Abstract
Rationale: Prior studies have shown an anticancer effect of metformin in patients with breast and colorectal cancer. It is unclear, however, whether metformin has a mortality benefit in lung cancer.
Objectives: To compare overall survival of patients with diabetes with stage IV non–small cell lung cancer (NSCLC) taking metformin versus those not on metformin.
Methods: Using data from the Surveillance, Epidemiology, and End Results registry linked to Medicare claims, we identified 750 patients with diabetes 65–80 years of age diagnosed with stage IV NSCLC between 2007 and 2009. We used propensity score methods to assess the association of metformin use with overall survival while controlling for potential confounders.
Measurements and Main Results: Overall, 61% of patients were on metformin at the time of lung cancer diagnosis. Median survival in the metformin group was 5 months, compared with 3 months in patients not treated with metformin (P < 0.001). Propensity score analyses showed that metformin use was associated with a statistically significant improvement in survival (hazard ratio, 0.80; 95% confidence interval, 0.71–0.89), after controlling for sociodemographics, diabetes severity, other diabetes medications, cancer characteristics, and treatment.
Conclusions: Metformin is associated with improved survival among patients with diabetes with stage IV NSCLC, suggesting a potential anticancer effect. Further research should evaluate plausible biologic mechanisms and test the effect of metformin in prospective clinical trials.
Keywords: lung neoplasms, diabetes, metformin, survival analysis
At a Glance Commentary
Scientific Knowledge on the Subject
Metformin has been found to have anticancer activity in preclinical models and seems to be associated with improved survival among patients with diabetes with breast, prostate, and colorectal cancer. Little is known about the potential effect of metformin on survival in patients with lung cancer.
What This Study Adds to the Field
Using a population-based cancer registry, we determine that metformin use is associated with improved survival among patients with diabetes with stage IV non–small cell lung cancer, after controlling for potential confounders. These findings suggest that metformin may have an anti–lung cancer effect.
There is increasing interest in investigating the repurposing of already-approved medications as possible cancer chemotherapeutic agents (1–3). The use of these medications offers several advantages to traditional drug development, including an established safety profile and shorter timeline for regulatory evaluation. Among the medications being evaluated, metformin, a biguanide derivative commonly used to treat type II diabetes, has been found to have anticancer activity in preclinical models (4, 5).
Patients with diabetes are at higher risk for developing cancer, including breast, colorectal, pancreatic, and lung malignancies (6–9). Furthermore, diabetes seems to worsen cancer-specific and overall survival in several cancers (10–13). Among patients with diabetes, metformin has been associated with a decreased overall risk for developing certain cancers (14, 15). Metformin also seems to be associated with improved survival among patients with diabetes with breast, prostate, and colorectal cancer (14, 16, 17).
There are little data regarding the effects of metformin use on lung cancer prognosis. One small retrospective study from China showed that patients with diabetes with lung cancer treated with metformin had improved overall and progression-free survival (18). However, the effect of metformin on lung cancer outcomes has not been validated in larger samples.
In this study, we used a nationally representative, population-based cancer data source in the United States to determine the effect of metformin use on survival outcomes among patients with diabetes with stage IV non–small cell lung cancer (NSCLC).
Methods
The study was conducting using the Surveillance, Epidemiology, and End Results (SEER) registry (2007–2009) linked to Medicare claims (19). We selected patients with diabetes greater than or equal to 65 years with histologically confirmed stage IV NSCLC. We excluded individuals in health care maintenance organizations or those without Part B Medicare insurance because of a lack of complete claims and those without Part D coverage for whom we could not ascertain outpatient medications (20). To avoid patients who would not have been metformin candidates, we excluded patients older than 80 years, or those with stage IV–V chronic kidney disease or end-stage renal disease, or those not taking any diabetic medication. We excluded patients living in a nursing home at time of diagnosis because they likely had limited functional status.
Sociodemographics and Comorbidities
Sociodemographic information was obtained from the SEER and Medicare databases. We used the Deyo adaptation of Charlson index to assess the burden of comorbidities (21–23) and data about use of home health services (restricted to homebound patients) as a proxy for poor performance status (24).
