Abstract
Rationale: In 2005, the lung allocation score (LAS) was implemented to prioritize organ allocation to minimize waiting-list mortality and maximize 1-year survival. It resulted in transplantation of older and sicker patients without changing 1-year survival. Its effect on resource use is unknown.
Objectives: To determine changes in resource use over time in lung transplant admissions.
Methods: Solid organ transplant recipients were identified within the Nationwide Inpatient Sample (NIS) data from 2000 to 2011. Joinpoint regression methodology was performed to identify a time point of change in mean total hospital charges among lung transplant and other solid-organ transplant recipients. Two temporal lung transplant recipient cohorts identified by joinpoint regression were compared for baseline characteristics and resource use, including total charges for index hospitalization, charges per day, length of stay, discharge disposition, tracheostomy, and need for extracorporeal membrane oxygenation.
Measurements and Main Results: A significant point of increased total hospital charges occurred for lung transplant recipients in 2005, corresponding to LAS implementation, which was not seen in other solid-organ transplant recipients. Total transplant hospital charges increased by 40% in the post-LAS cohort ($569,942 [$53,229] vs. $407,489 [$28,360]) along with an increased median length of stay, daily charges, and discharge disposition other than to home. Post-LAS recipients also had higher post-transplant use of extracorporeal membrane oxygenation (odds ratio, 2.35; 95% confidence interval, 1.56–3.55) and higher incidence of tracheostomy (odds ratio, 1.52; 95% confidence interval, 1.22–1.89).
Conclusions: LAS implementation is associated with a significant increase in resource use during index hospitalization for lung transplant.
Keywords: lung transplant, lung allocation score, lung transplant cost, solid-organ transplant, Nationwide Inpatient Sample
At a Glance Commentary
Scientific Knowledge on the Subject
Introduction of the lung allocation score (LAS) has led to transplantation of sicker and older patients without changing 1-year mortality. However, the effect of LAS on resource use is unknown.
What This Study Adds to the Field
LAS implementation is associated with a significant increase in resource use during index hospitalization for lung transplant.
Lung transplantation for patients with end-stage lung disease is associated with improved survival and quality of life (1, 2). In May 2005, the Organ Procurement and Transplantation Network (OPTN) implemented the lung allocation score (LAS; a composite score incorporating physiologic and comorbid variables that predict waitlist mortality and 1-year post-transplant survival) to maximize recipient population benefit by reducing waitlist mortality (3). Implementation of the LAS altered the recipient population leading to the listing and transplantation of older patients with greater illness severity (4). Although 1-year survival has been preserved in the post-LAS era, increasing LAS severity, which comprises a small but growing proportion of transplant recipients, has been associated with worse 1-year survival (4–10). Also, although long-term overall survival is preserved (4, 11), 1-year conditional survival has worsened post-LAS, suggesting potential negative effects of the LAS on long-term survival (11).
Whether greater resource use has been required to maintain short-term survival despite transplanting older and sicker recipients in the LAS era has not been adequately explored. In a single-institution study, significantly higher index hospital admission charges and 1-year post-transplant hospital charges were found in recipients in the highest LAS quartile (12). Similarly, after implementation of the Swiss organ allocation system that prioritized allocation based on medical urgency, there was a marked trend toward increased post-transplant hospital costs without a change in 1-year mortality (13). Although these single-institution studies suggest increased health care use is required in higher-acuity recipients, the overall impact of LAS implementation on healthcare resource use is unknown. Understanding the impact of LAS implementation on lung transplant resource use is integral to defining overall transplant benefit and optimizing organ allocation.
We hypothesized that implementation of the LAS increased health care use during the index lung transplant hospitalization. To answer this question, we analyzed hospital charges and discharge disposition data for all index hospitalizations for solid-organ transplant available in the Nationwide Inpatient Sample (NIS) database. Some of the results of this study have been previously presented in the form of an abstract (14).
Methods
The Stanford University Institutional Review Board granted an exemption from review because this research uses deidentified data. Administrative records were extracted from discharge datasets for the years 2000–2011 from the NIS, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. The NIS is the largest publicly available, all-payer database for inpatient care in the United States. Each dataset year includes records on 7–8 million admissions from approximately 1,000 hospitals in 37 states, which reflect a 20% stratified sample of all US nonfederal, nonrehabilitation hospitals (15). It contains discharge sample weights to facilitate nationally representative estimates based on the sampling design.
Healthcare Cost and Utilization Project–supplied Clinical Classifications Software for International Classification of Diseases, Ninth Revision, Clinical Modification was used to generate diagnostic, comorbidity, and procedural classification codes (16). Using International Classification of Diseases, Ninth Revision, Clinical Modification Volume 3 procedure codes, admission records in which a solid-organ transplant was performed were retained for further examination (see Appendix A in the online supplement) (17, 18). Admissions for patients younger than 18 years of age and admissions for cornea transplantation, skin grafting, and bone marrow transplantation were excluded. Discharge weights were used to create national estimates for transplant admissions within the NIS stratified sampling frame.
