Abstract
Purpose.
We determined whether the scleral spur is shorter in primary open-angle glaucoma (POAG) eyes compared to age-matched normal eyes and whether the collapse of Schlemm's canal (SC) is more prevalent in eyes with a shorter scleral spur.
Methods.
The anterior segments of normal (n = 20) and POAG eyes (n = 20) were fixed and processed for light microscopy. The scleral spur length, ratio of posterior trabecular meshwork (TM) insertion into the scleral spur to the posterior TM height, and the percentage of SC collapse were measured. Analysis using an existing mathematical model was conducted to estimate the distances that the scleral spur theoretically would move in vivo and to determine if these distances would be sufficient to keep SC open in POAG compared to normal eyes.
Results.
The mean scleral spur length was significantly shorter in POAG eyes compared to normal eyes (P < 0.0001). A higher mean percentage of SC collapse was found in POAG eyes than in normal eyes (P < 0.0001). Estimated posterior movement of scleral spur in POAG eyes was less than sufficient to prevent the collapse of SC. A significant negative correlation was found between the posterior scleral spur movement and percent collapse of SC (P < 0.0001).
Conclusions.
A shorter scleral spur found in POAG eyes was associated with a higher percent of SC collapse. Our data suggest that a shorter scleral spur may be a risk factor in the development of POAG by being insufficient to hold SC open.
Keywords: scleral spur, Schlemm's canal, trabecular meshwork, primary open-angle glaucoma, light microscopy
Shorter scleral spur length identified in POAG eyes compared to age-matched normal eyes, which contributes to collapse of Schlemm's canal, due to its inadequate posterior movement upon ciliary muscle contraction.
Introduction
Primary open-angle glaucoma (POAG) is the second leading cause of blindness in the world.1 The primary risk factor for POAG is elevated IOP, which is caused by increased outflow resistance somewhere in the trabecular meshwork (TM) outflow pathway.2–4 The main resistive site is believed to be within the inner wall endothelium of Schlemm's canal (SC), its basement membrane, and underlying extracellular matrix in the juxtacanilicular tissue.3–5 Nevertheless, other factors, including short scleral spur lengths,6–8 are believed to be involved in the pathogenesis of POAG.
The scleral spur is a shelf-like structure formed from a projection of the sclera, bordered anteriorly by the corneoscleral portion of the TM and posteriorly by the longitudinal fibers of the ciliary muscle.9 The scleral spur contains circumferentially oriented elastic and collagenous fibers, which give it its rigidity relative to other parts of the sclera.10 In addition, the scleral spur also contains spindle-shaped, circularly oriented contractile myofibroblast cells, also known as scleral spur cells.11,12 Elastic fiber tendons from the longitudinal fibers of the ciliary muscle project anteriorly into the scleral spur and join with the elastic fibers of the scleral spur.13 The elastic fibers of the scleral spur are continuous anteriorly with the elastic fibers in the TM, terminating in the juxtacanilicular tissue underneath the endothelium of SC.9–11 Given the scleral spur's rigid nature and the type of network that it forms with the neighboring ciliary muscle and TM, the scleral spur has long been hypothesized to have a key role in maintaining the patency of SC, and, thus, facilitating aqueous outflow. The scleral spur serves this role by acting as a means for the ciliary muscle to exert its force on the TM via posterior displacement of the scleral spur, and in doing so, facilitating aqueous egress by separating the trabecular beams, and, thus, opening and supporting SC.7,9,14
One treatment for lowering IOP in POAG is pilocarpine, a drug that increases aqueous outflow by inducing contraction of the ciliary muscle, which then opens up the beams of the TM. The drug's effectiveness is lost upon severing the anterior attachment of the ciliary muscle from the scleral spur, which demonstrates the importance of this network in the aqueous outflow pathway.15,16 In addition, the finding that the posterior part of SC where the scleral spur exerts the most force is wider than the anterior also is testament to this function of the scleral spur in keeping the canal open.17
One previous study found a shorter scleral spur in POAG eyes compared to normal eyes, but did not provide a detailed description of the methods of measurment.8 This finding led to the hypothesis that a short scleral spur compromises the “ciliary muscle–scleral spur–trabecular meshwork” network, which leads to the collapse of SC and subsequent increase in IOP in POAG patients.8,17–19 Only one other study to our knowledge reported a measurement for the length of the scleral spur in normal eyes; however, this study did not explicitly explain how the measurement was done.20 The reported values for the scleral spur length in normal eyes from these two studies were so different that additional confirmation is required.
In this study, we hypothesized that the scleral spur is shorter in eyes with POAG compared to age-matched normal eyes and that the shorter scleral spur may be insufficient to hold SC open, resulting in the collapse of SC in eyes with POAG. To test our hypothesis, we developed a clear and accurate method to measure the scleral spur length to assess differences between normal and POAG eyes, and we examined the relationships between scleral spur length and percentage of SC collapse and between scleral spur length and age of the POAG patients at diagnosis. In addition, a mathematical analysis using an existing model20 was conducted to estimate the distances that the scleral spur theoretically would move in vivo, and to determine if these distances would be sufficient to keep SC open in POAG eyes compared to normal eyes.
Materials and Methods
Materials
A total of 20 POAG eyes from 13 donors (67–90 years old) with clinically confirmed diagnoses of POAG and 20 age-matched normal eyes from 16 donors (63–92 years old) without any known ocular diseases were obtained from the National Disease Research Interchange (NDRI; Philadelphia, PA, USA) and San Diego Eye Bank (San Diego, CA, USA) within 24 hours postmortem. Normal eyes also were confirmed to be grossly normal by examination under a dissecting microscope. A summary of the donor characteristics is shown in Tables 1 and 2. The mean ages of normal and POAG eyes were not statistically different (unpaired t-test, P = 0.097). In four of the normal and seven of the POAG donors, both eyes were used. All donor eyes were used in accordance with the guidelines regarding use of human subjects and tissues as outlined in the Declaration of Helsinki.
Table 1.
Donor Information of Normal Eyes
|
Donor ID |
Age/Sex |
Eye(s) |
Cause of Death |
| 1 | 63 F | Right | End stage dementia |
| 2 | 66 F | Right | Cardiac arrest |
| 3 | 67 M | Right | Cardiac arrest |
| 4 | 68 M | Both | Lung cancer |
| 5 | 69 M | Right | Motor vehicle accident |
| 6 | 70 F | Right | Respiratory failure |
| 7 | 76 M | Right | Bowel obstruction |
| 8 | 78 M | Both | Liver failure |
| 9 | 78 F | Both | Unknown |
| 10 | 78 M | Right | COPD |
| 11 | 79 F | Right | Congestive heart failure |
| 12 | 83 M | Both | Respiratory failure |
| 13 | 83 F | Right | Congestive heart failure |
| 14 | 83 M | Right | Prostate cancer |
| 15 | 85 M | Right | Respiratory failure |
| 16 | 92 M | Right | GI bleed |
Table 2.
