Abstract
Rationale: Primary graft dysfunction (PGD) causes early mortality after lung transplantation and may contribute to late graft failure. No effective treatments exist. The pathogenesis of PGD is unclear, although both neutrophils and activated platelets have been implicated. We hypothesized that neutrophil extracellular traps (NETs) contribute to lung injury in PGD in a platelet-dependent manner.
Objectives: To study NETs in experimental models of PGD and in lung transplant patients.
Methods: Two experimental murine PGD models were studied: hilar clamp and orthotopic lung transplantation after prolonged cold ischemia (OLT-PCI). NETs were assessed by immunofluorescence microscopy and ELISA. Platelet activation was inhibited with aspirin, and NETs were disrupted with DNaseI. NETs were also measured in bronchoalveolar lavage fluid and plasma from lung transplant patients with and without PGD.
Measurements and Main Results: NETs were increased after either hilar clamp or OLT-PCI compared with surgical control subjects. Activation and intrapulmonary accumulation of platelets were increased in OLT-PCI, and platelet inhibition reduced NETs and lung injury, and improved oxygenation. Disruption of NETs by intrabronchial administration of DNaseI also reduced lung injury and improved oxygenation. In bronchoalveolar lavage fluid from human lung transplant recipients, NETs were more abundant in patients with PGD.
Conclusions: NETs accumulate in the lung in both experimental and clinical PGD. In experimental PGD, NET formation is platelet-dependent, and disruption of NETs with DNaseI reduces lung injury. These data are the first description of a pathogenic role for NETs in solid organ transplantation and suggest that NETs are a promising therapeutic target in PGD.
Keywords: acute lung injury, immunity, innate, inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
Primary graft dysfunction (PGD) is a major cause of morbidity and mortality after lung transplantation. The pathophysiology of PGD is incompletely understood, and although an inflammatory cascade involving neutrophils is thought to be important, the mechanism by which neutrophils cause injury is unknown.
What This Study Adds to the Field
We investigated the role of neutrophil extracellular traps (NETs), structures consisting of DNA complexed with neutrophil proteins, in two animal models of PGD. We found that NETs are formed during experimental PGD in a platelet-dependent manner and that disruption of NETs protects against PGD. We also show that NETs are present in clinical samples from patients with PGD. These findings are the first description of NETs after solid organ transplantation and suggest that NETs are a therapeutic target in PGD.
Lung transplantation is an increasingly used therapy for end-stage lung disease (1). Survival after lung transplantation, however, is lower compared with other solid organ transplants (2). Primary graft dysfunction (PGD) is a form of acute lung injury (ALI) that develops within 72 hours after lung transplantation and is a contributor to early mortality and morbidity after transplant (3, 4). In addition, PGD is a significant risk factor for the development of bronchiolitis obliterans syndrome, a form of chronic lung allograft dysfunction, which is the major limitation to long-term survival in lung transplant recipients (5–7). As with other forms of ALI, no specific therapies are available for PGD (8).
The mechanisms underlying the development of PGD are incompletely understood, although ischemia-reperfusion injury (IRI) resulting in an inflammatory cascade is thought to play a central pathophysiologic role (9, 10). This cascade compromises lung endothelial and alveolar epithelial barrier integrity, leading to flooding of alveolar airspaces with protein-rich edema fluid. Like other forms of ALI, the inflammatory response in PGD is characterized by a robust recruitment of neutrophils into the lung, and blunting this neutrophil response in animal models ameliorates lung injury (11, 12).
Among the effector functions of neutrophils is the formation of neutrophil extracellular traps (NETs), which are extracellular elaborations of DNA complexed with histones and neutrophil granular proteins (13). NETs are generated by a regulated cell death program termed “NETosis” (14). NETs ensnare and kill bacteria and fungi (13, 15–17), and may also contribute to antiviral defenses (18). Furthermore, NETs have been implicated in a growing number of autoimmune and inflammatory disease states including small vessel vasculitis and systemic lupus erythematosis (19–22). In the lung, our group and others have shown that NETs are formed in the lung during transfusion-related ALI, and that NETs are directly involved in causing tissue injury in animal models of transfusion-related ALI (23, 24). NETs have also been implicated in the pathogenesis of experimental ventilator-induced lung injury (22). Platelet–neutrophil interactions have been shown to cause lung injury (25–27) and to promote NETosis (16, 23). We hypothesized that NETs are formed in the lung during PGD in a platelet-dependent manner, and are pathogenic, contributing to lung injury and alveolar flooding.
