Abstract
Rationale: Patients with chronic obstructive pulmonary disease (COPD) are at high risk for lung cancer (LC) and represent a potential target to improve the diagnostic yield of screening programs.
Objectives: To develop a predictive score for LC risk for patients with COPD.
Methods: The Pamplona International Early Lung Cancer Detection Program (P-IELCAP) and the Pittsburgh Lung Screening Study (PLuSS) databases were analyzed. Only patients with COPD on spirometry were included. By logistic regression we determined which factors were independently associated with LC in PLuSS and developed a COPD LC screening score (COPD-LUCSS) to be validated in P-IELCAP.
Measurements and Main Results: By regression analysis, age greater than 60, body mass index less than 25 kg/m2, pack-years history greater than 60, and emphysema presence were independently associated with LC diagnosis and integrated into the COPD-LUCSS, which ranges from 0 to 10 points. Two COPD-LUCSS risk categories were proposed: low risk (scores 0–6) and high risk (scores 7–10). In comparison with low-risk patients, in both cohorts LC risk increased 3.5-fold in the high-risk category.
Conclusions: The COPD-LUCSS is a good predictor of LC risk in patients with COPD participating in LC screening programs. Validation in two different populations adds strength to the findings.
Keywords: COPD, screening, lung cancer
At a Glance Commentary
Scientific Knowledge on the Subject
Patients with chronic obstructive pulmonary disease (COPD) are at high risk for lung cancer and represent a potential target to improve the diagnostic yield of screening programs.
What This Study Adds to the Field
We developed and validated a COPD-specific score to predict lung cancer risk, the COPD-LUCSS, which may be useful to identify COPD patients with the highest risk for lung cancer.
Lung cancer (LC) and chronic obstructive pulmonary disease (COPD) are among the most important causes of death worldwide (1). Several studies have documented that patients with COPD are at high risk for the development of LC (2, 3) and LC is an important cause of death in these patients (4).
The National Lung Cancer Screening Trial (NLST) recently showed that LC screening with low-dose computed tomography (LDCT) of the thorax in a selected population of current or former smokers decreases LC-specific mortality by at least 20% (5). The NLST selection criteria included an age between 55 and 74 years, a smoking history of at least 30 pack-years, and for former smokers the quit date had to be within 15 years before recruitment. The U.S. Preventive Services Task Force and several professional organizations now recommend LC screening (6–9) and recommend using the NLST entry criteria for the selection of individuals to be screened.
Because patients with COPD are at a higher risk for developing LC than healthy smokers, it has been suggested to use the presence of airway obstruction on spirometry and of emphysema on LDCT as risk factors to select the highest risk populations for screening programs (10–14).
The use of clinical variables (age, body mass index [BMI], pack-years, family history of LC, or self-reported COPD) integrated in risk prediction scores has been previously described in the context of optimizing the selection of candidates for CT screening (15–17). The inclusion of some of these simple clinical parameters has demonstrated that the LC detection rate can be improved (10).
To that end, we analyzed the databases from two different LC screening studies from Europe and the United States to develop and validate a COPD-tailored score that could improve selection criteria to predict LC risk.
Methods
Participants included in this study were recruited into two different single-arm observational LC screening studies using LDCT: the Pamplona International Early Lung Cancer Detection Program (P-IELCAP) in Spain (18), and the Pittsburgh Lung Screening Study (PLuSS) in the United States (19).
Study Protocol
Derivation study (PLuSS cohort)
Details regarding subject enrollment and the protocol followed for LC assessment in the PLuSS have been described previously (19). The study enrolled subjects between January 2002 and April 2005 that were between 50 and 79 years of age, current or former smokers with at least 12.5 pack-years of smoking, and with no personal LC history. As explained in that previous work (19), “the presence of emphysema on LDCT was evaluated qualitatively (present versus absent): three readers, a pulmonologist, a general radiologist, and a chest radiologist, visually scored the baseline CT scan for emphysema presence and severity. Scoring procedures used a five-level semi quantitative scale, based on National Emphysema Treatment Trial criteria (20), to represent no, trace, mild, moderate, and severe emphysema. The latter four categories roughly correspond to emphysema affecting less than 10, 10–25, 25–50%, and greater than 50% of the lung, respectively. In a validation study involving 266 study subjects, a simple quadratic function of this five-level visual emphysema severity score explained 26% of the variance observed with a quantitative measure derived from CT densitometry (19).”