To establish if lung transplant admissions captured by the NIS closely approximate all transplant procedures performed in the United States, we compared summary statistics for all lung transplant procedures recorded in the comprehensive datasets from the OPTN through the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by members of the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. Using SRTR Standard Analysis Files, all adults (age ≥ 18 yr) who underwent lung transplant between January 1, 2000 and December 31, 2011 were included for analysis. Descriptive and outcome variables were generated from existing SRTR data fields to compare with those available through the NIS. The SRTR data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
The primary outcome from analysis of NIS records was total inpatient hospital charges for the index admission for solid-organ transplant. Secondary outcomes included median length of stay, mean hospital charges per day (as a measure of resource use density), and in-hospital mortality. Discharge disposition (for patients discharged alive) was defined as discharge to home, transfer to another acute-care hospital, or discharge to rehabilitation (including skilled nursing facility, long-term acute care hospital, or home-based nursing care), and then summarized as routine discharges to home or other. Procedure codes were used to identify performance of tracheostomy, renal failure requiring renal-replacement therapy, and the use of pretransplant or post-transplant extracorporeal membrane oxygenation (ECMO).
Hospital charges and charges per day were adjusted for inflation specific to inpatient care by adjusting all values to 2011 dollars using the Bureau of Labor Statistics Consumer Price Index. The Consumer Price Index subindex specific to inpatient hospital services was used with a baseline of December 1996 taken as 100, and yearly Consumer Price Index values used to generate a conversion factor to 2011 dollars (19).
Statistical Analysis
To determine whether a change in mean total charges occurred and to identify the timing of any changes, joinpoint regression of total charges was used and is described in detail in Appendix B. Joinpoint regression identified a time point of major change, which was used to divide the total population of lung transplant admissions into two temporal cohorts: January 1, 2000 through December 31, 2005 (pre-LAS); and January 1, 2006 through December 31, 2011 (post-LAS). A secondary analysis was performed to compare baseline, outcome, and resource use parameters between these two cohorts. To control for any temporal trends in increased resource use specific to transplantation, we used difference-in-differences analyses, applying interaction terms between type (lung vs. other) and time (pre-LAS vs. post-LAS) of transplant.
Because the stratified sampling frame of the NIS requires the use of advanced techniques to estimate variance, continuous variables are presented as mean (standard deviation) and categorical variables are described using percentages plus estimated national numbers. The Wilcoxon rank-sum, chi-square, or analysis of variance were used to compare variables between different cohorts. For outcome variables, odds ratios (ORs) with 95% confidence intervals (CIs) also were calculated. A predetermined α of 0.05 was used as the threshold of statistical significance for the primary outcome and the composite secondary outcome. Joinpoint regression was performed using dedicated software developed by the National Cancer Institute (Joinpoint Regression Program, version 4.1.0; Statistical Methodology and Applications Branch, National Cancer Institute Surveillance Research Program, Bethesda, MD). All other analyses were performed using SAS (SAS 9.3; SAS Institute, Cary, NC).
Results
The baseline demographics and clinical characteristics of the NIS lung transplant recipients as compared with all U.S. lung transplant recipients as captured by the SRTR registry are comparable and shown in Table 1. Within the NIS datasets (extrapolated to total U.S. hospital admissions), 6,361 lung transplant recipients were identified in the pre-LAS era (2000–2005) and 9,922 recipients in the post-LAS era (2006–2011). Recipients in the post-LAS era were older, were more likely to be male, were of increased ethnic diversity, were more likely to have major medical comorbidities, were more likely to be hospitalized prior to transplant, and were more likely to have restrictive lung disease (LAS group D) than obstructive lung disease (LAS group A). They were also more likely to have Medicare insurance and less likely to have private or health maintenance organization insurance (Table 2).
Table 1.