In the present study, we show that NETs are formed in the lung in two mouse models of pulmonary IRI: hilar clamp (HC), and orthotopic lung transplantation (OLT) with prolonged cold ischemia (PCI). NET formation is associated with platelet activation and sequestration within the lung, and platelet inhibition reduces NET formation and lung injury. Treatment with DNaseI, which disrupts NETs (13), also decreased lung injury in the mouse OLT model. In specimens from human lung transplant recipients, bronchoalveolar lavage fluid (BALF) from patients with PGD contained significantly more NETs than BALF from patients without PGD. These results suggest an important role for NETs in the pathogenesis of PGD.
Some of the results of these studies have been previously reported in the form of an abstract (28).
Methods
Additional detail on the mouse surgical procedures, ALI, arterial blood gas measurements, and NETs ELISA are provided in the online supplement.
Study Approvals
All animal experiments were approved by the Institutional Animal Care and Use Committee at University of California, San Francisco. Human BALF specimens were collected and analyzed with the approval of the University of California, Los Angeles institutional review board. Human plasma samples were collected as part of the Lung Transplant Outcomes Group cohort study, with institutional review board approval at each study center.
Mice
Male C57BL/6J mice, 8 to 12 weeks old, purchased from The Jackson Laboratory (Bar Harbor, ME), and housed under specific pathogen–free conditions, were used for all experiments.
Hilar Clamp Model
Briefly, mice underwent left thoracotomy and the left hilum was occluded with a silk suture tied in a slipknot, or left untied in sham surgery. Mice were awakened from anesthesia during a 2-hour ischemia period. The slipknot suture was then removed, the animal was killed after a 4-hour reperfusion period, and blood and lungs were collected. In selected experiments, 125I-labeled albumin (Iso-Tex Diagnostics, Inc., Friendswood, TX) was administered by intraperitoneal injection immediately before reperfusion for measurement of lung vascular permeability (extravascular plasma equivalents [EVPE]).
Orthotopic Lung Transplant Model
Left lung transplants in mice (C57BL/6J donor and recipient) were performed using the method described by Okazaki and colleagues (29). Two experimental lung transplant groups were studied. In the first group, on procurement, the donor lung was immediately transplanted into a recipient animal with no cold ischemia. In the second group, extended cold ischemia was introduced by storing the Perfadex (XVIVO Perfusion Inc., Englewood, CO)-perfused and air-inflated donor lung in Perfadex-soaked sterile gauze at 4°C for 18 hours before implantation (30). The recipient animal was killed 8 hours after transplantation, and blood and lungs were collected. In selected experiments, mice were treated with aspirin (ASA) (Sigma, St. Louis, MO; 100 μg/g, intraperitoneally) or vehicle control (dimethyl sulfoxide) 24 hours and again 2 hours before OLT-PCI. Thromboxane B2 levels were determined by enzyme immunoassay (Amersham, Pittsburgh, PA) in plasma and BAL obtained 8 hours after transplantation (25). CD41 immunohistochemistry was done in OCT-embedded lungs as previously described (25). Also, in selected experiments, DNaseI (Roche, Indianapolis, IN), 2,000 U in 15 μl diluent (20 mM Tris-HCl, 1 mM MgCl2) versus diluent alone, was directly instilled into the donor lung bronchus before bronchial anastomosis.
Immunofluorescence Microscopy
NETs were visualized in frozen lung sections by staining for DNA, histones, and neutrophil elastase (NE) as previously described (23).
Human BALF and Plasma Samples
Patient specimens from two independent cohorts were analyzed by ELISA for the presence of NETs, as detailed in the methods section of the online supplement. The first cohort underwent lung transplantation at the University of California, Los Angeles between March 2000 and August 2008. Banked BALF collected within 24 hours (Day 0) of transplantation was available for 10 patients who had persistent moderate or severe PGD (grade 2 or 3 at all-time points 24 through 72 h after transplant). Ten banked Day 0 BALF specimens from patients without PGD (PGD grade 0 at all of the above time points) were chosen at random as control subjects. The second cohort consisted of subjects enrolled in the Lung Transplant Outcomes Group prospective cohort study (31). Banked plasma collected before transplant, at 4–6 hours following, and 24 hours following bilateral transplant was analyzed from 23 patients who developed severe persistent PGD (grade 3 at all time points 24 through 72 h after transplant), and from 15 control subjects frequency matched on sex and pretransplant diagnosis who remained free of PGD at each time point (32). PGD was defined per international guidelines (4). Clinical characteristics of each cohort are presented in Table E1 in the online supplement.
