It is reassuring to know that in the 21st century, chronic obstructive pulmonary disease (COPD) is appropriately recognized not as a single disease but as a constellation of heterogeneous lung diseases with several distinct phenotypes (1, 2). Given how lipids and their bioactive metabolites provide essential structural and functional support in all living organisms (e.g., participating in cellular proliferation, apoptosis, senescence, migration, and organ vascularization), it is not surprising that their role has been actively investigated in the complex pathobiology of COPD. Specifically, among different classes of lipids, sphingolipids have been highlighted as key bioactive metabolites and potential biomarkers in COPD (3).
Ceramides (Cer), sphingomyelins (SM), and sphingosine-1-phosphate (S1P) are among the most common bioactive lipid mediators, some of which act as the extracellular ligands for G-protein-coupled receptors (4). S1P and its receptor, S1PR1 (expressed on lymphocytes), have been shown to be required for cell egression from the lymphoid system and into tissue under normal conditions (5). The discovery of S1P-mediated gradient formation and lymphocyte trafficking has stimulated the development of novel therapeutic agents (e.g., fingolimod) that suppress the immune system and treat some of the most recalcitrant autoimmune inflammatory diseases (6). Whether S1P or other bioactive lipids specifically promote acquired immune responses in smoke-induced lung inflammation, and whether their modulation could be used as novel therapeutics in different COPD phenotypes, remains unclear.
Smoking has been linked to increased lung Cer concentrations. This increase in Cer level has in turn been linked to the activation of apoptosis, impaired efferocytosis, and abnormal tissue repair that could collectively culminate in emphysema (7). Further, sphingolipids have been shown to be elevated in the sputum of smokers with COPD (8), suggesting that various sphingolipids might be associated with different COPD phenotypes.
In this issue of the Journal, Bowler and colleagues (pp. 275–284) examined the association between sphingolipids and different phenotypes in COPD (9). The authors used biological samples (plasma and peripheral blood mononuclear cells) collected from the COPDGene cohort to show that several sphingolipids found in the plasma are strongly associated with emphysema and COPD exacerbation phenotypes, but not with airflow obstruction and chronic bronchitis. They arrive at their conclusion by first performing a targeted study of 69 distinct sphingolipid species that were used for quantitative comparison in 129 current and former smokers. After adjusting for multiple covariates (e.g., age, sex, body mass index, and current smoking) and false discovery rate, they found that concentrations of Cer, SM, and gangliosides were strongly and inversely associated with emphysema phenotype in smokers (Figure 1). Employing receiver operating characteristic curves, they demonstrated that several sphingolipids improved diagnosis of moderate to severe emphysema beyond clinical and physiological covariates. Using a similar strategy, they identified 11 sphingolipids including four trihexosylceramides, three dihexosylceramides, sulfatide, and ganglioside that were positively, whereas S1P and SM were negatively, associated with severe COPD exacerbations. In support of these findings, receiver operating characteristic curve analyses showed that these 11 sphingolipids improved the ability to diagnose severe exacerbations beyond just clinical and physiologic covariates. The authors explored gene–metabolite association with their phenotypic analysis and show that sphingnosine–CYR61 is associated with severe emphysema whereas Cer–Acer is associated with severe COPD exacerbations.
Figure 1.
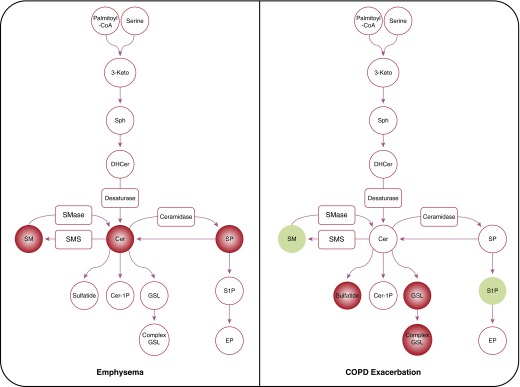
The sphingolipid metabolic pathway associated with emphysema (left) and COPD exacerbation (right). Circles and rectangles represent the metabolites and enzymes, respectively. Red and green colors indicate positive and negative associations, respectively. Concentration of Cer, SM, and gangliosides are strongly and inversely associated with emphysema phenotype and glycolipids (trihexosylceramides, dihexosylceramides, ganglioside). Sulfatides are positively and S1P and SM are negatively associated with severe COPD exacerbation phenotype. 3-Keto = 3-ketosphinganine; Cer = ceramide; Cer-1P = ceramide-1 phosphate; COPD = chronic obstructive pulmonary disease; DHCer = dihyroceramide; EP = ethanolamine phosphate; GSL = glycosphingolipid; SM = sphingomyeline; SMase = sphingomyelinase; SMS = sphingomyelin synthase; SP = sphingosine; S1P = sphingosine-1-phosphate; Sph = sphinganine.
