Key Points
Platelets from Hermansky-Pudlak syndrome models are less apt to secrete contents of multiple storage granules at sites of vascular injury.
The secretion defect contributes to poor hemostasis and perhaps to heightened colitis incidence in Hermansky-Pudlak syndrome patients.
Abstract
Hermansky-Pudlak syndrome (HPS) is characterized by oculocutaneous albinism, bleeding diathesis, and other variable symptoms. The bleeding diathesis has been attributed to δ storage pool deficiency, reflecting the malformation of platelet dense granules. Here, we analyzed agonist-stimulated secretion from other storage granules in platelets from mouse HPS models that lack adaptor protein (AP)-3 or biogenesis of lysosome-related organelles complex (BLOC)-3 or BLOC-1. We show that α granule secretion elicited by low agonist doses is impaired in all 3 HPS models. High agonist doses or supplemental adenosine 5′-diphosphate (ADP) restored normal α granule secretion, suggesting that the impairment is secondary to absent dense granule content release. Intravital microscopy following laser-induced vascular injury showed that defective hemostatic thrombus formation in HPS mice largely reflected reduced total platelet accumulation and affirmed a reduced area of α granule secretion. Agonist-induced lysosome secretion ex vivo was also impaired in all 3 HPS models but was incompletely rescued by high agonist doses or excess ADP. Our results imply that (1) AP-3, BLOC-1, and BLOC-3 facilitate protein sorting to lysosomes to support ultimate secretion; (2) impaired secretion of α granules in HPS, and to some degree of lysosomes, is secondary to impaired dense granule secretion; and (3) diminished α granule and lysosome secretion might contribute to pathology in HPS.
Introduction
Effective thrombus formation by platelets at sites of blood vessel injury requires the stimulus-dependent release of effectors from membrane-enclosed dense granules, α granules, and lysosomes.1-3 Dense granules harbor small molecules that upon release amplify platelet activation and adhesion, blood vessel constriction, and wound repair.4-6 α granules store protein factors that facilitate platelet adhesion, clot stabilization, fibrinolysis, angiogenesis, wound repair, and inflammation.7-9 Lysosomes store proteolytic enzymes that likely contribute to thrombus remodeling.3 Granule contents are normally released upon platelet stimulation.10 Disorders of granule secretion11-13 or granule formation1,14 result in excessive bleeding.
Hermansky-Pudlak syndrome (HPS) is a group of autosomal recessive disorders characterized by prolonged bleeding, oculocutaneous albinism, and other symptoms.15,16 Clinically significant bleeding diathesis in HPS has been ascribed to platelet dense granule malformation.5,16 Platelets in HPS patients and mouse models17 lack detectable dense granules by electron microscopy18 and do not effectively store serotonin and adenine nucleotides or release them upon stimulation.19-21 Consequently, platelet aggregation in vitro is impaired.22 These defects likely reflect impaired delivery of membrane contents to nascent dense granules within megakaryocytes or proplatelets. The genes that are mutated in the 9 known HPS variants and in 12 of 15 mouse HPS models encode subunits of distinct protein complexes (adaptor protein-3 [AP-3] and biogenesis of lysosome-related organelles complex [BLOC]-1, -2, and -3) that function in transmembrane cargo delivery to lysosome-related organelles (LROs) in other cell types.15,23-25 AP-3 sorts cargoes from endosomes into transport carriers toward lysosomes or LROs.26 BLOC-3 is a guanine nucleotide exchange factor for the tissue-restricted Rab GTPases RAB32 and RAB38,27 which function with BLOC-1 and BLOC-2 in as yet unclear ways to deliver cargoes from endosomes to LROs in melanocytes.25 RAB32 and RAB38 regulate cargo localization to dense granule–like compartments in a megakaryocytoid cell line,28 but how AP-3 and BLOCs function in megakaryocytes and platelets is not known.
Dense granules and α granules are both LROs29 and, like lysosomes, are proposed to derive from similar multivesicular precursors30,31 and to employ similar fusion machinery for secretion.11-13,32 Nevertheless, the formation of α and dense granules is differentially controlled. For example, NBEAL2, VPS16B, and VPS33B regulate the biogenesis of α granules but not dense granules.33-37 In platelets of HPS patients, the number, morphology, and content levels of α granules and lysosomes are normal.17,38,39,40 Thrombin-induced secretion of α granule and lysosome contents was impaired in platelets from 1 uncharacterized HPS patient,41 but has not been systematically analyzed in different HPS subtypes. Thrombin-induced lysosome secretion from platelets in mouse HPS models was reported to vary from modestly impaired to hyperactive.17,39
To assess platelet α granule and lysosome secretion in HPS variants, we exploited 3 congenic mouse HPS models with defects in different protein complexes: pearl (Ap3b1pe/pe; referred to here as AP3−/−), a model for HPS type 2 that lacks AP-342,43; pallid (Pldnpa/pa or BLOC-1−/−), a model for HPS type 9 that lacks BLOC-144,45; and light ear (Hps4le/le or BLOC-3−/−), a model for HPS type 4 that lacks BLOC-3.46,47 Platelets from each model were analyzed for agonist-dependent secretion from α granules and lysosomes using ex vivo assays and intravital imaging. We provide evidence that AP-3, BLOC-1, and BLOC-3 impact the release of multiple platelet granule types.
Materials and methods
Mouse strains
Experiments used 12- to 13-week-old male C57BL/6J (wild-type [WT]) and congenic B6-Cg-Pldnpa/J (pallid), B6-Cg-AP3b1pe/J (pearl), and B6.C3-Pde6brd1 Hps4le/J (light ear) mice (Jackson Laboratory, Bar Harbor, ME) bred at the University of Pennsylvania under guidelines of the University Laboratory Animal Resources. The light ear mice also carry a mutation in phosphodiesterase 6B, which is not expressed in hematopoietic cells. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania or Children’s Hospital of Philadelphia.
Antibodies and reagents
For details on the antibodies used for flow cytometry, immunofluorescence microscopy, and intravital imaging, see supplemental Methods available on the Blood Web site. For intravital imaging, antibodies or F(ab)′2 fragments were labeled using Alexa Fluor monoclonal antibody labeling kits according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Chemicals were from Sigma-Aldrich (St Louis, MO) and reagents were from Invitrogen unless otherwise specified. The DuoSet ELISA Development kit was from R&D Systems (Minneapolis, MN).
Platelet preparation and flow cytometry analysis
Platelets were isolated from blood by differential centrifugation as described by Pang et al48 (supplemental Methods). Platelets resuspended in Tyrode’s solution buffered with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid were incubated with or without agonist at 37°C for 10 minutes. For flow cytometry, treated platelets were incubated with fluorophore-conjugated antibodies at room temperature for 20 minutes, diluted with Tyrode’s solution buffered with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and analyzed using a FACSCalibur flow cytometer and CellQuest Pro 5.2 software (Becton-Dickinson). Data represent the percentage of cells that express the indicated surface marker at a level above the background of unstimulated cells (see supplemental Figures 1-4 for gating strategy).
