Abstract
Posttranslational protein modification by ubiquitination, a signal for lysosomal or proteasomal proteolysis, can be regulated and reversed by deubiquitinating enzymes (DUBs). This study examined the roles of UCHL1 and UCHL3, two members of ubiquitin C-terminal hydrolase (UCH) family of DUBs, in murine fertilization and preimplantation development. Before fertilization, these proteins were associated with the oocyte cortex (UCHL1) and meiotic spindle (UCHL3). Intracytoplasmic injection of the general UCH-family inhibitor ubiquitin-aldehyde (UBAL) or antibodies against UCHL3 into mature metaphase II oocytes blocked fertilization by reducing sperm penetration of the zona pellucida and incorporation into the ooplasm, suggesting a role for cortical UCHL1 in sperm incorporation. Both UBAL and antibodies against UCHL1 injected at the onset of oocyte maturation (germinal vesicle stage) reduced the fertilizing ability of oocytes. The subfertile Uchl1gad−/− mutant mice showed an intriguing pattern of switched UCH localization, with UCHL3 replacing UCHL1 in the oocyte cortex. While fertilization defects were not observed, the embryos from homozygous Uchl1gad−/− mutant females failed to undergo morula compaction and did not form blastocysts in vivo, indicating a maternal effect related to UCHL1 deficiency. We conclude that the activity of oocyte UCHs contributes to fertilization and embryogenesis by regulating the physiology of the oocyte and blastomere cortex.
Keywords: oocyte, sperm, ubiquitin, proteasome, UCH
INTRODUCTION
Fertilization is a well-orchestrated cascade of events, rather than a single, isolated reaction. Interruption of any step in the sequence will almost certainly cause fertilization failure. Only a fully mature oocyte will be recognized and penetrated by a fertilizing spermatozoon to ensure rapid and synchronous male and female pronuclear development (Plachot and Mandelbaum, 1990)
Numerous cellular processes such as DNA repair, cell cycle regulation, antigen presentation, cell-cell communication, cell differentiation and apoptosis, are regulated by protein ubiquitination. Ubiquitination is a stable posttranslational modification leading to substrate-protein degradation by the 26S proteasome, a multi-subunit protease with three distinct peptidase activities. Alterations in ubiquitination and proteasomal degradation lead to uncontrolled growth, causing tumors (Fuchs, 2002). Deubiquitination reverses this process, and is mediated by deubiquitinating enzymes (DUBs). DUBs are thiol proteases important for regulating different cellular processes (Wilkinson, 2009). Ubiquitin C-terminal hydrolases L1 (UCHL1) and L3 (UCHL3) belong to the family of DUBs responsible for hydrolyzing carboxyl-terminal esters and amides of ubiquitin (Fang et al., 2010). UCHL1 is a 223-amino acid protein. The overall structure of UCHL1 is very similar to that of UCHL3, which has a similar size and shares 51% of sequence identity (Boudreaux et al., 2010).
The UCHs participate in gametogenesis (Gu et al., 2009; Kwon et al., 2005; Susor et al., 2007) and components of the ubiquitin system are involved in early steps of mammalian fertilization, including sperm capacitation (Kong et al., 2009), acrosomal exocytosis (Chakravarty et al., 2008) and zona pellucida (ZP) penetration (Sakai et al., 2004; Sutovsky, 2004). A recent study of porcine fertilization revealed that sperm-acrosomal UCHL3 can participate in sperm-zona pelucida (ZP) interactions and inhibition of polyspermy (Yi et al., 2007b). UCHL1 is one of the most abundant proteins in mammalian oocytes (Ellederova et al., 2004; Massicotte et al., 2006). UCHL1 accumulates in the oocyte cortex, where it may also inhibit polyspermy defense (Yi et al., 2007b). Mutant mice defective in UCHL1 function (the subfertile Uchl1gad−/− mutants) display increased polyspermy after in vitro fertilization (Sekiguchi et al., 2006).
Recent studies revealed a complimentary UCH distribution in porcine, bovine and murine oocytes, with UCHL1 accumulation in the oocyte cortex, and UCHL3 association with oocyte spindle (Susor et al., 2010; Yi et al., 2007b). Based on these observations, we hypothesized that these respective UCHs may regulate sperm-oolemma interactions, completion of second meiosis and sperm incorporation in the cortical ooplasm during murine fertilization. To test the hypothesis, we injected antibodies specific to UCHL1 and UCHL3 and used a variety of UCH-inhibitors to alter their activities and localization during oocyte maturation and fertilization. Supplementing this approach with studies of the Uchl1gad−/− mutant mouse, we found that interference with these UCHs caused a reduction in fertilization rate, abnormal fertilization patterns and failure to undergo morula compaction after fertilization.
MATERIALS AND METHODS
Oocyte collection and in vitro maturation
Germinal vesicle (GV)-stage oocytes were collected from ovaries of B6D2F1 mice at 44-46 h after the females were injected intra-peritoneally (i.p.) with 5 IU Gonadotropin Pregnant Mare Serum (PMSG; Calbiochem, San Diego, CA). GV-intact follicular oocytes were released from the large antral follicles by puncturing with a needle in HEPES-buffered M2 medium supplemented with 0.1 mM of 3-isobutyl-1-methyl-xanthine (IBMX; Sigma-Aldrich, St. Louis, MO). All cultures were maintained in MEM-α medium supplemented with 10% FBS (Life Technologies Carlsbad, CA) at 37°C in a humidified atmosphere of 5% CO2 for 16h.
Metaphase II oocyte and embryo collection from wild type and Uchl1gad mice
Mice were superovulated by i.p. injection of 5 IU PMSG followed 46-48 h later by 5 IU human chorionic gonadotropin (hCG; Sigma-Aldrich). Oocytes were collected 13-14 h post hCG. The Uchl1gad+/+ (wild type) or Uchl1gad−/− homozygous mutant females were placed with B6D2F1 males. One cell embryos were collected 23 h post hCG. Embryos were placed in a sterile culture dish containing 200 μl of HEPES-Buffered M2 medium. Cumulus cells were partially removed by treatment in HEPES-buffered M2 medium containing 120 U/ml hyaluronidase (ICN Pharmaceuticals, Costa Mesa, CA; 500 U/mg). Nuclear status of zygotes was observed with the help of DAPI staining (Vector Labs). Blastocysts were collected at 108 h post-hCG.
In vitro fertilization
Spermatozoa were released from the caudae epididymis of B6D2F1 male mice into fertilization medium composed of 1 ml of MEM-α medium (Gibco) supplemented with 4 mg/ml BSA-Fraction V (Sigma) covered with mineral oil, and allowed to capacitate for 1 h before fertilization. Ten to twenty μl of sperm (5 × 106) were added to 500 μl of fertilization media and incubated at 37°C under 5% CO2 in humidified air for 6 h. Only morphologically normal oocytes with one polar body were used for IVF. Presumptive zygotes were washed in KSOM medium, cultured for 10h, and fixed to check pronucleus (PN) formation. Parthenogenetic embryos were produced by treating MII stage oocytes for 5.5 h in Ca2+-free CZB medium supplemented with 10 mM Sr2+ and cytochalasin B (Sigma), as described (O'Neill et al., 1991)
Intracytoplasmic sperm injection (ICSI)
The oocytes that were used for ICSI were pre-injected with ubiquitin aldehyde (UBAL) at MII stage. ICSI was performed in HEPES-CZB (HCZB) drops covered with mineral oil. Capacitated spermatozoa were suspended in a drop of 7% PVP (Sigma) in HCZB. Each spermatozoon was aspirated from the tail side and several piezo pulses (PrimeTech, Ltd., Ibaraki-Ken, Japan) were applied to detach the sperm tail from the sperm head. Up to 10 sperm heads were aspirated into injection pipette at one time. A holding pipette was used to hold the oocyte with its spindle oriented at the 6 or 12 o'clock position. The zona pellucida was penetrated by applying piezo pulses at 3 o'clock and one sperm head was injected per oocyte. Injected oocytes were transferred to KSOM medium for culture. Pronuclei were observed 6 h later and blastocysts were collected 120 h post ICSI.
Inhibitor and antibody treatments
To observe the function of UCHL1 and UCHL3 during fertilization and oocyte activation, we used UCH inhibitors (Ubiquitin-aldehyde and UCHL3-inhibitor), as well as antibodies specific for UCHL1 and UCHL3. Ubiquitin-aldehyde (UBAL; Enzo/Biomol, Plymouth Meeting, PA UW 8450) is a full-length ubiquitin protein in which the C terminus has been modified with an aldehyde-group. It is a highly potent, extremely stable inhibitor of most members of ubiquitin-C-terminal hydrolases/isopeptidase family (Melandri et al., 1996). We injected approximately 5pl of 100μM UBAL into MII oocytes (14 h post hCG) prior to IVF, ICSI or parthenogenetic activation. The cumulus cells were partially removed to facilitate injection without compromising oocyte quality (Vergara et al., 1997). Alternatively, UBAL was added to IVF medium during fertilization to treat zona-intact or zona-free oocytes. In the latter case, the zona pellucida was removed using 0.1% Pronase (Sigma; P-8811) in M2 media. For both experiments, the oocytes were left to recover for 2h in the incubator before proceeding with IVF. The UCHL3-inhibitor (EMD/Calbiochem, Gibbstown, NJ: Cat # 662069) was added during oocyte maturation or fertilization at the concentration of 100 μM. The UCHL3 inhibitor is specific to UCHL3 protein. We also injected approximately 5 pl of 1μg/ μl of anti-UCHL1 or anti-UCHL3 antibody into GV and Metaphase II stages oocytes, and performed fertilization. A non-immune rabbit/mouse serum was injected as a control. The specificity of antibodies was confirmed by Western blotting (see companion paper by Mtango et al.).