The interrater agreement for visual emphysema detection was kappa statistic (0.68; 95% confidence interval, 0.64–0.73). Airway obstruction was determined at baseline by spirometry according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendation (21), in this case without post-bronchodilation measurements. An annual or earlier follow-up LDCT was scheduled according to the established protocol. All subjects signed an informed consent and the institutional review board for the University of Pittsburgh approved the study.
Validation cohort (P-IELCAP cohort)
Individuals entering the P-IELCAP were enrolled between September 2001 and March 2013, as part of the International Early Lung Cancer Action Program (18). Detailed information regarding individual enrollment, emphysema, airway obstruction, and LC assessments has been previously described (22). Briefly, we included men and women, 40 years of age or older, who were current or former smokers with a tobacco history of greater than 10 pack-years, and without LC symptoms. LDCT and a spirometry were performed at baseline, and an annual or earlier follow-up was scheduled according to the established protocol (18). As previously explained (18), “the presence of emphysema on the LDCT was evaluated qualitatively (present versus absent): all images were read by two expert chest radiologists for visual assessment of the presence of emphysema, using validated criteria (23). Briefly, the extent of emphysema was graded from 0 to 4, with a grade of 0 indicating no emphysema, and a grade of 4 indicating the presence of emphysema in greater than 75% of the lung. For the purpose of this study, patients with a score greater than or equal to 1 were classified as having emphysema.” Our group has shown the reliability of this measurement with an excellent interrater agreement (kappa coefficient, 0.91) (23).
Airway obstruction was defined according to the GOLD recommendations by the presence of a FEV1/FVC ratio less than 0.70 after the administration of albuterol 400 μg (21). The ethics committee of the University of Navarra approved the study protocol and all subjects signed an informed consent before entry. Only individuals from both cohorts with spirometrically defined COPD (FEV1/FVC <0.70) were included in the present study.
Clinical Parameters
A personal interview with trained personnel in the P-IELCAP cohort and a self-administered questionnaire in the PLuSS cohort was conducted and the following data were registered: age, sex, smoking status, pack-years history, years since quit smoking for former smokers, and family history of LC. The BMI was calculated as the weight in kilograms divided by height in meters squared. Pulmonary function tests were performed following American Thoracic Society/European Respiratory Society guidelines (24). NLST inclusion criteria (age between 55 and 74 yr; at least 30 pack-years of smoking history; and, if former smokers, had quit within the previous 15 yr) were applied to both cohorts.
Outcomes
LC diagnosis was performed in the P-IELCAP cohort using a standardized diagnostic algorithm (22). As described previously (18), “patients with noncalcified nodules less than 10 mm were followed up with repeat LDCTs and further workup if growth of a nodule was detected. Subjects with suspicious nodules greater than or equal to 10 mm were immediately referred for positron emission tomography (PET), percutaneous needle biopsy, or intraoperative biopsy. For the purpose of the study, all cases of LC diagnosed as a consequence of screening or due to a new onset of symptoms (interval cases) were included in the analysis.”
In the PLuSS cohort as also stated in their original work (19) “to document diagnostic events and outcomes, a nurse practitioner maintained telephone contact with persons who had received a follow-up recommendation. At annual intervals, the investigators used brief telephone interviews and/or mailed questionnaires to update the vital and cancer status of study subjects and to ascertain interval lung biopsy procedures. Using information obtained over the telephone and signed consent, the investigators identified, acquired, and reviewed relevant medical records, including images and reports from imaging studies (thoracic CT, PET, or PET-CT), biopsy procedure reports, pathology reports, and death certificates.” Follow-up time was registered in months up to the time of the LC diagnosis.