Analysis of granule protein release
Platelet factor 4 (PF-4; CXCL4) and platelet basic protein (PBP; β-thromboglobulin; CXCL7) in whole-cell lysates and platelet releasates were quantified by enzyme-linked immunosorbent assay according to manufacturer’s protocols (R&D Systems). Platelets were lysed (8 × 105/μL) in phosphate-buffered saline/0.1% bovine serum albumin/1% Triton-X 100 and diluted up to 10 000× for assay within the linear range of the standard curve, generated concomitantly using purified protein to calculate protein concentrations. Lysosomal enzyme activity in whole-cell lysates and releasates was measured for β-hexosaminidase by a colorimetric assay using P-nitrophenyl-N-acetyl-α-d-glucosaminide as a substrate,49 and was measured for β-glucuronidase using the fluorogenic substrate 4-methylumbelliferyl-β-d-glucuronide (EMD Millipore, Billerica, MA).50
Laser-induced thrombus formation in mouse cremaster muscle arterioles
Thrombus formation was visualized in the cremaster muscle microcirculation of male WT, pearl, pallid, and light ear mice using Alexa-568-labeled anti-CD41 F(ab)′2 fragments and Alexa Fluor 488-labeled anti-CD62p antibody as described by Stalker et al51 (supplemental Methods). Data are reported as the area encompassed by CD41-positive and CD62p-positive cells surrounding laser-induced vascular damage.
Immunofluorescence microscopy
Details regarding isolation of platelet-rich plasma, fixation, and immunostaining are described in supplemental Methods. Immunolabeled cells were analyzed on a Leica DM16000B inverted microscope equipped with a 100× plan apo lens, Hamamatsu Orca Flash 4.0 V2 camera and Leica Application Suite software for image capture, deconvolution, and 3-dimensional rendering. Sequential z planes were captured at 0.2-μm intervals and deconvolved using Gold’s algorithm with 3 iterations. Images were assembled and optimized for brightness and contrast using Adobe Photoshop (Mountainview, CA).
Statistical analyses
Data were analyzed and graphed using Prism v.5 or 6 for MacIntosh (GraphPad, La Jolla, CA). Data from each HPS model platelet sample at each time point were compared with those from WT platelets by 1-way analysis of variance, and P values were determined by the Bonferroni multiple comparison test.
Results
Activation-induced cell surface expression of CD62p in response to low-dose agonist is impaired in HPS model platelets
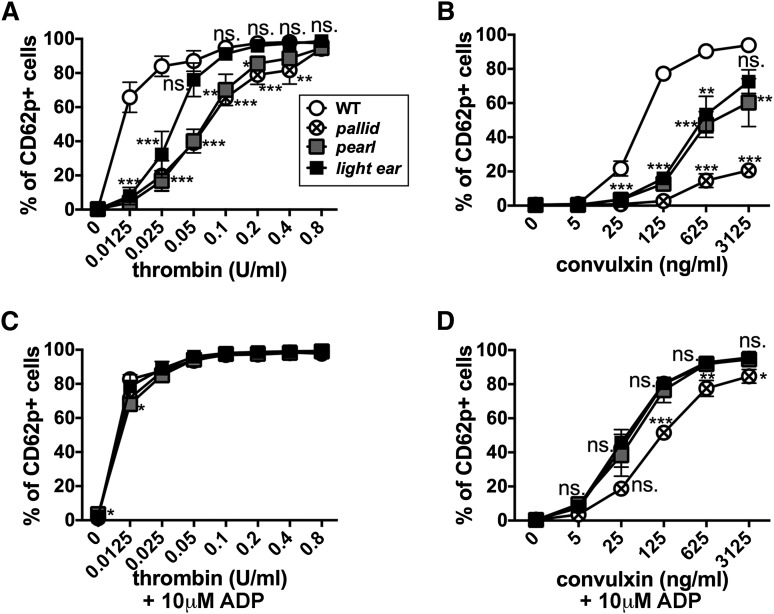
To assess α granule secretion, we first probed for cell surface exposure of CD62p (P-selectin) after fusion of α granule membranes with the plasma membrane.52,53 Platelets from C57BL/6J (WT), pearl, pallid, and light ear mice expressed CD62p at similar levels by flow cytometry after permeabilization (supplemental Figure 1A), indicating that loss of AP-3, BLOC-1, or BLOC-3 does not impact total CD62p accumulation. Washed platelets were stimulated with agonists for protease-activated receptors (thrombin) or for glycoprotein VI (convulxin) at varying concentrations, and surface CD62p exposure on intact cells was assessed by flow cytometry. As described by Berman et al,53 increasing doses of either agonist elevated the percentage of WT platelets that express surface CD62p, as well as CD62p levels on positive cells (Figure 1A-B; supplemental Figures 1B and 2C), such that 66% and 77% of cells expressed CD62p when exposed to even the lowest doses of thrombin (0.0125 U/mL) or convulxin (125 ng/mL), respectively. By contrast, the HPS model platelets were nearly unresponsive at these doses (Figure 1A-B; supplemental Figure 1C), and higher doses elicited CD62p surface expression with distinct dose-response profiles in platelets from each model. Light ear (BLOC-3−/−) platelets were only modestly less responsive to thrombin than WT, whereas pearl (AP-3−/−) and pallid (BLOC-1−/−) platelets required higher thrombin doses to achieve similar responses (Figure 1A). HPS model platelets were less sensitive to convulxin, such that only 72% of light ear and 60% of pearl platelets expressed surface CD62p in response to the highest dose (compared with 94% for WT). Very few pallid platelets (20%) responded to even the highest dose of convulxin (Figure 1B). The dose-response curves for percent of positive cells and for levels of CD62p on positive cells were similar (supplemental Figure 2C), and responses of platelets from heterozygous age-matched littermates were identical to those from WT platelets (unpublished data). These data indicate that α granule secretion in platelets from mouse models of HPS type 2, type 4, and type 9 is hyporesponsive to stimulation but can be overcome by a strong stimulus, particularly via protease-activated receptors. The more modest impairment in light ear platelets relative to other HPS models is commensurate with the storage pool deficiencies in these models.17,54
Figure 1.
Agonist-induced surface expression of the α granule membrane protein CD62p is impaired in HPS model platelets. Washed platelets (8 × 107) isolated from WT, pallid, pearl, or light ear mice were stimulated with the indicated concentrations of thrombin (A and C) or convulxin (B and D) for 10 minutes in the absence or presence of 10 μM ADP as indicated, and then analyzed by flow cytometry for CD62p surface expression. Shown is the percentage of cells with labeling above the background level observed on unstimulated cells (see supplemental Figures 1 and 2 for gating strategies and examples of flow cytometry profiles.). Data represent mean ± standard deviation from at least 3 experiments. *P < .05; **P < .01; ***P < .005 for HPS models vs WT. ns., nonsignificant.
Supplemental ADP restores a WT dose response of CD62p surface expression to agonist in HPS model platelets
Adenosine 5′-diphosphate (ADP), an agonist of platelet P2Y1 and P2Y12 receptors,55 is released from dense granules on platelet activation,4 but HPS platelets have diminished ADP storage pools. To test whether the impaired α granule secretion in HPS platelets reflected reduced ADP release, we assessed whether supplemental ADP could restore CD62p surface expression at low thrombin or convulxin doses. The addition of 10 μM ADP barely affected the dose-response of WT platelets to either agonist but completely restored the WT dose response to thrombin in platelets from all 3 HPS models, impacting both the percentage of responding cells and the level of CD62p surface expression on positive cells (Figure 1C-D; supplemental Fig. 2). Supplemental ADP also restored a WT response to convulxin by pearl and light ear platelets and dramatically enhanced the response by pallid platelets (Figure 1D). The response of pallid platelets to convulxin was dramatically enhanced by ADP but remained lower than that of the other HPS models. These data suggest that impaired α granule secretion in HPS platelets exposed to low agonist doses results from the inadequate supply of ADP and/or other effectors from dense granules.