Statistics
Unless noted otherwise, results are reported as mean ± SD and were analyzed using an unpaired t test (GraphPad PRISM version 5.0; GraphPad Software Inc., La Jolla, CA). Human ELISA data were analyzed using the Mann-Whitney rank-sum test, and for graphs of these data, the median is indicated, the box represents the 25th–75th percentiles, and whiskers represent the range from minimum to maximum values. P values of less than or equal to 0.05 were considered to be statistically significant.
Results
NETs Are Formed in Hilar Clamp Ischemia-Reperfusion Lung Injury
We first studied NETs in mouse lung IRI using the well-established HC model in which the left pulmonary hilum is transiently occluded to induce pulmonary IRI (Figure 1A). In pilot experiments, an occlusion time of 2 hours, followed by 4 hours of reperfusion, caused robust lung injury in the ischemic left lung, with increased extravascular lung water, EVPE, and BALF protein concentration compared with lungs from animals that underwent sham surgery (Figures 1B–1D). Shorter ischemic times resulted in less injury, consistent with a dose-response relationship between ischemic time and resultant injury (see Figure E1).
Figure 1.
(A) Schematic of the hilar clamp (HC) ischemia reperfusion model. (B) Extravascular lung water (ELW) and (C) extravascular plasma equivalents (EVPE) are increased after HC as compared with sham surgery, as is (D) bronchoalveolar lavage fluid protein concentration. n ≥ 5 for all groups, **P < 0.01.
Lungs from mice that underwent HC were evaluated for NETs using immunofluorescence microscopy. NETs, defined by the colocalization of DNA, histone, and NE, were visualized throughout the ischemic lung after HC but not after sham surgery (Figure 2A). We also assayed plasma and BALF from these animals for NE-DNA complexes by ELISA. Plasma NE-DNA complexes were more abundant in mice after HC as compared with sham surgery, whereas there was no difference in BALF NE-DNA complexes (Figures 2B and 2C).
Figure 2.
(A) Immunofluorescence microscopy showing neutrophil extracellular traps, defined as colocalized extracellular DNA (blue), neutrophil elastase (NE) (green), and histone (red), present in the lung after hilar clamp (HC) but not after sham surgery. Scale bar = 10 μm. Results are representative of at least three independent experiments. (B, C) NE-DNA complexes are increased in plasma after HC (C) but are not increased in bronchoalveolar lavage fluid (B). n ≥ 6 for all groups, *P < 0.05. BALF = bronchoalveolar lavage fluid.
NETs Are Formed in Experimental PGD after Lung Transplantation
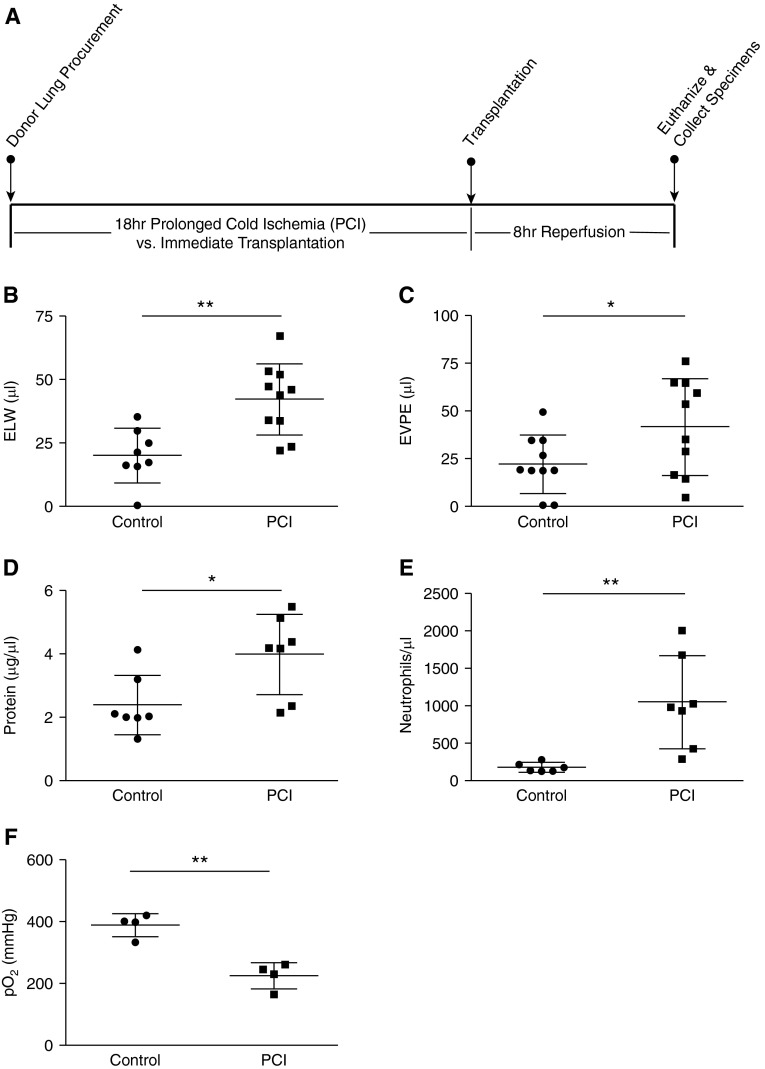