Donor Information of POAG Eyes
|
Donor ID |
Age/Sex |
Eye(s) |
Age at POAG Dx |
Medications |
Cause of Death |
| 17 | 67 F | Both | 65 | X | Congestive heart failure |
| 18 | 74 M | Right | 73 | T | CVA |
| 19 | 76 M | Both | 54 | B | CVA |
| 20 | 78 F | Right | 72 | Pi, T | Cardio-pulmonary arrest |
| 21 | 78 M | Right | 78 | Pr | Cardiac arrest |
| 22 | 79 F | Both | 76 | Al, L, T, X | CVA |
| 23 | 79 M | Both | 49 | Az, T, TZ | Acute renal failure |
| 24 | 80 M | Right | 70 | N/A | Unknown |
| 25 | 80 M | Both | N/A | N/A | Respiratory failure |
| 26 | 85 M | Left | 65 | X | Neck cancer |
| 27 | 88 F | Both | N/A | C | CVA |
| 28 | 90 M | Left | 60 | T, X | Congestive heart failure |
| 29 | 90 F | Both | N/A | X | Respiratory failure |
Al, Alphagan; Az, Azopt; B, Betoptic; C, Combigan; L, Lotemax; Pi, Pilocarpine; Pr, Propine; T, Timolol; TZ, Travatan Z; X, Xalatan.
Methods
Eye Processing.
Whole eyes with a small cut at the equator were immersion-fixed (0 mm Hg) with a modified Karnovsky's fixative (2% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer, pH 7.3) for three hours at room temperature. Each fixed eye was cut into anterior and posterior segments through the equator, followed by careful removal of the vitreous body and lens. Anterior segments of the eyes then were divided into four quadrants (designated temporal, nasal, superior, and inferior). Histological sections of 1 to 1.5 mm were cut radially. The sections were postfixed with 2% osmium tetroxide in 1.5% potassium ferrocyanide for two hours, dehydrated in an ascending series of ethanols, and embedded in Epon-Araldite (Electron Microscopy Sciences, Hatfield, PA, USA). Some tissue samples without known specific quadrants from previous studies, fixed similarly, were used in this study. Serial semithin sections (3 μm) were cut and stained with 1% toluidine blue (Fisher Scientific Co., Waltham, MA, USA) to identify the scleral spur, TM, and SC. Light micrographs were taken at a magnification of 10× to analyze any differences between the normal and POAG eyes. In 12 POAG eyes and 10 normal eyes, the images from all four quadrants were analyzed by repeated measures ANOVA, and no statistical difference was found (P = 0.972) between the quadrants within each eye in all of the characteristics measured. Post hoc analysis was performed with a Tukey HSD test to compare all pairs of quadrants, and no significant difference was observed in any pair of quadrants. In all other eyes, two images from different quadrants were analyzed.
Photographs of the histological slides were taken using QCapture (v2.73.0; Advanced Imaging Concepts, Inc., Princeton, NJ, USA) and examined. All measurements were taken three times using ImageJ (v1.46; National Institutes of Health [NIH], Bethesda, MD, USA), and the data analyzed were the means of the three measurements for each donor eye. Additionally, another trained, masked observer (JL) repeated all measurements to confirm the repeatability of the method. The percentage difference between two individuals was 4.68%, which demonstrated no significant statistical difference.
Measurement Methods.
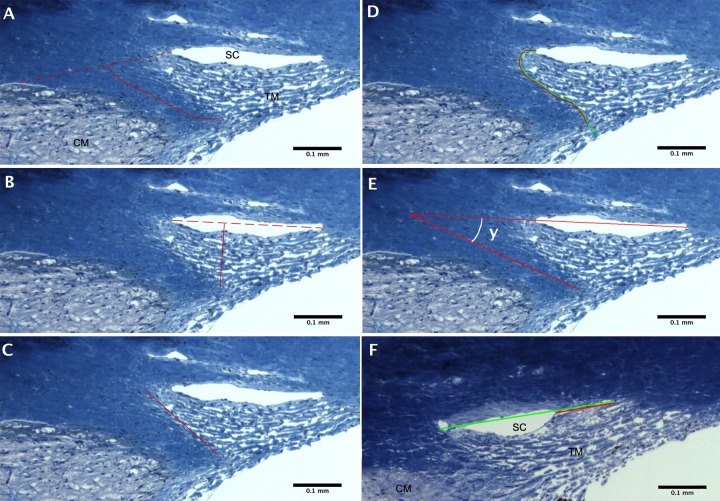
The length of the scleral spur was measured by three different methods, one developed in this study and two from previous literature.17,20 Since the two previous studies were not explicit in their methods and did not provide a figure,17,20 we developed a clear and accurate method to measure the true length of the scleral spur, including its slight curvature. In our method, a line was drawn from the point where the sclera curves out to form the spur, usually located near the posterior end of SC, to the point where the sclera begins again (Fig. 1A, dotted line). This line demarcated where the scleral spur ends and the sclera begins. Then, a curved line was drawn from the tip of the scleral spur to the dotted line, bisecting the width of the scleral spur at every point and representing the scleral spur length (Fig. 1A, solid line). In the second method (method of Nesterov and Batmanov17), we attempted to replicate their measurements of the scleral spur length, defined as the distance “from its tip to the level of the posterior end of SC,” by measuring from the tip of the scleral spur, directly to the level of the posterior end of SC (Fig. 1B). In the third method (method of Moses and Arnzen20), we attempted to interpret and replicate their estimation of the scleral spur length in normal eyes by measuring along the anterior side of the scleral spur from the tip to the level of the posterior end of SC in normal and POAG eyes (Fig. 1C). In addition to the length, the cross-sectional area of the scleral spur was measured by tracing the perimeter of the scleral spur, which includes the dotted line shown in Figure 1A as a border between sclera and scleral spur.
Figure 1.
Methods for measurements. (A–C) Measurements of the scleral spur length. Three methods were used to measure the scleral spur length, as shown in a 72-year-old normal eye. In our method (A) the solid line represents scleral spur length, drawn from the tip of the scleral spur to the middle of the red dotted line, which connects the anterior and posterior points where the sclera curves out to form the spur. In the method of Nesterov and Batmanov8 (B) the measurement was taken from the level of the anterior base of the scleral spur, directly to the level of the posterior end of SC. In the method of Moses and Arnzen20 (C) scleral spur length was measured by the red, solid line from the tip of the scleral spur to the level of the posterior end of SC, along the anterior side of the scleral spur. (D) Measurement of the ratio of TM insertion into the scleral spur to the total posterior height of TM, shown in the same eye as in (A–C). The red line represents the length of TM insertion in the scleral spur; the green line represents the total posterior height of TM from the anterior chamber to SC. (E) Measurement of the angle between the scleral sulcus and the scleral spur shown in the same eye as in (A–D). A straight, red line through the anterior and posterior ends of SC was drawn to designate the scleral sulcus as outlined by Grierson et al.15 The straight, red line from the tip of the scleral spur was drawn to designate the axis of the scleral spur and to identify the desired angle. The curved line indicates angle measured. (F) Measurement of the percentage of collapse of SC, shown in a POAG eye. The red line represents the width of SC collapse; the green line represents the total width of SC.