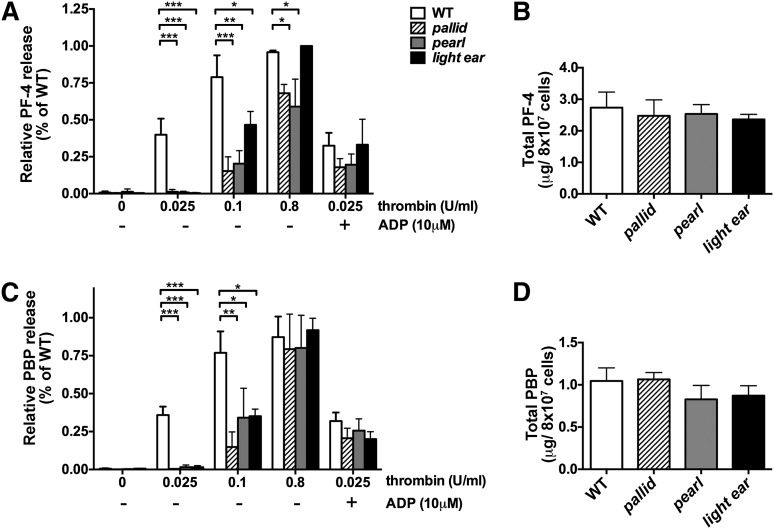
Impaired α granule content release in response to low-dose thrombin stimulation in HPS model platelets
To test whether agonist-dependent release of α granule contents was impaired in HPS models, releasates from WT and HPS model platelets were assayed for PF-4 and PBP following exposure to increasing doses of thrombin. Whereas all platelet populations contained similar levels of PF-4 and PBP prior to stimulation (Figure 2B,D), platelets from all 3 HPS models were nearly unresponsive to 0.025 U/mL of thrombin, a dose that induced 35% to 40% of the maximal release of PF-4 and PBP in WT platelets (Figure 2A,C). Increased thrombin doses stimulated higher PF-4 and PBP secretion from HPS platelets, with near WT levels of PBP secretion and substantial PF-4 secretion from all platelet populations at 0.8 U/mL of thrombin; light ear platelets were most similar to WT. Addition of ADP to 0.025 U/mL of thrombin restored PF-4 and PBP secretion to WT levels. These data show that CD62p expression and α granule content secretion are similarly impaired in HPS models.
Figure 2.
Induced secretion of α granule contents from HPS model platelets is impaired at low doses of thrombin. Washed platelets (8 × 107) isolated from WT, pallid, pearl, or light ear mice were stimulated with the indicated concentrations of thrombin in 100 μL of phosphate-buffered saline/0.1% bovine serum albumin for 10 minutes in the absence or presence of 10 μM adenosine 5′-diphosphate (ADP) as indicated, and then supernatants were collected and analyzed by enzyme-linked immunosorbent assay for PF-4 (A) or PBP (C). Untreated platelets (8 × 107) were lysed in 100 μL of lysis buffer, and lysates were analyzed directly for content of PF-4 (B) or PBP (D). In panels A,C, the percentage of PF-4 and PBP in releasates relative to untreated cell lysates for each sample was plotted relative to the highest percentage of release observed in a single assay for WT platelets (range: 50% to 84% for WT). Data represent mean ± standard deviation from at least 3 independent experiments. *P < .05; **P < .01; ***P < .005.
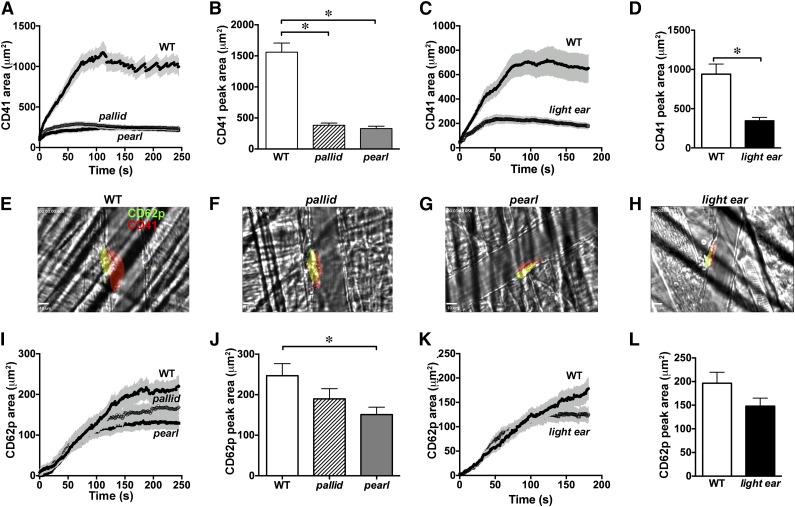
Abnormal thrombus formation in vivo in HPS model mice
To test whether α granule secretion from HPS model platelets is impaired during thrombus formation in vivo, we employed intravital microscopy during laser-induced blood vessel damage in the cremaster muscle. Accumulation of platelets labeled by fluorophore-conjugated antibodies to CD41 (total platelets) and to CD62p (α granule secretion) was imaged by video fluorescence microscopy (supplemental Movie 3a-d). In WT mice, CD41+ platelets accumulated immediately after injury and increased linearly for 3 minutes, leveling off by 4 minutes (Figure 3A-H). Thrombi contained a small “core” of CD62p-positive platelets immediately abutting the injury site and a distal “shell” of CD62p-negative platelets51 (Figure 3E-H). Total platelet accumulation in mice of all 3 HPS models was severely attenuated—by nearly 80% in pallid and pearl mice—reflecting primarily a loss of the CD62p-negative shell (Figure 3A-H). However, the peak size of the CD62p-positive core at 3 to 4 minutes was also decreased in all 3 HPS models (Figure 3I-L; but statistically significant only in pearl mice). Kinetic analyses showed that the initial rate of CD62p-positive platelet accumulation was identical in all mice but leveled off earlier in the HPS models, with the rate slowing earliest in pearl and latest in light ear (Figure 3I-L). These data suggest that in HPS models, (1) the excessive bleeding reflects small thrombi at blood vessel injury sites; (2) the thrombi are small primarily because they lack a shell; and (3) at least in pearl mice, α granule secretion distal to the site of injury within the core is impaired.
Figure 3.
Impaired thrombus formation and α granule secretion after laser injury in HPS model mice. WT, pallid, pearl, or light ear mice were injected with fluorophore-conjugated antibodies to CD41 (to detect total platelets) and to P-selectin (to detect α granule secretion) and then subjected to laser-induced injury in the cremaster muscle microvasculature. Accumulation of signal for CD41 and P-selectin at injury sites was imaged by live intravital video microscopy. The total area of CD41+ (A-D) and P-selectin+ (I-L) platelet accumulation at injury sites was quantified over time. The time course in separate sets of experiments is shown for WT vs pallid and pearl mice for 4 minutes (A and I) or WT vs light ear mice for 3 minutes (C and K). Panels B,D,J,L show the peak area of accumulation at the end of the time course (mean ± standard deviation from at least 3 independent experiments). (E-H) Frames from movies at the 3-minute time point of representative thrombi labeled for P-selectin (green) and CD41 (red) in WT, pallid, pearl, and light ear mice (red and green overlay is yellow). *P < .05.