To extend our findings of NET formation during IRI, we used the previously described mouse OLT model to study NETs in a model system that more closely mimics human PGD. Briefly, explanted left single donor lungs were transplanted immediately after harvest, or subjected to prolonged (18 h) cold ischemia (PCI) before transplantation into recipient animals (Figure 3A) (30). Lung injury and NET formation were then assessed after 8 hours of post-transplant reperfusion. Using this model, extravascular lung water and EVPE were significantly increased in transplanted lungs subjected to PCI as compared with control lungs transplanted after minimal ischemic time (Figures 3B and 3C). In addition, PCI resulted in increased BALF protein concentration and neutrophilia, and impaired oxygenation (Figures 3D–3F), all findings consistent with ALI.
Figure 3.
(A) Schematic of the orthotopic lung transplant with prolonged cold ischemia (PCI) model. (B) Extravascular lung water (ELW) and (C) extravascular plasma equivalents (EVPE) (n ≥ 8 in each group), (D) bronchoalveolar lavage fluid protein concentration (n = 7 in each group), and (E) bronchoalveolar lavage fluid neutrophil count (n ≥ 6 in each group) are all increased in lungs transplanted after PCI as compared with lungs transplanted immediately after harvest (control group). (F) The arterial blood partial pressure of oxygen (Po2) is lower in mice that received lung grafts subjected to PCI (n = 4 in each group). *P < 0.05, **P < 0.01.
Immunofluorescence microscopy was next used to examine transplanted lungs for the presence of NETs. NETs were readily visible in lungs subjected to PCI, but not in lungs transplanted immediately after harvest (Figure 4A). To quantify the effects of PCI on NET production in the transplanted lung, plasma from recipient animals, and BALF collected from transplanted lungs, was analyzed for NE-DNA complexes by ELISA. Plasma NE-DNA complex levels were similar between the two groups of recipient animals, but these complexes were present in greater abundance in BALF collected from lungs transplanted after PCI compared with control lungs transplanted immediately after harvest (Figures 4B and 4C).
Figure 4.
(A) Immunofluorescence microscopy showing neutrophil extracellular traps, defined as colocalized extracellular DNA (blue), neutrophil elastase (NE) (green), and histone (red), present in transplanted lungs subjected to prolonged cold ischemia (PCI), but not in those transplanted immediately after harvest (control group). Scale bar = 10 μm. Results are representative of at least three independent experiments. (B) NE-DNA complexes are increased in bronchoalveolar lavage fluid collected from lung grafts subjected to PCI as compared with lungs transplanted immediately after harvest (n = 6 in each group), whereas NE-DNA complexes are present at similar low levels in plasma from both groups of recipient mice (C) (n ≥ 12). *P < 0.05. BALF = bronchoalveolar lavage fluid.