To evaluate the effect of the scleral spur length on the TM, the ratio of TM inserted into the anterior border of the scleral spur to the entire posterior height of the TM also was measured. The entire posterior height of the TM was measured from the point where the sclera curves out to form the scleral spur, along the anterior edge of the spur, ending where the TM meets the anterior chamber (Fig. 1D, green line). The amount of TM attached to the anterior aspect of the scleral spur was measured from where the sclera curves out by the posterior end of SC, along the anterior part of the spur and ending at the tip of the scleral spur (Fig. 1D, red line).
To use a previously established mathematical model20 to estimate the theoretical in vivo change in scleral spur position after ciliary muscle contraction, the angle between the scleral spur and scleral sulcus was measured by the method outlined by Grierson et al.15 The scleral sulcus was demarcated as a line passing through the anterior and posterior aspects of SC. In instances where SC was collapsed, an attempt was made to find where the canal would be located. The angle was measured where the line demarcating the scleral sulcus meets the line traced from the tip of the scleral spur and along its axis (Fig. 1E). Then, this angle was used with the model of Moses and Arnzen20 to estimate the theoretical change in position of the scleral spur upon ciliary muscle contraction.
The total width of SC and the portion of SC that was collapsed were measured from the anterior to posterior aspects. The percentage of collapse then was determined by dividing the width of collapse (Fig. 1F, red line) by the entire width of SC (Fig. 1F, green line). The maximum height of SC also was measured from the inner wall to the outer wall at the most open point along SC. In some POAG eyes, SC was completely collapsed in the image. In these cases, the maximum SC height was recorded as 0.
Statistical Methods
All statistical analyses were performed using R statistical computing package (v3.0.1; R Foundation for Statistical Computing, Vienna, Austria). Unpaired Student's t-tests were computed to compare the differences between normal and POAG eyes in all of the observed characteristics. Multiple linear regression analyses were performed with the scleral spur length measurements and available clinical diagnosis data of POAG eyes, to assess the association between the independent variable, scleral spur length, and the dependent variable, age at diagnosis of POAG, while controlling for sex. In instances where the date of diagnosis was not specifically stated, an approximation was obtained from other portions of the patient's ocular history, such as start date of medication. Six POAG eyes were not included in this analysis because their diagnosis dates were unattainable from clinical medical history from NDRI or next-of-kin. Other correlations were investigated using the same linear regression procedure. Multiple logistic regression analysis was performed using all eyes (n = 40) and using only one eye (chosen randomly) from each donor (n = 29), to test the association between scleral spur length and disease status (normal versus POAG). Using the intercept (β) values from the two multiple logistic regression models, we calculated the odds ratios and their 95% confidence intervals (CI) to assess for possible bias from using both eyes from 11 donors. The odds ratio calculated with all eyes (0.9121; CI: [0.8562, 0.9716]) was not significantly different from the odds ratio calculated with only one eye from each donor (0.9246; CI: [0.8689, 0.9840]), which indicates that the use of two eyes from the same donor would not affect the results or conclusions of this study.
Results
Scleral Spur Length
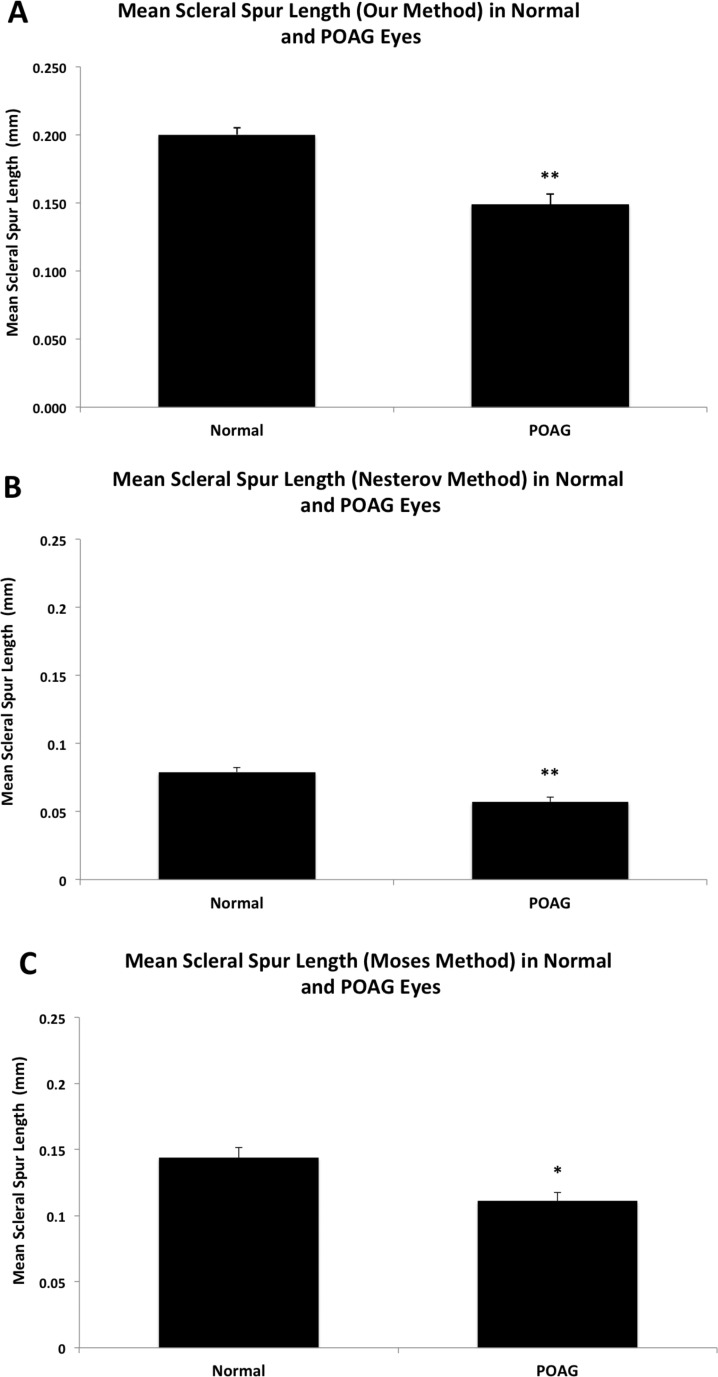
The length of the scleral spur in POAG eyes was significantly shorter than that in normal eyes using all three methods of measurement (Fig. 2). Using the method devised in this study, the mean length of the scleral spur in POAG eyes was 0.149 ± 0.008 mm (mean ± SE), which was significantly shorter than the mean length found in normal eyes of 0.200 ± 0.005 mm (P < 0.0001, Fig. 2A). Using the method of Nesterov and Batmanov,17 the mean length of the scleral spur in POAG eyes was 0.057 ± 0.003 mm, which was significantly shorter than the mean length of the scleral spur in normal eyes of 0.079 ± 0.003 mm (P < 0.0001; Figure 2B). Finally, using our interpretation of the normal length estimation of Moses and Arnzen,20 the mean length in POAG eyes was 0.111 ± 0.006 mm, which was significantly shorter than the mean length in normal eyes of 0.144 ± 0.008 mm (P = 0.002, Fig. 2C).
Figure 2.