Comparison of Baseline and Outcome Characteristics of National Estimates of Lung Transplant Admissions in the NIS and Those Represented in the SRTR Registry
| NIS (n = 16,283)* | SRTR (n = 16,412) | Delta | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Recipient age, yr | 53.0 | ±0.7 | 51.1 | ±0.1 | +1.9 |
| Male | 9,187 | (56.4%) | 9,047 | (55.1%) | +1.3% |
| Race | |||||
| White | 13,514 | (83.0%) | 14,028 | (85.5%) | −2.5% |
| Asian/Pacific Islander | 192 | (1.2%) | 198 | (1.2%) | −<0.1% |
| Black | 1,169 | (7.2%) | 1,289 | (7.9%) | −0.7% |
| Hispanic | 1,005 | (6.2%) | 789 | (4.8%) | +1.4% |
| Other | 403 | (2.5%) | 108 | (0.7%) | +1.8% |
| Payer | |||||
| Medicare | 5,831 | (35.8%) | 4,940 | (30.1%) | +5.7% |
| Medicaid | 1,071 | (6.6%) | 1,339 | (8.2%) | −1.6% |
| Private | 8,507 | (52.2%) | 9,610 | (58.6%) | −6.4% |
| Self-pay/other | 874 | (5.4%) | 523 | (3.2%) | +2.2% |
| Major medical comorbidities | |||||
| Hypertension | 4,498 | (27.6%) | 3,635 | (22.1%) | +5.5% |
| Diabetes | 3,600 | (22.1%) | 2,757 | (16.8%) | +5.3% |
| Clinical characteristics | |||||
| Medical status prior to transplant | |||||
| Hospitalized | 2,329 | (14.3%) | 2,378 | (14.5%) | −0.2% |
| On ECMO | 142 | (0.9%) | 184 | (1.1%) | −0.2% |
| LAS primary diagnosis group | |||||
| A (obstructive lung disease) | 5,925 | (36.4%) | 6,856 | (41.8%) | −5.4% |
| B (pulmonary vascular disease) | 558 | (3.4%) | 715 | (4.4%) | −1.0% |
| C (CF/immunodeficiency) | 2,160 | (13.3%) | 2,618 | (16.0%) | −2.7% |
| D (restrictive lung disease) | 6,806 | (41.8%) | 6,121 | (37.3%) | +4.5% |
| Other | 834 | (5.1%) | 102 | (0.6%) | +4.5% |
| Era | |||||
| 2000–2005 | 6,361 | (39.1%) | 6,803 | (41.5%) | −2.4% |
| 2006–2011 | 9,922 | (60.9%) | 9,609 | (58.5%) | +2.4% |
| Admission characteristics | |||||
| Length of stay, d | 24.9 | ±1.6 | 28.4 | ±0.4 | −3.5 |
| Inpatient mortality | 1,160 | (7.1%) | 1,156 | (7.0%) | +0.1% |
Definition of abbreviations: CF = cystic fibrosis; ECMO = extracorporeal membrane oxygenation; LAS = lung allocation score; NIS = National Inpatient Survey; SRTR = Scientific Registry of Transplant Recipients.
Extrapolated.
Table 2.
Comparison of Lung Transplant Admissions from Two Temporal Cohorts before and after Data-derived Joinpoint
| Pre-LAS (2000–2005) (n = 6,361) | Post-LAS (2006–2011) (n = 9,922) | P Value | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Recipient age, yr | 50.9 | ±0.7 | 54.4 | ±0.6 | <0.0001 |
| Male | 3,279 | (51.6%) | 5,908 | (59.5%) | <0.0001 |
| Race | 0.0024 | ||||
| White | 5,423 | (85.3%) | 8,091 | (81.5%) | |
| Asian/Pacific Islander | 45 | (0.7%) | 147 | (1.5%) | |
| Black | 447 | (7.0%) | 722 | (7.3%) | |
| Hispanic | 319 | (5.0%) | 686 | (6.9%) | |
| Other | 127 | (2.0%) | 276 | (2.8%) | |
| Payer | <0.0001 | ||||
| Medicare | 1,887 | (29.7%) | 3,944 | (39.8%) | |
| Medicaid | 499 | (7.8%) | 572 | (5.8%) | |
| Private/HMO | 3,669 | (57.7%) | 4,838 | (48.8%) | |
| Self-pay/other | 306 | (4.8%) | 568 | (5.7%) | |
| Major medical comorbidities | |||||
| Hypertension | 1,324 | (20.8%) | 3,174 | (32.0%) | <0.0001 |
| Diabetes | 746 | (11.7%) | 2,854 | (28.8%) | <0.0001 |
| Clinical characteristics | |||||
| Medical status prior to transplant | |||||
| Not hospitalized | 5,797 | (91.1%) | 8,157 | (82.2%) | |
| Hospitalized | 564 | (8.9%) | 1,765 | (17.8%) | <0.0001 |
| On ECMO | 39 | (0.6%) | 103 | (1.0%) | 0.25 |
| LAS primary diagnosis group | <0.0001 | ||||
| A (obstructive lung disease) | 2,874 | (45.2%) | 3,051 | (30.8%) | |
| B (pulmonary vascular disease) | 277 | (4.3%) | 281 | (2.8%) | |
| C (CF/immunodeficiency) | 933 | (14.7%) | 1,227 | (12.4%) | |
| D (restrictive lung disease) | 1,995 | (31.4%) | 4,811 | (48.5%) | |
| Other | 282 | (4.4%) | 552 | (5.6%) | |
Definition of abbreviations: CF = cystic fibrosis; ECMO = extracorporeal membrane oxygenation; LAS = lung allocation score; HMO = health maintenance organization.