Platelets Promote NET Formation in Experimental PGD
Because platelets have an important role in other models of lung injury, as well as in NET formation, we examined the contribution of platelets to lung injury in our model. Platelet accumulation within the lung circulation was assessed by immunohistochemical staining for the platelet-specific marker CD41. Compared with control transplants with no cold ischemia (Figure 5A), platelet sequestration was increased in lung grafts transplanted after PCI (Figure 5B). ASA treatment before lung transplantation with PCI reduced platelet sequestration (Figure 5C) and significantly reduced thromboxane levels in both plasma and BALF (Figures 5D and 5E). ASA treatment reduced NET formation (Figure 5F), reduced lung injury as measured by BALF neutrophilia and protein concentration (Figures 5G and 5H), and improved oxygenation (Figure 5I).
Figure 5.
(A–C) Photomicrographs (×40 magnification) showing immunohistochemical staining of lung tissue for the platelet-specific marker CD41 in (A) lung transplanted immediately after harvest (control subject), (B) lung subjected to prolonged cold ischemia (PCI) and transplanted into a recipient treated with diluent (dimethyl sulfoxide), and (C) lung subjected to PCI and transplanted into a recipient treated with aspirin (ASA) before transplant. Images are representative of at least three independent experiments. (D) Plasma and (E) bronchoalveolar lavage fluid (BALF) thromboxane B2 levels are reduced by ASA treatment in mice that received lung transplants after PCI. ASA treatment also reduces (F) BALF neutrophil elastase (NE)-DNA complexes, (G) neutrophils, and (H) protein concentration, and (I) increases recipient arterial partial pressure of oxygen (Po2). n = 4 for all groups, *P < 0.05, **P < 0.01.
Disrupting NETs Protects against Experimental PGD
Based on the above results demonstrating that NETs are present in the lung in two different mouse models of pulmonary IRI, we hypothesized that the NETs contribute directly to tissue injury in these models. We therefore reasoned that disrupting NETs using DNaseI, which degrades NETs (13, 23), could limit the extent of lung injury in the OLT model. To test this hypothesis, we treated mouse lung allografts subjected to PCI with DNaseI (vs. diluent control), administered by intrabronchial instillation immediately before bronchial anastomosis. Lungs were then assessed for injury and for the presence of NETs 8 h after transplantation. Because the exogenously administered DNaseI would confound BALF total protein measurements, we instead measured BALF albumin as an indicator of lung vascular permeability in these experiments.
DNaseI treatment reduced NE-DNA complexes in BALF collected from lung allografts subjected to PCI (Figure 6A). DNaseI treatment also reduced BALF neutrophilia and albumin leak (Figures 6B and 6C), and markedly improved oxygenation (Figure 6D). Histopathologic evidence of lung injury was also reduced by DNaseI administration (see Figure E2).
Figure 6.
In lungs transplanted after prolonged cold ischemia (PCI), intrabronchial DNaseI treatment before implantation reduces (A) bronchoalveolar lavage fluid neutrophil elastase (NE)-DNA complexes, (B) neutrophils, and (C) albumin concentration, and (D) increases recipient arterial partial pressure of oxygen (Po2). n ≥ 4 for all groups, *P < 0.05, **P < 0.01.
NETs Are Present in Human PGD Specimens
To assess the clinical relevance of our animal model findings that implicate NETs as major contributors to the pathogenesis of PGD and potential therapeutic targets, we examined banked plasma and BALF collected from human lung transplant recipients.
BALF NE-DNA complexes were present in much greater abundance in patients with moderate or severe PGD than in those who remained free of PGD (Figure 7A). In contrast, there was no difference between patients without PGD and those with severe PGD in plasma circulating NE-DNA complexes either before transplant, immediately following transplant, or 24 hours after transplant (Figure 7B).
Figure 7.
(A) Analysis of post-transplant Day 0 bronchoalveolar lavage fluid from patients who underwent lung transplant at University of California, Los Angeles and were either free of PGD (PGD-0) or had moderate to severe PGD (PGD-2/3). Bronchoalveolar lavage fluid neutrophil elastase (NE)-DNA complexes were significantly higher in PGD-2/3 patients. n = 10 for each group. (B) Analysis of plasma samples from subjects in the Lung Transplant Outcomes Group cohort who were free of PGD (PGD-0) or had severe PGD (PGD-3). Plasma NE-DNA complexes were no different between these groups either before transplant (Pre), 4–6 hours after transplant (Day 0), or 24 hours after transplant (Day 1) (Pre and Day 0, n = 12 PGD-0, 22 PGD-3; Day 1 n = 15 PGD-0, 23 PGD-3). **P < 0.01; ns = nonsignificant.