Mean scleral spur length in normal and POAG eyes. (A) Using our method, the mean scleral spur length was significantly shorter in POAG eyes (0.149 ± 0.008 mm) than in normal eyes (0.200 ± 0.005 mm, P < 0.0001). Error bars: SE. **P ≤ 0.001. (B) Using the method of Nesterov and Batmanov,17 mean scleral spur length was much shorter than the measurement by our method, but it also is significantly shorter in POAG eyes (0.057 ± 0.003 mm) compared to normal eyes (0.081 ± 0.003 mm, P < 0.0001). Error bars: SE. **P ≤ 0.001. (C) Using the method of Moses and Arnzen,20 mean scleral spur length was shorter than the measurement by our method, but longer than the method of Nesterov and Batmanov.17 Similarly, the length was significantly shorter in POAG eyes (0.111 ± 0.006 mm) than in normal eyes (0.144 ± 0.008 mm, P = 0.002). Error bars: SE. *P < 0.05.
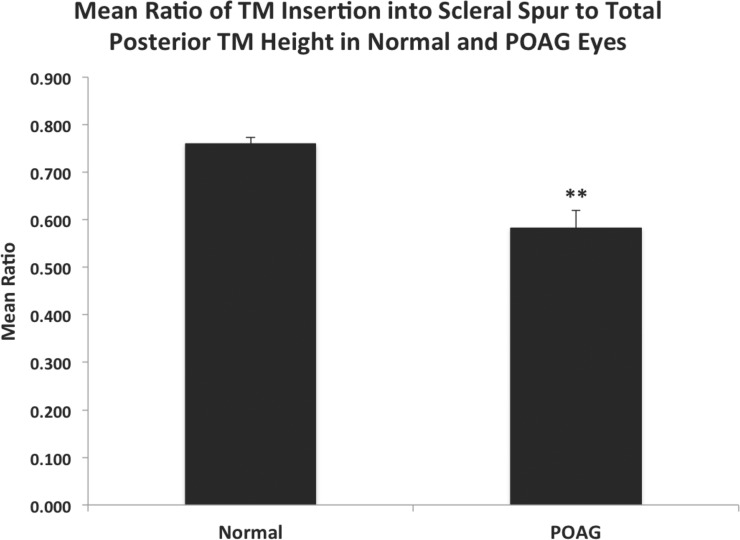
Observing the effect of the scleral spur length on the amount of TM insertion into the anterior aspect of the spur, the ratio of the TM inserted into the anterior aspect of the spur to the total posterior TM height was significantly smaller in POAG eyes (0.582 ± 0.037), compared to normal eyes (0.760 ± 0.013, P < 0.0001, Fig. 3).
Figure 3.
Mean ratio of TM insertion into scleral spur to total posterior TM height in normal and POAG eyes. The ratio of the TM insertion into the anterior aspect of the scleral spur to the total posterior TM height was significantly smaller in POAG eyes (0.582 ± 0.037), compared to age-matched normal eyes (0.760 ± 0.013, P < 0.0001). Error bars: SE. **P ≤ 0.001.
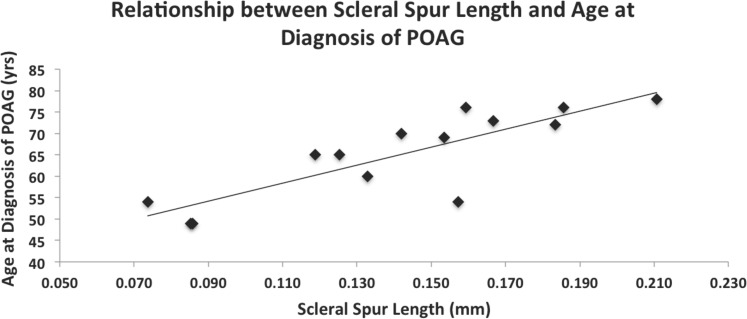
When analyzing the effect of scleral spur length on the age at diagnosis of POAG, a significant, positive linear trend was observed, using the data collected via the method developed in this study while controlling for sex (R2 = 0.768, n = 14; P < 0.001; Fig. 4). When the scleral spur length of POAG eyes measured using our interpretation of the Moses and Arnzen20 method was plotted against age at diagnosis, a weaker positive correlation was observed, which also was significant (R2 = 0.558, n = 14; P = 0.011). There was a weak, positive correlation between the lengths measured using the method of Nesterov and Batmanov17 and age at diagnosis; however, this correlation did not reach significance (R2 = 0.336, n = 14; P = 0.154).
Figure 4.
Relationship between scleral spur length and patient age at diagnosis of POAG. A significant, positive correlation was observed between scleral spur length (mm) and the patient's age in years at diagnosis of POAG (R2 = 0.768, n = 14; P < 0.001) by multiple linear regression analysis while controlling for sex.
Other Scleral Spur Dimensions
The maximum width of the scleral spur (measured using the dotted line in Fig. 1A) was significantly smaller in POAG eyes (0.174 ± 0.006) than that in normal eyes (0.233 ± 0.009, P < 0.0001). The mean cross-sectional area of the scleral spur in POAG eyes (0.0147 ± 0.0008 mm2) also was significantly smaller than that in normal eyes (0.0178 ± 0.0009 mm2, P = 0.013).
Scleral Spur Posterior Movement
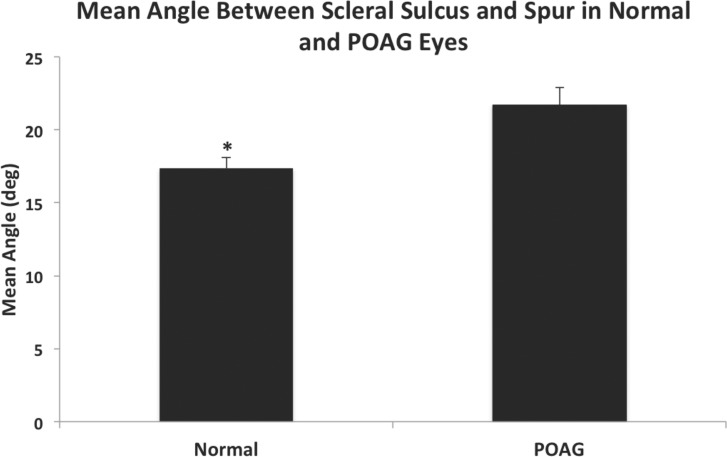
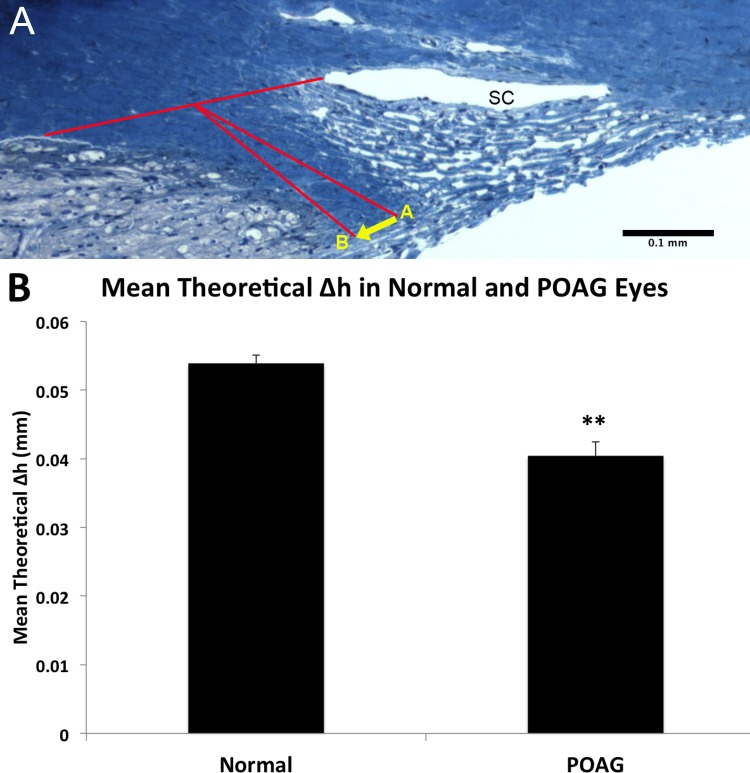
To use the mathematical model developed by Moses and Arnzen,20 the angle, γ, between the scleral sulcus and axis of the scleral spur was measured and was significantly wider in POAG eyes (21.70° ± 1.18°), compared to normal eyes (17.37° ± 0.74°, P = 0.004, Fig. 5). Using the Moses and Arnzen20 model to predict the posterior movement (Δh) of the scleral spur upon ciliary muscle contraction (Fig. 6A), the estimated mean distance that the scleral spur theoretically would move posteriorly in POAG eyes (0.040 ± 0.002 mm) was significantly smaller than the predicted posterior movement of the scleral spur in normal eyes (0.054 ± 0.001 mm, P < 0.0001, Fig. 6B). The majority of POAG eyes (16/20, 80%) had a calculated Δh value that did not meet the minimum requirement of 0.047 mm to open the TM fully and maintain patency of SC as determined by Moses and Arnzen,20 while the majority of normal eyes (19/20, 95%) met their minimum requirement.