Diabetes and Diabetic Regimen
We identified patients with diabetes using a validated algorithm based on presence of International Classification of Diseases, Ninth Revision codes (250.xx) prior to lung cancer diagnosis. Diabetes-related complications and end-organ damage were summarized using a validated, claims-based severity score (25). Diabetes medication use was ascertained from Medicare Part D claims. Patients were classified as using specific drugs if there was a pharmacy claim submitted within 6 months prior to cancer diagnosis. Medications were classified as follows: metformin, sulfonylureas, meglitinides, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors, and insulin.
Cancer-related Factors and Treatment
Tumor location and histology were obtained from SEER; histologic subtypes were classified using International Classification of Diseases for Oncology (26). We ascertained use of diagnosis and staging procedures from Medicare claims. Using validated claim-based algorithms, we classified patients as treated with chemotherapy if they received treatment within 4 months of NSCLC diagnosis (27). Receipt of radiation therapy was ascertained using SEER and Medicare data (28).
Study Outcome
The study outcome was overall survival determined from Medicare data. Survival times were calculated as the period from the date of diagnosis to the date of death; subjects alive as of December 15, 2011 were censored.
Statistical Analysis
Baseline characteristics were compared between patients using metformin versus other diabetes medications using the t test, chi-square test, or Wilcoxon test. Unadjusted Kaplan-Meier curves were plotted for patients treated with or without metformin and compared using the log-rank test.
We used propensity score methods to control for potential allocation bias (29) because differences in patient characteristics and diabetes severity may have influenced use of metformin. The propensity score represents the probability that a patient will receive a treatment based on their known characteristics. We calculated propensity scores using a logistic model that included patients’ sociodemographics, comorbidities, performance status, diabetes medications, and diabetes severity score and used regression analysis to evaluate whether covariates were balanced across treatment groups.
Cox regression was used to compare survival of patients on metformin versus other medications adjusting for propensity scores and use of positron emission tomography scan, tumor characteristics, and use of chemotherapy. Adjusted analyses were performed using inverse probability weighting, fitting a stratified Cox model according to propensity score quintiles, and matching patients by propensity scores (30). We conducted secondary stratified analyses by receipt of chemotherapy, insulin treatment, and other oral medication use. Additionally, to assess if the survival benefit was specific to metformin, we tested the effect of insulin or other oral medications among patients not receiving metformin. Analyses were performed with SAS 9.3 (SAS, Cary, NC) using two-tailed P values. Our study was deemed exempt following institutional review board evaluation.
Results
We identified 1,177 patients aged 65–80 years with diabetes at the time of diagnosis with stage IV NSCLC. We excluded 394 patients who were not on diabetes medications and 33 individuals who were in a nursing home at time of diagnosis; the final cohort consisted of 750 patients. Overall, 492 (61%) patients were treated with metformin at time of lung cancer diagnosis; median time of metformin use was 26.7 months among the cohort diagnosed in 2009 (who had Medicare Part D coverage since 2007). Metformin-treated patients were more likely to have fewer comorbidities (P = 0.02) and, as expected, were less likely to receive sulfonylureas (P = 0.04) or insulin (P < 0.01) (Table 1). All other baseline characteristics were not significantly different between the two groups and all covariates were well-balanced after adjustment for propensity scores (Table 1). Those in the metformin group were more likely to have had a positron emission tomography scan (P = 0.02) (Table 2); all other cancer-staging variables (mediastinoscopy, fine-needle biopsy, and bone scan) were similar between the two groups. Metformin-treated patients were also more likely to have been treated with chemotherapy (P = 0.02), but there was no difference between the groups for erlotinib therapy.
Table 1.
