX humoral responses in HB dogs following liver delivery of AAV-cFIX-Padua. Two University of Alabama HB dogs with no previous history of inhibitors were assessed for (A,D) cFIX antigen and activity, (B,E) anti-cFIX IgG2 and Bethesda units, and (C,F) whole blood clotting times after AAV8-cFIX-Padua administration. (A-C) Trex received 3 × 1012 vg/kg and (D-F) Wick received 1 × 1012 vg/kg. Black line, closed circles, cFIX activity and cFIX Bethesda units; gray line, open circles, cFIX antigen and anti-cFIX IgG2; horizontal dashed lines, positive levels of Bethesda units and IgG2; vertical dashed lines, time of challenge with recombinant cFIX; dark gray bar, WBCT of hemophiliac dogs (>45 minutes); light gray bar, WBCT of normal dogs (<8 minutes).
Table 1.
Summary of AAV8-cFIX-Padua treatment of 3 adult dogs with severe HB
| Dog | cFIX-Padua plateau levels | Bleeds per month | ||||||
|---|---|---|---|---|---|---|---|---|
| Body weight, kg | Vector dose, vg/kg | Vector dose, total dose | Activity, % mean ± standard deviation | Antigen, % mean ± standard deviation | Specific activity | Pretreatment | Posttreatment | |
| Wiley | 10.2 | 3 × 1012 | 30.6 × 1012 | 241.2 ± 31.9 | 28.8 ± 9.3 | 8.6 | 7/32 | 0/36 |
| Trex | 5.8 | 3 × 1012 | 17.4 × 1012 | 26.8 ± 5.5 | 3.81 ± 1.29 | 7.9 | 4/32 | 0/36 |
| Wick | 8.6 | 1 × 1012 | 8.6 × 1012 | 39.9 ± 5.8 | 3.33 ± 0.38 | 12 | 0/11 | 0/19 |
| Total | 11/75 | 0/91 | ||||||
There was no formation of inhibitory or noninhibitory antibodies to cFIX (Figure 1B,E; supplemental Figure 2) with cumulative long-term follow-up of ∼4.5 years (ongoing observations). We specifically assay for canine IgG2 subclass, which is associated with inhibitory antibody in this HB model on FIX protein concentrate injection or gene therapy,16,23 and is the equivalent of human IgG4 (the IgG subclass primarily associated with inhibitory antibody29). Although IgG4 is the predominant antibody subclass for human inhibitors, some patients also develop IgG1 and/or IgG229; therefore, we also assayed for total canine IgG and IgG1. No antibodies of any IgG subclass against cFIX were found in either dog (Figure 1B,E; supplemental Figure 2).
We challenged these dogs by intravenous injection of 0.5 mg cFIX-WT protein concentrate 11 months (Wick) or 2.3 years (Trex) after vector injection. We reported earlier that in this naïve HB dog strain, this strategy induces the formation of inhibitory antibodies to cFIX-WT within a week after challenge.16 Here, in the AAV-cFIX-Padua–expressing dogs, there was no detection of antibodies to cFIX by Bethesda assay or anti-cFIX IgG2 ELISA (Figure 1B,E and supplemental Figure 2). Thus, expression of the cFIX-Padua variant shows increased specific activity as observed in humans12 and immune responses even on challenge with cFIX-WT protein concentrates.
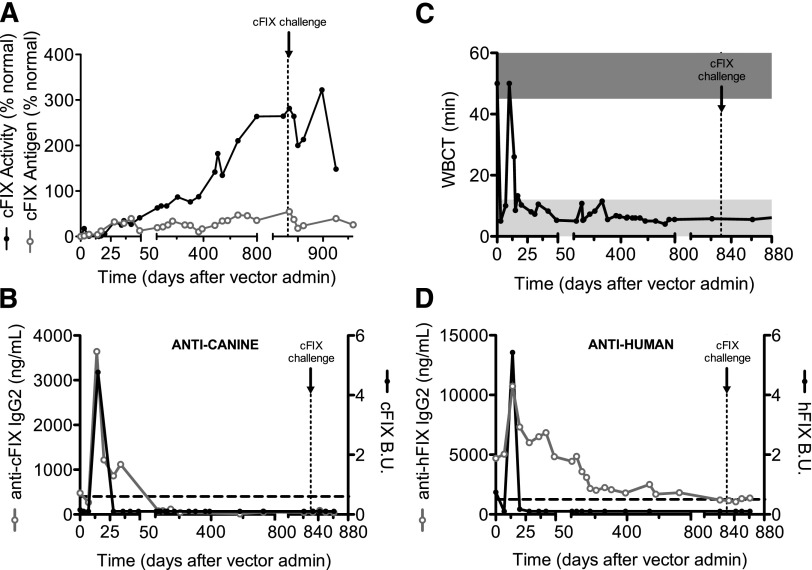
Eradication of inhibitory antibodies to FIX by liver gene therapy using AAV-cFIX-Padua
Wiley developed inhibitors after primary exposure to human FIX protein, and these inhibitory antibodies cross-reacted with cFIX. At the time of vector injection, the inhibitor titers against cFIX were undetectable by Bethesda assay but anti-cFIX IgG2 levels were ∼500 ng/mL. After AAV-cFIX-Padua injection, we observed a rapid increase in cFIX antigen and activity, which reached levels of 2% and 20% of normal, respectively. These levels quickly decreased to pretreatment levels, coinciding with an increase in inhibitor titers and anti-cFIX IgG2 (Figure 2; Table 1). These findings are consistent with the initial normalization of the WBCT by week 1 and a return to baseline levels at day 10 after vector injection. Wiley’s inhibitors showed an anamnestic response, with titers peaking