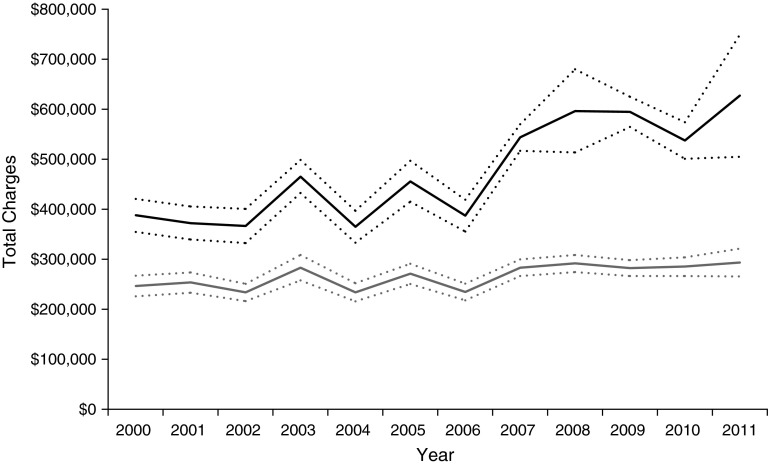
Total hospital charges per admission for lung transplant compared with other solid-organ transplant recipients are shown in Figure 1. Joinpoint regression selected a model with one joinpoint at 2005 (95% CI, 2003–2007) for lung transplant recipients identifying a significant temporal point of change in increased total hospital charges per admission. No joinpoint was identified for other solid-organ transplant recipients (P = 0.47). Appendix C shows the full details of model testing and the parameters of the final model.
Figure 1.
Total hospital charges per admission for patients undergoing lung transplant (black) and other solid-organ transplant (gray). Dotted lines denote ±standard error.
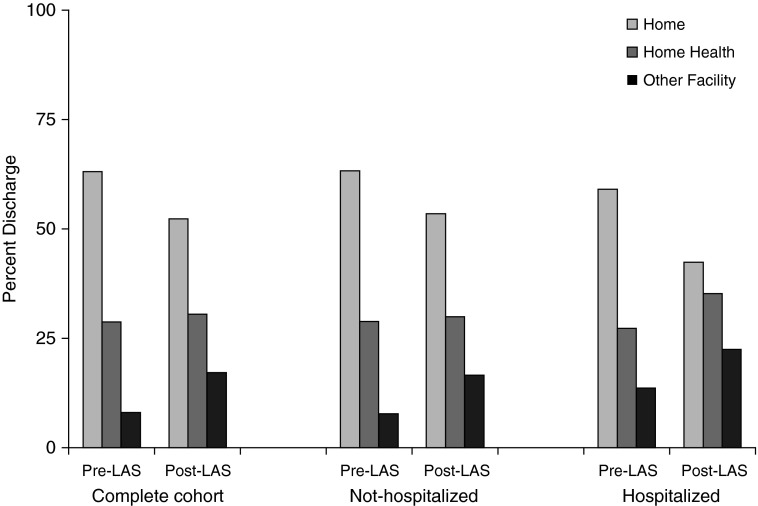
Among lung transplants, the post-LAS cohort had a significant 40% increase in total charges ($569,942 [53,229] vs. $407,489 [28,360]) compared with the pre-LAS cohort (difference-in-differences relative to nonlung transplants, $138,572; P < 0.0001) along with longer median length of stay (difference-in-differences, 6.3 d; P < 0.0001) and increased charges per day. Discharge disposition other than to home including in-hospital death, home nursing, rehabilitation, and skilled nursing facility discharges were increased in the post-LAS cohort (OR, 1.42; 95% CI, 1.23–1.63) as shown in Figure 2. Specifically, post-LAS recipients were more likely to be discharged to a skilled nursing facility or rehabilitation hospital (17.2% vs. 8.1%) or home with nursing care (30.5% vs. 28.8%) compared with pre-LAS recipients. Post-LAS recipients had higher post-transplant use of ECMO (OR, 2.35; 95% CI, 1.56–3.55) and higher incidence of tracheostomy (OR, 1.52; 95% CI, 1.22–1.89) compared with pre-LAS recipients. Difference-in-differences relative to nonlung transplants were also significant for all outcomes (P < 0.01) except for use of ECMO (P = 0.84). There was no difference in the incidence of hemodialysis between the two cohorts (Table 3).
Figure 2.
Percentage of admissions resulting in routine discharge to home, home health, or other facilities. LAS = lung allocation score.
Table 3.