Impaired agonist-induced lysosome secretion in mouse HPS model platelets is largely independent of the dense granule defect
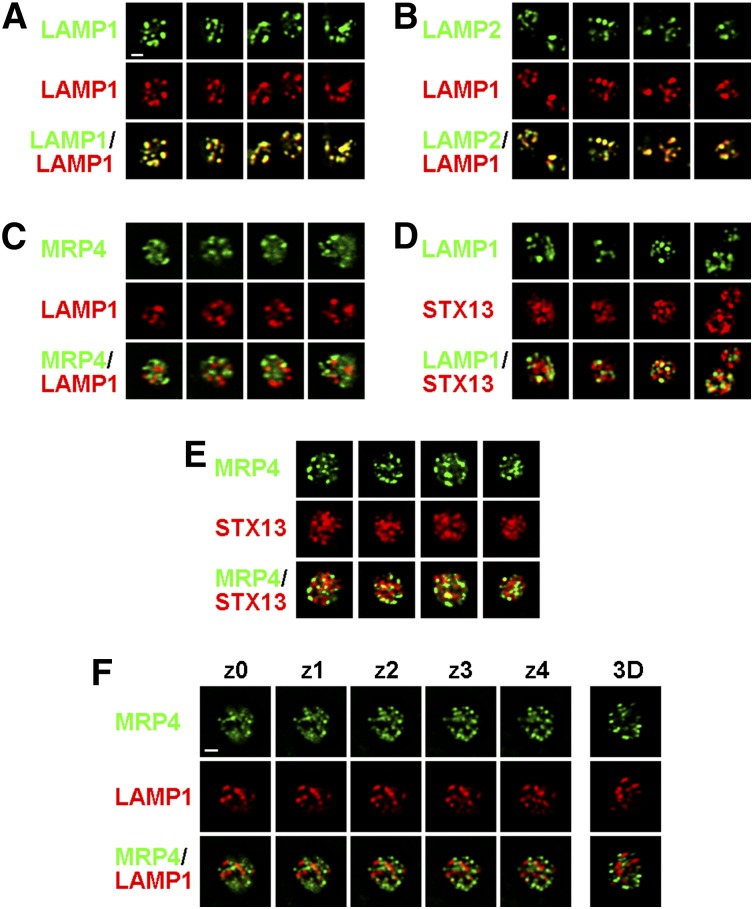
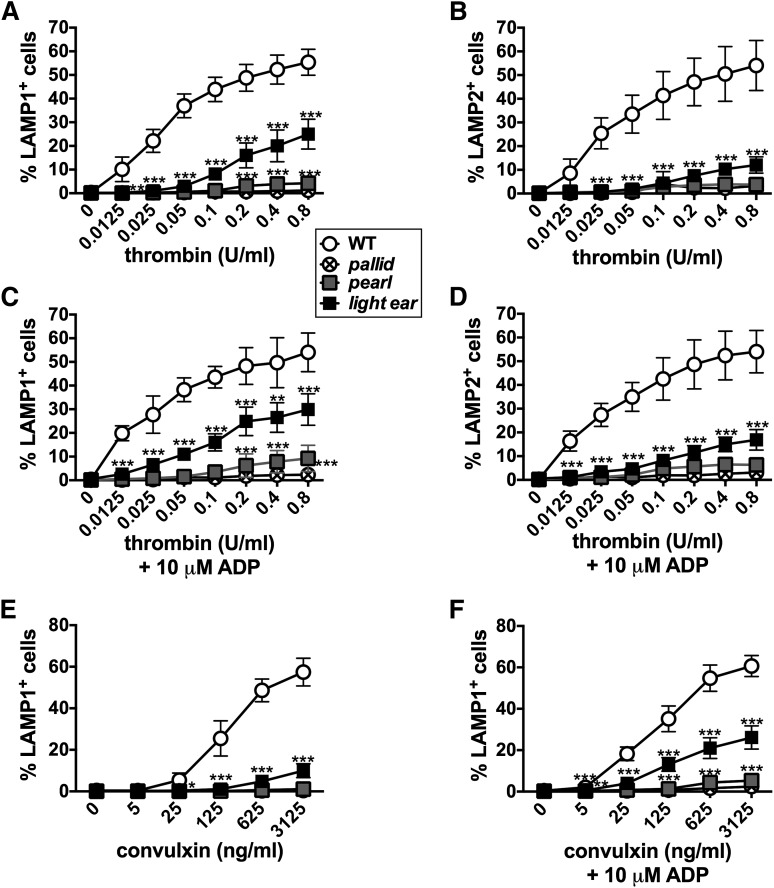
Varied degrees of agonist-dependent lysosomal enzyme release by HPS model platelets have been reported.17,41 To measure lysosome secretion, we assessed agonist-induced surface expression of the lysosomal membrane proteins LAMP1 and LAMP2, which become exposed at the plasma membrane after fusion with lysosomal membranes.56,57 By immunofluorescence microscopy in WT platelets, LAMP1 and LAMP2 colocalized to a few punctate structures that did not overlap with puncta harboring either the dense granule membrane protein multidrug resistance protein 4 (MRP4)58 or the early endosomal SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) syntaxin 13 (STX13) (Figure 4A-D,F). MRP4 and STX13 also did not overlap (Figure 4E). Thus, LAMP1 and LAMP2 predominantly localize to structures that are distinct from dense granules58,59 or early endosomes and are likely lysosomes, as in other cell types. Total cellular levels of LAMP1 and LAMP2 were identical in WT and HPS mice platelets as measured by flow cytometry after permeabilization (supplemental Figure 3). Thrombin treatment of WT platelets led to a dose-dependent increase in both the percentage of cells expressing surface LAMP1 and LAMP2, peaking at ∼50% at the highest dose tested (0.8 U/mL; Figure 5A-B; supplemental Figure 4A), and in the surface levels per cell (supplemental Figure 4C). However, platelets from all 3 HPS models were dramatically hyporesponsive to thrombin. None responded to 0.025 U/mL (compared with 20% to 25% of WT platelets), and pallid and pearl platelets barely responded to the highest dose (Figure 5A-B; supplemental Figure 4B-C). Higher doses of thrombin elicited a stronger but still muted response from light ear platelets. Similar responses were observed with convulxin; barely any pearl or pallid platelets and only 10% ± 3% of light ear platelets expressed LAMP1 at the highest dose compared with 57% ± 7% of WT (Figure 5E). Unlike for CD62p, supplementation with ADP had essentially no effect on the induced surface expression of LAMP1 or LAMP2 on pallid or pearl platelets in response to either agonist, and only modestly heightened the agonist sensitivity of light ear platelets (Figure 5C-D,F; supplemental Figure 4C). These results suggest that agonist-dependent lysosome release is severely impaired in all 3 HPS models (less so in light ear platelets), and that the impairment is largely independent of the defect in dense granule formation.
