Abstract
A xanthine amine congener (XAC), an amine-functionalized derivative of 1,3-dipropyl-8-phenylxanthine, is an antagonist ligand for A2 adenosine receptors of human platelets. XAC inhibited 5′-N-ethylcarboxamidoadenosine (NECA)-induced stimulation of adenylate cyclase activity with a KB of 24 nM. [3H]XAC exhibits saturable, specific binding with a Kd of 12 nM and a Bmax of 1.1 pmol/mg protein at 37°C. [3H]XAC binding in platelets is the first example of labeling of A2 adenosine receptors in which the potencies of adenosine agonists and antagonists in inhibiting binding are commensurate with their potencies at these receptors in functional studies. Furthermore, [3H]XAC is the first antagonist radioligand with high affinity at A2 adenosine receptors.
Keywords: Adenosine receptor, Platelet, Xanthine
1. INTRODUCTION
A variety of radioligands have been developed for the A1 adenosine receptors that mediate inhibition of adenylate cyclase: such ligands have been extensively used in investigation of A1 receptors (reviews [1,2]). Radioligands for the A2 adenosine receptors that mediate stimulation of adenylate cyclase have not proven entirely satisfactory. In striatal membranes, 5′-N-[3H]ethylcarboxamido-adenosine ([3H]NECA) appears to bind to both A1 and A2 adenosine receptors, and strategies for elimination of A1 receptor binding have been developed [3]. In peripheral tissues, binding studies with [3H]NECA have not been proven satisfactory, particularly with respect to structure-activity profiles for adenosine antagonism by adenosine analogs and xanthines [2].
1,3-[3H]Diethyl-8-phenylxanthine ([3H]DPX) has been the only antagonist radioligand for the study of adenosine receptors [4]. Based on a binding study with guinea pig brain, the suggestion has been made that [3H]DPX may label A2 adenosine receptors [4]. This appears unlikely, since N6-substituted adenosine analogs were very weak and compounds without intrinsic activity at adenosine receptors were relatively potent in competing for [3H]DPX binding [4]. Thus, a direct characterization of A2 adenosine receptors with an antagonist radioligand has still not been achieved, and even for A1 receptors [3H]DPX is not entirely satisfactory.
A ‘functionalized congener’ approach to adenosine receptor ligands has been developed [5,6]. In this approach 1,3-dipropyl-8-phenylxanthine was modified to provide a p-carboxy-methyloxy function for covalent attachment to amines, amino acids and oligopeptides [5,6]. One potent analog, a 1,3-dipropylxanthine amine congener (XAC), has been tritiated to high specific activity (103 Ci/mmol). [3H]XAC binds with Kd values of 0.17 and 1.2 nM to A1 adenosine receptors of calf and rat brain, respectively [7]. It represents the most satisfactory antagonist radioligand for the study of A1 adenosine receptors. Here, [3H]XAC was used as a radioligand for the A2 adenosine receptors in human platelets.
2. MATERIALS AND METHODS
Human platelet membranes were prepared as in [8]. Adenylate cyclase was assayed essentially as described [8]. Briefly stated, the medium contained 0.1 mM [α-32P]ATP (0.3 μCi/tube), 1 μM GTP, 1 mM MgCl2, 0.1 mM cyclic AMP, 1 μg/ml adenosine deaminase, 0.1 mM rolipram (4-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidinone, ZK 62,711), 1 mM EGTA, 5 mM creatine phosphate as the Tris salt, 0.4 μg/ml creatine kinase, 2 mg/ml bovine serum albumin and 50 mM Tris-HCl, pH 7.4, in a total volume of 100 μl. Incubations were initiated by the addition of 5–15 μg membrane protein and were conducted for 10 min at 37°C. Reactions were stopped by the addition of 0.4 ml of 125 mM zinc acetate and 0.5 ml of 144 mM Na2CO3. Cyclic AMP was purified as described in [8].
The binding of [3H]XAC to human platelet membranes was measured in a total volume of 1 ml containing 50 mM Tris-HCl, pH 7.4, 0.1 μg/ml adenosine deaminase and approx. 100–200 μg membrane protein. In competition experiments the radioligand was present in a final concentration of 1 nM. Other substances were added as indicated. Incubation was carried out at 37°C for 90 min. All assays were done in triplicate. Bound and free radioligand were separated by filtering the assay volume through Whatman GF/B glass-fiber filters that had been treated with 0.3% polyethylene imine for 90 min as described by Bruns et al. [9]. The filters were washed twice with 5 ml ice-cold incubation buffer. Nonspecific binding of [3H]XAC was determined in the presence of 5 mM theophylline.