Statistical Analysis
Quantitative data with a normal distribution were expressed using the mean and the SD. Quantitative data with nonnormal distribution were described with the median and the interquartile range. Qualitative data were described using relative frequencies. We first selected the predictor variables to include in the COPD LC screening score (COPD-LUCSS) from the PLuSS cohort. Cox regression analysis was used to explore the independent association of each predictor variable with LC diagnosis in the PLuSS cohort. Predictor variables that were statistically significant were included in a multiple Cox regression model to determine which of them best predicted LC diagnosis. The use of the predictors as continuous variables showed that the model has a similar statistical performance before and after categorization (see Table E1 in the online supplement). We believe, as research practitioners, that categorization of predictor variables can help clinicians apply the score in their daily practice.
To determine the threshold of a categorical variable to be included in the score, we first divided each continuous variable in quartile or quintiles. We then visually compared their Kaplan-Meier (KM) curves and selected the best cut off values of each predictor variable (data not shown): age greater than 60 years, pack-years greater than 60, and BMI less than 25.
To build the new COPD-LUCSS, we first determined the relative weight of each predictor variable in the score by calculating its value according to the ratio of their log(hazard ratios) (logHR) to the smallest one (in this case, BMI <25), obtained in the Cox analysis: logHR BMI <25/logHR BMI <25 = 1, logHR pack-years >60/logHR BMI <25 = 1.7 (rounded to 2 for its clinical application); logHR age >60 years old/logHR BMI <25 = 3.2 (rounded to 3 for its clinical application); and logHR emphysema yes versus no/logHR BMI <25 = 3.8 (rounded to 4 for its clinical application). To obtain the final value of the COPD-LUCSS, we added each predictor variable value, ranging from 0 to 10 points (Table 1).
Table 1.
COPD-LUCSS
| Score (Points) | |
|---|---|
| BMI <25 | 1 |
| Pack-years history >60 | 2 |
| Age >60 yr | 3 |
| Radiologic emphysema, yes | 4 |
| Total | 10 |
Definition of abbreviations: BMI = body mass index; COPD-LUCSS = chronic obstructive pulmonary disease lung cancer screening score.
COPD-LUCSS categories: low-risk category, 0–6 points; high-risk category, 7–10 points.
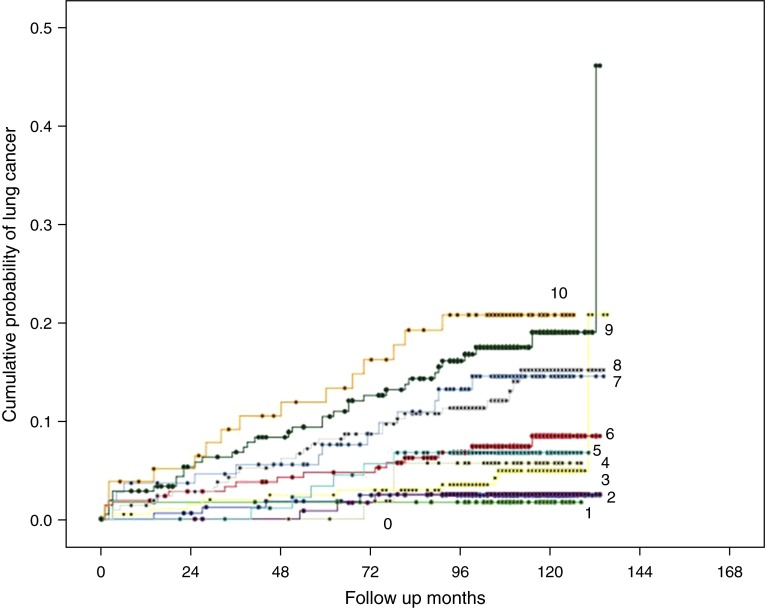
COPD-LUCSS risk categories were selected after visual inspection of the LC risk profile of each point of the COPD-LUCSS in the deriving cohort (Figure 1). Visually, groups 0–6 and 7–10 appear to be clustered, showing a gap between scores 6 and 7. Based on the grouping of the KM curves of each score, two categories were selected: high risk (scores 0–6) and low risk (scores 7–10). Cox regression was used to explore the association of (COPD-LUCSS) categories and the probability of LC diagnosis using low-risk category as reference.