Immunofluorescence
Oocytes were fixed and processed as described (Sutovsky et al., 2004; Yi et al., 2007b). A mix of anti-UCHL1 mouse IgG (ab20559, Abcam, Cambridge, MA; dil. 1/200) and affinity purified rabbit anti-UCHL3 IgG (LifeSpan Biosciences/MBL, Woburn, MA; LS-A8724; dil. 1/200) was applied overnight at 4°C, followed by washing and incubation for 40 min with a mixture of DAPI (blue DAN stain; Life Technologies/Molecular Probes; 2.5 μg/ml), goat anti-rabbit-TRIC and goat anti-mouse IgG (red and green fluorescent, respectively; both from Zymed, San Francisco, CA, both diluted 1/100). Spindle localization of UCHL3 was confirmed by using an alternative rabbit anti-UCHL3 (cat. #3525S, Cell Signaling Technology, Boston, MA). Oocytes were mounted on slides in VectaShield medium and examined under a Nikon Eclipse 800 microscope equipped with a CoolSnap HQ CCD camera operated by MetaMorph 4.6 software. Negative controls were performed by replacing of specific antibodies with non-immune rabbit and mouse sera (Sigma) and these slides were photographed at comparable settings.
Statistical analysis
A two-tailed unpaired Student's t test was used to compare the data between two groups. P < 0.05 was considered to be statistically significant
RESULTS
Experimental design and summary of results
A summary of experimental design and most significant results is shown in TABLE 1, with a detailed explanation in TABLE 2. Briefly we added UCHL3 inhibitor (L3i) in IVM medium alone, IVF medium alone or in both IVM and IVF medium (TABLE 1 A and B). Microinjection of UBAL into either GV-stage oocytes or MII oocytes was followed by IVF, performed with or without the zona pellucida (IVF + Zona/-zona; TABLE 1 C-F). Additional experiments included ICSI (TABLE 1 G) or parthenogenetic activation (PA; TABLE 1 H) of oocytes pre-injected with UBAL; addition of UBAL into fertilization medium using oocytes with or without zona (TABLE 1 I, J), injection of UCHL1 antibody in GV oocytes subjected to IVF without zona (TABLE 1 K), and UCHL1 antibody injection in MII oocytes followed by IVF (TABLE 1 L, M).
Table 1.
Summary of experimental design and fertilization rates
| TREATMENT | Stage | added/injected | Zona intact | Zona removed | % PN | |
|---|---|---|---|---|---|---|
| A | L3i in medium | GV | Y | 56.2 | ||
| B | UCHL3i in medium | MII | Y | 52.2 | ||
| C | UBAL injection | GV | Y | 45.4 | ||
| D | UBAL injection | GV | Y | 6.6 | ||
| E | UBAL injection | MII | Y | 7.9 | ||
| F | UBAL injection | MII | Y | 47.6 | ||
| G | UBAL injection | MII + ICSI | Y | 80.2 | ||
| H | UBAL injection | MII + PA | Y | 92.1 | ||
| I | UBAL in medium | MII | Y | 81.9 | ||
| J | UBAL in medium | MII | Y | 60.1 | ||
| K | UCHL1 Ab inj | GV | Y | 5.2 | ||
| L | UCHL1 Ab inj | MII | Y | 5.8 | ||
| M | UCHL1 Ab inj | MII | Y | 72.5 | ||
TABLE 2.
Significant results and outcome on effect of UCHs during in vitro maturation and fertilization of mouse oocytes
| Treatment | Replicates | N-oocyte treated | Treatment group | Control group | Main outcome & conclusion | |
|---|---|---|---|---|---|---|
| A | L3i during IVM, then IVF without L3i | 2 | 176 | Fertilization rate in L3i: 56.2 ± 17.6% | Fert. Rate in control: 85.5 ± 9.5 (P = 0.28) | Oocyte matured in the presence of L3i have somewhat reduced fertilizing ability; Conclusion: Inhibition of UCHL3 activity during maturation reduces fertilizing ability of the oocyte. |
| B | L3i during IVF | 3 | 486 | Fertilization rate in L3i: 52.2 ± 3.1% | Fertilization rate in control: 94.7 ± 2.7 P = 0.0005 | Presence of L3i during IVF reduces fertilization rate. Spindle and pronuclear abnormalities were observed in the non-fertilized and fertilized oocyte, respectively. Conclusion: Inhibition of UCHL3 activity during fertilization reduces fertilization rate and causes aberrant pronuclear development. |
| C | UBAL injection in GV oocyte, IVM & IVF | 2 | 164 | Fertilization rate 45.4 ± 14.8% | Fertilization rate 84.8 ± 1.95% (P = 0.12) | Fertilization rate reduced to 54 % of control. Conclusion: Ooplasmic UCHs sensitive to UBAL play a role in fertilization. |
| D | UBAL injection in GV oocyte, IVM & ZF-IVF | 2 | 169 | Fertilization rate: 6.6 ± 0.6% | Fert. rate in PBS- injected control: 82.2 ± 0.6% (P = 0.0001) | Near complete fertilization block in UBAL-injected oocyte (fert. rate in UBAL was 8 % of that in control). Conclusion: The effect of UBAL on fertilization is not due to its effect on ZP. |
| E | UBAL injection in MII-oocyte & IVF | 4 | 301 | Fertilization rate 7.9 ± 5.8% | Fertilization rate 69.9 ± 23.4% (P = 0.04) | Near complete block of fertilization; no sperm accumulation in periviteline space. Conclusion: Ooplasmic UCHs sensitive to UBAL play a role in fertilization. |
| F | UBAL injection in MII-oocyte & ZF-IVF | 3 | 660 | Fertilization rate 47.6 ± 20.7% | Fertilization rate PBS-injected control: 79.2 ± 10.2% (P = 0.56) | Some but not a significant reduction in fertilization rate for UBAL. Conclusion: The effect of UBAL is not immediate; perhaps there is not enough time for injected UBAL to act on its substrates when fertilization is accelerated by ZP removal. |
| G | UBAL injection in MII-oocyte & ICSI | 5 | 733 | Fertilization rate (% 2PN) of UBAL-ICSI oocyte: 80.2 ± 5.3; Fertilization rate of UBAL-IVF oocyte: 1.7 ± 1.7 (P < 0.0001) | Fertilization rate of control ICSI oocyte: 79.8 ± 6.7 | Normal fertilization and PN-development are observed after ICSI of UBAL-injected oocyte; no fertilization after IVF in the same batches of UBAL-injected oocyte. Conclusion: UBAL injection does now harm the oocytes. |
| H | UBAL injection in MII-oocyte & parthenogentic activation | 3 | 412 | All groups including UBAL and PBS injected and non-injected parthenotes, and the IVF control had near 100% fertilization/PN development rates (range 92.1 ± 7.9% or 100%). | Conclusion: UBAL des not affect oocyte viability assessed by their ability to undergo parthenogenetic activation, so UBAL has likely a more specific effect. | |
| I & J | IVF (ZP+ and ZP− free) with UBAL in IVF medium | 4 | 639 | Fert. Rate UBAL-ZP+ IVF: 81.9±7.5%; Fert Rate UBAL ZF-IVF: 60.1±12.8% | Fert rate control ZP+ IVF: 89.5± 5.6% (P=0.47); Fert. Rate control ZF-IVF 84.3 ± 10.3 % (P=0.19) | Addition of UBAL in IVF medium has no significant effect on fertilization rate, regardless of the presence/absence of ZP, though a somewhat reduced fertilization is observed in ZF-IVF of UBAL oocyte. |
| K & K’ | L1-AB injection in GV/MII oocyte & ZF-IV | 4 | 761 | Fertilization rates reduced in oocyte injected at GV stage (P = 0.1) but not in those injected at MII (P = 0.41) | Accelerating fertilization by zona removal reduces the inhibitory effect of AB injected at MII stage, but the inhibition is substantial when AB is injected at GV stage prior to IVM and ZF-IVF. | |
| L | L1-AB injection in MII & IVF | 4 | 544 | Fertilization rate 5.8 ± 5.5% in L1AB injected oocyte (P= 0.0009 between L1AB and NRS) | Fertilization rate 74.8 ± 10.0% in NRS control and 88.0 ± 8.3% in no-injection control. | Fertilization rate in L1AB group reduced to7.8 % of NRS-injected control. Conclusion: UCHL1 is essential for fertilization, may affect sperm-oolemma fusion, sperm incorporation or chemoattractant production by the oocyte |
| TOTAL | 33 | 4559 | N/A | N/A | ||
ABBREVIATIONS
GV-germinal vesicle oocyte
GVBD-germinal vesicle breakdown
IVF-in vitro fertilization
IVM-in vitro maturation
MII-metaphase II-oocyte
L1AB-mouse antibody against UCHL1 (Abcam, #ab20559,)
L3i-inhibitor of UCHL3 (Calbiochem #662069)
L3AB-rabbit antibody against UCHL3 (MBL #A8724)
UBAL-ubiquitin aldehyde
ZP-zona pellucida
ZF-IVF-zona-free IVF (ZP removed before fertilization)
2PN-two pronuclei (male=female) present in an ovum are consistent with normal fertilization by IVF or ICI (one sperm tail must be present in ooplasm in IVF 2PN oocyte for them to be considered fertilized; sperm tail is separated from sperm head prior to murine ICSI)
NRS- non-immune rabbit sera
Oocyte matured under the influence of UCH inhibitors have reduced potential for fertilization
Initial studies of in vitro maturation in the presence of UCHL3-inhibitor revealed a significant effect leading to aberrant oocyte meiosis and polar body (PB) extrusion (see companion paper, Mtango et al.,). We therefore decided to assess whether the UCHL3-inhibitor (L3i) had an effect on sperm-oocyte interactions and zygotic/pronuclear development. We examined fertilization rates of morphologically normal MII oocytes fertilized in the presence of L3i (Fig. 1; TABLE 2 B) as well as the fertilizing ability of oocytes matured in the presence of L3i (Suppl. Fig. 1; TABLE 2 A, Fig. 1). When L3i was present during IVF, the average fertilization rate in the treated group was reduced to 52% compared to 94% in control fertilization (TABLE 2 B, Fig. 1). This reduction was highly significant (P=0.0005) overall in three replicates, with 486 oocyte examined. A range of abnormalities was observed in the pronuclei of the fertilized oocyte and in the spindles of oocyte that remained unfertilized after this treatment (Fig. 2). In some oocytes, sperm-oolemma fusion apparently occurred normally, but sperm incorporation failed, resulting in parthenogenetic activation (e.g. Fig. 1 B), similar to fertilization failures observed in bovine oocyte in the presence of the microfilament disrupting drug cytochalasin B (Sutovsky et al., 2003; Sutovsky et al., 1996). Other patterns of aberrant fertilization in the presence of L3i included dispermy or polyspermy, presence of abnormal polar bodies and parthenogenetic activation with multiple female pronuclei/karyomeres (Fig. 2).