Figure 5.
Comparison of angle between scleral sulcus and spur in normal and POAG eyes. Mean angle was significantly wider in POAG eyes (21.70° ± 1.18°) compared to normal eyes (17.37° ± 0.74°, P = 0.004). Error bars: SE. *P < 0.05.
Figure 6.
Posterior movement of scleral spur upon ciliary muscle contraction. (A) Upon ciliary muscle contraction, the scleral spur moves from point A to point B. A horizontal distance (Δh) of at least 0.047 mm (represented by the yellow arrow) is needed to fully open up the TM and support SC to maintain adequate outflow, according to Moses and Arnzen.20 (B) Estimated mean theoretical distance (Δh) of scleral spur posterior movement in normal and POAG eyes. The scleral spur in normal eyes would move a distance of 0.054 ± 0.001 mm, which is greater than the required value of 0.047 mm, whereas the scleral spur in POAG eyes would move a distance of 0.040 ± 0.002 mm, which is less than the required value and significantly shorter than the distance in normal eyes (P < 0.0001). Errors bars: SE. **P < 0.0001.
State of Schlemm's Canal
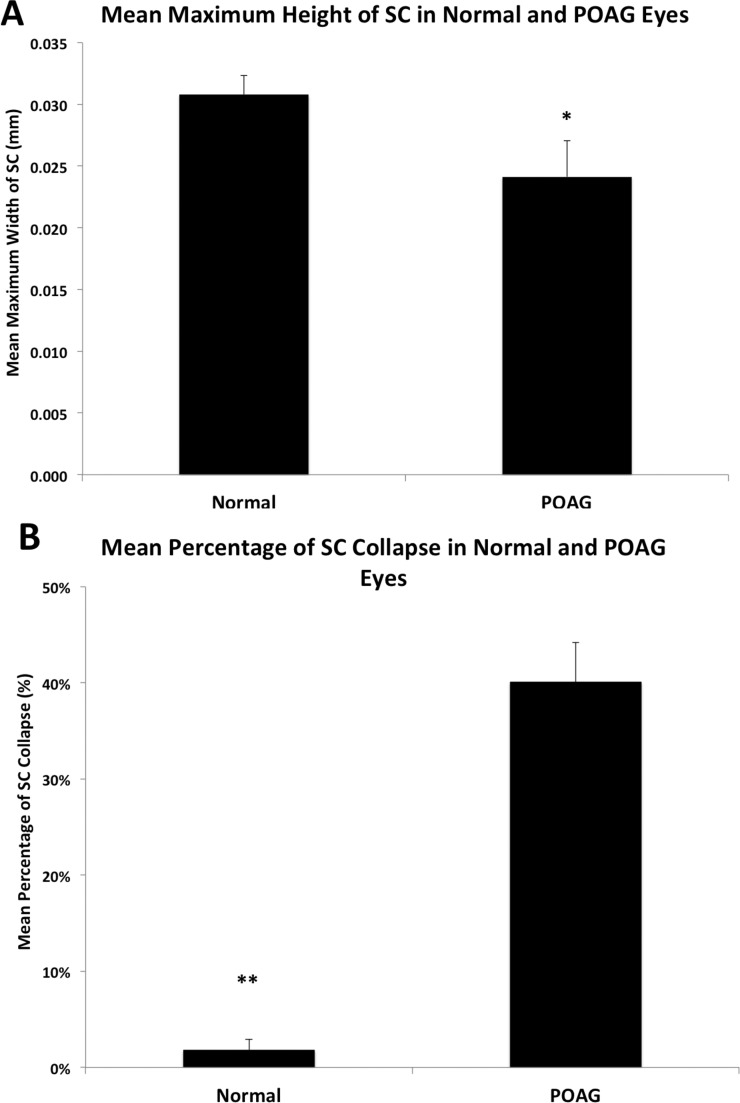
The anterior to posterior width of SC was not significantly different between normal (0.316 ± 0.012 mm) and POAG eyes (0.287 ± 0.010 mm, P = 0.074). The maximum height of SC was significantly narrower in POAG eyes (0.024 ± 0.003 mm), compared to normal eyes (0.031 ± 0.002 mm, P = 0.049, Fig. 7A).
Figure 7.
Comparison of Schlemm's canal maximum height and percentage of collapse in normal and POAG eyes. (A) Mean maximum height of SC was significantly smaller in POAG eyes (0.024 ± 0.003 mm) compared to normal eyes (0.031 ± 0.002 mm; P = 0.049). Error bars: SE. *P < 0.05. (B) Mean percentage of collapse of SC was significantly higher in POAG eyes (40.08 ± 4.13%) than in normal eyes (1.78 ± 1.12%, P < 0.0001). Error bars: SE. **P ≤ 0.001.