at ∼5 Bethesda units on day 15 (Figure 2B) followed by a spontaneous decline to complete eradication by day 28. Similarly, anti-cFIX IgG2 and total IgG levels peaked concomitant with the Bethesda titers but showed a slower decline, with total eradication at day 70. At no point were anti-cFIX IgG1 antibodies detected (supplemental Figure 2). No anti-cFIX antibodies have been observed over 2.8 years. Notably, Wiley's anti-human FIX inhibitors rose to 5.3 Bethesda units at day 14 and disappeared by day 20 (Figure 2D). The anti-hFIX IgG2 levels rose from a baseline of 5 μg/mL to a peak at day 14 of 11 μg/mL; although they are gradually declining, residual levels of ∼1.5 μg/mL remain detectable. These data suggest that Wiley developed immune tolerance to specific epitopes shared between the human and canine FIX proteins; however, noninhibitory antibodies specific for epitopes unique to hFIX remain. Systemic complications associated with inhibitory antibodies to FIX include anaphylactic reaction and nephrotic syndrome.30,31 Importantly, Wiley showed no evidence of abnormal kidney function or urinary protein loss (data not shown). To confirm the eradication of inhibitory antibody to cFIX and immune tolerance induction, Wiley was challenged with 0.5 mg cFIX-WT protein concentrate 2.3 years after vector injection. Again, no humoral responses were documented by Bethesda assay or anti-cFIX IgG1, IgG2, or total IgG ELISA (Figure 2B; supplemental Figure 2). Together these findings show that AAV liver expression of cFIX-Padua is efficacious in eradicating inhibitory antibodies to cFIX without systemic toxicity.
Figure 2.
cFIX expression and anti-cFIX humoral responses in an HB dog with preexisting anti-hFIX inhibitors after administration of AAV-cFIX-Padua. Wiley, a University of Alabama HB dog with a history of inhibitors against hFIX, was assessed for (A) cFIX antigen and activity, (B) anti-cFIX IgG2 and Bethesda units, (C) whole blood clotting time, and (D) anti-hFIX IgG2 and Bethesda units after administration of 3 × 1012 vg/kg AAV8-cFIX-Padua. Black line, closed circles, cFIX activity, cFIX Bethesda units, and hFIX Bethesda units; gray line, open circles, cFIX antigen, anti-cFIX IgG2 and anti-hFIX IgG2; horizontal dashed lines, positive levels of Bethesda units and IgG2; vertical dashed lines, time of challenge with recombinant cFIX; dark gray bar, WBCT of hemophiliac dogs (>45 minutes); light gray bar, WBCT of normal dogs (<8 minutes).
Cytokine profile following AAV-cFIX-Padua injection and FIX inhibitor eradication
We ran a multiplex array on plasma from all dogs looking at 8 cytokines, with a focus on T-cell cytokines (Figure 3). Wick did not have detectable cytokine levels for any of the time points assayed. Trex showed a spike on day 3 of GM-CSF, IL-15, TNF-α, IL-6, and IL-18, as well as small spikes of IL-10 and IFN-γ (Figure 3B-G). Interestingly, he also showed high levels of GM-CSF, IL-15, IL-6, and IL-18 on day 70 (Figure 3B-C,E-F). These levels did not correlate with any loss of cFIX expression or activity, detection of anti-cFIX antibodies, or prolongation of WBCT (Figures 1 and 3A; supplemental Figure 2). Wiley’s levels of GM-CSF, IL-15, IL-6, and IL-18 (Figure 3B-C,E-F) were inversely correlated to his cFIX activity (Figure 3A). Conversely, he had relatively low levels of TNF-α and IL-2 throughout, with a small spike in the latter at day 27 (Figure 3D,H). Wiley’s IL-10 levels climbed from weeks 2 to 4, generally falling thereafter until he was challenged on day 833, at which point levels began to rise again (Figure 3G). IFN-γ showed biphasic response, with an early peak at week 4 followed by a late peak following the recombinant protein challenge (Figure 3I).
Figure 3.