The strength of the work includes the study population (the well-phenotyped COPDGene cohort), as well as replication of some of the markers using untargeted mass spectrometry in an independent laboratory. Some of the caveats of the study include using plasma samples that were not collected at the time of COPD exacerbation to measure trihexosylceramide levels. Given this limitation, which could significantly affect their findings, this biomarker might not support the conclusion that a rapid flux of sphingosine-to-ceramide-to-glycosylated ceramide metabolism occurs in smokers with COPD exacerbation. Further, a large study using the Multi-Ethnic Study of Atherosclerosis (MESA) and mixed-effect models reported that higher plasma concentrations of sphingomyelin predicted increased annual progression of emphysema (10). These findings appear to be contradictory to the current report that found a negative correlation between most of the sphingolipid species and emphysema severity (9). However, there are several differences between these reports that might provide insight to the seemingly divergent results.
First, the report by Bowler and colleagues is based on cross-sectional analysis of many highly bioactive lipids; in contrast, the MESA study examined plasma SMs to evaluate longitudinal changes in emphysema progression using serial chest computed tomography scans (9, 10). Second, the sample size in the MESA study was much larger, and the cohort was drawn from the general population (never and ever smokers) without selecting for lung disease per se, whereas the COPDGene cohort was designed to recruit smokers with and without COPD. Finally, the current report measured a large number of SMs in relation to several clinical endpoints in a relatively small cohort, whereas the MESA study used a large cohort, and its single endpoint was focused on changes in emphysema progression. Barring differences in the methodology used to measure SMs (mass spectroscopy versus spectrophotometric assays), the differences in study design (cross-sectional versus longitudinal) could account for their divergent findings.
Overall, the current study adds significantly to our understanding of how metabolic pathways might be activated in smokers with different clinical phenotypes. Further, phenotypic characterization of this population in turn could identify novel bioactive lipids that could be linked to disease pathogenesis. More work is required to further clarify how quantitative measurement of bioactive lipids could be used to complement our current clinical and physiological assays to better diagnose emphysema and understand why patients with COPD exacerbate. Finally this work reminds us that ancillary studies in large, well-phenotyped cohorts (e.g., MESA, COPDGene, SPIROMICS, etc.) allow the testing of novel hypotheses to provide new insight into the conundrum of COPD.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35:1–6. doi: 10.1016/j.ccm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Vogelmeier C, Vestbo J. COPD assessment: I, II, III, IV and/or A, B, C, D. Eur Respir J. 2014;43:949–950. doi: 10.1183/09031936.00019714. [DOI] [PubMed] [Google Scholar]
- 3.Telenga ED, Hoffmann RF, Ruben t’Kindt, Hoonhorst SJM, Willemse BWM, van Oosterhout AJM, Heijink IH, van den Berge M, Jorge L, Sandra P, et al. Untargeted lipidomic analysis in chronic obstructive pulmonary disease. Uncovering sphingolipids. Am J Respir Crit Care Med. 2014;190:155–164. doi: 10.1164/rccm.201312-2210OC. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 5.Thangada S, Khanna KM, Blaho VA, Oo ML, Im D-S, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappos L, Radue E-W, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, et al. FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 7.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, Schweitzer KS, Skobeleva A, Rajashekhar G, Hubbard WC, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. 2010;181:344–352. doi: 10.1164/rccm.200906-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrache I, Kamocki K, Poirier C, Pewzner-Jung Y, Laviad EL, Schweitzer KS, Van Demark M, Justice MJ, Hubbard WC, Futerman AH.Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models PLoS ONE 20138e62968Electronic Resource. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowler RP, Jacobson S, Cruickshank C, Hughes GJ, Siska C, Ory DS, Petrache I, Schaffer JE, Reisdorph N, Kechris K. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am J Respir Crit Care Med. 2015;191:275–284. doi: 10.1164/rccm.201410-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed FS, Jiang XC, Schwartz JE, Hoffman EA, Yeboah J, Shea S, Burkart KM, Barr RG. Plasma sphingomyelin and longitudinal change in percent emphysema on CT. The MESA lung study. Biomarkers. 2014;19:207–213. doi: 10.3109/1354750X.2014.896414. [DOI] [PMC free article] [PubMed] [Google Scholar]