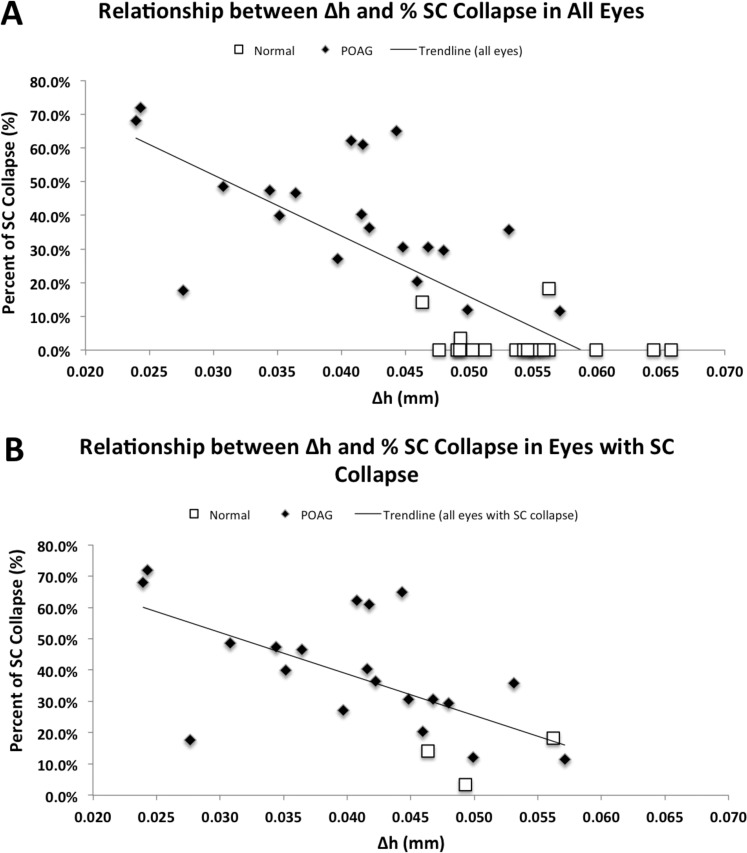
We found SC to be collapsed in the anterior portion of all POAG eyes, consistent with the findings of Nesterov et al.8 In normal eyes, the majority (17/20) of SC remained fully patent; three samples showed partial collapse, but none of them was completely collapsed. The average length of SC that remained patent in POAG eyes (0.159 ± 0.011 mm) was significantly shorter than the patent length in normal eyes (0.311 ± 0.001 mm, P < 0.0001). The mean percentage of collapse in POAG eyes was 40.1 ± 4.13%, which was significantly higher than the mean percentage of collapse of SC in all of the normal eyes (1.78 ± 1.12%, P < 0.0001, Fig. 7B). In the normal eyes that showed SC collapse (n = 3), the mean percentage was 11.90 ± 4.40%. Finally, we compared the length of the scleral spur measured using our method in eyes with collapse of SC (0.154 ± 0.007 mm) and those without collapse of SC (0.203 ± 0.005 mm), and eyes with collapse of SC had a significantly shorter scleral spur (P < 0.0001). Then, we investigated the possibility of a correlation between estimated distance of scleral spur posterior movement (Δh) and the percentage of collapse of SC; we found a statistically significant negative correlation using all eyes in a multiple linear regression analysis controlling for sex and age (R2 = 0.642, n = 40; P < 0.0001; Fig. 8A), showing that eyes with a smaller Δh usually exhibited more percent collapse of SC. Additionally, we examined the correlation between the Δh and percentage of SC collapse specifically in those eyes that exhibited SC collapse using a multiple linear regression analysis controlling for sex and age, and we found a significant, negative correlation (R2 = 0.527, n = 23; P < 0.001; Fig. 8B).
Figure 8.
Relationship between Δh and % SC collapse in all eyes and eyes with SC collapse. (A) Using all eyes, a significant, negative correlation was observed between Δh (mm) and the percent collapse of SC (R2 = 0.642, n = 40; P < 0.0001). (B) Using only eyes that exhibited SC collapse, a significant, negative correlation was observed between Δh (mm) and the percent collapse of SC (R2 = 0.528, n = 23; P < 0.001).
Discussion
We investigated whether the scleral spur is shorter in POAG eyes compared to normal eyes using three different methods, and whether a shorter scleral spur has any effect on the patency of SC, to test our hypothesis that a shorter scleral spur is insufficient to hold SC open, leading to subsequent collapse in POAG.
Our major findings are that a significantly shorter scleral spur was, indeed, detected in eyes with POAG compared to scleral spurs in normal eyes; a significant, positive correlation between the length of the scleral spur and age at diagnosis of POAG was detected; using a previously established mathematical model,20 the mean theoretical posterior movement of the scleral spur was insufficient to support and maintain patency of SC in POAG eyes, but was sufficient in normal eyes; eyes with POAG experienced significantly more percent collapse of SC than normal eyes; and a significant, negative correlation was found between theoretical posterior movement of the scleral spur and percent collapse of SC. Our results supported our hypotheses that a shorter scleral spur exists in POAG eyes and is insufficient to hold SC open, resulting in more prevalent collapse of SC in eyes with a shorter scleral spur.
One previous study by Nesterov et al.8 reported the presence of a significantly shorter scleral spur in POAG eyes compared to normal eyes; however, we found that the method of Nesterov and Batmanov17 actually measured the height of scleral spur, not the length, as their values for the scleral spur length in normal eyes were similar to the values of the scleral spur height reported by Hamanaka et al.6 In our attempt to replicate the values of Nesterov and Batmanov17 for their interpretation of the scleral spur length, sometimes called the diameter by Nesterov and Batmanov,17 we used the method of Hamanaka6 to measure the scleral spur height in normal eyes (Fig. 1B), and we obtained a value (0.081 ± 0.003 mm) that was similar to the Nesterov and Batmanov17 value of scleral spur length in normal eyes (0.085 ± 0.003 mm, 1-sample t-test, P = 0.276), but significantly different from the values of Hamanaka6 of the scleral spur height in normal eyes (0.093 ± 0.022 mm, 1-sample t-test, P = 0.039). The difference between the Hamanaka6 value and ours could be a result of differences in sample patient population. These values are very different from the value of scleral spur length obtained by our method and that of Moses and Arnzens.20 In POAG eyes, although both of the studies by Nesterov et al.8,17 and our interpretation of the method of Nesterov and Batmanov17 obtained significantly smaller values, there was a significant difference between their and our observations (1-sample t-test, P < 0.0001). Again, this difference could be a result of differences in patient population or our relatively small sample size compared to the report of Nesterov and Batmanov.17 Nevertheless, the method of Nesterov and Batmanov17 actually measures the height of the scleral spur (as described by Hamanaka,6 from its tip to the level of the posterior end of SC). Thus, their method (Fig. 1B) does not provide as accurate a measure of the actual scleral spur length as our method developed in this study. Using the method devised in this study, which measures the length of the scleral spur more accurately and takes into account its curvature, we were able to come to the same conclusion as Nesterov and Batmanov,17 that POAG eyes have significantly shorter scleral spurs compared to normal eyes (P < 0.0001, Fig. 2A).
A previous study by Moses and Arnzen20 reported a length for the scleral spur (0.150 mm) in normal eyes, without examining POAG eyes. We applied their method in this study and obtained a similar value in normal eyes (0.144 ± 0.008 mm, P = 0.419), and we observed a significantly shorter scleral spur length in POAG eyes compared to normal eyes (0.111 ± 0.006 mm, P = 0.002). However, Moses and Arnzen20 did not take into account the curvature of the scleral spur; thus, our method provides the most accurate measurement of the scleral spur length and is able to detect greater statistical difference between normal and POAG eyes (P < 0.001) compared to their method (P < 0.05).
