Abstract
The placebo effect is a complex phenomenon occurring across a variety of clinical conditions. While much placebo research has been conducted in diseases defined by self-report such as depression, chronic pain, and irritable bowel syndrome (IBS), asthma has been proposed as a useful model because of its easily measured objective outcomes. Studies examining the placebo response in asthma have not only contributed to an understanding of the mechanisms behind the placebo response but also shed an interesting light on the current treatment and diagnosis of asthma. This paper will review current literature on placebos in general and specifically on the placebo response in asthma. It focuses on what we know about the mechanisms behind the placebo effect, whether there is a specific portion of the population who responds to placebos, which patient outcomes are influenced by the placebo effect, and whether the effect can be augmented.
Keywords: Placebo effect, Asthma, Clinical outcomes, Expectancy, Clinical trial design
Introduction
A placebo is an inactive substance or sham form of therapy used either as a control in clinical trials or in an attempt to elicit a positive response from a patient. Until the first half of the twentieth century, the use of placebos was widespread [1]; some have argued that placebos reflect the entire history of medicine prior to evidence-based interventions [2]. In the early 1900s, Richard Cabot, a leading physician at Harvard Medical School, stated that he “was brought up, as I suppose every physician is, to use placebo, bread pills, water subcutaneously, and other devices” [3]. Despite their ubiquity, for centuries, placebos were dismissed as inert interventions that did not result in significant therapeutic benefits or actual pathophysiological changes, but merely mollified the patient by deceptive means [4]. Though lacking pharmacologic activity, placebos are cited as improving signs and symptoms of a wide variety of human diseases within research trials and in actual clinical practice [5, 6, 7•]. On this basis, accepted standards for design of clinical trials specify that effects of active treatments are compared against those of a placebo [8–10]. In spite of this common practice, it is frequently not known whether the observed placebo response differs from the natural history of the disease under study or from regression to the mean [11, 12] and whether all subjects or a select population are susceptible to placebo effects [10]. It also remains unclear whether subjects who do manifest true placebo responses on a single occasion manifest a placebo response to the repeated administration of a similar placebo (i.e., have reliable placebo responses) [13]. In addition, in subjects who do manifest a reliable placebo response, it has been difficult to determine whether this response is consistent across a variety of placebo modalities (e.g., pharmacological vs. device placebos). These difficulties are due, in part, to the scarcity of experimental models allowing for repeated administration of specific placebos.
The Placebo Effect in Asthma
Asthma is a chronic and often debilitating inflammatory disorder affecting over 25 million individuals in the USA [14]. It is characterized by airway hyperresponsiveness, airflow obstruction, and chronic inflammation. One of the hallmarks of asthma is that changes in baseline lung function can occur rapidly with medical intervention and these changes can be rigorously and objectively quantified using well-established techniques. Furthermore, repeated assessments of lung function response can be performed over relatively short periods of time, and observation of stable asthmatic individuals without acute intervention does not pose logistical or ethical barriers. In this regard, asthma is an excellent model in which to study placebo effects. Studies documenting a placebo-like response in asthma date back to at least the late 1800s, when Mackenzie et al. [15] demonstrated that cold symptoms, including pulmonary distress, could be elicited after deceptive presentation of an artificial rose. Studies examining the placebo response in asthma have not only contributed to an understanding of the mechanisms behind the placebo response but also shed an interesting light on the current treatment and diagnosis of asthma. This paper will review current literature on placebos in general and specifically on the placebo response in asthma. We focus on what we know about the mechanisms behind the placebo effect, whether there is a specific portion of the population who responds to placebos, which patient outcomes are influenced by the placebo effect, and whether the effect can be augmented. Continued research in these areas should focus on how to use this knowledge to inform decisions in future clinical trial design and whether the placebo effect can and should be harnessed in clinical practice to improve outcomes for asthmatics who remain uncontrolled despite conventional therapies.
What Are the Mechanisms Behind the Placebo Effect?
One of the difficulties in studying the placebo effect relates to the complexity of the psychological and biological mechanisms involved. While several psychological phenomena, such as expectancy and conditioning, have been consistently observed across a variety of clinical conditions [16–18], evidence illustrates that biologically, the placebo response is modulated by the release of a wide variety of disease-specific neurotransmitters [19•, 20•]. Additionally, multiple studies have shown that placebo responses are associated with regionally specific, quantifiable effects on relevant brain structures [20•]. Patient expectancy is one of the most well-researched psychological contributors to the placebo response. Expectancy is the theory that an individual’s response to an intervention is directly related to their perception of the anticipated outcome. Despite the pharmacologically inactive nature of placebos, multiple clinical trials have shown that administration of inert substances can induce positive outcomes. This improvement has been postulated to relate to patient expectations of benefit, either owing to the patient’s belief that they are receiving active medication or through the positive experience—including embodied cognition—of the ritual of treatment and the patient-doctor relationship [21]. Studies have shown that 30–50 % of asthmatics demonstrate reproducible bronchoconstriction to inhaled saline presented as an irritant; this effect is reversible when inhaled saline is then identified as a bronchodilator [22, 23]. More recently, in a study by Castro et al. in which patients were randomized to undergo active or sham bronchial thermoplasty, 64 % of sham-treated patients showed improvement in quality of life [24]. Furthermore, administration of active treatment (e.g., opioids to treat pain) unbeknownst to patients resulted in a reduced global drug effect as compared to open administration [25], highlighting the fact that even with pharmacologically active treatment, patient expectation plays a vital role in the observed treatment response.