Baseline Characteristics of Patients with Stage IV Non–Small Cell Lung Cancer with Diabetes in the SEER-Medicare Database, 2007–2009
| Characteristic | Metformin (n = 458) | No Metformin (n = 292) | P Value | Adjusted P Value* |
|---|---|---|---|---|
| Age, yr, mean ± SD | 72.2 ± 4.0 | 72.7 ± 4.0 | 0.09 | 0.98 |
| Male, n (%) | 233 (52.8) | 164 (57.8) | 0.19 | 0.99 |
| Married, n (%) | 212 (48.1) | 135 (47.5) | 0.89 | 0.99 |
| Race/ethnicity, n (%) | 0.25 | 0.99 | ||
| White | 297 (67.4) | 187 (65.9) | ||
| Black | 57 (12.9) | 50 (17.6) | ||
| Hispanic | 45 (10.2) | 21 (7.4) | ||
| Other | 42 (9.5) | 26 (9.2) | ||
| Income, n (%) | 0.23 | 0.99 | ||
| First quartile | 153 (34.8) | 101 (35.6) | ||
| Second quartile | 91 (20.7) | 70 (24.7) | ||
| Third quartile | 99 (22.5) | 67 (23.6) | ||
| Fourth quartile | 97 (25.1) | 46 (16.2) | ||
| Diabetes severity index score, median (IQR) | 4 (3) | 5 (4) | 0.17 | 0.99 |
| Comorbidity score, n (%) | 0.02 | 0.96 | ||
| <1 | 194 (42.4) | 115 (39.4) | ||
| 1–2 | 121 (26.4) | 59 (20.2) | ||
| >2 | 143 (31.2) | 118 (40.4) | ||
| Diabetes medications, n (%) | ||||
| Sulfonylureas | 234 (51.1) | 172 (58.9) | 0.04 | 0.98 |
| Meglitinides | 25 (5.5) | 24 (8.2) | 0.14 | 0.91 |
| Thiazolidinediones | 159 (34.7) | 108 (37.0) | 0.53 | 0.99 |
| DPP-4 inhibitors | 34 (7.4) | 19 (6.5) | 0.63 | 0.97 |
| α-Glucosidase inhibitors | ≤11 (≤2.5) | ≤11 (≤4.0) | 0.93 | 0.99 |
| Insulin | 121 (26.4) | 112 (38.4) | <0.01 | 0.77 |
Definition of abbreviations: DPP-4 = dipeptidyl peptidase-4; IQR = interquartile range.
P values for analysis adjusting for propensity scores.
Table 2.
Lung Cancer and Treatment Characteristics of Study Subjects
| Characteristic | Metformin (n = 458) | No Metformin (n = 292) | P Value |
|---|---|---|---|
| Tumor histology, n (%) | 0.69 | ||
| Adenocarcinoma | 276 (62.6) | 172 (60.6) | |
| Squamous cell | 120 (27.2) | 82 (28.9) | |
| Large cell or other | 45 (10.2) | 30 (10.5) | |
| Tumor site, n (%) | 0.80 | ||
| Upper lobe | 202 (45.8) | 133 (46.8) | |
| Middle/lower lobe | 139 (31.5) | 81 (28.5) | |
| Other | 100 (22.7) | 70 (24.7) | |
| Mediastinoscopy, n (%) | 12 (2.8) | ≤11 (≤4.0) | 0.25 |
| PET scan, n (%) | 163 (37.9) | 81 (29.5) | 0.02 |
| Bone scan, n (%) | 83 (19.3) | 43 (15.6) | 0.22 |
| Fine-needle aspiration, n (%) | 113 (25.6) | 73 (25.7) | 0.98 |
| Chemotherapy, n (%) | 227 (51.5) | 122 (43.0) | 0.03 |
| Erlotinib, n (%) | 66 (14.4) | 36 (12.3) | 0.42 |
Definition of abbreviation: PET = positron emission tomography.