Figure 1.
Kaplan-Meier survival curves comparing each chronic obstructive pulmonary disease lung cancer screening score lung cancer point risk profile in the derivation Pittsburgh Lung Screening Study chronic obstructive pulmonary disease cohort. Visually, groups 0–6 and 7–10 appear to be clustered, showing a gap between scores 6 and 7. Thus, risk groups were classified as low risk (0–6 points) and high risk (7–10 points).
To validate the score we then calculated the COPD-LUCSS in the validating cohort (P-IELCAP) using the previously defined score. Patients with COPD from P-IELCAP were then classified as low (those with scores 0–6) and high risk (those with scores 7–10) and their KM curves constructed for the cumulative risk of LC diagnosis.
To determine the accuracy of COPD-LUCSS, we compared its predictive capacity with that of the NLST entry criteria that have been chosen as selection criteria for screening by numerous approved guidelines. To that end, we compare the KM curves of those patients with and without NLST criteria with those in the highest and lowest risk categories of the COPD-LUCSS. Significant levels for all tests were established as a two-tailed P less than or equal to 0.05. Calculations were made with SPSS version 20.0 Inc. (IBM, Chicago, IL).
Results
The clinical and physiologic characteristics of both cohorts are shown in Table 2. In comparison with PLuSS, the COPD cohort in P-IELCAP was characterized by a lower proportion of women, fewer pack-years, more former smokers with longer abstinence times, a higher FEV1%, a higher proportion of patients in GOLD stages I-II, and fewer individuals with emphysema on LDCT. The groups have similar age, proportions of active smokers, and proportions of individuals with family history of LC. The frequency of diagnosis of LC was greater in PLuSS.
Table 2.
Clinical and Physiologic Characteristics of Both Cohorts
| P-IELCAP (n = 572) | PLuSS (n = 1,553) | P Value | |
|---|---|---|---|
| Age, yr* | 61 ± 9 | 61 ± 7 | 0.66 |
| Sex (female/male), % | 17/83 | 43/56 | <0.001 |
| BMI, kg/m2* | 27 ± 4 | 27 ± 5 | 0.05 |
| Pack-years† | 43 (30–60) | 53 (39–70) | <0.001 |
| Active smoker, % | 34 | 33 | 0.47 |
| FEV1%† | 79 (66–91) | 71 (58–83) | <0.001 |
| FEV1/FVC† | 62 (56–66) | 62 (55–66) | 0.97 |
| Years of former smoker† | 7 (3–15) | 4 (1–8) | <0.001 |
| Family history LC (yes), % | 17 | 17 | 0.89 |
| GOLD I-II vs. III-IV, % | 93 vs. 7 | 85 vs. 15 | <0.001 |
| LDCT emphysema, yes % | 45 | 62 | <0.001 |
| LC diagnosis, n (%) | 33 (6%) | 134 (9%) | 0.03 |
| LC incidence density | 2.29/100 | 1.05/100 | <0.001 |
| LC annual detection rate | 5.34 | 0.9 | |
| Follow-up, mo† | 13 (1–48) | 108 (95–118) | <0.001 |
Definition of abbreviations: BMI = body mass index; GOLD = Global Initiative for Obstructive Lung Disease; LC = lung cancer; LDCT = low-dose computed tomography; P-IELCAP = Pamplona International Early Lung Cancer Detection Program; PLuSS = Pittsburgh Lung Screening Study.
Mean ± SD.
Median (25th–75th percentiles).
No correlation was found between emphysema presence and FEV1% in the P-IELCAP cohort (Spearman correlation coefficient, 0.04; P = 0.31) and weak in the PLuSS cohort (Spearman correlation coefficient, −0.06; P = 0.01).
A total of 48% and 30% of the subjects with COPD in P-IELCAP and PLuSS, respectively, did not meet NLST criteria. These subjects would have not been screened if NLST criteria had been used. Of those diagnosed with LC, all of the individuals from P-IELCAP and 70% of those from PLuSS were in GOLD stages I-II.