Outcome Comparisons of Lung Transplant Admissions from Two Temporal Cohorts
| Pre-LAS (2000–2005) |
Post-LAS (2006–2011) |
P Value | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|
| n = 6,361 | n = 9,922 | ||||||
| Total charges, 2011
dollars |
$407,489 |
±$28,360 |
$569,942 |
±$53,229 |
<0.0001 |
|
|
| Length of stay, d | 22.1 | ±1.4 | 26.6 | ±2.0 | <0.0001 | ||
| Length of stay ≥30 d | 1,151 | (18.1%) | 2,598 | (26.2%) | <0.0001 | 1.61 | (1.35–1.91) |
| Length of stay ≥60 d | 356 | (5.6%) | 877 | (8.8%) | <0.0001 | 1.64 | (1.23–2.17) |
| Charges per day, 2011 dollars | $29,330 | ±$2,764 | $29,788 | ±$1,431 | <0.0001 | ||
| Hemodialysis during admission | 173 | (2.7%) | 261 | (2.6%) | 0.91 | 0.97 | (0.63–1.01) |
| Tracheostomy performed during admission | 652 | (10.3%) | 1,463 | (14.8%) | <0.0001 | 1.52 | (1.22–1.89) |
| Post-transplant ECMO | 148 | (2.3%) | 528 | (5.3%) | <0.0001 | 2.35 | (1.56–3.55) |
| In-hospital death | 539 | (8.5%) | 621 | (6.3%) | 0.018 | 0.72 | (0.55–0.94) |
| Discharge disposition* | <0.0001 | ||||||
| Home | 3,672 | (63.1%) | 4,865 | (52.3%) | |||
| Home with nursing | 1,676 | (28.8%) | 2,834 | (30.5%) | |||
| Skilled nursing facility/rehab hospital | 474 | (8.1%) | 1,602 | (17.2%) | |||
| Discharge to other than home† | 2,689 | (42.3%) | 5,057 | (51.0%) | <0.0001 | 1.42 | (1.23–1.63) |
Definition of abbreviations: CI = confidence interval; ECMO = extracorporeal membrane oxygenation; LAS = lung allocation score; OR = odds ratio.
Of those alive at discharge.
Of all admissions; includes in-hospital death, home nursing, rehabilitation, and skilled nursing facility discharges.
Because a greater proportion of patients in the post-LAS cohort were inpatients at the time of transplant, we stratified our analysis by inpatient status at the time of transplant to address confounding by the inclusion of pretransplant charges impacting charges for the entire hospital admission. The increase in average total hospital charges, charges per day, use of tracheostomy, use of post-transplant ECMO, and proportion of patients discharged to other than home remains in both subgroups in the post-LAS era (see Appendix D). Given that the LAS was implemented in May 2005 and that the NIS database does not reliably distinguish within-year charges, an analysis of three temporal cohorts (2000–2004, pre-LAS; 2005, peri-LAS; and 2006–2011, post-LAS) was performed that demonstrates an intermediate state of resource use in the 2005 cohort (see Appendix E).
Discussion
This study demonstrates a novel association between LAS implementation and an increase in healthcare resource use among lung transplant recipients. We identified a significant point of change in total hospital charges for the index admission for transplant in lung transplant recipients but not other solid-organ transplants and this time coincides with implementation of the LAS in 2005. In the post-LAS era, total hospital charges during the index hospitalization for lung transplant increased by 40%. Length of stay, daily charges, disposition other than to home, use of ECMO, and use of tracheostomy also increased. Conversely, in-hospital mortality significantly decreased.
Post-LAS recipients were older with greater comorbidities and illness severity so it is notable that in-hospital survival improved. However, although we found a 2% absolute decrease in mortality, discharge to a skilled nursing facility or acute rehabilitation hospital increased by 9%. Although increased discharge to a rehabilitation facility represents increased resource use, we cannot comment on whether this represents an extension of inpatient care to speed recovery post-transplant, is a measure of increased disability post-transplant requiring extended specialized care, or reflects a shifting of mortality beyond the index hospitalization. Further characterization of these recipients and their long-term outcomes is important in assessing the value of this resource use.
Although higher LAS scores have been associated with decreased 1-year survival (9, 10), 1-year survival has been preserved in the post-LAS era despite increasing recipient age and illness severity (4, 6–8, 20). We have previously identified a transient increase in mortality in months 13 and 14 post-transplant and a decreased overall survival among patients surviving beyond 1 year in the post-LAS era (11). Although limited to the index hospitalization for transplant, our current findings suggest that increased resource use is an important component of sustaining 1-year survival under the LAS. Longitudinal tracking of lung transplant recipient resource use beyond the index hospitalization through use of payers’ data is needed to further explore this association.