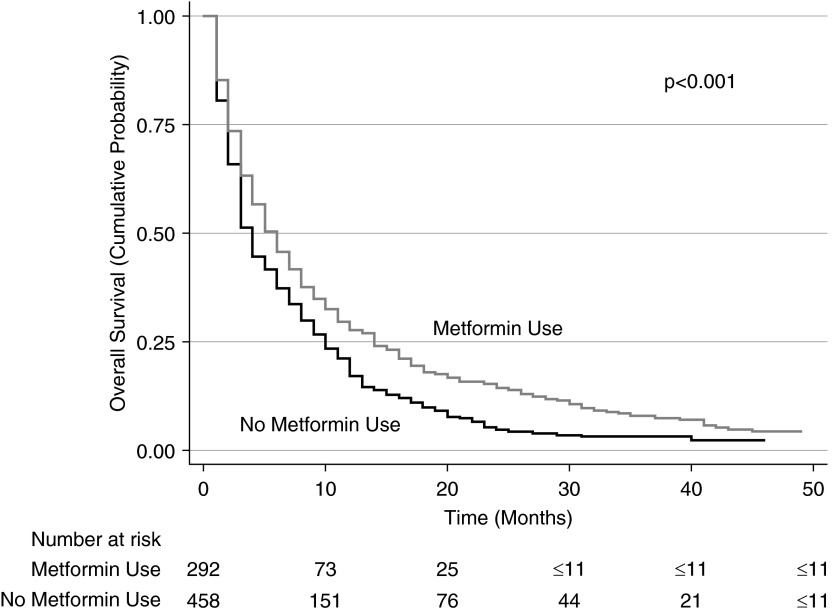
Unadjusted median overall survival for those in the metformin group was 5 months (interquartile range, 12 mo) compared with 3 months among those not treated with metformin (interquartile range, 8 mo; P < 0.001) (Figure 1). Inverse probability weighting analyses using Cox regression (and adjusted for staging work-up, cancer characteristics, and chemotherapy use) showed that metformin was associated with significantly better overall survival (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.71–0.89) (Table 3). The survival advantage with metformin persisted when the analyses were repeated using stratification (HR, 0.79; 95% CI, 0.61–0.93) or matching (HR, 0.73; 95% CI, 0.62–0.87) of study patients by propensity scores.
Figure 1.
Kaplan-Meier survival curves for all patients in cohort. Patients in the metformin group have better overall survival than those in the nonmetformin group.
Table 3.
Propensity Score Analysis: Comparison of Survival of Patients Treated with and without Metformin
| Model | Hazard Ratio (95% CI) |
|---|---|
| Primary analysis: entire cohort | |
| Inverse probability weighted | 0.80 (0.71–0.89) |
| Stratified by propensity score quintiles | 0.79 (0.61–0.93) |
| Matched analysis (n = 708) | 0.73 (0.62–0.87) |
| | |
| Secondary analyses | |
| Chemotherapy-treated patients | |
| Inverse probability weighted | 0.77 (0.65–0.92) |
| Stratified by propensity score quintiles | 0.77 (0.60–0.98) |
| Matched analysis (n = 340) | 0.75 (0.58–0.97) |
| Nonchemotherapy-treated patients | |
| Inverse probability weighted | 0.83 (0.71–0.97) |
| Stratified by propensity score quintiles | 0.86 (0.69–1.08) |
| Matched analysis (n = 368) | 0.77 (0.61–0.99) |
| Insulin-treated patients | |
| Inverse probability weighted | 0.76 (0.61–0.95) |
| Stratified by propensity score quintiles | 0.70 (0.52–0.93) |
| Matched analysis (n = 218) | 0.65 (0.48–0.88) |
| Noninsulin-treated patients | |
| Inverse probability weighted | 0.79 (0.69–0.92) |
| Stratified by propensity score quintiles | 0.83 (0.67–1.02) |
| Matched analysis (n = 490) | 0.77 (0.62–0.95) |
| Other oral medication–treated patients | |
| Inverse probability weighted | 0.74 (0.65–0.84) |
| Stratified by propensity score quintiles | 0.71 (0.59–0.85) |
| Matched analysis (n = 520) | 0.65 (0.53–0.80) |
Definition of abbreviation: CI = confidence interval.
The hazard ratio represents the risk of death of a patient treated with metformin compared with a patient who did not receive metformin. All models were also adjusted for tumor histology, tumor site, receipt of positron emission tomography scan, and use of chemotherapy.