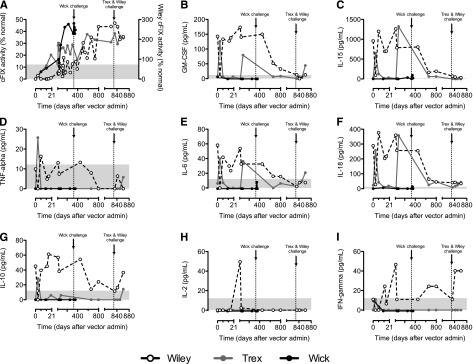
Cytokine profiles of dogs following AAV-cFIX-Padua liver gene therapy. A cytokine multiplex array was run to assess the levels of (B) GM-CSF, (C) IL-15, (D) TNF-α, (E) IL-6, (F) IL-18, (G) IL-10, (H) IL-2, and (I) IFN-γ compared with the (A) cFIX expression levels as measured by percentage cFIX activity in the 3 HB dogs (Wiley: red, right y axis for % activity; Trex: blue; Wick, green) at baseline and following delivery of AAV. Gray bars indicate level of detection of the assay.
Risk of thrombogenicity of long-term expression of FIX-Padua
Expression of FIX-Padua is associated with improvement of the disease phenotype as noted by the normalization of WBCT (Figures 1 and 2), TEG parameters (supplemental Figure 1; supplemental Table 1), and the lack of bleeding episodes (Table 1).
In both dogs expressing FIX in the range of mild hemophilia (Wick and Trex), TAT complex and d-dimer levels at multiple time points remained in the normal range,26,32 consistent with a lack of clinically recognized thrombosis (supplemental Figure 3). Notably, data from the dog Wiley showed continuous expression of supraphysiologic levels of FIX-Padua after inhibitor eradication for >400 days and yet presented no clinical evidence of peripheral thrombosis. TAT and d-dimer levels remain within normal limits26,32 (supplemental Figure 3). These data suggest that the risk of thrombogenicity of FIX-Padua is low at a wide range of expression levels.
Humoral responses to AAV8 capsid
All dogs developed a robust anti-AAV8 capsid immune response measured by increase in IgG2 and, to a lesser extent, IgG1 levels (Figure 4). These data indicate that these dogs are capable of generating sustained immune responses to other antigens excluding the possibility of a general state of defective immune responses.
Figure 4.
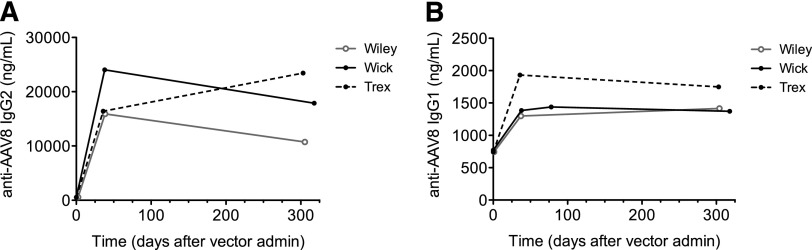
Humoral responses to AAV8 capsid in HB dogs following liver delivery of AAV-cFIX-Padua. All 3 dogs were assessed for anti-AAV8 (A) IgG1 and (B) IgG2 antibodies at baseline and time points following AAV administration using anti-AAV8 IgG ELISAs. Wick, black solid line, closed circles; Trex, black dotted line, closed circles; Wiley, solid gray line, open circles.
Murine studies
Lack of immune responses to hFIX-Padua by AAV liver gene therapy
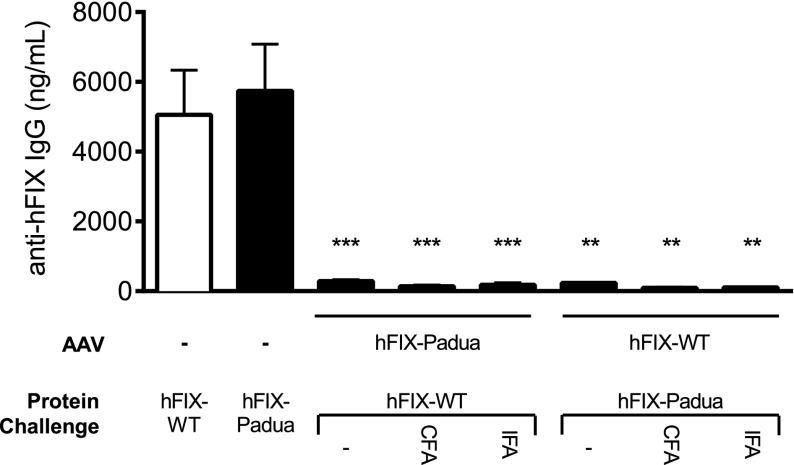
Adult HB mice (n = 9-11 mice per group) received injections of AAV encoding either hFIX-Padua or hFIX-WT resulting in similar circulating FIX plateau antigen levels (724 ± 116 and 850 ± 96 ng/mL, respectively). Mice expressing hFIX-WT received recombinant hFIX-Padua protein and, conversely, mice expressing hFIX-Padua were challenged with hFIX-WT. Initial challenges started at week 10 after vector administration, followed by an additional dose weekly for 3 weeks. No antibody to hFIX was detected by anti-hFIX total IgG (Figure 5) or Bethesda assay (data not shown). Further challenges with hFIX-Padua or hFIX-WT in CFA or in IFA failed to trigger immune responses to either hFIX forms (Figure 5). In contrast, naïve HB mice (n = 4-5) develop antibodies to hFIX upon exposure to protein alone, even without adjuvant (Figure 5). Thus, on stringent immunologic challenges with reciprocal protein, no antibody formation was detected in these mice, suggesting that the immunogenicity of FIX-Padua is comparable to that of FIX-WT.