There is a scarcity of literature regarding cost and resource use in lung transplantation, particularly in the post-LAS era. Pre-LAS studies demonstrated that despite high costs associated with transplantation there was an improvement in quality-adjusted life years and identified cost of care and marginal gains in life expectancy as the greatest limitation to overall cost-effectiveness (21). Furthermore, patients with cystic fibrosis had the greatest benefit with the highest quality-adjusted life years gained among recipient disease groups, with patients with restrictive lung disease among the lowest in mean life years and quality-adjusted life years gained (22). The effect of the LAS on cost-effectiveness and cost-utility is beyond the scope of this article and deserves future study. Within the LAS era, Arnaoutakis and colleagues (12) demonstrated increased index admission charges, length of hospital stay, and first year hospital charges in high LAS lung transplant recipients at a single center. Our study adds to their work by identifying a significant nationwide increase in health care use during the index admission for lung transplant under the LAS system.
The benefit of lung transplantation is to prolong survival and/or improve quality of life in patients with advanced lung disease. The current LAS system prioritizes survival benefit over other post-transplant outcomes, such as graft survival and health-related quality of life, by disproportionately weighing waiting-list mortality over post-transplant survival. Transplant benefit is exclusively defined as survival benefit without consideration of other nonsurvival outcomes, such as health-related quality of life. Although the LAS system has reduced waiting-list mortality without impacting 1-year survival, there is appropriate concern regarding its negative affect on long-term survival and whether it truly optimizes the benefit derived from scarce donor organs (11, 23). When transplant benefit was redefined by equally weighing median graft survival and median waiting-list survival, Russo and colleagues (24) demonstrated that recipients with LAS between 50 and 79 have the greatest transplant benefit. This suggests that the current disproportionate weight on waiting-list survival and prioritization of sicker patients may adversely affect post-transplant morbidity and mortality. This is supported by prior association between higher LAS scores and diminished 1-year transplant survival, higher post-transplant hospital charges, and increased resource use (9, 10, 12).
The association between LAS implementation and increased health care use reveals a significant impact of the LAS on an important nonmortality outcome that has not been previously reported and is not currently captured by transplant registries. This finding alone carries important ramifications given the current economic strain on the U.S. healthcare system and limited donor supply. Solid-organ transplant funding, including elimination of lung transplantation funding, was targeted previously in Arizona Medicaid cost savings legislation (25). Further research focused on the economics and cost-utility surrounding lung transplantation is needed to ensure universal recipient coverage and appropriate transplant center reimbursement of this life-saving procedure.
In addition to costs, the increase in resource use, specifically prolonged hospital length of stay, disposition other than to home, and tracheostomy, likely impact health-related quality of life. This is an important benefit that patients weigh heavily in pursuing lung transplant but is not currently considered in defining transplant benefit. Further exploration of the effect of LAS implementation on both long-term resource use and health-related quality of life is integral to informing decisions on how to measure transplant benefit and design organ allocation policy. Historically, these efforts have been limited by absence of validated patient-centered outcome assessment tools in lung transplantation patients; however, a validated questionnaire has recently been developed (26). Transplant registries should begin capturing pertinent nonmortality outcomes in both waiting list and recipient patients.
Although the NIS sampling frame is designed to produce nationally representative estimates, little prior literature exists to establish whether admissions types that do not occur across a full range of hospitals (e.g., transplant procedures) are adequately captured and represented by the NIS sampling frame. We compared summary statistics between the NIS lung transplant cohort and the complete universe of U.S. lung transplant recipients recorded in the SRTR registry to assess the validity of the NIS for organ transplant analysis. We found that the NIS’s stratified sampling methods closely approximate the full population of national lung transplant admissions (Table 1) and allow analysis of resource use not captured in the SRTR and other registries.
Inherent limitations of the NIS database do exist. First, the admissions-based design of the NIS precludes recipient-level tracking through multiple inpatient encounters. Because our analysis included only the initial admission for the transplant procedure, it underestimates the cumulative resources expended on lung transplant patients through multiple admissions. Using diagnosis codes for the presence of a transplanted organ (e.g., V42.6, “Lung replaced by transplant”) would capture cumulative longitudinal hospital charges for subsequent admissions in lung transplant patients, but would be confounded by the accrual over time of more lung transplant patients alive in the United States. Analyzing all hospitalizations of transplant patients would not indicate an increase in charges per patient or allow a meaningful analysis of resource use across different indications for admission.
Second, charges and procedure codes are available only for the entire hospital admission limiting isolation of contributors to the total resources directly related to lung transplant. We performed a sensitivity analyses stratified by inpatient status at time of transplant and found similar trends among both inpatients and outpatients. We also analyzed multiple secondary endpoints (e.g., tracheostomy, hemodialysis, ECMO use) to describe trends in these contributing comorbidities. Among other solid-organ transplant recipients, there was no significant change in tracheostomy use; however, there was a significant nationwide increase in ECMO use, as previously described (27).
