Abstract
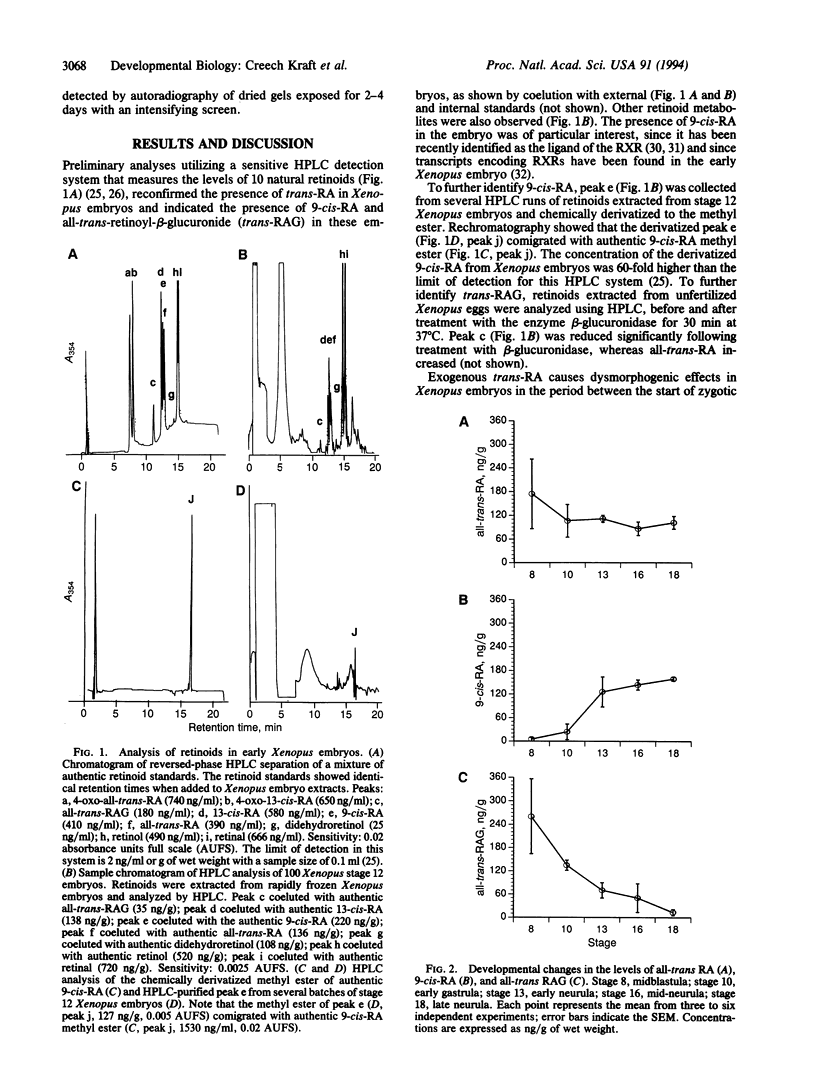
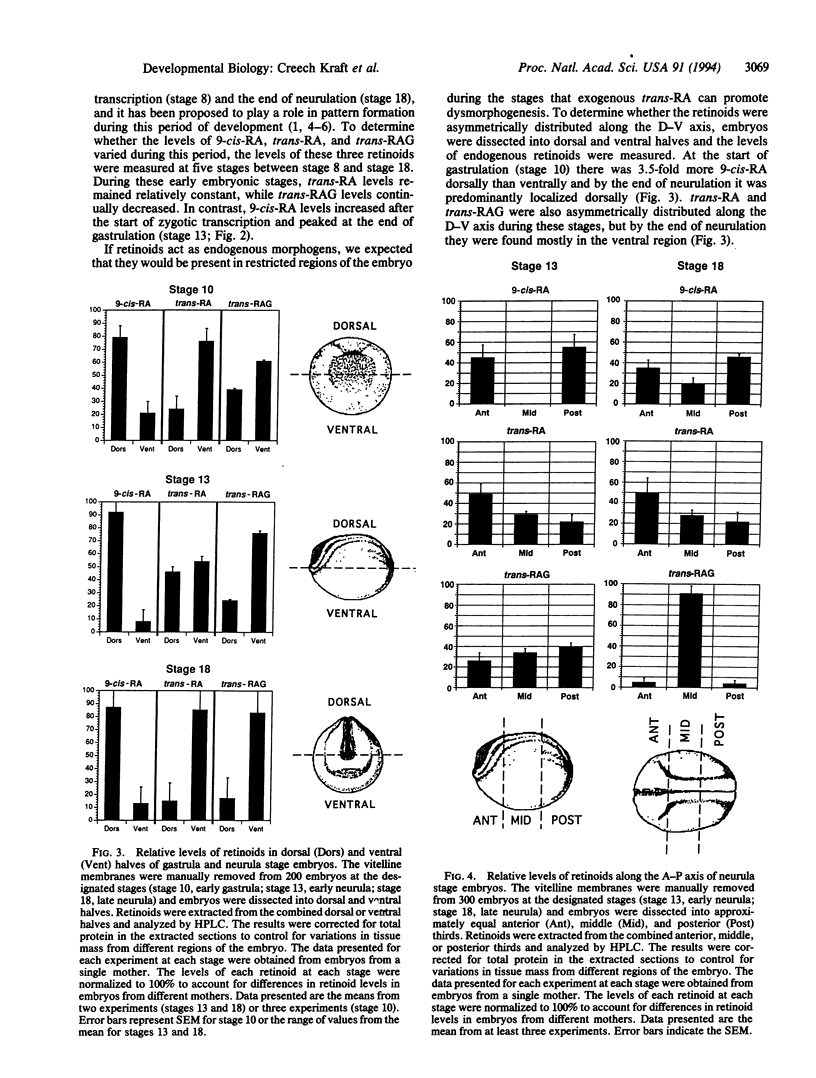
Endogenous retinoids are potential regulators of vertebrate embryogenesis that have been implicated in early anterior-posterior patterning and limb-bud development. We have characterized the temporal and spatial distribution of 9-cis-retinoic acid in the Xenopus embryo and compared it to two other retinoids, all-trans-retinoic acid and all-trans-retinoyl-beta-glucuronide. 9-cis-Retinoic acid is first detected after the midblastula transition and by the end of gastrulation is localized primarily within the anterior and posterior dorsal regions of the embryo. Since 9-cis-retinoic acid is a 6-fold more potent dysmorphogen than trans-retinoic acid, we suggest that it is involved in the early specification of the Xenopus anterior-posterior axis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkan W., Colbert M., Bock C., Linney E. Transgenic indicator mice for studying activated retinoic acid receptors during development. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3347–3351. doi: 10.1073/pnas.89.8.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B., Mangelsdorf D. J., Dyck J. A., Bittner D. A., Evans R. M., De Robertis E. M. Multiple retinoid-responsive receptors in a single cell: families of retinoid "X" receptors and retinoic acid receptors in the Xenopus egg. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2321–2325. doi: 10.1073/pnas.89.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Huang L., Russo A. F., Solursh M. Retinoic acid is enriched in Hensen's node and is developmentally regulated in the early chicken embryo. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10056–10059. doi: 10.1073/pnas.89.21.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Dreyer C. A retinoic acid receptor expressed in the early development of Xenopus laevis. Genes Dev. 1991 Jan;5(1):94–104. doi: 10.1101/gad.5.1.94. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Thaller C., Eichele G. Evidence that Hensen's node is a site of retinoic acid synthesis. Nature. 1992 Sep 17;359(6392):237–241. doi: 10.1038/359237a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991 Oct 4;67(1):89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M. Limb development in mouse embryos. I. Analysis of teratogenic effects of retinoic acid. Teratology. 1973 Jun;7(3):289–298. doi: 10.1002/tera.1420070310. [DOI] [PubMed] [Google Scholar]
- Kraft J. C., Juchau M. R. Correlations between conceptal concentrations of all-trans-retinoic acid and dysmorphogenesis after microinjections of all-trans-retinoic acid, 13-cis-retinoic acid, all-trans-retinoyl-beta-glucuronide, or retinol in cultured whole rat embryos. Drug Metab Dispos. 1992 Mar-Apr;20(2):218–225. [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Maden M., Ong D. E., Summerbell D., Chytil F. The role of retinoid-binding proteins in the generation of pattern in the developing limb, the regenerating limb and the nervous system. Development. 