Figure 5.
Humoral responses to hFIX-Padua following challenge. Ten to 15 weeks after administration of 5 × 1010 vg/kg AAV8-hFIX-R338L or AAV-hFIX-WT, HB mice were assessed for development of anti-hFIX IgG antibodies following subcutaneous challenges with 2 μg per mouse recombinant hFIX-WT or hFIX-Padua with protein alone, protein with CFA, and protein with IFA. Untreated HB mice challenged with protein alone served as a positive control. **P < .01; ***P < .001.
Long-term expression and toxicity of FIX-Padua is comparable to FIX-WT
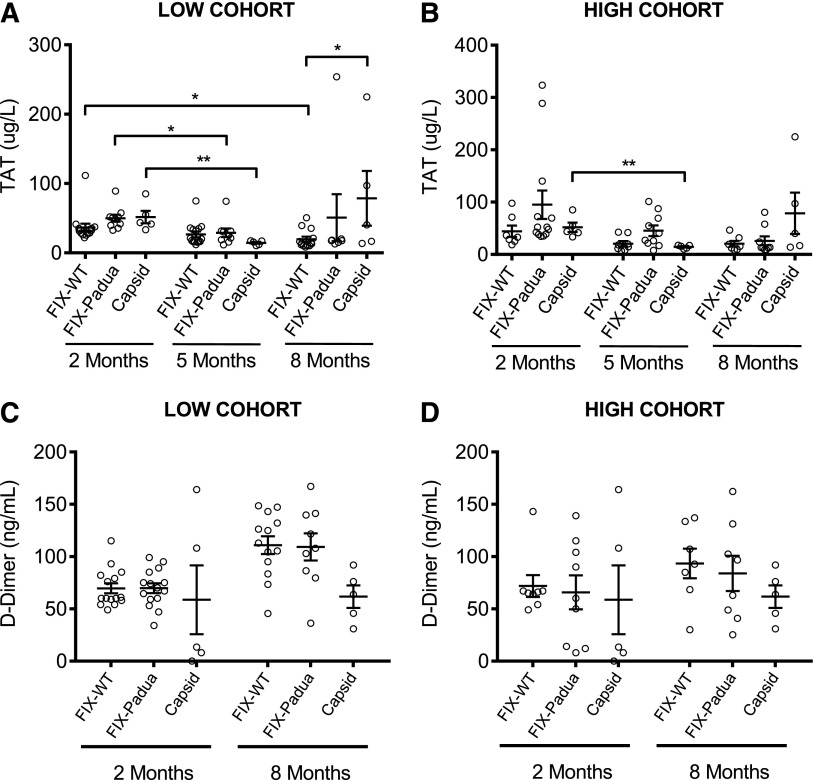
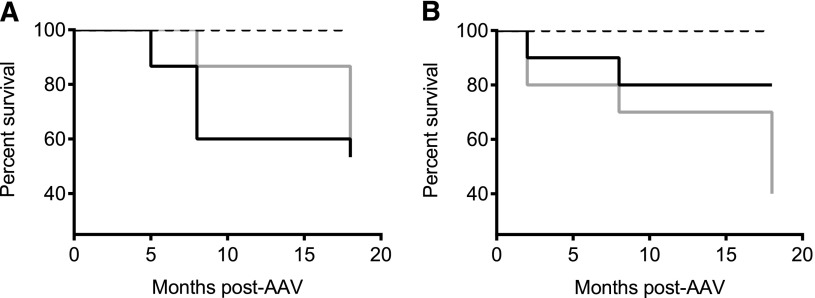
To assess whether expression of hFIX-Padua is associated with abnormal activation of coagulation or early death, hemostatically normal C57Bl/6 male mice received increasing vector doses encoding hFIX-Padua or hFIX-WT. As expected, hFIX specific activity was 7- to 10-fold higher in the hFIX-Padua compared with hFIX-WT–expressing mice (Table 2). We grouped mice expressing FIX-Padua or FIX-WT according to the FIX activity at low (100-230%) and high (480-2020%) cohorts. A time course of TAT complex levels show no difference between the FIX forms and no increase in TAT levels over time (Figure 6A-B). d-dimer levels increased as a function of time only for the low dose cohorts of hFIX-WT (P = .0002) and hFIX-Padua (P = .006), but there was no difference in d-dimer levels between the 2 FIX variants (Figure 6C-D). No changes in d-dimer levels were observed over time for the control group. Kaplan-Meier survival analysis showed no difference between mice expressing hFIX-Padua or hFIX-WT (Figure 7). Early death was associated with supraphysiologic levels that typically started only 8 to 10 months after vector injection. Moreover, data from the control group show no death during the study. Together, these findings suggest that the long-term toxicity in mice expressing a wide range of expression of hFIX-Padua is comparable of that of hFIX-WT.