Third, we acknowledge that the LAS was implemented on May 4, 2005. However, because of limitations of NIS data reporting we cannot accurately distinguish monthly or daily charges and thereby provide a clean pre-LAS cohort (January 1, 2000–May 3, 2005) and post-LAS cohort (May 4, 2005–December 31, 2011). Therefore, we conservatively included all of 2005 in the pre-LAS cohort, an approach that would theoretically decrease the magnitude of the effect of the LAS on resource use. To further address this limitation, we performed a sensitivity analysis of three temporal cohorts (2000–2004, pre-LAS; 2005, LAS; and 2006–2011, post-LAS), as shown in Appendix E, which supports our assumption that this limitation minimizes the magnitude of the LAS effect on resource use.
Finally, total hospital charges as reported in the NIS represent hospital billing and cannot be interpreted as actual expenditures or true costs. However, assuming a fixed relationship between charges and costs over time, this trend in charges allows temporal comparisons of resource use national level data. This approach has been previously established (28–32). Additionally, we performed difference-in-differences analyses for lung relative to nonlung transplants to control for potential temporal trends unique to organ transplantation not accounted for by adjustment for inflation specific to nationwide inpatient hospital charges.
Conclusions
The association between LAS implementation and a significant point of change in total hospital charges and resource use for the index admission for transplant in lung transplant recipients highlights the importance of ongoing assessment of organ allocation systems. Increased understanding of relevant nonmortality outcomes will be a critical component in defining overall transplant benefit and optimizing allocation policy of scarce donor lungs.
Footnotes
Supported in part by HL095686 (M.R.N.) and the Ranzetta Family Foundation.
Author Contributions: B.G.M. and J.J.M. contributed equally to the design of the study, data analysis and interpretation, and drafting and revising of the article. P.H.U.L., J.E.L., L.C., M.R.N., M.R.Z., V.V., and D.W. made substantial contributions to interpretation of data and revising the article. G.S.D. was responsible for conception and design of study, acquisition and interpretation of the data, and contributed critically to drafting and revising the article. B.G.M., J.J.M., and G.S.D. take responsibility for the integrity of the data and the accuracy of the data analysis. All authors provided final approval of the version to be published.
Originally Published in Press as DOI: 10.1164/rccm.201408-1562OC on December 17, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gross CR, Savik K, Bolman RM, III, Hertz MI. Long-term health status and quality of life outcomes of lung transplant recipients. Chest. 1995;108:1587–1593. doi: 10.1378/chest.108.6.1587. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, et al. The registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, et al. Development of the new lung allocation system in the United States. Am J Transplantation. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 4.Valapour M, Skeans MA, Heubner BM, Smith JM, Schnitzler MA, Hertz MI, Edwards LB, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 annual data report: lung. Am J Transplantation. 2014;14(Suppl. 1):139–165. doi: 10.1111/ajt.12584. [DOI] [PubMed] [Google Scholar]
- 5.Lingaraju R, Blumenthal NP, Kotloff RM, Christie J, Ahya VN, Sager JS, Pochettino A, Hadjiliadis D. Effects of lung allocation score on waiting list rankings and transplant procedures. J Heart Lung Transplant. 2006;25:1167–1170. doi: 10.1016/j.healun.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Gries CJ, Mulligan MS, Edelman JD, Raghu G, Curtis JR, Goss CH. Lung allocation score for lung transplantation: impact on disease severity and survival. Chest. 2007;132:1954–1961. doi: 10.1378/chest.07-1160. [DOI] [PubMed] [Google Scholar]
- 7.Kozower BD, Meyers BF, Smith MA, De Oliveira NC, Cassivi SD, Guthrie TJ, Wang H, Ryan BJ, Shen KR, Daniel TM, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. J Thorac Cardiovasc Surg. 2008;135:166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 8.McCue JD, Mooney J, Quail J, Arrington A, Herrington C, Dahlberg PS. Ninety-day mortality and major complications are not affected by use of lung allocation score. J Heart Lung Transplant. 2008;27:192–196. doi: 10.1016/j.healun.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, Shah AS. Impact of U.S. Lung allocation score on survival after lung transplantation. J Heart Lung Transplant. 2009;28:769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Liu V, Zamora MR, Dhillon GS, Weill D. Increasing lung allocation scores predict worsened survival among lung transplant recipients. Am J Transplantation. 2010;10:915–920. doi: 10.1111/j.1600-6143.2009.03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell BG, Levitt JE, Goldstein BA, Mooney JJ, Nicolls MR, Zamora M, Valentine V, Weill D, Dhillon GS. Impact of the lung allocation score on survival beyond 1 year. Am J Transplantation. 