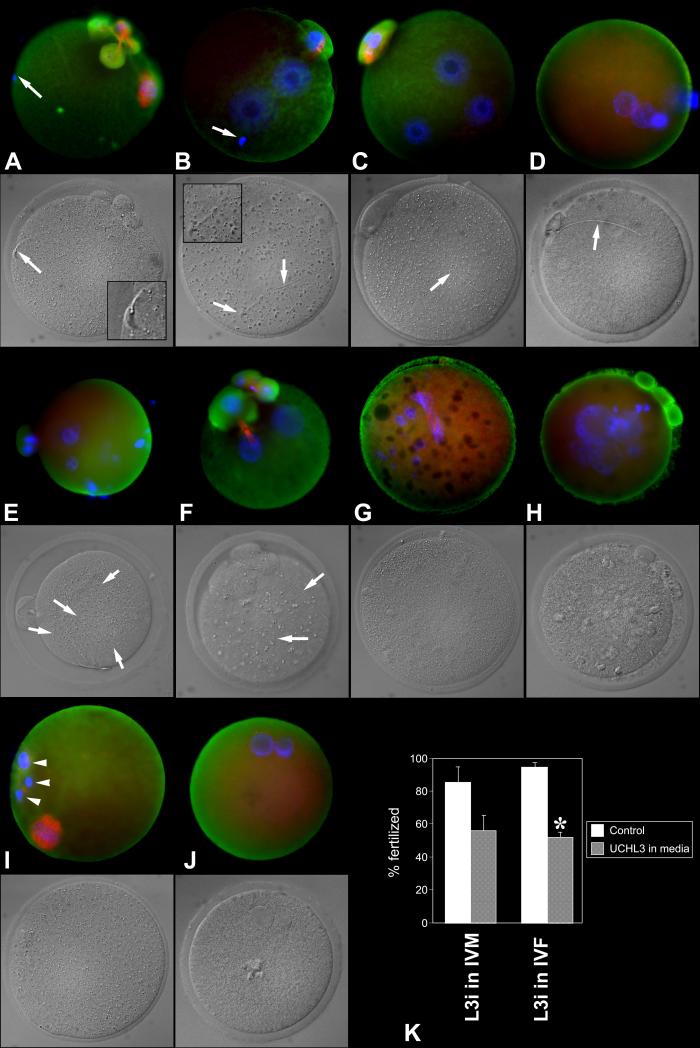
Fig. 1.
Fertilization anomalies in the oocyte matured under control conditions (no inhibitor) and fertilized in the presence of UCHL3-inhibitor. A. Spermatozoon (arrow) penetrated ZP but failed to fuse with the oolemma and/or become incorporated. Oocyte displays abnormal spindle and a fragmented PB1. B. Sperm incorporation failed (arrow) but the ovum was activated, forming two female PN. C. Monospermic zygote with two female PN. D. Monospermic zygote with supernumerary, abnormally small pronuclei. E. Polyspermic egg showing multiple sperm tails in ooplasm. F. Dispermic ovum with supernumerary pronuclei and PBs. G. Fertilization failure with disrupted spindle and multiple female pronuclei/karyomeres. H. Fertilization failure with multiple parthenogenetic female pronuclei/karyomeres. I. Fertilization failure with a fragmented first polar body (arrowheads; incomplete PB2 extrusion). J. Spontaneous parthenogenote showing two abnormally small pronuclei. K. Diagram summarizing fertilization rates of ova exposed to UCHL3-inhibitor during in vitro maturation (two left columns; n=176 ova, two replicates) or fertilization (two right columns; n=486 ova, three replicates). Asterisk denotes a statistically significant difference between control and treatment, at P < 0.05.
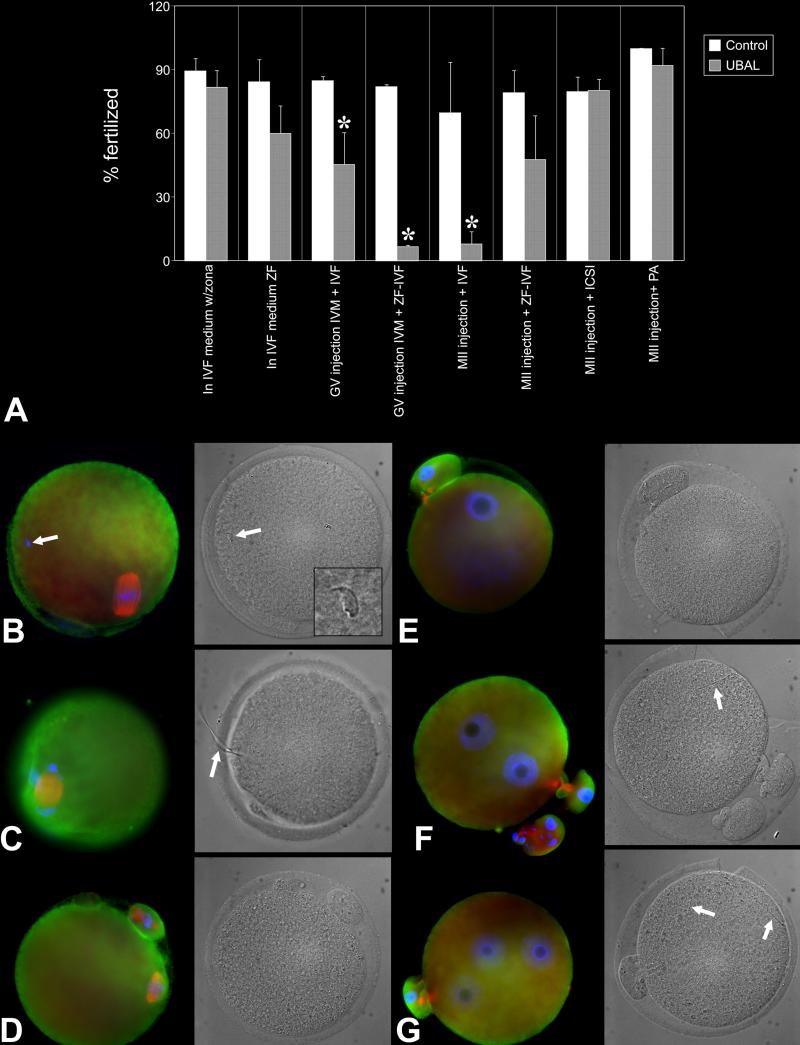
Fig. 2.
Intracytoplasmic injection of ubiquitin-aldehyde (UBAL) prevents murine fertilization but does not cause sperm accumulation in the perivitelline space. A. Diagram summarizing the effect of UBAL on fertilization rate under various scenarios including addition of UBAL in IVF medium (n=639 ova, four replicates), injection of UBAL in GV or MII oocytes followed by IVF (n= 164 ova and 301 ova, respectively; two replicates for GV, four for MII oocytes), injection of UBAL in GV or MII oocytes followed zona-free IVF (ZF-IVF; n=169 ova, two replicates for GV; n=660 ova, three replicates for MII), injection of UBAL in MII oocytes followed ICSI (n=733 ova, five replicates) or injection of UBAL in MII oocytes followed parthenogenetic activation (PA; n=412 ova, three replicates). Asterisk denotes a statistically significant difference between control and treatment, at P < 0.05.B. Metaphase II-stage, UBAL preinjected ovum. Only one spermatozoon is visible in perivitelline space (arrow). C. An UBAL-preinjected ovum in which the sperm head became incorporated in the oocyte, but the sperm flagellum (arrow) incorporation has not been completed. D-G. Control oocytes that remained unfertilized at metaphase-II (D), failed to fertilize but underwent spontaneous parthenogenetic activation (E; one PN is visible), were fertilized by a single spermatozoon (F, arrow) or fertilized by two spermatozoa (G, two sperm tails are identified in ooplasm by arrows).
The in vitro fertilization rate of oocyte matured in the presence of L3i tended to be reduced (56% compared to 85% controls), but the difference did not reach statistical significance (p=0.28; TABLE 2 A). However, various abnormalities were observed in these oocyte, such as abnormal pronuclei and second polar bodies, and the aggregation of UCHL3 in the spindles of unfertilized oocyte (Suppl. Fig. 1). Altogether, these data indicate that proper functioning of UCHs, and UCHL3 in particular, is required for normal oocyte maturation (companion paper) and fertilization (this paper).