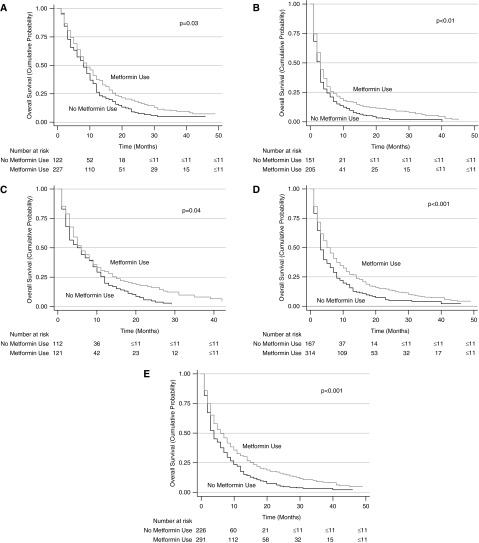
Unadjusted (Figure 2) and adjusted (Table 3) secondary analysis stratified by receipt of chemotherapy (HR, 0.77; 95% CI, 0.65–0.92), insulin treatment (HR, 0.76; 95% CI, 0.61–0.95), or other oral diabetes medications (HR, 0.73; 95% CI, 0.65–0.83) also showed a significant survival advantage conferred by metformin use. The survival benefit of metformin remained significant when secondary analyses were repeated using stratification or matching of patients by propensity scores. However, analyses excluding those on metformin showed that use of other oral diabetes medications did not confer a survival benefit (HR, 0.88; 95% CI, 0.65–1.20) in the insulin-treated group. Similarly, insulin was not associated with improved survival (HR, 0.97; 95% CI, 0.79–1.20) in patients on other (nonmetformin) oral medications.
Figure 2.
Stratified Kaplan-Meier survival curves. Stratified by patients who received chemotherapy (A), patients who did not receive chemotherapy (B), patients who were treated with insulin (C), patients who were not treated with insulin (D), and patients who were treated with other oral medications (E).
Discussion
Prior studies have reported a survival benefit with metformin use in breast, prostate, and colorectal cancer; however, there are limited data regarding the potential effectiveness of this drug among patients with lung cancer. Using population-based data, we found that among patients with stage IV NSCLC with preexisting diabetes, metformin compared with other diabetic medications was associated with significantly better survival. Our results contribute further evidence supporting the potential anticancer effects of metformin. Randomized clinical trials of metformin as an adjunctive treatment of lung cancer, and deeper investigation into its underlying biologic mechanisms, are likely warranted.
There have been few publications examining metformin’s influence on lung cancer risk (31–34). These studies show varying results regarding metformin and risk for developing lung cancer and lung cancer prognosis. In a retrospective case-control study, Mazzone and colleagues (31) found that metformin was associated with a more aggressive lung cancer phenotype and worse prognosis. Conversely, two observational studies, a small Chinese study with patients with lung cancer and a larger UK study that included many cancer types, showed that metformin was associated with improved survival in patients with lung cancer (17, 18). A major limitation of these studies is the lack of adjustment for baseline patient characteristics that might account for metformin use. Moreover, in these studies, all stages of lung cancer were included and the analyses did not control for differences in lung cancer treatment. Our study is the first to show the association of metformin with improved survival in a large, unselected sample of patients with stage IV NSCLC, after controlling and stratifying by use of other diabetic medications and chemotherapy. Consistent with the prior studies, we found that metformin was associated with an almost 20% improvement in overall survival among subjects with diabetes with metastatic lung cancer.
Although the mechanisms are not yet fully understood, there are two potential pathways through which metformin may have an anticancer effect. NSCLC expresses the insulin receptor, and high levels of insulin receptor expression have been associated with faster cancer progression and decreased survival (35). Metformin reduces insulin resistance and circulating insulin levels, thereby attenuating the stimulatory effect of insulin (36). Mouse models of lung cancer, using doses of metformin that are physiologically relevant to humans, have found that metformin administration led to decreased circulating concentrations of insulin and insulin-like growth factor-1 and in lung tissue, reduced activation of insulin receptor/insulin-like growth factor-1 receptor, decreased downstream signaling, and diminished cancer cell proliferation (37). Supporting this theory, we found that among patients treated with insulin, metformin use conferred a significant survival benefit and may therefore attenuate the cancer-promoting effects of insulin. Conversely, other oral medications were not associated with an improved survival, suggesting that the effect is specific to metformin.