Discussion
In this study, we show that NETs form in the lung in two different experimental models of PGD, and that NET formation after experimental lung transplantation is driven by a platelet-dependent mechanism. Furthermore, we show that more NET components are present in BALF from human lung transplant recipients with PGD than those free of PGD. Finally, we demonstrate that in experimental mouse PGD, disruption of NETs with DNaseI abrogates the development of lung injury. Collectively, these data implicate platelet-driven NET formation in the pathogenesis of PGD, and suggest that disruption of NETs may be a promising therapeutic strategy to prevent or treat PGD.
Our study adds to a growing literature on the role of NETs in sterile inflammation to now include PGD, and is the first study to our knowledge to demonstrate a pathogenic role for NETs in solid organ transplantation. Our study has a number of strengths. First, the use of two distinct mouse models of pulmonary IRI, as well as multiple methods to assay for the presence of NETs, demonstrates that our findings are robust and reproducible. Second, our animal results were validated in human lung transplant recipients, establishing the clinical importance of our findings. Finally, the robust treatment effect seen with DNaseI in the prevention of experimental PGD strongly supports a pathogenic, as opposed to epiphenomenal, role for NETs in PGD, and suggests a possible therapeutic target.
These findings, suggestive of a potential therapeutic role for DNase and ASA in PGD, are of particular relevance because PGD is common after lung transplantation, with incidence estimates ranging from 10 to 25% (33). Furthermore, PGD onset is predictably within the immediate post-transplant period. Unlike most other forms of ALI, therefore, PGD is uniquely amenable to interventions intended to prevent the development of injury. Our study suggests that DNaseI treatment holds promise as such an intervention.
Limitations of our study include the lack of matched plasma and BALF specimens from the same patients. This limitation, however, does not impact our fundamental findings regarding the presence and potential pathogenic role of NETs in both experimental and clinical PGD. In addition, although our fundamental finding that NETs are present in the lung during IRI was evident in both the HC and OLT mouse models, there were differences in these models that warrant mention. Specifically, BALF NE-DNA complexes were more elevated in the OLT model, whereas in the HC model NE-DNA complexes were more readily detected in plasma. A number of possibilities could explain this discrepancy; NETs may be produced in different compartments within the lung in each model (i.e., in the airspaces in the OLT model vs. in the lung vasculature in the HC model), or plasma DNases may be more active in the OLT model. Differences between warm ischemia (as induced in the HC model) and combined warm and cold ischemia (as in the OLT model) may also be a mechanism underlying this finding. It is important to note that our analysis of human lung transplant recipient BALF and plasma was most concordant with the results in the mouse OLT model, suggesting that this model better reproduces the pathophysiology of PGD than the HC model.
We have previously shown that activated platelets directly stimulate NET formation by neutrophils (23). Our current study builds on literature suggesting an important role for platelets in PGD. In humans, platelet aggregation is known to occur in lungs during PGD, and elevated levels of P-selectin, a marker of platelet activation, are associated with an increased risk of PGD (34, 35). Other markers of platelet activation are also increased after lung transplantation, as is the presence of platelet-leukocyte aggregates (36). In animal studies, antibody blockade of P-selectin, or deletion of the P-selectin gene, protects against PGD (37).
In the current study, ASA prevented NET formation and reduced lung injury, implicating a platelet-dependent mechanism for NET formation in experimental PGD. Other methods of platelet inhibition, including antibody-mediated depletion, proved technically unfeasible because of bleeding complications incurred during surgery. Despite this limitation, our findings support platelet-induced NETosis as a pathogenic mechanism in PGD, which may be a common pathophysiologic pathway for IRI in other organs.