Our study investigated the possible effects that a shorter scleral spur would have on the development of POAG; we found a significant, positive correlation between scleral spur length and the age at diagnosis of POAG, indicating that patients with shorter scleral spurs were diagnosed with POAG at a younger age (R2 = 0.768, n = 14; P < 0.001). While this association does not automatically imply causation, it is probable that individuals with a shorter scleral spur may be at a greater predisposition to POAG compared to their normal counterparts with typically longer scleral spurs. In addition, individuals with shorter scleral spurs may be predisposed to develop the disease at an earlier age relative to someone who has a longer scleral spur. Further clinical studies are needed to verify this association.
We also found that the ratio of the length of TM inserted into the anterior aspect of the scleral spur to the total posterior TM height was significantly smaller in POAG eyes compared to normal eyes (P < 0.0001, Fig. 3). This indicates that less TM inserts into the scleral spur in POAG eyes and a shorter spur would open significantly less TM upon ciliary muscle contraction compared to a longer scleral spur in normal eyes; this could increase outflow resistance and subsequently increase IOP.20 The amount of ciliary muscle attached to the scleral spur was not measured, because the extent of muscle attachment is difficult to define after using a toluidine blue stain; however, this is a possible future study.
Further analysis of the effectiveness of the scleral spur's posterior movement in opening up the TM and supporting SC required a previously established, mathematical model developed by Moses and Arnzen.20 To use this model, we measured the angle, γ, between the scleral sulcus and the axis of the scleral spur, as detailed by Grierson et al.15 The mean angle in POAG eyes was significantly wider than the mean angle in normal eyes (P = 0.004, Fig. 5), indicating that the initial position of the scleral spurs of POAG eyes is more posterior than the position in normal eyes and that the scleral spur of POAG eyes cannot move as much to the posterior as normal eyes to open the TM and support SC, thus, leading to collapse of SC. Collapse of SC could increase outflow resistance and IOP, which may contribute to subsequent development of POAG.18,19
We used the mathematical model of Moses and Arnzen,20 which determined that the scleral spur must move a distance of at least 0.047 mm posteriorly to fully open the TM and maintain patency of SC, to evaluate and compare the movement of the scleral spur in normal and POAG eyes using the values that we have obtained from our measurements. In our analysis, we assumed that all other parameters that these investigators examined were the same between normal and POAG eyes, except for the length of the scleral spur and the angle, γ, between the scleral sulcus and spur. We found that the mean theoretical posterior displacement (Δh) of the scleral spur upon ciliary muscle contraction was 0.054 ± 0.001 mm in normal eyes, which was greater than the 0.047 mm distance needed to produce the inward bowing of the TM described by Moses and Arnzen.20 In POAG eyes, the distance (0.040 ± 0.002 mm) did not meet the minimum requirement and was significantly smaller than that in normal eyes (P < 0.0001), indicating that the scleral spur of a POAG eye may be unable to open the TM and support the SC sufficiently. We used Δh as a measure of the effectiveness of posterior displacement by the scleral spur, since the measure accounted for the angle and the scleral spur length. Compared to the calculated minimum of Moses and Arnzen,20 POAG eyes have too wide angles and too short scleral spur lengths to open the TM and support SC sufficiently. This was supported by our observation that most POAG eyes (16/20) in this study demonstrated an insufficient posterior displacement. Only one normal eye did not meet the minimum required distance; however, the calculated Δh for this eye (0.0463 mm) was very close to the minimum. The limitation in using this model to compare normal and POAG eyes in our current study is that the difference in TM stiffness between normal and POAG eyes was not taken into consideration. Higher TM stiffness was reported previously in POAG eyes21 and may be caused by a progressive loss of cellularity.22 Increased stiffness in the TM may decrease flexibility and cause an inability to return to normal resting position after SC collapse. This would affect how effectively the scleral spur movement could stretch the TM and increase outflow. In addition, the observation that stiffer or denser tissue may shrink differently (or less) in response to fixation also was not taken into the consideration.23 Nevertheless, the Δh value determined by Moses and Arnzen20 provides a fairly confident measure of the effectiveness of the scleral spur's posterior movement in maintaining patency of SC and opening the TM. Therefore, the shorter scleral spur lengths and wider angles in POAG eyes, combined with significantly less TM insertion, may have a role in the pathogenesis of POAG.
Finally, we examined whether an association exists between collapse of SC and incidence of POAG. We discovered a significantly smaller maximum height of SC in POAG compared to normal eyes (P = 0.049, Fig. 7A), and a significantly higher percentage of collapse of SC in POAG, compared to normal eyes (P < 0.0001, Fig. 7B). These findings suggested that the longer scleral spur of normal eyes provides support for SC, whereas in POAG eyes, SC collapse is more prevalent, due to lack of support from the shorter scleral spur, as shown by the Δh values of nearly all POAG eyes falling below the required minimum value. Another possible explanation as to why a shorter scleral spur in a POAG eye might lead to SC collapse is that shorter scleral spurs have less TM attachment than normal eyes. Thus, when the ciliary muscle contracts in POAG eyes and pulls on the scleral spur, it moves a shorter distance to the posterior, opening up fewer layers of meshwork beams and failing to support SC; thus, outflow resistance would increase, leading to increased IOP, which could cause further SC collapse in an ever worsening cycle. Also, it was known that SC becomes narrower and collapses with increasing IOP18,19,24 and in POAG eyes compared to normal eyes.25 In addition, since no outer wall of SC exists at the collector channel ostia, the TM can herniate into these regions with increasing IOP.26,27 The obstruction of the collector channel ostia also was reported in the eyes with POAG histologically28 and clinically,29 which could further decrease aqueous humor outflow and increase IOP.
In this study, we found that a shorter scleral spur could compromise the “ciliary muscle-scleral spur-trabecular meshwork” network that normally works to maintain patency of SC. This hypothesis was supported by our finding that the mean scleral spur length was significantly shorter in eyes with SC collapse compared to eyes with a fully patent SC (P < 0.0001). Finally, we examined Δh, as a measure of the effectiveness of the scleral spur in maintaining patency of SC and opening the TM, alongside percent collapse of SC to observe any possible correlation. With all eyes, we found a significant, negative correlation (R2 = 0.642, n = 40; P < 0.0001; Fig. 8A), indicating that an eye with a smaller Δh value, which accounts for the spur length and angle, is likely to experience more percent collapse of SC than an eye with a higher Δh value. This also indicates that if the scleral spur is significantly shorter or if the angle is significantly wider than the average normal value, the eye would be more likely to experience SC collapse. We also examined the same correlation using only the eyes that exhibited SC collapse, and the correlation was still significant (R2 = 0.528, n = 23; P < 0.001; Fig. 8B). This demonstrated that the scleral spur does have an important role in keeping SC open and that this relationship between Δh and percent SC collapse was significant, although a relatively small number of eyes was examined.
There are several limitations in this histological study using immersion-fixed enucleated eye tissue. We do not know how the changes in IOP, episcleral venous pressure, and normal ciliary body tone would affect the position of the scleral spur and SC. Further in vivo studies will be needed to determine whether our findings would be comparable to in vivo physiological conditions.