Synthesis and purification of 3H-labeled 8-{4-[([{(2 - aminoethyl)amino}carbonyl]methyl)oxy] - phenyl}-1,3-dipropylxanthine ([3H]XAC) with a specific activity of 103 Ci/mmol are described elsewhere [7]. Other compounds were synthesized as in [5–7]. Materials and chemicals were from standard sources as described [5,7].
EC50 and IC50 values were obtained from concentration-response curves by linear regression after logit-log transformation. IC50 values were transformed into Ki values according to Cheng and Prusoff [10]. Slope factors of inhibition curves (nH) were calculated from indirect Hill plots.
3. RESULTS
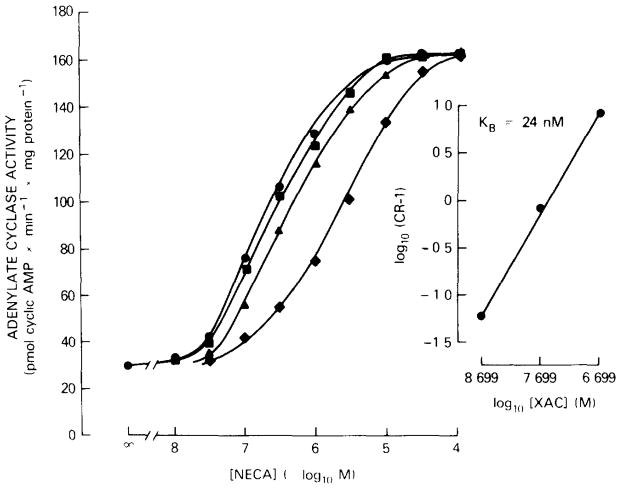
The potency of XAC as an antagonist at A2 adenosine receptors of human platelets was determined vs the NECA-stimulated adenylate cyclase activity in membranes. In human platelets, NECA has been shown to be a potent agonist at A2 receptors that mediate inhibition of aggregation via an activation of adenylate cyclase [8,11]. NECA stimulates adenylate cyclase activity about 4-fold over basal values with an EC50 of 0.255 μM (fig. 1). XAC shifted the concentration-response curve of NECA in a concentration-dependent manner to the right without changing the slope or the degree of stimulation by NECA (fig. 1) suggesting competitive antagonism. The dissociation constant, KB, for XAC, calculated from the Schild plot, was 24 nM as shown in the right panel of fig. 1. The slope of the line near unity confirms the competitive antagonism between NECA and XAC.
Fig. 1.
Stimulation of adenylate cyclase activity of human platelet membranes by NECA in the absence (
 ) and presence of XAC (2 nM,
) and presence of XAC (2 nM,
 ; 20 nM,
; 20 nM,
 ; 200 nM,
; 200 nM,
 ). Adenylate cyclase activity was determined at 37°C for 90 min. Right: Schild plot of the same data with the concentration ratio (CR) of the EC50 values for NECA in the presence and absence of XAC vs the XAC concentration (slope, 1.05; r = 0.9993). EC50 values for NECA: 0.255 μM in the absence and 0.27, 0.46 and 2.29 μM in the presence of 2, 20 and 200 nM XAC, respectively. Values are means of a typical experiment done in triplicate.
). Adenylate cyclase activity was determined at 37°C for 90 min. Right: Schild plot of the same data with the concentration ratio (CR) of the EC50 values for NECA in the presence and absence of XAC vs the XAC concentration (slope, 1.05; r = 0.9993). EC50 values for NECA: 0.255 μM in the absence and 0.27, 0.46 and 2.29 μM in the presence of 2, 20 and 200 nM XAC, respectively. Values are means of a typical experiment done in triplicate.