NETs, once formed, may cause lung injury via a number of mechanisms. First, the molecular components of the NETs (histones in particular, but also neutrophil granule proteins) are directly cytotoxic to pulmonary epithelial and endothelial cells (38, 39). Our group has also previously shown that NETs are directly toxic to cell monolayers (23). During states of sterile inflammation, like PGD, these components may be held in place by the DNA “backbone” of NETs, achieving high local concentrations and becoming directly toxic to cells within the lung. Second, the weblike structure of NETs may occlude small vessels and cause impairment to local blood circulation, resulting in additional tissue ischemia and resultant injury. Indeed, NETs have been shown to play a role in thrombus formation in animal models and have significant thrombogenic potential (40–42). Third, we observed an unexpected decrease in BALF neutrophils in the DNaseI-treated animals (Figure 6B), suggesting that NETs are mechanistically involved in the recruitment of additional neutrophils into the alveolar spaces in PGD, which may perpetuate injury. These findings suggest that NET production leads to a positive feedback loop of tissue injury, release of inflammatory mediators, recruitment of neutrophils, and production of additional NETs. By limiting injury, NET degradation may blunt the inflammatory cascade that results in additional neutrophil recruitment. The lung injury caused by NETs may therefore be the result of multiple pathogenic mechanisms working in concert.
Our findings in pulmonary PGD may well extend to IRI in other solid organ transplants, since similar mechanisms of injury in other organs have been proposed. DNase, the agent we used to disrupt NETs in the OLT mouse model, is of particular relevance because it is already an effective and widely used inhaled therapy for the treatment of cystic fibrosis (43, 44). Disruption of DNA present in cystic fibrosis mucus by DNase is believed to reduce mucus viscosity and therefore improve mucus clearance. Much of the DNA in cystic fibrosis mucus may derive from NETs, likely elaborated in response to chronic airway infection (45). Therefore, cystic fibrosis may be the first disease in which pathogenic NETs have been effectively treated with DNase. DNase may hold similar promise for the prevention or treatment of PGD, a condition with no effective pharmacologic treatments.
Acknowledgments
Acknowledgment
The authors are grateful to the participating centers and investigators in the Lung Transplant Outcomes Group.
Lung Transplant Outcomes Group Investigators are as follows: Jason Christie, M.D., M.S. (PI), Steven M. Kawut, M.D., M.S., Edward Cantu III, M.D., Joshua Diamond, M.D., M.S., Rupal Shah, M.D., M.S., Ejigayehu Demissie, M.S.N., Robert M. Kotloff, M.D., Vivek N. Ayha, M.D., James Lee, M.D., Denis Hadjiliadis, M.D., M.H.S., and Melanie Ruschefski, B.S., University of Pennsylvania (coordinating site). David J. Lederer, M.D., M.S. (PI), Selim M. Arcasoy, M.D., Joshua R. Sonett, M.D., Jessie Wilt, M.D., Frank D’Ovidio, M.D., Matthew Bacchetta, M.D., Hilary Robbins, M.D., Lori Shah, M.D., Nilani Ravichandran, N.P., Nadine Al-Naamani, M.D., Nisha Philip, M.B. B.S., Debbie Rybak, B.A., Matthew Lippell, B.S., Shefali Sanyal, B.S., Michael Koeckert, B.A., Amisha Desai, B.A., Megan Larkin, M.P.H., Brian Lim, Justin Shin, and Robert Sorabella, B.A., Columbia University. Lorraine Ware, M.D. (PI), and Stephanie Logan, R.N., Vanderbilt University. Ann Weinacker, M.D. (PI), Gundeep Dillon, M.D., Susan Spencer Jacobs, M.S.N., and Val Scott, M.S.N., Stanford University. Keith Wille, M.D. (PI), David McGiffin, M.D., and Necole Harris, B.S., University of Alabama, Birmingham. Jonathan Orens, M.D. (PI), Ashish Shah, M.D., Pali Shah, M.D., and John McDyer, M.D., Johns Hopkins University. Vibha Lama, M.D., M.S. (PI), Fernando Martinez, M.D., M.S., and Emily Galopin, B.S., University of Michigan. Scott M. Palmer, M.D., M.H.S. (PI), Jamie Todd, M.D., Laurie Snyder M.D., R. Duane Davis, M.D., and Ashley Finlen-Copeland, M.S.W., Duke University. Sangeeta Bhorade, M.D. (PI), University of Chicago. Maria Crespo, M.D. (PI), and Cynthia Gries M.D., M.S., University of Pittsburgh.