Metformin may also act directly on tumor cells by altering intracellular signaling pathways leading to a decrease in cell proliferation (38–43). In vitro and animal-based studies have reported that metformin inhibits NSCLC growth by activation of adenosine monophosphate–activated protein kinase in tumor cells (38). Activation of adenosine monophosphate–activated protein kinase down-regulates protein, cholesterol, and glycogen synthesis pathways and inhibits cell proliferation (44, 45). Combination therapy with metformin and doxorubicin also has been shown to inhibit lung tumor growth in animal studies, even when doxorubicin doses were reduced to subtherapeutic levels (46). Similarly, we found that even after controlling for receipt of chemotherapy, those in the metformin group had better overall survival, thus suggesting a potential independent cancer therapeutic effect of metformin.
Several strengths and limitations of this study should be noted. First, because of the observational nature of the study, treatment with metformin was not randomized. To address this limitation, we excluded patients who would not have been eligible for metformin therapy and adjusted our analyses for potential confounders, such as comorbidities, other diabetes medications, and diabetes severity to create a comparison group that would have had similar likelihoods of receiving metformin. Patients with NSCLC who were treated with metformin were more likely to receive chemotherapy and less likely to be on insulin; however, the survival benefit of metformin was consistent even after stratifying by these factors. We were also unable to assess glycemic control and therefore could not evaluate the potential benefit of metformin through its effect of diabetes outcomes. For this reason, we studied a cohort of patients with stage IV NSCLC, because most of these patients die of cancer progression rather than competing risks of death; thus, the potential effect of diabetes control should be marginal. We were also unable to assess the effect of metformin in younger patients and we did not assess the effect in patients without diabetes. Moreover, our cohort included many older patients with considerable number of comorbidities, many of whom did not receive chemotherapy. Thus, these findings may not be generalizable to healthier individuals with lung cancer.
Use of diabetes medications was determined using pharmacy claims, and therefore we have no information on adherence to therapy. Despite this, pharmacy claims data have been shown to have high concordance with pill counts (47). We used a conservative estimate of medication treatment because some patients receive a 3-month supply of their chronic medications. Furthermore, lack of adherence would have biased our results toward the null. Finally, we were not able to assess whether there was a dose-dependent effect of metformin on overall survival. Consistent with an intention-to-treat analysis, we did not include metformin use after lung cancer diagnosis because ongoing use of metformin after cancer diagnosis may suggest a healthier population.
In summary, these data suggest that among patients with diabetes and stage IV NSCLC, metformin use was associated with improved survival. This effect is consistent with the survival benefit of metformin observed in other cancer types. Further prospective studies evaluating the use of metformin in conjunction with chemotherapy for stage IV lung cancer can help determine if metformin is an effective treatment for lung cancer.
Acknowledgments
Acknowledgment
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services, and the Office of Strategic Planning, Health Care Finance Administration; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of the Surveillance, Epidemiology, and End Reults–Medicare Database. The interpretation and reporting of these data are the sole responsibilities of the authors.
Footnotes
Supported by the National Institutes of Health (1K07CA166462-01, J.J.L.; 1K07CA180782-01, K.S.). The funding source had no role in the study design, implementation, or interpretation of results.
Author Contributions: Conception and design, J.J.L. and J.P.W. Analysis and interpretation of the data, J.J.L., G.M., K.S., M.D.G., and J.P.W. Drafting of the article, J.J.L., E.J.G., and J.P.W. Statistical expertise, J.P.W. Administrative, technical, or logistic support, G.M., M.D.G., C.B.S., and D.L. All authors were involved in the critical revision of the manuscript and gave approval of the version submitted. J.P.W. is the guarantor.