2014;14:2288–2294. doi: 10.1111/ajt.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnaoutakis GJ, Allen JG, Merlo CA, Sullivan BE, Baumgartner WA, Conte JV, Shah AS. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;30:14–21. doi: 10.1016/j.healun.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinzing S, Brandi G, Raptis DA, Wenger U, Weber D, Stehberger PA, Inci I, Béchir M. Influence on ICU course, outcome and costs for lung transplantation after implementation of the new Swiss transplantation law. Transp Res. 2014;3:9. doi: 10.1186/2047-1440-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee P, Maxwell B, Levitt J, Valentine V, Sheikh A, Weill D, Dhillon G. Lung allocation score increases health care resource utilization after lung transplantation. Chest. 2013;144:110A. [Google Scholar]
- 15.Nationwide Inpatient Sample HCUP. (NIS)Healthcare cost and utilization project (HCUP)2000–2011 [accessed 2014 Mar 14]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp
- 16.HCUP Clinical Classifications Software (CCS) for ICD-9-CM Healthcare cost and utilization project (HCUP)2000–2011 [accessed 2014 Mar 14]. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
- 17.Herring AA, Woolhandler S, Himmelstein DU. Insurance status of U.S. organ donors and transplant recipients: the uninsured give, but rarely receive. Int J Health Serv. 2008;38:641–652. doi: 10.2190/HS.38.4.d. [DOI] [PubMed] [Google Scholar]
- 18.Pant C, Anderson MP, O’Connor JA, Marshall CM, Deshpande A, Sferra TJ. Association of Clostridium difficile infection with outcomes of hospitalized solid organ transplant recipients: results from the 2009 Nationwide Inpatient Sample database. Transpl Infect Dis. 2012;14:540–547. doi: 10.1111/j.1399-3062.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 19.Bureau of Labor Statistics Consumer price index detailed reports 1998–2011 2014[accessed 2014 May 15]Available fromhttp://www.bls.gov/cpi/cpi_dr.htm
- 20.Hachem RR, Trulock EP. The new lung allocation system and its impact on waitlist characteristics and post-transplant outcomes. Semin Thorac Cardiovasc Surg. 2008;20:139–142. doi: 10.1053/j.semtcvs.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey SD, Patrick DL, Albert RK, Larson EB, Wood DE, Raghu G University of Washington Medical Center Lung Transplant Study Group. The cost-effectiveness of lung transplantation. A pilot study. Chest. 1995;108:1594–1601. doi: 10.1378/chest.108.6.1594. [DOI] [PubMed] [Google Scholar]
- 22.Vasiliadis HM, Collet JP, Penrod JR, Ferraro P, Poirier C. A cost-effectiveness and cost-utility study of lung transplantation. J Heart Lung Transplant. 2005;24:1275–1283. doi: 10.1016/j.healun.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Egan TM, Kotloff RM. Pro/Con debate: lung allocation should be based on medical urgency and transplant survival and not on waiting time. Chest. 2005;128:407–415. doi: 10.1378/chest.128.1.407. [DOI] [PubMed] [Google Scholar]
- 24.Russo MJ, Worku B, Iribarne A, Hong KN, Yang JA, Vigneswaran W, Sonett JR. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg. 2011;141:1270–1277. doi: 10.1016/j.jtcvs.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Pondrom S. Death by budget cuts? Arizona targets transplantation for Medicaid reductions. Am J Transplantation. 2011;11:641–642. doi: 10.1111/j.1600-6143.2011.03520.x. [DOI] [PubMed] [Google Scholar]
- 26.Singer JP, Blanc PD, Dean YM, Hays S, Leard L, Kukreja J, Golden J, Katz PP. Development and validation of a lung transplant-specific disability questionnaire. Thorax. 2014;69:437–442. doi: 10.1136/thoraxjnl-2013-204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell BG, Powers AJ, Sheikh AY, Lee PH, Lobato RL, Wong JK.Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the nationwide inpatient sample 1998–2009 J Thorac Cardiovasc Surg 2014148416–421., e411 [DOI] [PubMed] [Google Scholar]
- 28.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. doi: 10.1016/j.jacc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Al-Rawajfah OM, Hewitt JB, Stetzer F, Cheema J. Length of stay and charges associated with health care-acquired bloodstream infections. Am J Infect Control. 2012;40:227–232. doi: 10.1016/j.ajic.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Baaj AA, Uribe JS, Nichols TA, Theodore N, Crawford NR, Sonntag VK, Vale FL. Health care burden of cervical spine fractures in the United States: analysis of a nationwide database over a 10-year period. J Neurosurg Spine. 2010;13:61–66. doi: 10.3171/2010.3.SPINE09530. [DOI] [PubMed] [Google Scholar]
- 31.Kozma CM, Dickson M, Raut MK, Mody S, Fisher AC, Schein JR, Mackowiak JI. Economic benefit of a 1-day reduction in hospital stay for community-acquired pneumonia (CAP) J Med Econ. 2010;13:719–727. doi: 10.3111/13696998.2010.536350. [DOI] [PubMed] [Google Scholar]
- 32.Eslami MH, McPhee JT, Simons JP, Schanzer A, Messina LM. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53:307–315. doi: 10.1016/j.jvs.2010.08.080. [DOI] [PubMed] [Google Scholar]