Footnotes
Supported by U.S. National Institutes of Health grants R01 HL107386 (M.R.L.), R01 HL112990 and CTOT 11,532,596 (J.A.B.), HL088263 (L.B.W.), HL103836 (L.B.W.), R01HL087115 (J.D.C.), R01HL081619 (J.D.C.), R01HL096845 (J.D.C.), K24HL115354 (J.D.C.), R01HL114626 (J.D.C.), and the Nina Ireland Program in Lung Health (M.R.L.).
Author Contributions: All authors have made a substantial contribution to the acquisition, analysis, or interpretation of data, and revision and final approval of the manuscript to be published. Each author made the following contributions: conception and design, D.M.S., B.M., F.L., G.O.-M., A.C., A.D., L.B.W., J.D.C., J.A.B., and M.R.L.; acquisition of data, analysis, and interpretation, D.M.S., B.M., F.L., A.C., A.D., J.A.B., and M.R.L.; and drafting and editing of the manuscript, D.M.S., B.M., G.O.-M., A.D., D.J.R., J.P.L., R.S., A.A., L.B.W., J.D.C., J.A.B., and M.R.L.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201406-1086OC on December 8, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report–2013; Focus Theme: Age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Annual Data Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) Introduction. Am J Transplant. 2013;13:8–10. doi: 10.1111/ajt.12018. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24:1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, Dahlberg PS. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 7.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, Trulock EP, Hachem RR. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8:2454–2462. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24:1489–1500. doi: 10.1016/j.healun.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299:H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 10.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 11.Ross SD, Tribble CG, Gaughen JR, Jr, Shockey KS, Parrino PE, Kron IL. Reduced neutrophil infiltration protects against lung reperfusion injury after transplantation. Ann Thorac Surg. 1999;67:1428–1433, discussion 1434. doi: 10.1016/s0003-4975(99)00248-9. [DOI] [PubMed] [Google Scholar]
- 12.Kreisel D, Sugimoto S, Tietjens J, Zhu J, Yamamoto S, Krupnick AS, Carmody RJ, Gelman AE. Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis. J Clin Invest. 2011;121:265–276. doi: 10.1172/JCI42596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 17.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossaint J, Herter JM, Van Aken H, Napirei M, Doring Y, Weber C, Soehnlein O, Zarbock A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood. 2014;123:2573–2584. doi: 10.1182/blood-2013-07-516484. [DOI] [PubMed] [Google Scholar]
- 23.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz-Munoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124:2625–2634. doi: 10.1182/blood-2014-03-562876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayah DM, Liu F, Caudrillier A, Looney MR. Neutrophil extracellular traps in ischemia-reperfusion lung injury [abstract] Am J Respir Crit Care Med. 2012;185:A5123. [Google Scholar]
- 29.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7:1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 30.Jungraithmayr W, De Meester I, Matheeussen V, Inci I, Augustyns K, Scharpe S, Weder W, Korom S. Inhibition of CD26/DPP IV attenuates ischemia/reperfusion injury in orthotopic mouse lung transplants: the pivotal role of vasoactive intestinal peptide. Peptides. 2010;31:585–591. doi: 10.1016/j.peptides.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, Milstone A, Orens J, Weinacker A, Demissie E, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175:69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med. 2010;31:161–171. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 34.Kawut SM, Okun J, Shimbo D, Lederer DJ, De Andrade J, Lama V, Shah A, Milstone A, Ware LB, Weinacker A, et al. Soluble P-selectin and the risk of primary graft dysfunction after lung transplantation. Chest. 2009;136:237–244. doi: 10.1378/chest.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombat M, Castier Y, Leseche G, Rufat P, Mal H, Thabut G, Fournier M, Groussard O, Degott C, Couvelard A. Early expression of adhesion molecules after lung transplantation: evidence for a role of aggregated P-selectin-positive platelets in human primary graft failure. J Heart Lung Transplant. 2004;23:1087–1092. doi: 10.1016/j.healun.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Sternberg DI, Shimbo D, Kawut SM, Sarkar J, Hurlitz G, D'Ovidio F, Lederer DJ, Wilt JS, Arcasoy SM, Pinsky DJ, et al. Platelet activation in the postoperative period after lung transplantation. J Thorac Cardiovasc Surg. 2008;135:679–684. doi: 10.1016/j.jtcvs.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci USA. 1997;94:757–761. doi: 10.1073/pnas.94.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, Wiedenbauer EM, Krautgartner WD, Stoiber W, Belohradsky BH, et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 45.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]