1989;107 (Suppl):109–119. doi: 10.1242/dev.107.Supplement.109. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Ruberte E., LeMeur M., Morriss-Kay G., Chambon P. Developmental analysis of the retinoic acid-inducible RAR-beta 2 promoter in transgenic animals. Development. 1991 Nov;113(3):723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay G. M., Murphy P., Hill R. E., Davidson D. R. Effects of retinoic acid excess on expression of Hox-2.9 and Krox-20 and on morphological segmentation in the hindbrain of mouse embryos. EMBO J. 1991 Oct;10(10):2985–2995. doi: 10.1002/j.1460-2075.1991.tb07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992 Sep 18;70(6):1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Reynolds K., Mezey E., Zimmer A. Activity of the beta-retinoic acid receptor promoter in transgenic mice. Mech Dev. 1991 Dec;36(1-2):15–29. doi: 10.1016/0925-4773(91)90068-h. [DOI] [PubMed] [Google Scholar]
- Rossant J., Zirngibl R., Cado D., Shago M., Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991 Aug;5(8):1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. M. Retinoic acid modifies the pattern of cell differentiation in the central nervous system of neurula stage Xenopus embryos. Development. 1991 Aug;112(4):945–958. doi: 10.1242/dev.112.4.945. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. Retinoic acid modifies mesodermal patterning in early Xenopus embryos. Genes Dev. 1991 Feb;5(2):175–187. doi: 10.1101/gad.5.2.175. [DOI] [PubMed] [Google Scholar]
- Shenefelt R. E. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972 Feb;5(1):103–118. doi: 10.1002/tera.1420050115. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Cheng P. F. Retinoic acid perturbs the expression of Xhox.lab genes and alters mesodermal determination in Xenopus laevis. Genes Dev. 1991 Aug;5(8):1321–1332. doi: 10.1101/gad.5.8.1321. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990 Jun;4(6):932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Tannahill D. Mechanism of anteroposterior axis specification in vertebrates. Lessons from the amphibians. Development. 1992 Feb;114(2):285–302. doi: 10.1242/dev.114.2.285. [DOI] [PubMed] [Google Scholar]
- Summerbell D. The effect of local application of retinoic acid to the anterior margin of the developing chick limb. J Embryol Exp Morphol. 1983 Dec;78:269–289. [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982 Apr 8;296(5857):564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Wagner M., Han B., Jessell T. M. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992 Sep;116(1):55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]
- Warkany J., Nelson R. C. APPEARANCE OF SKELETAL ABNORMALITIES IN THE OFFSPRING OF RATS REARED ON A DEFICIENT DIET. Science. 1940 Oct 25;92(2391):383–384. doi: 10.1126/science.92.2391.383. [DOI] [PubMed] [Google Scholar]