Oocyte pre-injection with ubiquitin-aldehyde blocks mouse fertilization in vitro
To evaluate further the roles of UCHs in fertilization, mouse oocytes were pre-injected with ubiquitin-aldehyde (UBAL), a specific inhibitor of UCH-family hydrolases, before IVF. This yielded a near-complete inhibition of fertilization in the zona-intact oocyte injected with UBAL at the MII-stage (TABLE 2 E, and a partial inhibition of fertilization in oocyte pre-injected with UBAL at the GV-stage (TABLE 2 C, D). There was no a noticeable accumulation of spermatozoa in the perivitelline space or morphological anomalies in the oocyte cortex, but incomplete sperm incorporation into the oocyte cortex (Fig. 2) was frequent. There was no difference between control and UBAL-injected oocyte when we used rhodamine-phalloidin staining to examine the integrity of cortical microfilaments (Suppl. Fig. 2). Studies in the porcine model demonstrated increased polyspermy when UBAL was added to the IVF medium (Yi et al., 2007b). In contrast, our mouse IVF data did not yield significant changes in fertilization and polyspermy rates with UBAL present during IVF of either zona-intact or zona-free oocytes. Fertilization rates were not significantly reduced by the addition of UBAL in the IVF medium (TABLE 2 I, J). Altogether, our observations provide evidence that UCHs sensitive to UBAL play a major role in fertilization. An inhibitory effect of UBAL could act at the level of the oolemma or at the level of the zona pellucida.
Effect of UBAL on zona-free and zona-intact oocytes during fertilization
To test the possibility that UBAL affected the zona ability to bind spermatozoa and be penetrated (e.g. by causing premature zona-hardening), we compared effects of UBAL-pre-injection and IVF with zona-intact and zona-free oocyte. The pre-injection of GV-stage oocyte with UBAL had reduced the zona-free fertilization rate it by over 80% compared to vehicle-injected controls (TABLE 2 D).
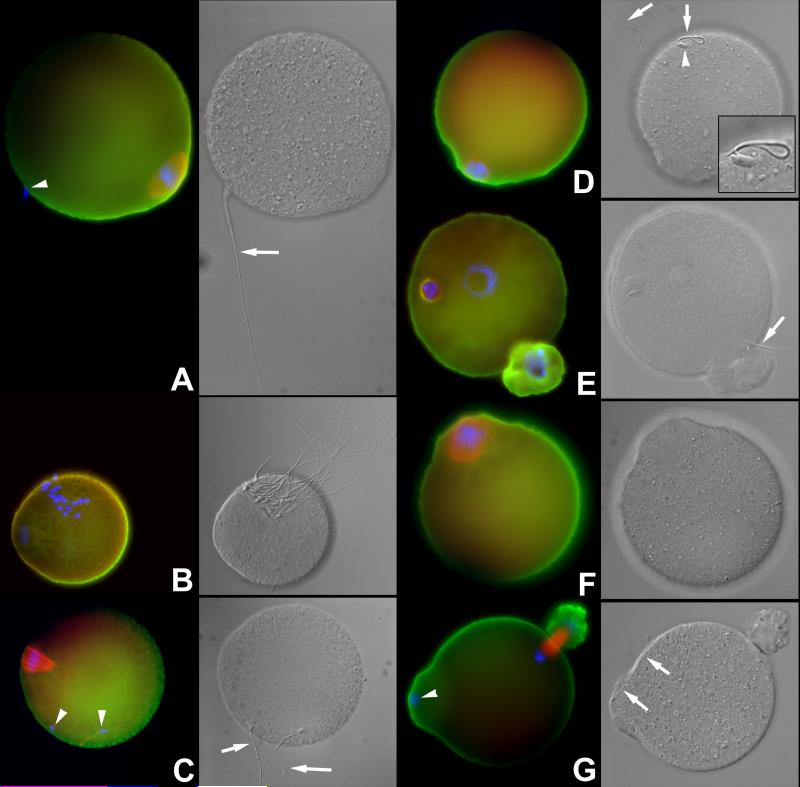
There was no significant effect on the fertilization when the MII oocyte were pre-injected with UBAL and fertilized without zonae (TABLE 2 F). This finding contrasts with the low fertilization rates of MII stage oocyte pre-injected with UBAL but fertilized with the zona intact. A possible explanation is that zona removal accelerated fertilization by reducing the time necessary for penetration, so that UBAL would not have time to saturate its substrate UCHs in the ooplasm. Alternatively, modifications of the zona that may occur during mouse oocyte maturation as a result of localized cortical granule discharge (Liu et al., 2003), could have been altered by the injection of UBAL at the GV-stage. Patterns indicative of delayed or incomplete sperm incorporation into the ooplasm were observed in the UBAL-preinjected oocytes that were fertilized zona-free (Fig. 3).
Fig. 3.
A. Fertilization /sperm incorporation anomalies in the oocytes pre-injected with UBAL at the GV or MII-stage of maturation, and fertilized zona-free. Arrows point to sperm tails, arrowheads to sperm nuclei. A Failed sperm-oolemma fusion and sperm incorporation documented by the presence of oolemma-bound intact spermatozoon and lack of oocyte activation (metaphase-II plate still present). B. Rare case in which multiple oolemma-bound spermatozoa were observed in an oocyte preinjected with UBAL. C Sperm incorporation and oocyte activation failure with two oolemma-bound spermatozoa. D. Sperm incorporation and oocyte activation failure, with a single oolemma-bound spermatozoon. E. Incomplete sperm tail incorporation in the presence of a male and female pronucleus and PB2 extrusion. The male pronucleus is abnormal. F. Complete fertilization failure in an UBAL-preinjected ovum. G. Control, vehicle-injected ovum showing normal incorporation/fertilization cone with concomitant incorporation of the sperm head and tail. Labeling of UCHL1 is green, UCHL3 red and DNA blue. Corresponding DIC images are shown in grayscale.
These data indicate that prolonged effects of UCH-inhibitors in the ooplasm can be mediated at the level of sperm-oolemma and/or sperm-oocyte cortex interactions. However, a more immediate effect can be induced at the level of sperm-zona binding or zona penetration by spermatozoa.
Pre-injection with UBAL is not detrimental to oocyte viability and pronuclear development, but affects cortical granule distribution and blastocyst size
To rule out that UBAL inhibition of fertilization was due to simple toxicity, we tested the ability of oocyte to respond to ICSI or parthenogenetic activation. High fertilization rates were achieved in both the ICSI oocytes preinjected with UBAL (80.2 ±13; 5.3% of oocyte with 2PN), and in control ICSI oocytes (79.8 ± 6.7% with 2PN; TABLE 2 G; Suppl. Fig. 3). Similar to previous UBAL-IVF experiments, the IVF controls for these ICSI experiments showed a high fertilization rate (80.0 ± 1.9%) while the UBAL-IVF controls performed in the same trials with ICSI displayed a nearly complete block of fertilization (1.7 ±1.7 % fertilized; p<0.0001, TABLE 2 G). The ICSI-UBAL oocyte did not show any visible morphological anomalies or altered distribution of UCHL1 and UCHL3. Those oocyte that did not develop 2PN after UBAL-ICSI or control ICSI frequently displayed one parthenogenetic female PN (Suppl. Fig. 3) Furthermore, both control and UBAL-pre-injected oocyte were easily activated parthenogenetically (TABLE 2 H). In that set of experiments, all groups, including UBAL and PBS injected parthenotes, non-injected parthenotes and the IVF control, had near 100% fertilization and PN development rates (range 92.1 ±7.9% to 100%)(TABLE 2 H). Thus, oocytes remained viable and could be activated following UBAL injection, indicating the effects on fertilization were not due to simple toxicity.
We also examined the ability of the UBAL pre-injected ICSI oocyte to form blastocysts. The percentage of blastocyst development was not significantly different (p=0.13) between UBAL (89.5 ± 4.1%) and control (74.8 ± 3.1%) oocyte in two replicates of this experiment. Cell number per blastocyst was also not significantly different (48.2 ± 2.5 UBAL vs. 51.3 ± 2.5 Ctrl; p=0.39; n= 78 UBAL + 52 Ctrl; two replicates). However, ICSI blastocysts grown from the UBAL-preinjected MII oocyte had a significantly smaller diameter (77.3 ± 1.4 μm UBAL vs. 90.9 ± 1.8 Ctrl; p<0.0001; n= 78 UBAL + 52 Ctrl; two replicates), indicating a possible long-term effect on blastocoel expansion rate.