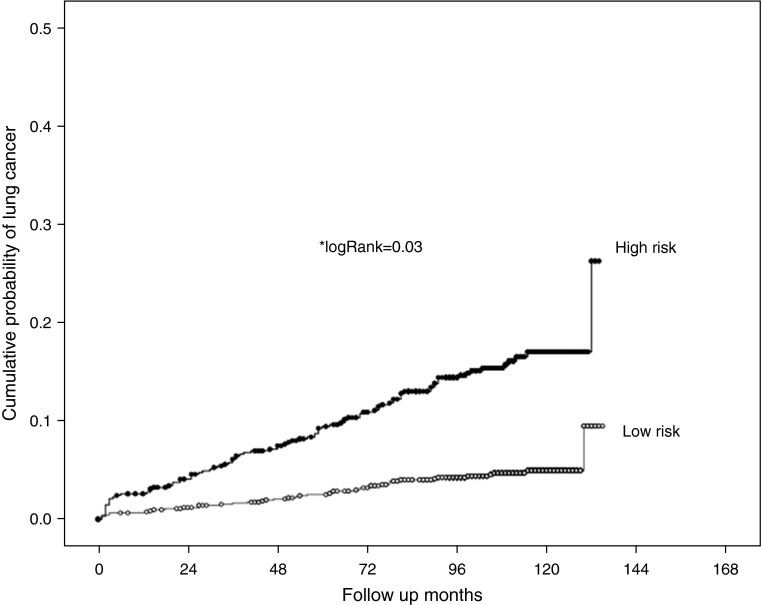
We then chose the PLuSS database to develop the COPD-LUCSS: an age greater than 60 years, a BMI less than 25 kg/m2, an accumulated smoking history of more than 60 pack-years, being in GOLD stages I or II, and the presence of emphysema on the LDCT were all independently associated with a diagnosis of LC on a Cox regression analysis (Table 3). After a multivariable analysis (Table 3), only age older than 60 years, a BMI less than 25 kg/m2, more than 60 pack-years of smoking, and emphysema on the LDCT remained independently associated with a diagnosis of LC. To build the COPD- LUCSS score, we assigned points to each parameter according to their HR values in the Cox regression (see Table E4). COPD-LUCSS scores range from 0 to 10 and proposed scores categories according to their KM risk profile are low risk (0–6 points) and high risk (7–10 points) (Figures 1 and 2). We used the Harrell C index to calculate the C statistics of our Cox regression model. The C statistics value for the model using continuous predictor variables was 0.698, whereas when using the 0–10 score with discretized predictor variables it was 0.691. The proposed score was then externally validated in the P-IELCAP validating cohort (see Figure E2) showing a similar risk profile. Cox regression analysis showed that patients in the highest risk category have a 3.5-fold greater risk of developing LC, both in the deriving and the validating cohorts (Table 4).
Table 3.
Univariable and Multivariable Cox Analysis Exploring the Independent Association of the Studied Variables with LC Diagnosis in the PLuSS Cohort
| P Value | HR | 95% CI | |
|---|---|---|---|
| Univariable Cox analysis | |||
| Age >60 vs. <60 | 0.001 | 2.5 | 1.6–3.7 |
| Sex, male vs. female | 0.88 | 0.9 | 0.7–1.4 |
| BMI <25 vs. >25 | 0.026 | 2.1 | 1.1–4.1 |
| Pack-years >60 vs. <60 | 0.001 | 1.9 | 1.3–2.6 |
| Active smoker | 0.37 | 1.2 | 0.8–1.6 |
| Years of former smoker | 0.47 | 1.6 | 0.5–5.0 |
| Family history LC, yes vs. no | 0.29 | 1.2 | 0.8–1.8 |
| GOLD I-II vs. III-IV | 0.04 | 1.4 | 1.04–1.6 |
| Emphysema, yes vs. no | 0.001 | 3.5 | 2.3–5.2 |
| Multivariable Cox analysis | |||
| Age >60 vs. <60 | 0.005 | 2.3 | 1.5–3.5 |
| BMI <25 vs. >25 | 0.15 | 1.2 | 0.9–1.8 |
| Pack-years >60 vs. <60 | 0.001 | 1.5 | 1.1–2.2 |
| Emphysema, yes vs. no | 0.001 | 2.7 | 1.7–4.3 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; GOLD = Global initiative for Obstructive Lung Disease; HR = hazard ratio; LC = lung cancer; PLuSS = Pittsburgh Lung Screening Study.