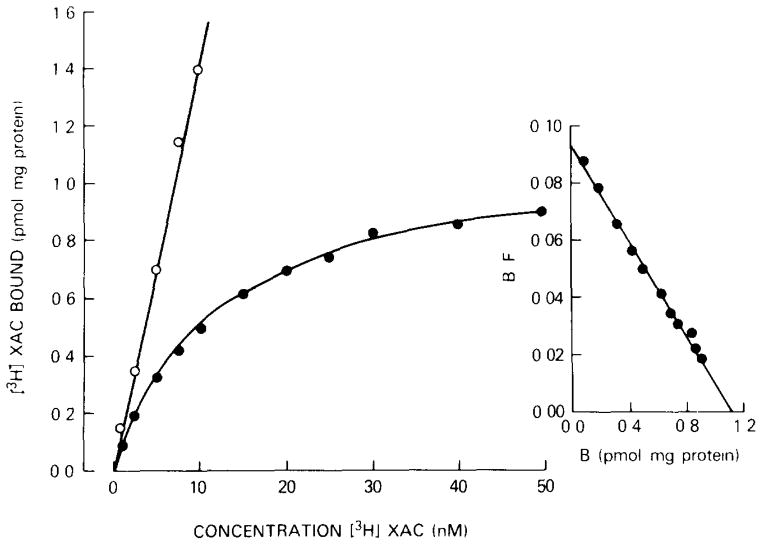
Radioligand binding experiments with tritiated XAC were performed at 37°C. A typical saturation experiment is shown in fig. 2. Specific [3H]XAC binding is saturable with a binding capacity (Bmax) of 1.1 pmol/mg protein. The Scatchard plot of the data is linear, indicating a homogeneous population of noncooperative binding sites with a Kd of 12 nM. Nonspecific binding determined in the presence of 5 mM theophylline was very high and amounted to about 75% of total binding at Kd. The same amount of nonspecific binding was obtained in the presence of 1 mM NECA. At 4°C, similar Kd and Bmax values were obtained, and nonspecific binding amounted to 60% of total binding at Kd (not shown).
Fig. 2.
Saturation of [3H]XAC binding to human platelet membranes. Specific (
 ) and nonspecific (
) and nonspecific (
 ) binding were determined for 90 min at 37°C. Values are means of a typical experiment done in triplicate. Right: Scatchard plot of the same data. Kd, 12 nM; Bmax, 1.1 pmol/mg protein.
) binding were determined for 90 min at 37°C. Values are means of a typical experiment done in triplicate. Right: Scatchard plot of the same data. Kd, 12 nM; Bmax, 1.1 pmol/mg protein.
Other known agonists and antagonists at adenosine receptors competitively displaced specific [3H]XAC binding to platelet membranes (fig. 3, table 1). The competition curves are monophasic with slope factors near unity. XAC itself was the most potent inhibitor of [3H]XAC binding with a Ki value of 17 nM. This value is in good agreement with the Kd values derived from the saturation experiments with [3H]XAC. DPX was about 10-fold less potent than XAC, but about 5-fold more potent than 1,3-dipropyl-8-phenyl-xanthine. The carboxylic acid congener (XCC) and the p-sulfo derivative of the latter compound were as potent as 8-phenyltheophylline in competing for [3H]XAC-binding sites. Theophylline and caffeine have Ki values of 29 and 51 μM, respectively, whereas ATP, adenine and inosine were inactive at [3H]XAC-binding sites. The agonist NECA has a Ki of 0.33 μM, which is in good agreement with the EC50 value for the stimulation of adenylate cyclase activity in these membranes. R-PIA was about 20-fold less potent than NECA as an inhibitor of [3H]XAC binding and is 10-fold less potent than NECA in stimulation of adenylate cyclase activity.
Fig. 3.
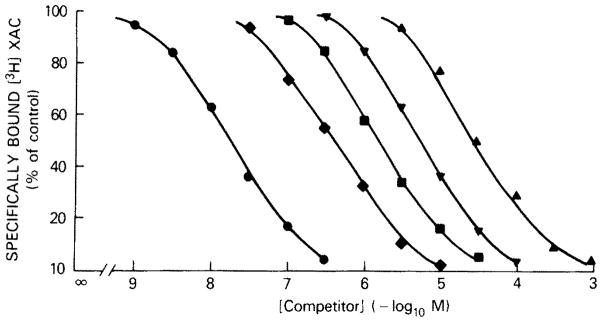
Competition for [3H]XAC binding to human platelet membranes. Binding of 1 nM [3H]XAC was measured for 90 min at 37°C. Slope factors: 0.94 for XAC (
 ), 0.91 for NECA (
), 0.91 for NECA (
 ), 0.97 for 8-phenyltheophylline (
), 0.97 for 8-phenyltheophylline (
 ), 0.99 for R-PIA (
), 0.99 for R-PIA (
 ) and 0.91 for theophylline (
) and 0.91 for theophylline (
 ). Values are means of a typical experiment done in triplicate.