Table 2.
Summary of AAV8-hFIX-WT and hFIX-Padua expression in C57BL/6 mice
| Dose (vg/kg) | hFIX-WT | hFIX-Padua | ||||
|---|---|---|---|---|---|---|
| Activity, % mean ± SEM | Antigen, % mean ± SEM | Specific activity | Activity, % mean ± SEM | Antigen, % mean ± SEM | Specific activity | |
| 1 × 1010 | 39.9 ± 0.9 | 2.8 ± 0.3 | NA* | 53.8 ± 6.4 | 3.0 ± 0.2 | NA* |
| 4 × 1010 | 63.6 ± 10.4 | 33.5 ± 2.4 | 1.9 | 173.5 ± 27.8 | 16.1 ± 3.2 | 10.8 |
| 1 × 1011 | 115.4 ± 21.9 | 62.0 ± 7.1 | 1.9 | 424.1 ± 59.8 | 45.9 ± 11.3 | 9.2 |
| 4 × 1011 | 456.3 ± 15.2 | 439.1 ± 17.4 | 1.0 | 1978.5 ± 344.1 | 271.2 ± 51.9 | 7.3 |
| 2 × 1012 | 698.6 ± 110.8 | 1020.4 ± 129.8 | 0.7 | ND | ND | ND |
| Average | 1.4 | 9.1 | ||||
ND, not determined; SEM, standard error of the mean.
Low antigen level detection limits impact specific activity calculation.
Figure 6.
Markers of coagulation activation in WT C57BL/6 mice expressing hFIX-WT and hFIX-Padua at supraphysiologic levels following AAV. Mice expressing hFIX-WT (white bars) and hFIX-Padua (black bars) at levels resulting in (A,C) 100% to 230% activity and (B,D) 480% to 2020% activity were assessed for (A,B) thrombin antithrombin complexes (TAT) and (C,D) d-dimers as markers of coagulation 2, 5 (TAT only), and 8 months following vector administration. At no point were there statistically significant differences between the hFIX-WT– and hFIX-Padua–expressing groups. Gray bars, empty capsid-treated control animals with normal FIX levels.
Figure 7.
Survival of WT C57BL/6 mice expressing hFIX-WT and hFIX-Padua at supraphysiologic levels following AAV. Mice expressing hFIX-WT (gray lines) and hFIX-Padua (black lines) at levels resulting in (A) 100% to 230% activity and (B) 480% to 2020% activity did not have different survival rates. Black dotted line, empty capsid-treated control animals with normal FIX levels.
Discussion
One of the major safety concerns in developing novel therapies based on protein, cell, or gene therapy for hemophilia is the formation of antibodies to the missing clotting factor. The presence of these antibodies renders the replacement therapy ineffective with increased morbidity and mortality.33-35 Experiences with protein replacement therapy demonstrate that manufacturing procedures, mismatches between the therapeutic protein sequence and host rare haplotypes, and modifications on the amino acid sequence could all influence the rates of inhibitor formation.2,36-38 Here we sought to test the safety of liver-restricted expression of the cFIX-Padua variant. HB dogs with underlying F9-null mutation exhibit high risk of antibody formation on exposure to cFIX-WT and thus provide an excellent model to test immune responses to the neotransgene. The data presented in HB dogs clearly demonstrate that AAV liver gene therapy ensures sustained expression of cFIX-Padua (25-300% of normal) with high specific activity (8- to 12-fold) consistent with the findings in humans hemizygous for FIX-Padua.12 We observed complete normalization of WBCT and TEG values concomitant with a lack of bleeding episodes over a long-term follow-up in naïve HB dogs and after eradication of cFIX inhibitors in the dog Wiley.
Clinical trials with severe HB using AAV2 or AAV8 vectors showed that in the high dose cohort (2 × 1012 vg/kg), therapeutic levels of hFIX-WT were achieved with transient liver immune-mediated toxicity10,11 controlled by a short-term course of immunosuppression.11 Here we achieved therapeutic levels of FIX activity (25-300%) at vector doses of 1 × 1012 or 3 × 1012 vg/kg. Therefore, we suggest that lower doses of AAV vectors will be able to reach therapeutic levels using FIX-Padua as the transgene. Expression in Trex was relatively poor; 2 things may account for this relatively low expression. First, Trex is notably smaller, resulting in a lower total vector dose. Second, the spike in Trex of proinflammatory Th2 and Th1 cytokines at day 3 is suggestive of a memory T-cell response to AAV. This could indicate low titers of anti-AAV antibodies that could partially inhibit transduction.