UCHL1 has a specific role in the functioning of the oocyte cortex during fertilization
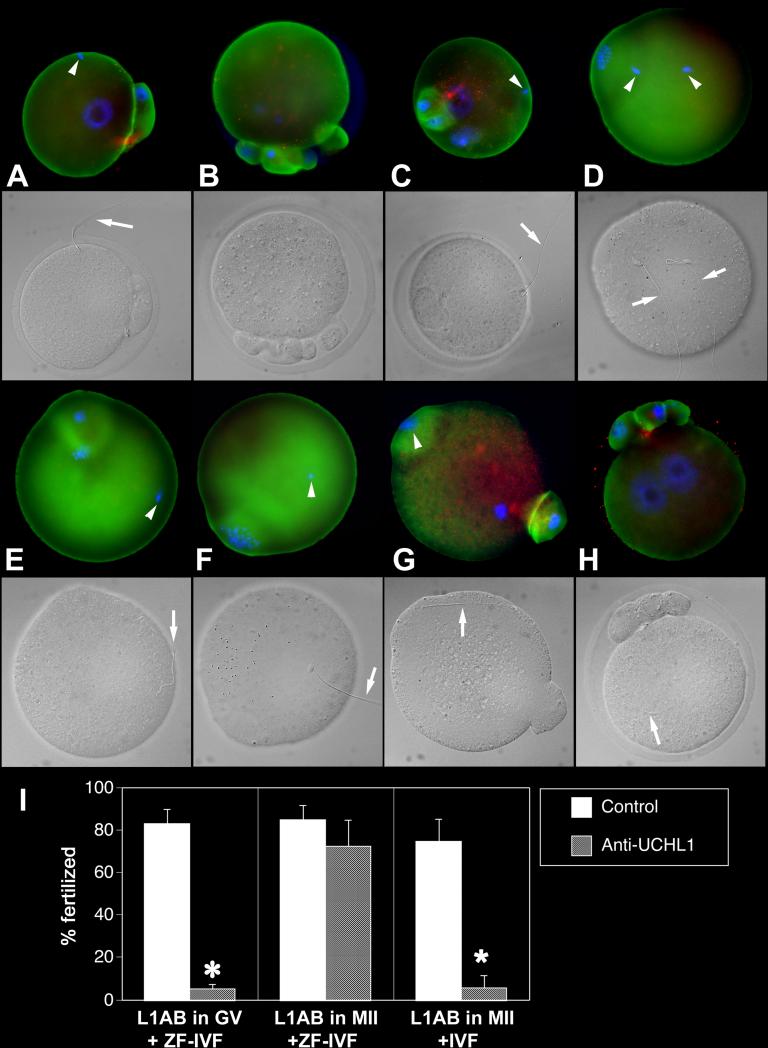
As a UCH family-specific enzyme inhibitor, UBAL could target multiple oocyte UCHs. Because of its prominent localization in the oocyte cortex, we focused on UCHL1. We pre-injected sets of GV-stage and MII oocyte with anti-UCHL1 antibody or control non-immune serum and found that pre-injection with anti-UCHL1 antibody at the MII-stage reduced the fertilization rate to 7.8% of non-immune serum (NRS) injected controls (TABLE 2 L). We found varied patterns of delayed or blocked sperm incorporation (Fig. 4), not unlike those observed in UBAL-injected oocyte. Similar to UBAL experiments, we seldom observed accumulation of spermatozoa in the perivitelline space of oocytes pre-injected with anti-UCHL1 antibody and fertilized with zonae intact. Similar to UBAL pre-injection, ZP-removal before IVF eliminated the inhibitory effect of anti-UCHL1 antibody injection at the MII stage, while the preinjection at GV-stage reduced fertilization (TABLE 2 K, K’). Possible explanation is that the acceleration of fertilization process by zona removal reduces the inhibitory effect of antibody injected at MII stage, as the shortened fertilization time does not allow for sufficient dissipation of the injected antibody across ooplasm. The specific inhibitory effect of anti-UCHL1 injection into zona-intact MII stage oocytes indicates that UCHs in the mouse oocyte cortex are involved in the regulation of zona function. Zona free oocyte-fertilization block following the injection of anti-UCHL1 antibody into GV-stage oocytes indicates the requirement of UCHL1 for the function of oocyte cortex during fertilization.
Fig. 4.
Fertilization failures of oocytes pre-injected with anti-UCHL1-antibody (L1AB) at the MII-stage of maturation. Labeling of UCHL1 is green, UCHL3 red and DNA blue. Arrows point to sperm tails, arrowheads to sperm nuclei. Corresponding DIC images are shown in grayscale. A. Injection of L1AB followed by IVF resulted in a sperm incorporation failure. B. An oocyte pre-injected with L1AB at MII shows abnormal PB1 extrusion and maternal chromosome partition. C. A complete sperm incorporation block is observed in the presence of oocyte activation and female PN-development (sperm-oolemma fusion occurred normally) after the L1AB injection. D-F. An ovum injected with L1AB at MII-stage, showing sperm incorporation and oocyte activation failure. G. Non-immune serum injection at MII and zona-free-IVF; normal sperm incorporation features a fertilization cone with concomitant incorporation of the sperm head and tail. H. Ovum pre-injected with non-immune serum and fertilized with intact zona, shows two normal pronuclei; PB1 is already fragmented H. Diagram summarizing fertilization rates of oocytes pre-injected with L1AB at GV-stage and fertilized zona-free (ZF-IVF), oocytes pre-injected with L1AB at MII stage and fertilized zona free (total of four replicates using 761 ova), and oocytes pre-injected with L1Ab at MII stage and fertilized with an intact zona (IVF; four replicates, 544 ova). Asterisk denotes a statistically significant difference between control and treatment, at P < 0.05.
Oocytes of UCHL1-deficient mice fertilize normally but have reduced development to blastocyst
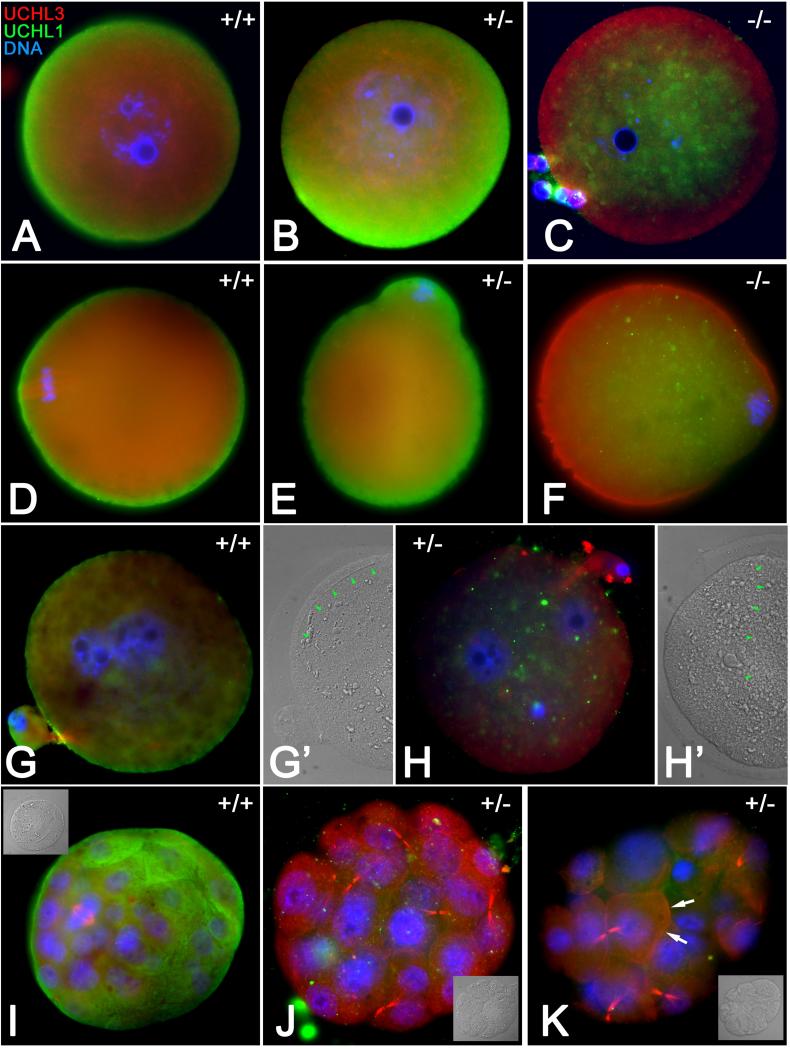
The gracile axonal dystrophy (gad) mice carry an in-frame deletion of exons 7 and 8 of the Uchl1 gene (Uchl1gad allele), resulting in neurodegeneration and reduced female fertility (Saigoh et al., 1999). In our mating trials, Uchl1gad−/− homozygous mutant females (n=7) produced an average of 3.6 pups per liter, compared to 7.3 pups per litter in wild type females. We also observed an average three-weak delay of pregnancy establishment in Uchl1gad−/− homozygous mutant females, and an increased perinatal/neonatal mortality of Uchl1gad−/−pups. To examine the localization patterns of UCHL1 and UCHL3 proteins in the oocytes of these mutants, Uchl1gad−/−, Uchl1ga+−/−and Uchl1gad+/+females were generated by heterozygous inter se crosses of Uchl1ga+−/− males and females, genotyped and used to harvest oocytes, zygotes and embryos. Contrary to UCHL distribution pattern in wild type mice (UCHL1 in cortex, UCHL3 in spindle), the GV stage and metaphase-II oocytes of Uchl1gad−/−females generated by heterozygous inter se crosses displayed a distinct absence of UCHL1 in oocyte cortex, where it was replaced with UCHL3 (Fig. 5 A-F). The association of UCHL3 with the meiotic spindle was not diminished in MII oocytes from Uchl1gad−/− females. In order to examine fertilization and embryo development to the blastocyst stage, while eliminating the possible contribution of a paternal infertility component from males bearing the Uchl1gadmutant allele, female Uchl1gad−/− mice were crossed with homozygous wild type males to produce female offspring carrying a maternally contributed Uchl1gad mutant allele. The zygotes isolated from Uchl1gad+/+or Uchl1gad−/− females, and fertilized in vivo by spermatozoa of Uchl1gad+/+ males, displayed comparable, near-exclusively monospermic fertilization rates (Fig. 5 G, H; Suppl. Fig. 4 A). However, 84 % of embryos recovered on day 4.5 post-hCG from Uchl1gad−/− females mated with wild type males were abnormal, arrested at the stage of morula compaction (Fig. 5 J, K; Suppl. Fig. 4 B). We did not observe a significant difference in numbers of MII oocytes, zygotes, or morula stage embryos obtained from Uchl1gad+/+ or Uchl1gad−/− females. 341 Over 80% of wild type embryos reached the blastocyst stage and showed accumulation of UCHL1 in the apical cortex of trophoblast cells (Fig. 5 I). The UCHL3 protein was observed in mitotic spindles and midbodies both in embryos both from wild type and Uchl1gad−/− females (Figure 6 I, J), while the morula-arrested embryos from Uchl1gad−/− females occasionally displayed UCHL3 accumulation in the blastomere cortex (Fig. 5 K). The reduced/failed blastocyst formation in the Uchl1ga+−/− embryos from homozygous mutant females suggests that the subfertility in these females is most likely due to developmental defects at the morula-to-blastocyst transition of preimplantation embryo development, arising from a deficiency of UCHL1 function in the oocyte, and thus a strong maternal effect of this mutation.