). Values are means of a typical experiment done in triplicate.
Table 1.
Competition for [3H]XAC binding to human platelet membranes
| Compound | Ki (μM) |
|---|---|
| 1,3-Dipropyl-8-(H2N(CH2)2NHCOCH2-O-phenyl)xanthine (XAC) | 0.017 |
| 1,3-Diethyl-8-phenylxanthine (DPX) | 0.19 |
| 5′-N-Ethylcarboxamidoadenosine | 0.33 |
| 1,3-Dipropyl-8-phenylxanthine | 1.0 |
| 8-Phenyltheophylline | 1.7 |
| 1,3-Dipropyl-8-(p-HO2CCH2O-phenyl)-xanthine (XCC) | 1.8 |
| 1,3-Dipropyl-8-(p-sulfophenyl)xanthine | 2.4 |
| N6-R-Phenylisopropyladenosine (R-PIA) | 5.7 |
| 8-(p-Sulfophenyl)theophylline | 7.2 |
| Theophylline | 29 |
| Caffeine | 51 |
| ATP | > 100 (25%) |
| Adenine | > 100 (19%) |
| Inosine | > 100 (1%) |
Binding of 1 nM [3H]XAC was measured for 90 min at 37°C. Ki values are means of 2 experiments done in triplicate. For Ki values above 100 μM, the percentage inhibition of [3H]XAC binding at 100 μM is given in parentheses
4. DISCUSSION
Several attempts have been made to characterize A2 adenosine receptors in radioligand-binding studies. [3H]NECA has been used often as an agonist radioligand for A2 adenosine receptors [12–15]. However, the binding characteristics of [3H]NECA to membranes containing only A2 receptors are not fully consonant with that expected of A2 receptors, since N6-substituted adenosine analogs, which do activate adenylate cyclase in these membranes, are weak or inactive as antagonists of [3H]NECA binding [12–15]. Furthermore, antagonists like DPX and 8-phenyl-theophylline, which are potent inhibitors of NECA-induced cyclic AMP accumulation or NECA-stimulated adenylate cyclase activity, did not compete for [3H]NECA-binding sites [13,15,16]. At least in peripheral tissues, therefore, the results with [3H]NECA have not been satisfactory.
The antagonist radioligand [3H]DPX has been reported to label A2 adenosine receptors in guinea pig brain membranes [4]. Again, several discrepancies in the structure-activity profile were observed. Compounds that are neither agonists nor antagonists at A2 receptors in brain, such as 8-bromoadenosine, competed for [3H]DPX binding, whereas the potent receptor against 5′-N-cyclopropylcarboxamidoadenosine was a very weak displacer of [3H]DPX binding [4]. Therefore, the nature of the [3H]DPX-binding sites in guinea pig brain remains unclear [2,4]. In addition, [3H]DPX has several shortcomings: it has only a low affinity for adenosine receptors in most tissues and a relatively low specific activity.
Recently, we have introduced a tritiated xanthine amine congener ([3H]XAC) as new antagonist radioligand for adenosine receptors [7]. [3H]XAC overcomes many of the limitations of the [3H]DPX radioligand. The Kd value for [3H]XAC binding to A1 receptors in rat brain membranes is 50-fold lower than that of [3H]DPX [7], and the specific activity is 8-fold higher than that of [3H]DPX. In addition to the usefulness of [3H]XAC as a radioligand for A1 receptors, the present study demonstrates that [3H]XAC is also useful for labeling of A2 adenosine receptors. XAC has a KB value of 24 nM for the antagonism of NECA-induced stimulation of adenylate cyclase activity in human platelet membranes. Thus, XAC is the most potent xanthine antagonist at A2 adenosine receptors yet reported.