Figure 2.
Kaplan-Meier survival curves showing the lung cancer risk profile of patients in the low- and high-risk groups from the derivation cohort. Patients in the high-risk group (chronic obstructive pulmonary disease lung cancer screening scores 7–10 points) have a significant higher probability of lung cancer diagnosis than those in the lower risk group.
Table 4.
Cox Regression Analysis Comparing Each COPD-LUCSS Lung Cancer Category Risk
| P Value | HR | 95% CI | |
|---|---|---|---|
| P-IELCAP cohort | |||
| Low-risk vs. high-risk category | 0.001 | 3.5 | 1.7–7.1 |
| PLuSS cohort | |||
| Low-risk vs. high-risk category | 0.001 | 3.5 | 2.4–5.1 |
Definition of abbreviations: CI = confidence interval; COPD-LUCSS = chronic obstructive pulmonary disease lung cancer screening score; HR = hazard ratio; P-IELCAP = Pamplona International Early Lung Cancer Detection Program; PLuSS = Pittsburgh Lung Screening Study.
In both cohorts, KM curves of patients included in the highest COPD-LUCSS LC risk category allows a better identification of patients at risk (by visual comparison) than that obtained by NLST criteria (see Figures E2A and E2B).
Discussion
This study of the value of LC screening in spirometrically defined COPD in two international cohorts had several novel findings. First, using easily obtainable variables a LC risk score (COPD-LUCSS) was developed in the PLuSS cohort and then validated in the P-IELCAP cohort. Second, two LC risk categories were identified to help in the selection of high-risk patients with COPD that could benefit from specific preventive strategies, such as LC screening. Lastly, application of the current NLST entry criteria results in a high proportion of missed LCs in both sites.
COPD-Lung Cancer Score (COPD-LUCSS)
One of the most difficult challenges of screening programs is the appropriate selection of patients with an optimal risk-benefit ratio (25–28). The use of simple clinical risk factors, such as age, pack-years history, BMI, family history of LC, or self-reported COPD, integrated in a risk prediction score has been previously reported in the context of selecting the best candidates for LC screening (13–15). Kovalchick and coworkers (29) made an initial attempt to improve NLST's predictive capacity for a general population of current and former smokers by including emphysema among the parameters to be evaluated. These authors reported that including this radiologic feature better defined the LC risk profile, resulting in the prevention of more deaths. Tammemagi and coworkers (30) suggested that the use of spirometric values (FEV1%) in smokers could be a useful predictor for early LC detection, with a greater effect in men than women.
Because COPD and emphysema are among the strongest risk factors associated with LC in the setting of LC screening, we decided to expand LC screening criteria in a specific population of smokers or former smokers by including spirometrically proved COPD. Patients with COPD, especially those with mild to moderate lung function impairment and radiologically detected emphysema, have a twofold to threefold risk for LC development as compared with smokers without these characteristics (18, 19). Several mechanisms have been proposed to explain the association between COPD and LC, including genetic risk factors (31), chronic local and systemic inflammatory processes (32), decreased immune surveillance (33), uncontrolled stimulation of bronchioalveolar stem cells (32), and common epigenetic processes (34). Because of this association, and because LC is one of the most frequent causes of death in patients with COPD (35), it has been suggested that screening for LC in this population may have an excellent risk-benefit ratio for LC detection (10–12).
We developed COPD-LUCSS using two different COPD cohorts participating in different LC screening studies. We first identified predictor variables independently associated with LC risk in one of the cohorts (PLuSS) and used them to develop a score. We then validated the ability of this score to predict LC risk in the second cohort (P-ILECAP). The predictor variables included in the score included age greater than 60 years, a BMI less than 25 kg/m2, a smoking history greater than 60 pack-years, and the presence of emphysema on the LDCT. Two categories were defined according to the points sum on the COPD-LUCSS. Compared with low-risk category, patients with COPD in the high-risk category had a significantly higher risk of developing LC (Table 4). This finding has potential important clinical implications considering that patients with COPD in low-risk category have a twofold to threefold risk (unpublished data) of LC compared with active or former smokers without airway obstruction (18, 19).