Figure 4.
LAMP1 and LAMP2 do not localize predominantly to dense granules in platelets. Platelets from WT mice were fixed, permeabilized, and labeled with a rabbit antibody to the LAMP1 cytoplasmic domain together with rat monoclonal antibodies to LAMP1 (A), LAMP2 (B), or multidrug resistance protein 4 (MRP4) (C and F), or with a rabbit antibody to syntaxin 13 (STX13) and rat monoclonal antibodies to LAMP1 (D) or MRP4 (E) and fluorophore-conjugated secondary antibodies. Platelets were then analyzed by deconvolution immunofluorescence microscopy. Shown are 4 single-plane images of 1 or 2 platelets each labeled by each antibody combination. (F) Shown are 5 sequential z planes (separated by 0.2 μm) and a 3-dimensional (3D)-rendered model of a single platelet labeled for LAMP1 and MRP4. Bar represents 1 μm.
Figure 5.
Agonist-induced surface expression of lysosomal membrane proteins is impaired in HPS model platelets. Washed platelets from WT, pallid, pearl, or light ear mice were stimulated as indicated with thrombin (A-D) or convulxin (E and F) for 10 minutes in the absence or presence of 10 μM ADP and then analyzed by flow cytometry for surface LAMP1 or LAMP2. Shown is the percentage of cells with labeling above the background observed on unstimulated cells (see supplemental Figure 4A,B for gating strategy and examples of flow cytometry profiles). Data represent mean ± standard deviation from at least 3 independent experiments. *P < .05; **P < .01; ***P < .005 for HPS models vs WT.
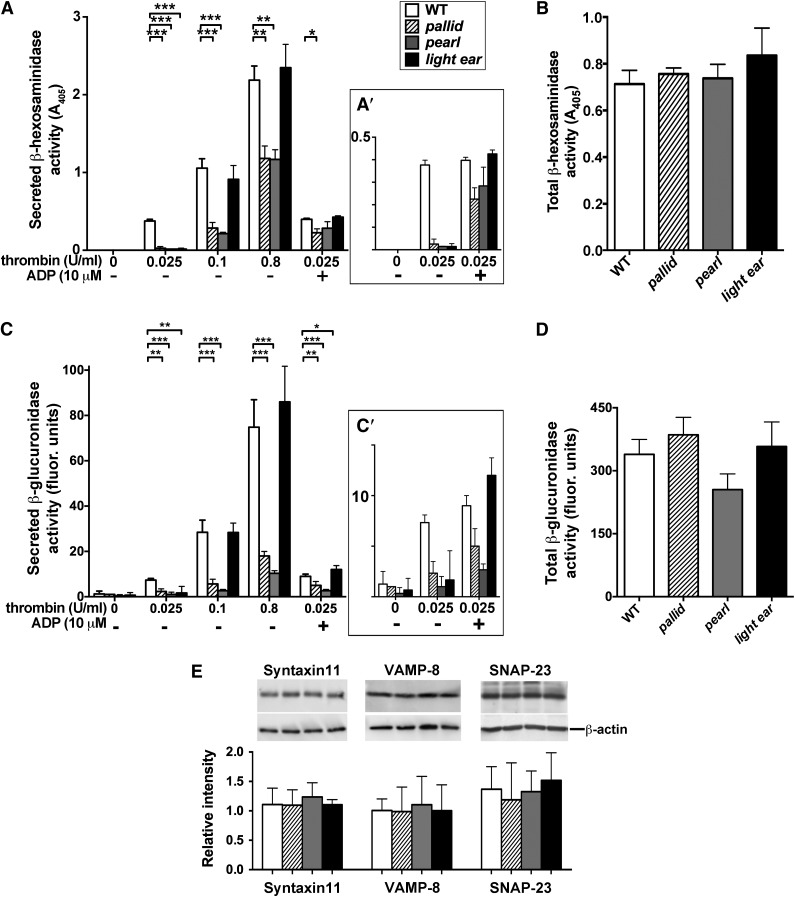
To affirm these results, we assayed thrombin-stimulated platelet releasates for the activity of the lysosomal enzymes β-hexosaminidase and β-glucuronidase. Total β-hexosaminidase activity in whole cell lysates did not differ significantly among WT, pallid, pearl, or light ear platelets, and the activity of β-glucuronidase was reduced by only 28% in pearl platelets (Figure 6B,D), suggesting that lysosomal content is not substantially affected by HPS. However, stimulation with a low dose of thrombin (0.025 U/mL; Figure 6A,C) elicited muted release of both enzymes from HPS model platelets relative to WT. In pearl and pallid platelets, the defect in β-hexosaminidase release was largely mitigated at higher thrombin doses or by addition of ADP at lower doses, but β-glucuronidase secretion was impaired at all doses and only modestly mitigated by excess ADP (Figure 6A,C). Enzyme release from light ear platelets was similar to that of WT at higher thrombin doses, and supplemental ADP restored a WT response to low-dose thrombin. These data support the conclusions that (1) lysosomal secretion is impaired in HPS model platelets, (2) the defect in pearl and pallid is at least partially independent of ADP release and thus of the dense granule biogenesis defect, and (3) AP-3 and BLOC-1 are more essential than BLOC-3 for efficient lysosomal secretion in platelets.
Figure 6.
Impaired lysosomal enzyme release upon thrombin simulation of HPS model platelets. Washed WT, pallid, pearl, or light ear platelets (8 × 107) were stimulated with varying concentrations of thrombin (A and C) for 10 minutes in the absence or presence of 10 μM ADP as indicated. Supernatants were collected and analyzed for activity of the lysosomal enzymes β-hexosaminidase (A) or β-glucuronidase (C) using colorimetric or fluorogenic substrates, respectively. Panels A′,C′ (insets) show values at 0.025 U/mL of thrombin with or without ADP on an expanded scale. Untreated platelets (8 × 107) were lysed in 100 μL of lysis buffer and analyzed directly for β-hexosaminidase (B) or β-glucuronidase (D) activity. Data represent mean corrected A405 (A, A′, and B) or fluorescence (C, C′, and D) values (mean ± standard deviation)for undiluted supernatant, normalized to the highest value in a given experiment, from at least 3 separate experiments. *P < .05; **P < .01; ***P < .005. (E) Lysates from washed WT, pallid, pearl, or light ear platelets were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by immunoblotting for syntaxin-11, VAMP-8, or SNAP-23 (top row), or β-actin (bottom row) as a control. Bands from 3 separate experiments were quantified and plotted as the mean signal ± standard deviation for syntaxin-11, VAMP-8, or SNAP-23 relative to β-actin.
HPS platelets express normal levels of granule fusion proteins
Stimulus-dependent secretion of α granules, dense granules, and lysosomes is mediated by a similar cohort of SNARE fusion proteins—syntaxin 11, VAMP8, and SNAP-2313,32,49,60,61—and defects in SNARE-dependent fusion cause bleeding diathesis as in HPS patients.11 Because BLOC-1 and AP-3 regulate SNARE distribution in neurons,62-64 we tested whether these SNARE components were depleted from HPS model platelets. However, levels of each SNARE component were not significantly different in lysates from WT, pearl, pallid, or light ear platelets (Figure 6E). Although these data do not rule out missorting of SNAREs within HPS model platelets and are confounded by the overabundance of α granules relative to lysosomes and dense granules in normal platelets, they indicate that changes in total SNARE content are not responsible for the granule secretion defects.