In platelet membranes, [3H]XAC exhibits saturable, specific binding with a Kd of 12 nM, which is about 2-fold lower than the KB determined in cyclase experiments. The Ki value of XAC for inhibition of [3H]XAC binding is in good agreement with the Kd value from the saturation experiments and the KB value from the cyclase experiments. Several xanthines compete for [3H]XAC binding in the known order of potency. The Ki values of these xanthines are in good agreement with the KB values for antagonism of NECA-induced adenylate cyclase stimulation in the same membrane preparation [16,17]. Only the Ki value for theophylline is 2–3-fold higher than that determined in cyclase and aggregation studies [16,17]. The potencies of the adenosine agonists NECA and R-PIA in inhibiting [3H]XAC binding are similar to the EC50 values for the stimulation of adenylate cyclase activity [8]. In contrast to the results with [3H]NECA binding in platelets [14], therefore, the profile of agonists and antagonists for the inhibition of [3H]XAC binding is commensurate with the data from functional studies like adenylate cyclase and aggregation [8,16,17].
A relative high level of filter binding was evident with [3H]XAC. The problem was solved in part by pretreatment of the glass-fiber filters with polyethylene imine according to Bruns et al. [9]. The filter binding is the main reason for the high nonspecific binding as shown in the saturation experiment (fig. 2). A further reduction in filter binding should permit the use of [3H]XAC for more detailed studies on A2 adenosine receptors in other tissues.
Acknowledgments
One of the authors (D.U.) is on leave from the Pharmakologisches Institut der Universität Heidelberg with support of the Deutsche Forschungsgemeinschaft (Uk 4/1-1).
References
- 1.Daly JW. J Med Chem. 1982;25:197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- 2.Daly JW. In: Adenosine: Receptors and Modulation of Cell Function. Stefanovich V, et al., editors. IRL Press; Washington, DC: 1985. pp. 31–46. [Google Scholar]
- 3.Yeung SMH, Green RD. Naunyn-Schmiedeberg’s Arch Pharmacol. 1984;325:218–225. doi: 10.1007/BF00495947. [DOI] [PubMed] [Google Scholar]
- 4.Bruns RF, Daly JW, Snyder SH. Proc Natl Acad Sci USA. 1980;77:5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson KA, Kirk KL, Padgett WL, Daly JW. J Med Chem. 1985;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KA, Kirk KL, Padgett WL, Daly JW. Mol Pharmacol. 1986 in press. [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson KA, Ukena D, Kirk KL, Daly JW. Proc Natl Acad Sci USA. 1986 doi: 10.1073/pnas.83.11.4089. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ukena D, Boehme E, Schwabe U. Naunyn-Schmiedeberg’s Arch Pharmacol. 1984;327:36–42. doi: 10.1007/BF00504989. [DOI] [PubMed] [Google Scholar]
- 9.Bruns RF, Lawson-Wendling K, Pugsley TA. Anal Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YC, Prusoff WH. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 11.Cusack NJ, Hourani SMO. Br J Pharmacol. 1981;72:443–447. doi: 10.1111/j.1476-5381.1981.tb10995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schütz W, Tuisl E, Kraupp O. Naunyn-Schmiedeberg’s Arch Pharmacol. 1982;319:34–39. doi: 10.1007/BF00491475. [DOI] [PubMed] [Google Scholar]
- 13.Schütz W, Steuerer G, Tuisl E. Eur J Pharmacol. 1982;85:177–184. doi: 10.1016/0014-2999(82)90463-0. [DOI] [PubMed] [Google Scholar]
- 14.Hüttemann E, Ukena D, Lenschov V, Schwabe U. Naunyn-Schmiedeberg’s Arch Pharmacol. 1984;325:226–233. doi: 10.1007/BF00495948. [DOI] [PubMed] [Google Scholar]
- 15.Ukena D, Schirren CG, Klotz KN, Schwabe U. Naunyn-Schmiedeberg’s Arch Pharmacol. 1985;331:310–316. doi: 10.1007/BF00498856. [DOI] [PubMed] [Google Scholar]
- 16.Ukena D, Boehme E, Schwabe U. In: Adenosine: Receptors and Modulation of Cell Function. Stefanovich V, et al., editors. IRL Press; Washington, DC: 1985. pp. 343–349. [Google Scholar]
- 17.Ukena D, Daly JW, Kirk KL, Jacobson KA. Life Sci. 1986;38:797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]