In summary, we found a shorter scleral spur in POAG eyes compared to those in age-matched normal eyes. We observed a high incidence of SC collapse in POAG eyes, which we believe is due to insufficient support of SC and TM by the short scleral spur. In addition, a strong positive correlation was found between scleral spur length and age at POAG diagnosis. Our data also suggested that SC collapse is much more prevalent in eyes with Δh values that do not meet the theoretical minimum of Moses and Arnzen.20 If this value were measurable in vivo, it might provide additional means for management of POAG.
Anterior segment optical coherence tomography (OCT) has been used extensively in vivo to identify the scleral spur and other structures of the aqueous outflow pathway in normal and POAG eyes.30–34 However, the resolution of current OCT images does not appear sufficient to provide precise and reproducible measurements of the scleral spur length. With improvement in this technology, further in vivo imaging studies evaluating the morphology of the scleral spur in different stages of POAG would likely provide a better understanding of its role in the pathogenesis of POAG.
Acknowledgments
We thank Rozanne Richman, MS, and Rui Jin, MS, for the technical assistance, and Andrew Lai, MPH, Li Deng, PhD, and Lisa M. Sullivan, PhD, for the statistical assistance.
Supported by NIH Grants EY022634 and EY018712, the Student Research Award of the Undergraduate Research Opportunities Program at Boston University (DLS), Boston University School of Medicine Summer Research Fellowship (JH), and the Massachusetts Lions Eye Research Fund.
Disclosure: D.L. Swain, None; J. Ho, None; J. Lai, None; H. Gong, None
References
- 1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783–801. [DOI] [PubMed] [Google Scholar]
- 3. Johnson M. ‘What controls aqueous humour outflow resistance?'. Exp Eye Res. 2006; 82: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007; 52 (suppl 2): S101–S104. [DOI] [PubMed] [Google Scholar]
- 5. Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009; 88: 648–655. [DOI] [PubMed] [Google Scholar]
- 6. Hamanaka T. Scleral spur and ciliary muscle in man and monkey. Jpn J Ophthalmol. 1989; 33: 221–236. [PubMed] [Google Scholar]
- 7. Moses RA, Grodzki WJ. The scleral spur and scleral roll. Invest Ophthalmol Vis Sci. 1977; 16: 925–931. [PubMed] [Google Scholar]
- 8. Nesterov AP, Hasanova NH, Batmanov YE. Schlemm's canal and scleral spur in normal and glaucomatous eyes. Acta Ophthalmol (Copenh). 1974; 52: 634–646. [DOI] [PubMed] [Google Scholar]
- 9. Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech. 1996; 33: 336–367. [DOI] [PubMed] [Google Scholar]
- 10. Tamm ER, Koch TA, Mayer B, Stefani FH, Lütjen-Drecoll E. Innervation of myofibroblast-like scleral spur cells in human monkey eyes. Invest Ophthalmol Vis Sci. 1995; 36: 1633–1644. [PubMed] [Google Scholar]
- 11. Tamm E, Flügel C, Stefani FH, Rohen JW. Contractile cells in the human scleral spur. Exp Eye Res. 1992; 54: 531–543. [DOI] [PubMed] [Google Scholar]
- 12. Lütjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999; 18: 91–119. [DOI] [PubMed] [Google Scholar]
- 13. Nishida S, Mizutani S. [Topography of the human ciliary muscle]. Nihon Ganka Gakkai Zasshi. 1991; 95: 1044–1056. [PubMed] [Google Scholar]
- 14. Rohen JW, Lütjen E, Bárány E. The relation between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. A study in two contrasting monkey species, Macaca irus and Cercopithecus aethiops. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1967; 172: 23–47. [DOI] [PubMed] [Google Scholar]
- 15. Grierson I, Lee WR, Abraham S. Effects of pilocarpine on the morphology of the human outflow apparatus. Br J Ophthalmol. 1978; 62: 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufman PL, Bárány EH. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976; 15: 793–807. [PubMed] [Google Scholar]
- 17. Nesterov AP, Batmanov YE. Study on morphology and function of the drainage area of the eye of man. Acta Ophthalmol (Copenh). 1972; 50: 337–350. [DOI] [PubMed] [Google Scholar]
- 18. Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol. 1975; 14: 286–292. [PubMed] [Google Scholar]
- 19. Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest Ophthalmol Vis Sci. 1982; 22: 625–632. [PubMed] [Google Scholar]
- 20. Moses RA, Arnzen RJ. The trabecular mesh: a mathematical analysis. Invest Ophthalmol Vis Sci. 1980; 19: 1490–1497. [PubMed] [Google Scholar]
- 21. Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011; 52: 2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camras LJ, Stamer WD, Epstein D, Gonzalez P, Yuan F. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 2014; 55: 814–823. [DOI] [PubMed] [Google Scholar]
- 23. Hayat MA. Specimen shrinkage. In: Hayat MA. ed Fixation for Electron Microscopy. Philadelphia, PA: Elsevier; 2012. [Google Scholar]
- 24. Zhang Y, Toris CB, Liu Y, Ye W, Gong H. Morphological and hydrodynamic correlates in monkey eyes with laser induced glaucoma. Exp Eye Res. 2009; 89: 748–756. [DOI] [PubMed] [Google Scholar]
- 25. Allingham RR, de Kater AW, Ethier CR. Schlemm's canal and primary open angle glaucoma: correlation between Schlemm's canal dimensions and outflow facility. Exp Eye Res. 1996; 62: 101–109. [DOI] [PubMed] [Google Scholar]
- 26. Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008; 49: 5346–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnstone MA. Pressure-dependent changes in configuration of the endothelial tubules of Schlemm's canal. Am J Ophthalmol. 1974; 78: 630–638. [DOI] [PubMed] [Google Scholar]
- 28. Gong H, Francis A. Schlemm's canal and collector channels as therapeutic targets. In: Samples J, Ahmed I. eds Innovations in Glaucoma Surgery. New York: Springer; 2014: 3–25. [Google Scholar]
- 29. Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci. 2010; 51: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 30. Cumba RJ, Radhakrishnan S, Bell NP, et al. Reproducibility of scleral spur identification and angle measurements using Fourier domain anterior segment optical coherence tomography. J Ophthalmol. 2012; 2012: 487309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakata LM, Lavanya R, Friedman DS, et al. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch Ophthalmol. 2008; 126: 181–185. [DOI] [PubMed] [Google Scholar]
- 32. McKee H, Ye C, Yu M, Liu S, Lam DS, Leung CK. Anterior chamber angle imaging with swept-source optical coherence tomography: detecting the scleral spur, Schwalbe's line, and Schlemm's canal. J Glaucoma. 2013; 22: 468–472. [DOI] [PubMed] [Google Scholar]
- 33. Seager FE, Wang J, Arora KS, Quigley HA. The effect of scleral spur identification methods on structural measurements by anterior segment optical coherence tomography. J Glaucoma. 2014; 23: e29–e38. [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Li H, Dorairaj S, et al. Assessment of scleral spur visibility with anterior segment optical coherence tomography. J Glaucoma. 2010; 19: 132–135. [DOI] [PubMed] [Google Scholar]