We also showed that liver expression of cFIX-Padua induced tolerance in these dogs and did not generate any anti-FIX humoral immune responses, even after challenge with cFIX-WT protein concentrate at doses associated with inhibitor formation in naïve HB dogs from this colony.17 FIX-Padua expression showed no increased immunogenicity on exposure to FIX-WT protein, which is the clinical scenario in which patients expressing FIX-Padua by AAV vectors may require protein replacement to control major hemostatic challenges.
Notably, expression of AAV-cFIX-Padua was successful in eradicating inhibitory antibodies to cFIX. We documented an amnestic response (∼5 Bethesda units) at early time points after AAV injection followed by eradication of the anti-cFIX antibodies and a gradual increase in the circulating levels of fully active cFIX-Padua. The lack of immune response to the transgene was maintained after challenge with FIX-WT protein concentrate, reinforcing the strength with which AAV liver gene transfer induces transgene-specific immune tolerance. No kidney, liver, or systemic toxicity was observed in this dog over a period of 3 years (ongoing observations). Of note, inhibitory antibodies to hFIX were completely eradicated, whereas residual non-neutralizing antibodies (IgG2) declined but are still detectable. This probably reflects that immune tolerance occurred to epitopes shared by canine and human FIX but not those restricted to hFIX, and thus this strategy is likely to epitope specific. The findings in this HB dog are the first demonstration of eradication of inhibitors to FIX in a large and immune competent HB model with preexisting antibodies to FIX.
The time course for eradication of inhibitor and detection of circulating FIX levels in this dog was similar to our early data in immune tolerance induction by AAV expressing canine FVIII in hemophilia A dogs with preexisting inhibitors.22 Despite the fact that detectable anti-FIX IgG2 resolved in 70 days, FIX activity took nearly 800 days to plateau. This suggests there were anti-FIX antibodies that our assay was unable to detect, which likewise reached the limit of detection and plateau around day 800. Wiley’s cytokine profile may reflect this. Prior to vector administration, he had high levels of inflammatory cytokines, including IL-6, suggesting a Th2 immune response as would be expected for inhibitors. These cytokines decreased following vector administration over the next 800 days concurrent with a rise in FIX activity, suggesting an anti-FIX response that is slowly resolving. Wiley’s cytokine responses were dramatically different from those of Trex and Wick, the latter of which had no detectable cytokines throughout the period monitored. We did note elevated cytokines in Trex on day 70, which is unlikely related cFIX-Padua.
Despite the inflammatory T-cell response occurring at the early time points in Wiley, there is a striking lack of IL-2. With the expression of IL-10, this is suggestive of a regulatory T-cell (Treg) response. In our FVIII inhibitor dog model,22 we likewise observed a transient increase in the pool of canine Treg cells (CD4+CD25+FoxP3+ T cells) peaking 1 to 2 weeks after vector delivery and immediately preceding the disappearance of antibodies. In Wiley, IL-10 expression rose weeks 2 to 4 and following the protein challenge, suggesting Tregs were again required to control an anti-cFIX immune response. Also of interest is the kinetics of the IFN-γ immune response seen in Wiley. There were 2 distinct peaks: one in the weeks immediately following vector administration and another following challenge with FIX protein. Although normally considered to be a cytotoxic T cell and Th1 cytokine, recent data suggested that IFN-γ can also be transiently produced by or required for the conversion of induced Tregs in the periphery.39,40 The second peak in IFN-γ after challenge, like the rise in IL-10, suggests that Tregs were again called on to squelch an anti-cFIX immune response. This could partially explain the remaining question of the mechanism of long-lasting AAV-induced tolerance.
In the hemophilia A model, we showed that IgG2 was also the predominant IgG subclass.22 The human equivalent of canine IgG2 is IgG4, and both are associated with a Th2 helper CD4+ T-cell response.16 Human IgG4 is produced only on prolonged or repeated exposure to antigen, and evidence suggests its production is promoted by Treg cell cytokines. Moreover, IgG4 has poor affinity to Fc receptor and for C1q of the complement pathway, thus preventing cell and complement activation, respectively.41 IgG4 production has been linked with activation/generation of CD4+ regulatory T cells and thus with a potential for down-modulated immune responses.42,43 Therefore, it is possible that in the context of inhibitors to FIX in this canine model, a similar immune tolerance induction mechanism was associated with inhibitor eradication.
Data on additional dogs with preexisting inhibitors to FIX are certainly needed to confirm this early successful observation and to more clearly define the underlying mechanism of immune tolerance. The success rates of immune tolerance induction protocols using frequent injections of FIX protein concentrates are lower than those for hemophilia A, and potential complications such nephrotic syndrome, allergic, and anaphylactic reactions all hamper the overall safety.1,31,44 It is possible that AAV-based immune tolerance induction could also be indicated for a selected group of HB patients with inhibitors to FIX.