Whether only patients with COPD in high-risk categories should be screened remains to be seen. However, it is possible that individuals in low-risk category may need less frequent screenings than those in high-risk category, resulting in a significant impact on the costs of screening. The development of a simple to use risk score based mainly on clinical parameters (age, smoking history, BMI) and the presence of CT-detected emphysema, could have important implications in the clinical management of patients with COPD. The identification of those at higher risk could tackle two important problems of this lethal association: the early detection of LC in patients with COPD, and the selection of individuals with the best risk-benefit ratio for LC screening programs. The latter may help in defining the optimal frequency of LC screenings in different populations.
Strengths and Limitations
The main strengths of the present study are the inclusion of two geographically different COPD populations and the development of a novel COPD-LUCSS, subsequently validated in a different COPD population. The study also has limitations. First, the findings should be restricted to patients with COPD similar to the ones included in these screening programs: individuals with mild to moderate degrees of airway obstruction. Further studies should be performed in patients with COPD not regularly seen in screening programs with more severe disease. Second, emphysema was detected qualitatively by LDCT. Unfortunately, we did not have information on diffusion capacity (an indirect measurement of emphysema presence, although not that specific) to include in the generation of this score without using LDCT because diffusing capacity of carbon monoxide has been shown to be an independent predictor of LC deaths in patients with COPD (36). Further studies should include this measurement to validate our findings.
Third, COPD participants from the P-IELCAP have a short follow-up time, an aspect that could limit our findings. However, if the patients would have been followed for a longer period of time, it is likely that a higher number of LC would have been diagnosed, thus enhancing our findings. Lastly, we should consider that patients with COPD have frequently associated comorbidities (37), such as cardiovascular disease, other types of cancers, and others that may impact on their morbidity and mortality. This could represent a competing cause of death or a limitation for lung resection of LC in early stages.
In conclusion, a COPD-specific score to predict LC risk, the COPD-LUCSS, may be useful to identify patients with COPD with the highest risk for LC. LC screening may have an impact on the high mortality rate from LC seen in patients with COPD.
Footnotes
Supported in part by a grant (RD12/0036/0062) from Red Temática de Investigación Cooperativa en Cáncer, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness and European Regional Development Fund “Una manera de hacer Europa,” Spanish Ministry of Health FIS Projects (PI04/2404, PI07/0792, PI10/01652, PI11/01626), the University of Pittsburgh Lung Cancer SPORE (NCI P50-CA90440, 1P50 HL084948), University of Pittsburgh Cancer Institute, and University of Pittsburgh Medical Center.
Author Contributions: J.P.d.-T., M.G.-G., D.O.W., P.S.-S., B.R.C., and J.J.Z., conception and design, analysis and interpretation, and drafting the manuscript for important intellectual content. J.L.W., J.B., A.B.A., and A.C., conception and design and analysis and interpretation.