Discussion
Platelets from HPS patients and mouse models are well documented to have malformed and largely nonfunctional dense granules.17,18,54,65,66 Here, we show that in mouse models of 3 HPS isoforms (types 2, 9, and 4), reflecting defects in AP-3, BLOC-1, and BLOC-3, stimulus-dependent release of α granules and lysosomes is also impaired. Our findings document roles for AP-3 and BLOCs in the secretion of multiple platelet granules and impact our understanding of the basis for hemostatic and vascular disruption in HPS patients.
HPS is primarily a disorder of LRO biogenesis.15,29,67 Although α granules are considered LROs,29,30,68 α granule biogenesis appears to be unaffected in HPS.38,66 Accordingly, our data show that total cellular levels of P-selectin and the α granule contents PF-4 and PBP are normal in platelets from 3 HPS models. Moreover, the impairment of α granule secretion at low-agonist doses in all 3 HPS models (more dramatic in platelets that lack AP-3- or BLOC-1 rather than BLOC-3) is normalized by high agonist doses or by supplemental ADP. Similar observations were made in platelets from an uncharacterized HPS patient,41 but to our knowledge, no other study has analyzed multiple HPS isoforms. We conclude that α granule biogenesis and the signaling pathways required for α granule secretion are largely intact in all 3 HPS models, and that the impaired α granule secretion is secondary to the absence of released ADP (with consequent failure to activate platelet P2Y receptors, consistent with effects of P2Y12 agonists and antagonists on α granule secretion69) and/or other dense granule contents. The hyporesponsiveness likely reflects an increased threshold for agonist signaling, as indicated by reductions in both the percentage of secreting cells and the number of granules secreted per cell.
Our intravital analyses in the laser injury model show that α granule secretion in response to low-dose agonist is impaired during a platelet response in vivo. As previously observed in the ruby-eye model of HPS type 6,60 which lacks a distinct protein complex (BLOC-2),70-72 thrombus area in pearl, pallid, and light ear mice was dramatically reduced at the injury site, primarily reflecting loss of the platelet shell. Because all known HPS isoforms lack AP-3, BLOC-1, BLOC-2, or BLOC-3, our results thus suggest that bleeding diathesis in HPS results largely from defective platelet accumulation and consequent thrombus instability. The reduced shell size likely reflects the loss of ADP release and mirrors the effects of P2Y12 receptor signaling antagonists in WT mice.51 Importantly, the CD62p+ core within the thrombus also tended to be small in pearl, pallid, and light ear mice, resulting from a premature slowing in the accumulation of CD62p+ (α granule–secreting) platelets. This premature tapering likely reflects the decreased sensitivity of α granule secretion to thrombin that we observed ex vivo. Thrombin activity in this model extends as a gradient from the site of vascular injury73-76 and is necessary for full platelet activation and α granule secretion.51,77 Our results indicate that ADP signaling reinforces platelet activation at the edge of this gradient where thrombin activity starts to decline. The apparent discrepancy of our results with the failure of a P2Y12 antagonist to block accumulation of α granule–secreting platelets in this model system51 likely reflects the reversible nature and short half-life of the antagonist, effects of ADP signaling via the P2Y1 receptor, and/or contributions of additional components released from dense granules to platelet activation in vivo. We speculate that limited agonist exposure at sites of more modest physiological injury might alter the balanced release of factors from α granules that stimulate or antagonize inflammation and/or angiogenesis, and could potentially account for unexplained HPS symptoms such as granulomatous colitis.16
Our data extend published observations that lysosomal hydrolase release from activated platelets is impaired in pallid and pearl mice17,39 and in storage pool deficiency patients78,79 after strong thrombin stimulation, and indicate that secretion from light ear platelets is also hyposensitive to low-dose agonist stimulation. In pallid and pearl platelets, the impairment of thrombin-stimulated β-glucuronidase release was similar to that of LAMP1 and LAMP2 surface expression and was more severe and less responsive to additional ADP than β-hexosaminidase release. LAMP1 and LAMP2 localized in WT platelets to structures distinct from MRP4-labeled dense granules, excluding the possibility that they largely derive from residual immature dense granule membranes in HPS models54 as previously suggested.59,80 The reason for the discrepant response of β-hexosaminidase is not clear, but we speculate that it reflects localization to a distinct class of lysosomes or perhaps to α granules. Alternatively, LAMP1, LAMP2, and β-glucuronidase might be partially mislocalized in HPS model platelets to structures other than lysosomes, consistent with the surface redistribution of LAMP1 in other AP-3- and BLOC-1-deficient cell types.43,64,81 Unlike α granule secretion, the impaired agonist response of LAMP1, LAMP2, and β-glucuronidase mobilization in all 3 HPS models was only modestly mitigated at higher agonist doses and could not be fully corrected by the addition of excess ADP in pearl and pallid platelets. This finding indicates that the defect in lysosome secretion in these HPS models is at least partly intrinsic to lysosomes and not secondary to impaired dense granule release. The restoration of normal lysosomal enzyme release from BLOC-3-deficient light ear platelets by excess ADP is consistent with findings from HPS patients,41,82 most of whom have HPS type 1 and therefore also lack BLOC-3,15 and suggests that BLOC-3 plays a minor role in cargo delivery to lysosomes. Notably, the impaired surface expression of LAMP1, LAMP2, or CD62p in response to low-dose thrombin stimulation observed here can potentially be incorporated into a diagnostic regimen for HPS in patients with bleeding diathesis.
What is the molecular basis for impaired lysosomal enzyme release from HPS platelets? Given that BLOC-1, BLOC-3, and AP-3 impact integral membrane protein sorting and delivery to LROs in melanocytes,15,29 we propose that this defect results from missorting or impaired activation of fusion proteins within precursor megakaryocytes. Lysosomes, dense granules, and α granules share a cohort of SNAREs and associated proteins that facilitate activation-induced fusion with the plasma membrane.11-13,32,49 Although HPS model platelets had normal levels of these proteins and we were unable to detect consistent alterations in SNAP-23 phosphorylation,83 it is likely that 1 of these components is mistargeted or fails to become activated in HPS megakaryocytes or platelets. High-resolution imaging relative to markers of lysosomes and dense granules will be necessary to effectively test this hypothesis.
Acknowledgments
The authors thank Sidney Whiteheart, Andrew Peden, Donald Siegel, Yair Argon, Margaret Chou, Santiago Di Pitro, Adriana Mantegazza, and Sang Min for helpful advice, reagents, and discussions; and Li Zhai for technical help.