Fig. 5.
Oocyte maturation, fertilization and embryo development in Uchl1gad mice. A-F Homozygous Uchl1 gad−/− oocytes (−/−), heterozygous Uchl1gad−/− oocytes (+/−) and wild type Uchl1gad+/+ oocytes (+/+) were obtained from the properly phenotyped daughters of Uchl1gad+/− female mice mated with Uchl1gad+/− males. Red labeling denotes UCHL3 while UCHL1 is shown in green. A-C. The GV-stage oocytes; note the replacement of cortical UCHL1 with UCHL3 in Uchl1gad−/− ovum. D-F. Metaphase-II oocyte; UCHL3 translocation to oocyte cortex becomes even more obvious in Uchl1gad−/− oocyte. G-K. Wild type Uchl1gad+/+ zygotes/embryos (G, G’, I) were obtained from Uchl1gad+/+ females mated with Uchl1gad+/+ males. Heterozygous Uchl1gad+/− zygotes and later stage embryos (H, H’, J and K) were obtained from Uchl1gad−/− females mated with Uchl1gad+/+ males; G, H. Neither the wild type nor the Uchl1gad+/− oocyte showed polyspermy or fertilization failure. Normal fertilization is revealed by the presence of two pronuclei (blue) and one sperm tail (arrows in panels G’ and H’, showing corresponding DIC images). I-K. Day 4 embryos; wild type, Uchl1gad+/+ embryo has a normal blastocyst appearance with a dividing blastomere within its inner cell mass. The Uchl1gad+/− embryos failed to reach blastocyst stage and became arrested at pre-compaction morula stage. Note the accumulation of UCHL3 in blastomere cortex in Uchl1gad+/− morula (arrows, panel K). Insets show corresponding DIC images.
DISCUSSION
Single gene deletion of Uchl1 or Uchl3 genes is neither infertility causing nor overtly embryo lethal (although the number of progeny is reduced), presumably due to a mutual compensating ability between these closely related Uch genes (Kurihara et al., 2000). In contrast, we find that the inhibitors and antibodies targeting oocyte UCHs have a profound negative effect on zona penetration and incorporation of sperm during mouse fertilization (see summary of fertilization data, TABLE 1 and TABLE 2). For instance, the inhibition of ooplasmic UCHs by UBAL reduces fertilization rates in zona-intact oocytes and to a lesser extent in the zona free oocytes. The effect of UBAL on fertilization/sperm incorporation is most likely mediated by UCHL1, since intracytoplasmic injection of anti-UCHL1 antibody almost completely abolished fertilization of zona-enclosed oocyte. No such effect on fertilization was observed in oocyte pre-injected with antibody against UCHL3. These results suggest an effect of UCHL1 on post-fertilization modification of the zona pellucida. For example, UCHL1 activity may control cortical granule release, with premature cortical granule release causing premature cleavage of ZP proteins to block sperm binding and sperm-zona penetration. Other effects on the zona are also possible.
Interestingly, inhibiting UCH function at the GV stage can also prevent fertilization, indicating additional targets of UCH action that control fertilization process. This could include changes in the oolemma and oocyte cortex, and potentially changes in the release of chemoattractant molecules from oocytes and/or cumulus cells. If UCHs only controlled oolemma and oocyte cortex, one would expect a fertilization block by intracytoplasmic injection of UBAL or anti-UCHL1 antibody to result in the accumulation of spermatozoa in the perivitelline space. This, however, was not the case in our studies. Furthermore, we have not observed differences in cortical microfilament distribution in control and UBAL injected oocyte stained with rhodamine-phalloidin. Altered distribution or function of cortical granules, as observed in bovine oocytes matured in the presence of UCH-inhibitors (Susor et al., 2010), could also alter fertilization rate in UCH-manipulated mouse oocyte. However, such a treatment, preventing cortical granule exocytosis and post-fertilization zona alterations, would be expected to actually increase fertilization/polyspermy rate, not reduce it, as we observed. The inability of the UBAL - injected oocytes to attract spermatozoa thus remains a distinct possibility. A recent study in ascidians indicated that the valosin containing protein (VCP/p97), an OTU-class deubiquitinating enzyme found in the asicidian oocyte cortex, interacts with the oocyte-produced sperm attracting factor SAAF and may be essential for fertilization (Kondoh et al., 2008).
Apart from a double knockout, the most efficient strategy to eliminate or reduce the compensation between UCHL1 and UCHL3 is by targeting these respective UCHs with specific antibodies and inhibitors once they already occupy their preferred subcellular locality. Such a strategy had an obvious effect on murine fertilization. Targeting of UCHL1 in the oocyte cortex inhibited fertilization. Interestingly, we find partial compensation in function between members of the UCH family. Loss of UCHL1 from the egg cortex of Uchl1gad−/− mice is accompanied by a potentially compensatory translocation of UCHL3 to the cortex. This suggests that UCH functions associated with the egg cortex and oolemma may be at least partially restored by UCHL3 function, if opportunity for this is presented during oogenesis.
Such a compensatory translocation of UCHL3 may be a long term process occurring during oogenesis in the Uchl1gad−/− mice. In contrast, the inhibition of both proteins after maturation does not allow this compensation, resulting in more severe effects of UCHL1 deficiency. The translocation of UCHL3 and possibly other related UCHs to the oocyte cortex may not be fast enough when inhibitors and antibodies, injected into the ooplasm, target UCHL1 that is already in oocyte cortex. The UCHL1 and UCHL3 are very closely related, sharing high amino acid sequence homology, similar size, and similar ability to deubiquitinate substrate proteins. Consequently, the UCHL1 and UCHL3 proteins may be, under certain experimental conditions, functionally overlapping (Kurihara et al., 2000).
High polyspermy has been demonstrated in porcine oocytes fertilized with the addition of UBAL in IVF medium. In this case, the most likely target of UBAL was UCHL3 associated with boar sperm acrosome (Yi et al., 2007b). High polyspermy was also proposed to be the cause of subfertility in Uchl1gad−/− mice in vitro (Sekiguchi et al., 2006). Neither our studies using UBAL addition in mouse IVF medium nor our findings in zygotes fertilized in vivo and recovered from Uchl1gad−/− females provide indication of increased polyspermy. However, it is possible that the Uchl1gad−/− oocyte would show polyspermy if challenged by higher number of spermatozoa during IVF, as reported previously (Sekiguchi et al., 2006). Alternatively, polyspermy might only be an in vitro phenomenon in the Uchl1gad−/− mutants, one that may be ameliorated by the exposure of spermatozoa to seminal plasma and oviductal fluid in vivo. We might speculate that deficiency in functional UCHL1 (Sekiguchi et al., 2006) could lead to different phenotypes in vitro vs. in vivo. Even so, the subfertility in Uchl1gad−/− mice (Sekiguchi et al., 2006; Yamazaki et al., 1988b) is most likely due to impaired preimplantation embryo development at the morula stage, as demonstrated by our data.
While rodents and other mammalian species seem to use the ubiquitin-proteasome pathway during sperm-oocyte interactions, our data hint at differences between taxa in how this pathway contributes to the fertilization process. Based on our studies in ungulate models, we anticipated that the inclusion of UBAL and antibodies against UCHL1 or UCHL3 in the IVF medium would stimulate murine fertilization and cause polyspermy. In ungulates, UCHL3 in the sperm acrosome is thought to participate in the process of sperm passage through zona pellucida by interacting with the sperm acrosome-borne proteasomes. In accord with the stimulatory effect of UBAL on proteasomal proteolysis, inhibition of UCHs increased sperm-zona penetration rate in porcine (Yi et al., 2007a) and bovine (Susor et al., 2010) oocytes. This is in line with the observation that the inhibition of whole 20S proteasomal core activities, an intervention that has opposite effect to that of UCH-inhibition, blocks sperm-ZP penetration in higher mammals (Sutovsky, 2004). In these experiments, zona free porcine oocytes were readily fertilized in the presence of proteasomal inhibitors (MG132 and lactacystin) or anti-proteasome antibodies. In the mouse, however, proteasomal inhibitors such as ALLN block sperm-oolemma fusion and sperm incorporation in the ooplasm (Wang et al., 2002). Our data on UCH inhibitors and antibodies affecting sperm incorporation in the ooplasm but not sperm-ZP penetration in the mouse are consistent with this observation. Thus, caution should be exercised when hypotheses on mammalian fertilization are generalized based solely on mouse data.