Originally Published in Press as DOI: 10.1164/rccm.201407-1210OC on December 18, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986;105:503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 3.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, Hagan G, Knobil K, Lomas DA, MacNee W, et al. ECLIPSE investigators. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 5.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Lung Association Providing guidance on lung cancer screening to patients and physicians 2012. Apr 23 [accessed 2013 Oct 17]. Available from: http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf
- 7.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e78S–92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaklitsch MT, Jacobson FL, Austin JHM, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 9.Wender R, Fontham ETH, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RP, Hopkins RJ. Targeted CT image screening and its effect on lung cancer detection rate. Chest. 2013;144:1419–1420. doi: 10.1378/chest.13-1321. [DOI] [PubMed] [Google Scholar]
- 11.Celli BR. Chronic obstructive pulmonary disease and lung cancer: common pathogenesis, shared clinical challenges. Proc Am Thorac Soc. 2012;9:74–79. doi: 10.1513/pats.201107-039MS. [DOI] [PubMed] [Google Scholar]
- 12.de-Torres JP, Casanova C, Marín JM, Zagaceta J, Alcaide AB, Seijo LM, Campo A, Carrizo S, Montes U, Cordoba-Lanus E, et al. Exploring the impact of screening with low-dose CT on lung cancer mortality in mild to moderate COPD patients: a pilot study. Respir Med. 2013;107:702–707. doi: 10.1016/j.rmed.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Young RP, Hopkins RJ. Diagnosing COPD and targeted lung cancer screening. Eur Respir J. 2012;40:1063–1064. doi: 10.1183/09031936.00070012. [DOI] [PubMed] [Google Scholar]
- 14.Young RP, Hopkins RJ.CT screening in COPD: impact on lung cancer mortality: de Torres JP, Casanova C, Marin JMet al. Exploring the impact of screening with low-dose CT on lung cancer mortality in mild to moderate COPD patients: a pilot study. Respir Med 2013; 107: 702–707 Respir Med 2014108813–814. [DOI] [PubMed] [Google Scholar]
- 15.Young RP, Hopkins RJ, Hay BA, Epton MJ, Mills GD, Black PN, Gardner HD, Sullivan R, Gamble GD. A gene-based risk score for lung cancer susceptibility in smokers and ex-smokers. Postgrad Med J. 2009;85:515–524. doi: 10.1136/pgmj.2008.077107. [DOI] [PubMed] [Google Scholar]
- 16.Young RP, Hopkins RJ, Duan F, Deng X, Gamble GD, Chiles C, Aberle DH. Lung cancer susceptibility SNPs add to risk prediction score for lung cancer: a sub-analysis of the ACRIN-Biomarker arm of the NLST. Am J Respir Crit Care Med. 2014;189:A5603. [Google Scholar]
- 17.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 19.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345:1075–1083. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 22.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 23.Bastarrika G, Wisnivesky JP, Pueyo JC, Díaz L, Arraiza M, Villanueva A, Alcaide AB, Campo A, Seijo L, de Torres JP, et al. Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. J Thorac Imaging. 2009;24:206–211. doi: 10.1097/RTI.0b013e3181a65263. [DOI] [PubMed] [Google Scholar]
- 24.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 25.Young RP, Hopkins RJ.Lung cancer risk prediction to select smokers for screening CT—letter Cancer Prev Res (Phila) 20125697–698.author reply 699 [DOI] [PubMed] [Google Scholar]
- 26.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 27.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157:571–573. doi: 10.7326/0003-4819-157-8-201210160-00524. [DOI] [PubMed] [Google Scholar]
- 28.Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, Klippenstein D, Lackner RP, Leard L, Leung AN, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10:240–265. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, Silvestri GA, Chaturvedi AK, Katki HA. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tammemagi MC, Lam SC, McWilliams AM, Sin DD. Incremental value of pulmonary function and sputum DNA image cytometry in lung cancer risk prediction. Cancer Prev Res (Phila) 2011;4:552–561. doi: 10.1158/1940-6207.CAPR-10-0183. [DOI] [PubMed] [Google Scholar]
- 31.Young RP, Hopkins RJ, Gamble GD, Etzel C, El-Zein R, Crapo JD. Genetic evidence linking lung cancer and COPD: a new perspective. Appl Clin Genet. 2011;4:99–111. doi: 10.2147/TACG.S20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 33.Rama I, Grinyó JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010;6:511–519. doi: 10.1038/nrneph.2010.102. [DOI] [PubMed] [Google Scholar]
- 34.Bowman RV, Yang IA, Semmler AB, Fong KM. Epigenetics of lung cancer. Respirology. 2006;11:355–365. doi: 10.1111/j.1440-1843.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 35.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 36.de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Dávila R, Zulueta JJ, Aguirre-Jaime A, Saetta M, et al. Lung cancer in patients with chronic obstructive pulmonary disease: incidence and predicting factors. Am J Respir Crit Care Med. 2011;184:913–919. doi: 10.1164/rccm.201103-0430OC. [DOI] [PubMed] [Google Scholar]
- 37.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake G, et al. BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]