This work was supported by National Institutes of Health National Eye Institute grant R01 EY015625 (M.S.M.), National Heart, Lung, and Blood Institute grants R01 HL121323 (M.S.M.), R01 HL119070 (T.J.S. and L.F.B), R01 HL040387 (T.J.S., L.F.B., and C.S.A.), P01 HL110860 (M.P.), and R01 HL120846 (C.S.A.), and National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK090969 (M.P.); and American Heart Association Research Grant 11SDG5720011 (T.J.S.) and Postdoctoral Fellowship 10POST3870044 (R.M.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
R.M. participated in all aspects of the study and contributed to project conception and experimental design, performed most of the experiments, analyzed and formatted most of the data, prepared most of the figures, and drafted and edited the manuscript; J.W., D.C.H., Y.W., and M.A.K. designed and performed some of the experiments and prepared some of the figures; T.J.S., C.S.A., L.F.B., and M.P. conceived of parts of the project, contributed to experimental design and data analysis, oversaw experimental work, and edited the manuscript; and M.S.M. conceived of and oversaw the project, contributed to experimental design, oversaw experimental work, performed data analysis, prepared and edited the figures, and cowrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael S. Marks, Department of Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, 1107B Abramson Research Center, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: marksm@mail.med.upenn.edu.
References
- 1.Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Curr Opin Hematol. 2013;20(5):464–471. doi: 10.1097/MOH.0b013e3283632e6b. [DOI] [PubMed] [Google Scholar]
- 2.Ren Q, Ye S, Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Curr Opin Hematol. 2008;15(5):537–541. doi: 10.1097/MOH.0b013e328309ec74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12(5):261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 4.McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res. 1999;95(1):1–18. doi: 10.1016/s0049-3848(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 5.Masliah-Planchon J, Darnige L, Bellucci S. Molecular determinants of platelet delta storage pool deficiencies: an update. Br J Haematol. 2013;160(1):5–11. doi: 10.1111/bjh.12064. [DOI] [PubMed] [Google Scholar]
- 6.Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312(5770):104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 7.Blair P, Flaumenhaft R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiano JEJ, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 10.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120(26):5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH, Whiteheart SW. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120(12):2493–2500. doi: 10.1182/blood-2012-05-430629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Q, Wimmer C, Chicka MC, et al. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116(6):869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH, Whiteheart SW. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120(12):2484–2492. doi: 10.1182/blood-2012-05-430603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nurden A, Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J Thromb Haemost. 2011;9(Suppl 1):76–91. doi: 10.1111/j.1538-7836.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 15.Wei A-H, Li W. Hermansky-Pudlak syndrome: pigmentary and non-pigmentary defects and their pathogenesis. Pigment Cell Melanoma Res. 2013;26(2):176–192. doi: 10.1111/pcmr.12051. [DOI] [PubMed] [Google Scholar]
- 16.Seward SL, Jr, Gahl WA. Hermansky-Pudlak syndrome: health care throughout life. Pediatrics. 2013;132(1):153–160. doi: 10.1542/peds.2012-4003. [DOI] [PubMed] [Google Scholar]
- 17.Novak EK, Hui SW, Swank RT. Platelet storage pool deficiency in mouse pigment mutations associated with seven distinct genetic loci. Blood. 1984;63(3):536–544. [PubMed] [Google Scholar]
- 18.Witkop CJ, Krumwiede M, Sedano H, White JG. Reliability of absent platelet dense bodies as a diagnostic criterion for Hermansky-Pudlak syndrome. Am J Hematol. 1987;26(4):305–311. doi: 10.1002/ajh.2830260403. [DOI] [PubMed] [Google Scholar]
- 19.Gerritsen SM, Akkerman JW, Nijmeijer B, Sixma JJ, Witkop CJ, White J. The Hermansky-Pudlak syndrome. Evidence for a lowered 5-hydroxytryptamine content in platelets of heterozygotes. Scand J Haematol. 1977;18(3):249–256. [PubMed] [Google Scholar]
- 20.White JG. Serotonin storage organelles in human megakaryocytes. Am J Pathol. 1971;63(3):403–410. [PMC free article] [PubMed] [Google Scholar]
- 21.Rao GH, White JG. An improved method for measuring endogenous serotonin in platelets of patients with Hermansky-Pudlak syndrome. Thromb Res. 1988;51(2):225–227. doi: 10.1016/0049-3848(88)90066-7. [DOI] [PubMed] [Google Scholar]
- 22.White JG, Witkop CJ. Effects of normal and aspirin platelets on defective secondary aggregation in the Hermansky-Pudlak syndrome. A test for storage pool deficient platelets. Am J Pathol. 1972;68(1):57–66. [PMC free article] [PubMed] [Google Scholar]
- 23.Di Pietro SM, Dell’Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6(7):525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 24.Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19(1):19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 25.Sitaram A, Marks MS. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology (Bethesda) 2012;27(2):85–99. doi: 10.1152/physiol.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dell’Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol. 2009;21(4):552–559. doi: 10.1016/j.ceb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Gerondopoulos A, Langemeyer L, Liang J-R, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22(22):2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120(19):4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks MS, Heijnen HFG, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25(4):495–505. doi: 10.1016/j.ceb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91(7):2313–2325. [PubMed] [Google Scholar]
- 31.Youssefian T, Cramer EM. Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood. 2000;95(12):4004–4007. [PubMed] [Google Scholar]
- 32.Ren Q, Barber HK, Crawford GL, et al. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell. 2007;18(1):24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urban D, Li L, Christensen H, et al. The VPS33B-binding protein VPS16B is required in megakaryocyte and platelet α-granule biogenesis. Blood. 2012;120(25):5032–5040. doi: 10.1182/blood-2012-05-431205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo B, Li L, Gissen P, et al. Requirement of VPS33B, a member of the Sec1/Munc18 protein family, in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2005;106(13):4159–4166. doi: 10.1182/blood-2005-04-1356. [DOI] [PubMed] [Google Scholar]
- 35.Albers CA, Cvejic A, Favier R, et al. Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nat Genet. 2011;43(8):735–737. doi: 10.1038/ng.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, et al. NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet α-granules. Nat Genet. 2011;43(8):732–734. doi: 10.1038/ng.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahr WH, Hinckley J, Li L, et al. Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nat Genet. 2011;43(8):738–740. doi: 10.1038/ng.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huizing M, Parkes JM, Helip-Wooley A, White JG, Gahl WA. Platelet alpha granules in BLOC-2 and BLOC-3 subtypes of Hermansky-Pudlak syndrome. Platelets. 2007;18(2):150–157. doi: 10.1080/13576500600936039. [DOI] [PubMed] [Google Scholar]
- 39.Feng L, Novak EK, Hartnell LM, Bonifacino JS, Collinson LM, Swank RT. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS2 genes independently contribute to the production and function of platelet dense granules, melanosomes, and lysosomes. Blood. 2002;99(5):1651–1658. [PubMed] [Google Scholar]