The immune competent status of these 3 dogs was confirmed by the humoral responses to the vector capsid proteins (IgG1 and IgG2). Similar findings observed in humans following AAV2 or AAV8 delivery to the liver.10,11,45 Thus, this strategy to use AAV for the transgene-specific immune tolerance does not induce a general status of immunosuppression.
Finally, studies in mice allow us to compare the immunogenicity of FIX-Padua with FIX-WT in a stringent manner. Mice expressing FIX-Padua or FIX-WT failed to mount an immune response to the reciprocal recombinant FIX proteins, even when mixed with adjuvants to enhance immune responses.
The potential thrombogenicity FIX-Padua expression appears to be minimal, as no evidence of pathological activation of coagulation or clinical evidence of thrombosis was noted in any of the 3 dogs with a cumulative observation of ∼6 years. Together with the lack of thrombosis in another group of 3 HB dogs expressing FIX-Padua following AAV delivery to skeletal muscle for a cumulative 5 years,24 there is no evidence of thrombosis in a total of 6 dogs in an 11-year period, suggesting a very low risk for such a complication. This is expected considering that thrombosis occurred only in FIX-Padua patients with activity levels >700% of normal, which is significantly higher than the FIX levels in these dogs.12
This is further supported by longitudinal studies in mice expressing FIX-Padua or FIX-WT, which showed similar profiles of activation of coagulation and survival rates. These data are agreement with previous short-term gene therapy studies in mice.46,47 The main determinant of death rates was the supraphysiologic levels of either FIX forms that occur in a time-dependent manner, ie, 8 to 10 months after vector injection, thus providing more stringent risk assessment than short-term46,47 or long-term studies expressing FIX at relatively lower levels.48 These data suggest that the safety of continuous expression of FIX-Padua does not differ from the FIX-WT expression and, thus FIX-Padua is not inherently more thrombogenic per se, but at extremely high levels behaves in a similar fashion as FIX-WT.
Collectively the efficacy and safety data from dogs and mice expressing FIX-Padua form the basis for translational studies. The safe immunological profile of FIX-Padua in the inhibitor-prone dogs would allow the inclusion of HB patients with F9 gene null mutations in gene therapy clinical trials.
Acknowledgments
The authors thank Alex Tai, Yifeng Chen, Holly Bachus, and Robert French for assistance, Dr Michael Betts for advice, and Heather Collins of the Radioimmunoassay and Biomarkers Core of the Penn Diabetes Research Center for running the canine cytokine arrays. This work was funded by National Institutes of Health, National Heart, Lung, and Blood Institute grants P01 HL64190 Project 1 (to V.R.A.), HL086944 (to C.D.L.), and PO1 HL64190, and National Blood Foundation Scientific Research grant 10-20 (to J.D.F.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.M.C., J.D.F., and J.I.S. planned and execute animal work; N.B.M. performed vector immune responses; G.P.N. and C.D.L. carried out animal care; S.Z. provided the AAV vectors; F.M. provided insights on experimental design; and V.R.A. directed experimental design, conducted data analysis and interpretation, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valder R. Arruda, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, 5056 Colket Translational Research Center, Philadelphia, PA 19104; e-mail: arruda@email.chop.edu.
References
- 1.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138(3):305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Lozier J, Johnson G, et al. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat Biotechnol. 2008;26(8):901–908. doi: 10.1038/nbt.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrier I. Inhibitor in haemophilia B. In: Lee C, Bertorp E, Hoots WK, editors. Textbook of Hemophilia. Malden: Blackwell Publishing, Inc; 2005. pp. 97–100. [Google Scholar]
- 4.Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127(4):379–391. doi: 10.1111/j.1365-2141.2004.05168.x. [DOI] [PubMed] [Google Scholar]
- 5.High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost. 2011;9(Suppl 1):2–11. doi: 10.1111/j.1538-7836.2011.04369.x. [DOI] [PubMed] [Google Scholar]
- 6.Löfqvist T, Nilsson IM, Berntorp E, Pettersson H. Haemophilia prophylaxis in young patients—a long-term follow-up. J Intern Med. 1997;241(5):395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- 7.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 8.Pipe SW. Hemophilia: new protein therapeutics. Hematology Am Soc Hematol Educ Program. 2010;2010:203-209. [DOI] [PubMed]
- 9.Powell JS, Pasi KJ, Ragni MV, et al. B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323. doi: 10.1056/NEJMoa1305074. [DOI] [PubMed] [Google Scholar]
- 10.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 11.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009;361(17):1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 13.Evans JP, Brinkhous KM, Brayer GD, Reisner HM, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci USA. 1989;86(24):10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauser AE, Whitlark J, Whitney KM, Lothrop CD., Jr A deletion mutation causes hemophilia B in Lhasa Apso dogs. Blood. 1996;88(9):3451–3455. [PubMed] [Google Scholar]