In spite of the aforementioned species differences, the participation of ubiquitin system in fertilization is extremely conserved, with communalities found between mammals (Morales et al., 2003), ascidians (Sawada et al., 2002), echinoderms (Yokota and Sawada, 2007) and even plants (Doelling et al., 2007). While the mechanism of their action may vary, the expression and localization patterns of UCHs appear to be evolutionarily conserved between rodents and primates. High expression levels of proteasomal subunits and enzymes of the ubiquitin system appear to be a common feature of mammalian oocytes and early embryos (Assou et al., 2009; Mtango and Latham, 2007). Importantly, aberrant expression of genes encoding for UCHs and other components of ubiquitin system has been associated with reduced developmental potential of primate embryos (Mtango and Latham, 2007). Our data confirm a conserved localization of UCHs between rodents, ungulates and primates. It is plausible that some etiologies of human infertility and developmental failure after assisted fertilization may arise from abnormal expression/functioning of human oocyte UCHs.
Beyond fertilization and polyspermy, we observe a strong maternal effect on preimplantation embryo development, wherein heterozygous Uchl1ga+−/− embryos produced by mating homozygous Uchl1gad−/− females to homozygous wild type males undergo developmental arrest between the morula and blastocyst stage. Such strong maternal effects could arise due to haploinsufficieny; however previous reports indicated a matrilineal origin of the fertility defect (Sekiguchi et al., 2006; Yamazaki et al., 1988a). This suggests that lethality most likely would arise from triploidy/multiplody (e.g., from polyspermy) or from aneuploidy resulting perhaps from meiotic defects in the Uchl1gad−/−oocytes. Because we find that polyspermy is not increased with the mutant oocytes, it is most likely that the increased embryo lethality is due to decreased developmental competence of Uchl1gad−/− oocytes, which could be exacerbated by altered oviductal environment. While UCHL1 protein does not seem to associate with the meiotic spindle, it could influence cytokinesis during oocyte maturation and also have an effect on the association of UCHL3 with the spindle. Consequently, mis-segregation of chromosomes during meiosis could arise from defects in polar body extrusion or spindle function in the oocyte. Thus, although UCHL3 may compensate for UCHL1 functions in the oocyte by translocating to the cortex, this compensation may come at a high cost, namely the incorrect chromosome segregation. The resulting aneuploidy would yield embryos that are unable to progress beyond the morula stage, as observed. Some reduction in blastocyst formation and quality has also been observed in oocyte fertilized by ICSI after preinjection with UBAL (see ICSI Data in Results). The aberrant blasctocyst phenotype was less robust than that of Uchl1gad−/− mice, possibly because the injected UBAL was metabolized by the ICSI zygotes prior to morula stage.
Altogether, our data demonstrate the importance of UCHL1 and UCHL3 for mouse fertilization. Our data also reveal for the first time a key maternal effect of the Uchl1 gene, such that oocyte-derived UCHL1 protein contributes to successful development to blastocyst stage, as well as a role for UCH proteins in blastocoels expansion. These results signify new, key roles for these proteins during early development.
Supplementary Material
Suppl. Fig. 1. Fertilization anomalies in the oocyte matured in the presence of UCHL3-inhibitor and fertilized under control conditions (no inhibitor). A. The spermatozoon penetrated the ovum, induced the development of female PN (arrowhead) and extrusion of PB2, but failed to decondense its own nucleus to form a male PN (arrow). B. Fertilization occurred normally but two female PN (arrowheads) formed, probably as a result of a failure to extrude PB2. Note cortical, polarized distribution of UCHL3 (red). C. Monospermic fertilization with multiple pronuclei. D. Two cell embryo entering second mitosis, with irregular chromosome arrangement in metaphase plates. E. A two-cell embryo with normal appearance. F. Fertilization failure with an abnormally large PB1 and lack of UCHL3 labeling in the spindle. G. Fertilization failure with an abnormal spindle; note the aggregates of UCHL3 in the spindle equator (arrowheads). H, I, J. Fertilization failures with disarrayed metaphase plates and unfocused spindle poles. The UCHL1 is green, UCHL3 red and DNA is blue. Arrows in DIC point to sperm tails.
Suppl. Fig. 2: Labeling of cortical actin microfilaments (F-actin; red) in the mouse oocytes preinjected with ubiquitin-aldehyde and fertilized in vitro. A. Control fertilized oocytes with two apposed pronuclei. B. Failed-fertilized oocyte, preinjected with UBAL at metaphase II. C. Failed-fertilized oocyte, preinjected with UBAL at the germinal vesicle stage. D. Oocyte that was fertilized following preinjection with UBAL at GV-stage; note an incomplete apposition of male and female pronuclei.
Suppl. Fig. 3: Fertilization of the UBAL-preinjected oocyte by ICSI shows that UBAL injection in ooplasm does not damage the oocytes. A. Percentages of oocytes showing normal 2PN configuration after ICSI (first and second column) or sperm penetration and PN-formation after IVF (third and fourth column) with/without preinjection with UBAL. B. A two-pronuclear zygote after UBAL preinjection and ICSI. C Two-cell embryo created by UBAL preinjection and ICSI. D. Two-pronuclear zygote produced by control ICSI without preinjection. E. Parthenogenetic control oocyte; spermatozoon escaped from ooplasm during ICSI. Labels: UCHL1 is in green, UCHL3 in red and DNA in blue. Corresponding DIC images are shown in grayscale.
Suppl. Fig. 4: In vivo fertilization and embryo development in Uchl1gad−/− and Uchl1gad+/+ females mated with wild type, Uchl1gad+/+ males. One cell zygotes and embryos were isolated 23 h post hCG; blastocysts were isolated 108 h post hCG. A. Diagram shows no differences between Uchl1gad+/+ and Uchl1gad−/− zygotes in the rates of polyspermy of the in vivo fertilization. B. Preimplantation embryo developmental process of the Uchl1gad−/− embryos obtained from Uchl1gad−/− females is severely impaired in vivo. The Uchl1gad−/− embryos do not develop to blastocyst stage.
ACKNOWLEDGEMENTS
This project was supported by National Research Initiative Competitive Grants no. 2007-35203-18274 and no. 2011-67015-20025 from the USDA National Institute of Food and Agriculture, to P.S. and grants from the National Center for Research Resources (RR15253, RR18907) and National Institute for Child Health and Human Development (HD41440, HD52788 and RR15253) to K.E.L. Additional funding was provided by the Food for the 21st Century Program of the University of Missouri-Columbia to P.S., and Institutional Research Concept Award IAPG-AVOZ-50450515 and C.A.V. grant GACR 524/07/1087 to A.S. Heterozygous Uchl1gad+/− mice were kindly provided by Professor Keiji Wada (National Institute of Neuroscience, Tokyo, Japan).
REFERENCES
- Assou S, Cerecedo D, Tondeur S, Pantesco V, Hovatta O, Klein B, Hamamah S, De Vos J. A gene expression signature shared by human mature oocytes and embryonic stem cells. BMC Genomics. 2009;10:10. doi: 10.1186/1471-2164-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreaux DA, Maiti TK, Davies CW, Das C. Ubiquitin vinyl methyl ester binding orients the misaligned active site of the ubiquitin hydrolase UCHL1 into productive conformation. Proc Natl Acad Sci U S A. 2010;107(20):9117–9122. doi: 10.1073/pnas.0910870107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Bansal P, Sutovsky P, Gupta SK. Role of proteasomal activity in the induction of acrosomal exocytosis in human spermatozoa. Reprod Biomed Online. 2008;16(3):391–400. doi: 10.1016/s1472-6483(10)60601-3. [DOI] [PubMed] [Google Scholar]
- Doelling JH, Phillips AR, Soyler-Ogretim G, Wise J, Chandler J, Callis J, Otegui MS, Vierstra RD. The ubiquitin-specific protease subfamily UBP3/UBP4 is essential for pollen development and transmission in Arabidopsis. Plant Physiol. 2007;145(3):801–813. doi: 10.1104/pp.106.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellederova Z, Halada P, Man P, Kubelka M, Motlik J, Kovarova H. Protein patterns of pig oocytes during in vitro maturation. Biol Reprod. 2004;71(5):1533–1539. doi: 10.1095/biolreprod.104.030304. [DOI] [PubMed] [Google Scholar]
- Fang Y, Fu D, Shen XZ. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806(1):1–6. doi: 10.1016/j.bbcan.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Fuchs SY. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol Ther. 2002;1(4):337–341. [PubMed] [Google Scholar]
- Gu YQ, Chen QJ, Gu Z, Shi Y, Yao YW, Wang J, Sun ZG, Tso JK. Ubiquitin carboxyl-terminal hydrolase L1 contributes to the oocyte selective elimination in prepubertal mouse ovaries. Sheng Li Xue Bao. 2009;61(2):175–184. [PubMed] [Google Scholar]
- Kondoh E, Konno A, Inaba K, Oishi T, Murata M, Yoshida M. Valosin-containing protein/p97 interacts with sperm-activating and sperm-attracting factor (SAAF) in the ascidian egg and modulates sperm-attracting activity. Dev Growth Differ. 2008;50(8):665–673. doi: 10.1111/j.1440-169X.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- Kong M, Diaz ES, Morales P. Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol Reprod. 2009;80(5):1026–1035. doi: 10.1095/biolreprod.108.073924. [DOI] [PubMed] [Google Scholar]