- 40.Holada K, Glierova H, Simak J, Vostal JG. Expression of cellular prion protein on platelets from patients with gray platelet or Hermansky-Pudlak syndrome and the protein’s association with alpha-granules. Haematologica. 2006;91(8):1126–1129. [PubMed] [Google Scholar]
- 41.Rendu F, Maclouf J, Launay JM, et al. Hermansky-Pudlak platelets: further studies on release reaction and protein phosphorylations. Am J Hematol. 1987;25(2):165–174. doi: 10.1002/ajh.2830250206. [DOI] [PubMed] [Google Scholar]
- 42.Feng L, Seymour AB, Jiang S, et al. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum Mol Genet. 1999;8(2):323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 43.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell. 1999;3(1):11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 44.Cullinane AR, Curry JA, Carmona-Rivera C, et al. A BLOC-1 mutation screen reveals that PLDN is mutated in Hermansky-Pudlak Syndrome type 9. Am J Hum Genet. 2011;88(6):778–787. doi: 10.1016/j.ajhg.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Falcón-Pérez JM, Starcevic M, Gautam R, Dell’Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem. 2002;277(31):28191–28199. doi: 10.1074/jbc.M204011200. [DOI] [PubMed] [Google Scholar]
- 46.Martina JA, Moriyama K, Bonifacino JS. BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. J Biol Chem. 2003;278(31):29376–29384. doi: 10.1074/jbc.M301294200. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T, Li W, Zhang Q, et al. Hermansky-Pudlak syndrome is caused by mutations in HPS4, the human homolog of the mouse light-ear gene. Nat Genet. 2002;30(3):321–324. doi: 10.1038/ng835. [DOI] [PubMed] [Google Scholar]
- 48.Pang L, Xue H-H, Szalai G, et al. Maturation stage-specific regulation of megakaryopoiesis by pointed-domain Ets proteins. Blood. 2006;108(7):2198–2206. doi: 10.1182/blood-2006-04-019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen D, Lemons PP, Schraw T, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000;96(5):1782–1788. [PubMed] [Google Scholar]
- 50.Brandt EJ, Elliott RW, Swank RT. Defective lysosomal enzyme secretion in kidneys of Chediak-Higashi (beige) mice. J Cell Biol. 1975;67(3):774–788. doi: 10.1083/jcb.67.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet signaling network. Blood. 2013;121(10):1875-1885. [DOI] [PMC free article] [PubMed]
- 52.Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddington M, Novak EK, Hurley E, Medda C, McGarry MP, Swank RT. Immature dense granules in platelets from mice with platelet storage pool disease. Blood. 1987;69(5):1300–1306. [PubMed] [Google Scholar]
- 55.Cunningham MR, Nisar SP, Mundell SJ. Molecular mechanisms of platelet P2Y(12) receptor regulation. Biochem Soc Trans. 2013;41(1):225–230. doi: 10.1042/BST20120295. [DOI] [PubMed] [Google Scholar]
- 56.Febbraio M, Silverstein RL. Identification and characterization of LAMP-1 as an activation-dependent platelet surface glycoprotein. J Biol Chem. 1990;265(30):18531–18537. [PubMed] [Google Scholar]
- 57.Silverstein RL, Febbraio M. Identification of lysosome-associated membrane protein-2 as an activation-dependent platelet surface glycoprotein. Blood. 1992;80(6):1470–1475. [PubMed] [Google Scholar]
- 58.Jedlitschky G, Tirschmann K, Lubenow LE, et al. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 2004;104(12):3603–3610. doi: 10.1182/blood-2003-12-4330. [DOI] [PubMed] [Google Scholar]
- 59.Niessen J, Jedlitschky G, Grube M, et al. Subfractionation and purification of intracellular granule-structures of human platelets: an improved method based on magnetic sorting. J Immunol Methods. 2007;328(1-2):89–96. doi: 10.1016/j.jim.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Graham GJ, Ren Q, Dilks JR, Blair P, Whiteheart SW, Flaumenhaft R. Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114(5):1083–1090. doi: 10.1182/blood-2009-03-210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95(3):921–929. [PubMed] [Google Scholar]
- 62.Newell-Litwa K, Chintala S, Jenkins S, et al. Hermansky-Pudlak protein complexes, AP-3 and BLOC-1, differentially regulate presynaptic composition in the striatum and hippocampus. J Neurosci. 2010;30(3):820–831. doi: 10.1523/JNEUROSCI.3400-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell. 2009;20(5):1441–1453. doi: 10.1091/mbc.E08-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salazar G, Craige B, Styers ML, et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell. 2006;17(9):4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hermansky F, Pudlak P. Albinism associated with hemorrhagic diathesis and unusual pigmented reticular cells in the bone marrow: report of two cases with histochemical studies. Blood. 1959;14(2):162–169. [PubMed] [Google Scholar]
- 66.White JG, Edson JR, Desnick SJ, Witkop CJ., Jr Studies of platelets in a variant of the Hermansky-Pudlak syndrome. Am J Pathol. 1971;63(2):319–332. [PMC free article] [PubMed] [Google Scholar]
- 67.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14(10):1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- 69.Quinton TM, Murugappan S, Kim S, Jin J, Kunapuli SP. Different G protein-coupled signaling pathways are involved in alpha granule release from human platelets. J Thromb Haemost. 2004;2(6):978–984. doi: 10.1111/j.1538-7836.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Q, Zhao B, Li W, et al. Ru2 and Ru encode mouse orthologs of the genes mutated in human Hermansky-Pudlak syndrome types 5 and 6. Nat Genet. 2003;33(2):145–153. doi: 10.1038/ng1087. [DOI] [PubMed] [Google Scholar]
- 71.Di Pietro SM, Falcón-Pérez JM, Dell’Angelica EC. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic. 2004;5(4):276–283. doi: 10.1111/j.1600-0854.2004.0171.x. [DOI] [PubMed] [Google Scholar]
- 72.Gautam R, Chintala S, Li W, et al. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2). J Biol Chem. 2004;279(13):12935–12942. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- 73.Welsh JD, Colace TV, Muthard RW, Stalker TJ, Brass LF, Diamond SL. Platelet-targeting sensor reveals thrombin gradients within blood clots forming in microfluidic assays and in mouse. J Thromb Haemost. 2012;10(11):2344–2353. doi: 10.1111/j.1538-7836.2012.04928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stalker TJ, Welsh JD, Tomaiuolo M, et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124(11):1824–1831. doi: 10.1182/blood-2014-01-550319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomaiuolo M, Stalker TJ, Welsh JD, Diamond SL, Sinno T, Brass LF. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood. 2014;124(11):1816–1823. doi: 10.1182/blood-2014-01-550343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welsh JD, Stalker TJ, Voronov R, et al. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124(11):1808–1815. doi: 10.1182/blood-2014-01-550335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci USA. 2007;104(1):288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiss HJ, Rogers J. Thrombocytopathia due to abnormalities in platelet release reaction—studies on six unrelated patients. Blood. 1972;39(2):187–196. [PubMed] [Google Scholar]
- 79.Holmsen H, Setkowsky CA, Lages B, Day HJ, Weiss HJ, Scrutton MC. Content and thrombin-induced release of acid hydrolases in gel-filtered platelets from patients with storage pool disease. Blood. 1975;46(1):131–142. [PubMed] [Google Scholar]
- 80.Israels SJ, McMillan EM, Robertson C, Singhory S, McNicol A. The lysosomal granule membrane protein, LAMP-2, is also present in platelet dense granule membranes. Thromb Haemost. 1996;75(4):623–629. [PubMed] [Google Scholar]
- 81.Setty SRG, Tenza D, Truschel ST, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18(3):768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lages B, Dangelmaier CA, Holmsen H, Weiss HJ. Specific correction of impaired acid hydrolase secretion in storage pool-deficient platelets by adenosine diphosphate. J Clin Invest. 1988;81(6):1865–1872. doi: 10.1172/JCI113532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karim ZA, Zhang J, Banerjee M, et al. IκB kinase phosphorylation of SNAP-23 controls platelet secretion. Blood. 2013;121(22):4567–4574. doi: 10.1182/blood-2012-11-470468. [DOI] [PMC free article] [PubMed] [Google Scholar]