Originally Published in Press as DOI: 10.1164/rccm.201407-1395OC on December 18, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stenvang J, Kümler I, Nygård SB, Smith DH, Nielsen D, Brünner N, Moreira JM. Biomarker-guided repurposing of chemotherapeutic drugs for cancer therapy: a novel strategy in drug development. Front Oncol. 2013;3:313. doi: 10.3389/fonc.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina-Franco JL, Giulianotti MA, Welmaker GS, Houghten RA. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov Today. 2013;18:495–501. doi: 10.1016/j.drudis.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta SC, Sung B, Prasad S, Webb LJ, Aggarwal BB. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–480. doi: 10.1016/j.tem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 7.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 8.Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17:616–628. doi: 10.4158/EP10357.RA. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35:1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, Saquib N, Rock CL, Pierce JP. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29:54–60. doi: 10.1200/JCO.2010.29.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YC, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, Chang SC, Lan YT, Wang HS, Liu CY, et al. Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. J Cancer Res Clin Oncol. 2011;137:211–220. doi: 10.1007/s00432-010-0879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder CF, Stein KB, Barone BB, Peairs KS, Yeh HC, Derr RL, Wolff AC, Carducci MA, Brancati FL. Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis. 2010;13:58–64. doi: 10.1038/pcan.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135:639–646. doi: 10.1007/s10549-012-2170-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:707–710. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 17.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, Yao B, Xie K, Li LH, Dong H, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117:5103–5111. doi: 10.1002/cncr.26151. [DOI] [PubMed] [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(Suppl. 8):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and Medicare claims for cancer patients. Med Care. 2006;44:921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.Wisnivesky JP, Smith CB, Packer S, Strauss GM, Lurslurchachai L, Federman A, Halm EA. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II-IIIA lung cancer: observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Fritz AG.editorInternational classification of diseases for oncology: ICD-O 3rd edGeneva, Switzerland: World Health Organization; 2000 [Google Scholar]
- 27.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knopf KB. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(Suppl. 8):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 28.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(Suppl. 8):IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 29.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(Suppl. 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 30.Wei L, Lin D, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 31.Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, Sasidhar M. The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer. 2012;12:410. doi: 10.1186/1471-2407-12-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodmer M, Becker C, Jick SS, Meier CR. Metformin does not alter the risk of lung cancer: a case-control analysis. Lung Cancer. 2012;78:133–137. doi: 10.1016/j.lungcan.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Smiechowski BB, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care. 2013;36:124–129. doi: 10.2337/dc12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JS, Kim ES, Liu D, Lee JJ, Solis L, Behrens C, Lippman SM, Hong WK, Wistuba II, Lee HY. Prognostic impact of insulin receptor expression on survival of patients with nonsmall cell lung cancer. Cancer. 2012;118:2454–2465. doi: 10.1002/cncr.26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen—induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108:2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu N, Gu C, Gu H, Hu H, Han Y, Li Q. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma. 2011;58:482–490. doi: 10.4149/neo_2011_06_482. [DOI] [PubMed] [Google Scholar]
- 40.Tseng SC, Huang YC, Chen HJ, Chiu HC, Huang YJ, Wo TY, Weng SH, Lin YW. Metformin-mediated downregulation of p38 mitogen-activated protein kinase-dependent excision repair cross-complementing 1 decreases DNA repair capacity and sensitizes human lung cancer cells to paclitaxel. Biochem Pharmacol. 2013;85:583–594. doi: 10.1016/j.bcp.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Do MT, Kim HG, Khanal T, Choi JH, Kim DH, Jeong TC, Jeong HG. Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol Appl Pharmacol. 2013;271:229–238. doi: 10.1016/j.taap.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitazono S, Takiguchi Y, Ashinuma H, Saito-Kitazono M, Kitamura A, Chiba T, Sakaida E, Sekine I, Tada Y, Kurosu K, et al. Effect of metformin on residual cells after chemotherapy in a human lung adenocarcinoma cell line. Int J Oncol. 2013;43:1846–1854. doi: 10.3892/ijo.2013.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–839. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 46.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44:471–477. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]