- Kurihara LJ, Semenova E, Levorse JM, Tilghman SM. Expression and functional analysis of Uch-L3 during mouse development. Mol Cell Biol. 2000;20(7):2498–2504. doi: 10.1128/mcb.20.7.2498-2504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J, Mochida K, Wang YL, Sekiguchi S, Sankai T, Aoki S, Ogura A, Yoshikawa Y, Wada K. Ubiquitin C-terminal hydrolase L-1 is essential for the early apoptotic wave of germinal cells and for sperm quality control during spermatogenesis. Biol Reprod. 2005;73(1):29–35. doi: 10.1095/biolreprod.104.037077. [DOI] [PubMed] [Google Scholar]
- Massicotte L, Coenen K, Mourot M, Sirard MA. Maternal housekeeping proteins translated during bovine oocyte maturation and early embryo development. Proteomics. 2006;6(13):3811–3820. doi: 10.1002/pmic.200500803. [DOI] [PubMed] [Google Scholar]
- Melandri F, Grenier L, Plamondon L, Huskey WP, Stein RL. Kinetic studies on the inhibition of isopeptidase T by ubiquitin aldehyde. Biochemistry. 1996;35(39):12893–12900. doi: 10.1021/bi9612935. [DOI] [PubMed] [Google Scholar]
- Morales P, Kong M, Pizarro E, Pasten C. Participation of the sperm proteasome in human fertilization. Hum Reprod. 2003;18(5):1010–1017. doi: 10.1093/humrep/deg111. [DOI] [PubMed] [Google Scholar]
- Mtango NR, Latham KE. Ubiquitin proteasome pathway gene expression varies in rhesus monkey oocytes and embryos of different developmental potential. Physiol Genomics. 2007;31(1):1–14. doi: 10.1152/physiolgenomics.00040.2007. [DOI] [PubMed] [Google Scholar]
- O'Neill GT, Rolfe LR, Kaufman MH. Developmental potential and chromosome constitution of strontium-induced mouse parthenogenones. Mol Reprod Dev. 1991;30(3):214–219. doi: 10.1002/mrd.1080300308. [DOI] [PubMed] [Google Scholar]
- Plachot M, Mandelbaum J. Oocyte maturation, fertilization and embryonic growth in vitro. Br Med Bull. 1990;46(3):675–694. doi: 10.1093/oxfordjournals.bmb.a072424. [DOI] [PubMed] [Google Scholar]
- Sakai N, Sawada MT, Sawada H. Non-traditional roles of ubiquitin-proteasome system in fertilization and gametogenesis. Int J Biochem Cell Biol. 2004;36(5):776–784. doi: 10.1016/S1357-2725(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Sawada H, Sakai N, Abe Y, Tanaka E, Takahashi Y, Fujino J, Kodama E, Takizawa S, Yokosawa H. Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proc Natl Acad Sci U S A. 2002;99(3):1223–1228. doi: 10.1073/pnas.032389499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi S, Kwon J, Yoshida E, Hamasaki H, Ichinose S, Hideshima M, Kuraoka M, Takahashi A, Ishii Y, Kyuwa S, Wada K, Yoshikawa Y. Localization of ubiquitin C-terminal hydrolase L1 in mouse ova and its function in the plasma membrane to block polyspermy. Am J Pathol. 2006;169(5):1722–1729. doi: 10.2352/ajpath.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susor A, Ellederova Z, Jelinkova L, Halada P, Kavan D, Kubelka M, Kovarova H. Proteomic analysis of porcine oocytes during in vitro maturation reveals essential role for the ubiquitin C-terminal hydrolase-L1. Reproduction. 2007;134(4):559–568. doi: 10.1530/REP-07-0079. [DOI] [PubMed] [Google Scholar]
- Susor A, Liskova L, Toralova T, Pavlok A, Pivonkova K, Karabinova P, Lopatarova M, Sutovsky P, Kubelka M. Role of ubiquitin C-terminal hydrolase-l1 in antipolyspermy defense of mammalian oocytes. Biol Reprod. 2010;82(6):1151–1161. doi: 10.1095/biolreprod.109.081547. [DOI] [PubMed] [Google Scholar]
- Sutovsky P. Visualization of sperm accessory structures in the mammalian spermatids, spermatozoa, and zygotes by immunofluorescence, confocal, and immunoelectron microscopy. Methods Mol Biol. 2004;253:59–77. doi: 10.1385/1-59259-744-0:059. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc Res Tech. 2003;61(4):362–378. doi: 10.1002/jemt.10350. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Navara CS, Schatten G. Fate of the sperm mitochondria, and the incorporation, conversion, and disassembly of the sperm tail structures during bovine fertilization. Biol Reprod. 1996;55(6):1195–1205. doi: 10.1095/biolreprod55.6.1195. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Van Leyen K, McCauley T, Day BN, Sutovsky M. Degradation of paternal mitochondria after fertilization: implications for heteroplasmy, assisted reproductive technologies and mtDNA inheritance. Reprod Biomed Online. 2004;8(1):24–33. doi: 10.1016/s1472-6483(10)60495-6. [DOI] [PubMed] [Google Scholar]
- Vergara GJ, Irwin MH, Moffatt RJ, Pinkert CA. In vitro fertilization in mice: Strain differences in response to superovulation protocols and effect of cumulus cell removal. Theriogenology. 1997;47(6):1245–1252. doi: 10.1016/s0093-691x(97)00104-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Song C, Duan C, Shi W, Li C, Chen D, Wang Y. Effects of ubiquitinproteasome pathway on mouse sperm capacitation, acrosome reaction and in vitro fertilization. Chinese Sci Bulletin. 2002;47(2):127–132. [Google Scholar]
- Wilkinson KD. DUBs at a glance. J Cell Sci. 2009;122(Pt 14):2325–2329. doi: 10.1242/jcs.041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Wakasugi N, Sakakibara A, Tomita T. Reduced fertility in gracile axonal dystrophy (gad) mice. Jikken Dobutsu. 1988a;37(2):195–199. doi: 10.1538/expanim1978.37.2_195. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Wakasugi N, Tomita T, Kikuchi T, Mukoyama M, Ando K. Gracile axonal dystrophy (GAD), a new neurological mutant in the mouse. Proc Soc Exp Biol Med. 1988b;187(2):209–215. doi: 10.3181/00379727-187-42656. [DOI] [PubMed] [Google Scholar]
- Yi YJ, Manandhar G, Oko RJ, Breed WG, Sutovsky P. Mechanism of sperm-zona pellucida penetration during mammalian fertilization: 26S proteasome as a candidate egg coat lysin. Soc Reprod Fertil Suppl. 2007a;63:385–408. [PubMed] [Google Scholar]
- Yi YJ, Manandhar G, Sutovsky M, Li R, Jonakova V, Oko R, Park CS, Prather RS, Sutovsky P. Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization. Biol Reprod. 2007b;77(5):780–793. doi: 10.1095/biolreprod.107.061275. [DOI] [PubMed] [Google Scholar]
- Yokota N, Sawada H. Effects of proteasome inhibitors on fertilization of the sea urchin Anthocidaris crassispina. Biol Pharm Bull. 2007;30(7):1332–1335. doi: 10.1248/bpb.30.1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. Fertilization anomalies in the oocyte matured in the presence of UCHL3-inhibitor and fertilized under control conditions (no inhibitor). A. The spermatozoon penetrated the ovum, induced the development of female PN (arrowhead) and extrusion of PB2, but failed to decondense its own nucleus to form a male PN (arrow). B. Fertilization occurred normally but two female PN (arrowheads) formed, probably as a result of a failure to extrude PB2. Note cortical, polarized distribution of UCHL3 (red). C. Monospermic fertilization with multiple pronuclei. D. Two cell embryo entering second mitosis, with irregular chromosome arrangement in metaphase plates. E. A two-cell embryo with normal appearance. F. Fertilization failure with an abnormally large PB1 and lack of UCHL3 labeling in the spindle. G. Fertilization failure with an abnormal spindle; note the aggregates of UCHL3 in the spindle equator (arrowheads). H, I, J. Fertilization failures with disarrayed metaphase plates and unfocused spindle poles. The UCHL1 is green, UCHL3 red and DNA is blue. Arrows in DIC point to sperm tails.
Suppl. Fig. 2: Labeling of cortical actin microfilaments (F-actin; red) in the mouse oocytes preinjected with ubiquitin-aldehyde and fertilized in vitro. A. Control fertilized oocytes with two apposed pronuclei. B. Failed-fertilized oocyte, preinjected with UBAL at metaphase II. C. Failed-fertilized oocyte, preinjected with UBAL at the germinal vesicle stage. D. Oocyte that was fertilized following preinjection with UBAL at GV-stage; note an incomplete apposition of male and female pronuclei.
Suppl. Fig. 3: Fertilization of the UBAL-preinjected oocyte by ICSI shows that UBAL injection in ooplasm does not damage the oocytes. A. Percentages of oocytes showing normal 2PN configuration after ICSI (first and second column) or sperm penetration and PN-formation after IVF (third and fourth column) with/without preinjection with UBAL. B. A two-pronuclear zygote after UBAL preinjection and ICSI. C Two-cell embryo created by UBAL preinjection and ICSI. D. Two-pronuclear zygote produced by control ICSI without preinjection. E. Parthenogenetic control oocyte; spermatozoon escaped from ooplasm during ICSI. Labels: UCHL1 is in green, UCHL3 in red and DNA in blue. Corresponding DIC images are shown in grayscale.
Suppl. Fig. 4: In vivo fertilization and embryo development in Uchl1gad−/− and Uchl1gad+/+ females mated with wild type, Uchl1gad+/+ males. One cell zygotes and embryos were isolated 23 h post hCG; blastocysts were isolated 108 h post hCG. A. Diagram shows no differences between Uchl1gad+/+ and Uchl1gad−/− zygotes in the rates of polyspermy of the in vivo fertilization. B. Preimplantation embryo developmental process of the Uchl1gad−/− embryos obtained from Uchl1gad−/− females is severely impaired in vivo. The Uchl1gad−/− embryos do not develop to blastocyst stage.