- 15.Nichols TC, Raymer RA, Franck HW, et al. Prevention of spontaneous bleeding in dogs with haemophilia A and haemophilia B. Haemophilia. 2010;16(Suppl 3):19–23. doi: 10.1111/j.1365-2516.2010.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog RW, Fields PA, Arruda VR, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13(11):1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 17.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4(3):192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 18.Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5(1):56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 19.Niemeyer GP, Herzog RW, Mount J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 21.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24(3):257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 22.Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116(26):5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99(8):2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 24.Finn JD, Nichols TC, Svoronos N, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012;120(23):4521–4523. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arruda VR, Stedman HH, Nichols TC, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105(9):3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewhurst E, Cue S, Crawford E, Papasouliotis K. A retrospective study of canine D-dimer concentrations measured using an immunometric “Point-of-Care” test. J Small Anim Pract. 2008;49(7):344–348. doi: 10.1111/j.1748-5827.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- 27.Haurigot V, Mingozzi F, Buchlis G, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2010;18(7):1318–1329. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanciu L, Toso R, Margaritis P, et al. A zymogen-like factor Xa variant corrects the coagulation defect in hemophilia. Nat Biotechnol. 2011;29(11):1028–1033. doi: 10.1038/nbt.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whelan SF, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121(6):1039–1048. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 30.Dimichele D. Immune tolerance therapy for factor VIII inhibitors: moving from empiricism to an evidence-based approach. J Thromb Haemost. 2007;5(Suppl 1):143–150. doi: 10.1111/j.1538-7836.2007.02474.x. [DOI] [PubMed] [Google Scholar]
- 31.Warrier I, Ewenstein BM, Koerper MA, et al. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediatr Hematol Oncol. 1997;19(1):23–27. doi: 10.1097/00043426-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Mehta JL, Chen L, Nichols WW, Mattsson C, Gustafsson D, Saldeen TG. Melagatran, an oral active-site inhibitor of thrombin, prevents or delays formation of electrically induced occlusive thrombus in the canine coronary artery. J Cardiovasc Pharmacol. 1998;31(3):345–351. doi: 10.1097/00005344-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Donfield SM, Lynn HS, Lail AE, Hoots WK, Berntorp E, Gomperts ED Hemophilia Growth and Development Study Group. Delays in maturation among adolescents with hemophilia and a history of inhibitors. Blood. 2007;110(10):3656–3661. doi: 10.1182/blood-2007-05-088062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay CR, DiMichele DM International Immune Tolerance Study. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119(6):1335–1344. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 35.Soucie JM, Miller CH, Kelly FM, et al. CDC Inhibitor Surveillance Working Group. National surveillance for hemophilia inhibitors in the United States: Summary report of an expert meeting. Am J Hematol. 2014;89(6):621–625. doi: 10.1002/ajh.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouw SC, van der Bom JG, Ljung R, et al. PedNet and RODIN Study Group. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231–239. doi: 10.1056/NEJMoa1208024. [DOI] [PubMed] [Google Scholar]
- 37.Peerlinck K, Rosendaal FR, Vermylen J. Incidence of inhibitor development in a group of young hemophilia A patients treated exclusively with lyophilized cryoprecipitate. Blood. 1993;81(12):3332–3335. [PubMed] [Google Scholar]
- 38.Viel KR, Ameri A, Abshire TC, et al. Inhibitors of factor VIII in black patients with hemophilia. N Engl J Med. 2009;360(16):1618–1627. doi: 10.1056/NEJMoa075760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenecke C, Lee CW, Thamm K, et al. IFN-γ production by allogeneic Foxp3+ regulatory T cells is essential for preventing experimental graft-versus-host disease. J Immunol. 2012;189(6):2890–2896. doi: 10.4049/jimmunol.1200413. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Hong J, Sun W, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest. 2006;116(9):2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigal LH. Basic science for the clinician 58: IgG subclasses. J Clin Rheumatol. 2012;18(6):316–318. doi: 10.1097/RHU.0b013e318269446b. [DOI] [PubMed] [Google Scholar]
- 42.Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23(1):119–124. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 43.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 44.Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood. 1997;89(3):1115–1116. [PubMed] [Google Scholar]
- 45.Murphy SL, Li H, Mingozzi F, et al. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol. 2009;81(1):65–74. doi: 10.1002/jmv.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantore A, Nair N, Della Valle P, et al. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120(23):4517–4520. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 47.Monahan PE, Sun J, Gui T, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: Preclinical evaluation supporting an ongoing AAV clinical trial [published online ahead of print November 24, 2014]. Hum Gene Ther. doi: 10.1089/hum.2014.106. doi:10.1089/hum.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao CY, Yang SJ, Tao MH, Jeng YM, Yu IS, Lin SW. Incorporation of the factor IX Padua mutation into FIX-Triple improves clotting activity in vitro and in vivo. Thromb Haemost. 2013;110(2):244–256. doi: 10.1160/TH13-02-0154. [DOI] [PubMed] [Google Scholar]