Multiple studies have shown conditioning and associative learning paradigms also play crucial roles in the placebo response. In a study by Goebel et al., patients with allergic rhinitis underwent a conditioning protocol, receiving desloratadine, an H1 receptor antagonist, paired with a novel drink, for five consecutive days. After the washout period, patients who were reexposed to the novel drink plus placebo showed improved symptom scores, decreased wheal size on the skin prick test, and diminished basophil activation [26, 27]. Numerous other pharmacological conditioning trials have shown that these placebo responses mimic active drug effects and that prior exposure to an effective treatment is an important part of the conditioned placebo response [20•, 26, 28]. These findings highlight the specificity of the placebo response and the difficulty in generalizing results of placebo trials across clinical conditions. Despite these complexities, research has begun to identify various neurotransmitters and brain regions involved in disease-specific placebo responses. Neuroimaging studies examining placebo administration in chronic pain have confirmed brain changes similar to those observed with opioid treatment [19•]. Additionally, placebo administration for analgesia has been shown to act on the endogenous opioid system, with its effects reversed by the opioid antagonist naloxone [29]. In Parkinson’s disease, the placebo response has been linked to dopamine release in the striatum and neuronal activity in the subthalamic nucleus, both of which are key brain regions affected by Parkinson’s disease and are associated with motor control [30–32]. Research on the conditioned immune response has demonstrated involvement of the insular cortex, amygdala, and ventromedial nucleus of the hypothalamus [33]. While the majority of the pathways and mechanisms behind the placebo response are not yet fully understood, research continues to demonstrate that neurobiological and pathophysiological changes accompany the placebo response.
Who Are Placebo Responders?
It has been postulated that another reason behind the complexity of studying the placebo response may be that only a portion of the population are “placebo responders.” The inability to definitively demonstrate the existence or nonexistence of a subset of individuals who predictably respond to placebo administration has constrained placebo research for 50 years [2, 13]. Improved sophistication of clinical trial design and more precise basic research experiments would be possible with the capability to discriminate a placebo-responsive population. To this end, research has sought to identify personality traits that contribute to the likelihood that a person will respond to placebo administration. In various trials, placebo responders have been found to be more anxious, self-centered, talkative, and spiritual [34], sociable and extroverted [35], suggestible [36], and younger in age [37, 38]. However, despite these continued efforts to identify particular traits associated with the placebo response, trials have not yet been able to consistently replicate findings for any particular attribute [13].
In addition to examining personality and demographic characteristics, research has commenced looking at genetic polymorphisms that could potentially play a role in identifying placebo responders. In a recent study of patients with inflammatory bowel syndrome (IBS), patients homozygous for the catechol-O-methyltransferase (COMT) val158met methionine allele (met/met) were found to be more responsive to placebo than their val/met or val/val counterparts [39]. The study showed a linear relationship between number of met alleles and placebo responsiveness. COMT, an essential enzyme in dopamine catabolism, has been shown to play a vital role in pathways involving reward, pain, memory, and learning. As evidence has implicated dopamine as a key player in patient expectancy via these pathways, it follows that a polymorphism affecting dopamine transport might reasonably alter the placebo response. Larger replications are critical to verify this genetic association, and the authors have noted that it is unlikely this polymorphism is wholly responsible for as complex a phenomenon as the placebo response; further research, including neuroimaging, is warranted. Most neuroimaging studies have examined neural activity during placebo administration in analgesia; there is a paucity of neuroimaging studies looking at the placebo response in peripheral organs [20•]. Future studies examining the neurobiological and genetic bases of the placebo response will determine whether science can truly identify placebo responders from nonresponders and whether these traits are consistent across individuals and illnesses. Additional findings in these areas can direct future clinical trial design and studies examining the placebo response.
Can the Placebo Response Be Augmented?
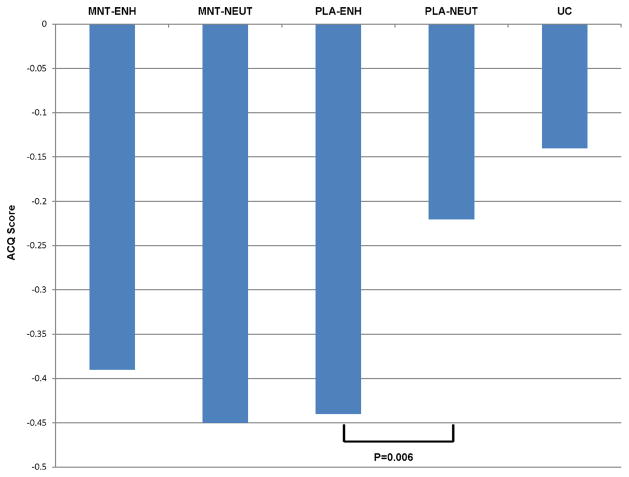
As discussed above, one of the most well-researched areas of placebo medicine relates to the contribution of the psychological concept of expectancy [4]. Knowing the significance of the role expectancy plays in the placebo response, research has focused on ways to modify and enhance patient expectation and thus subjective response to placebo administration. Verbal suggestions and environmental cues are some of the most important factors that contribute to expectations [25]. Many studies have shown that modifying expectations, through the use of verbal cues suggestive of positive outcomes, results in an increased placebo response [19•]. Evidence has shown that verbal cues or previous experience may work through the same neurobiological pathways as certain pharmacological agents by causing the release of endogenous opioids, dopamine [40], and cannabinoids [41]. A study by Wise et al. [42] sought to determine whether enhancing expectation of treatment benefit through positive verbal and written cues would improve response to placebo and/or active treatment. This multicentered clinical trial enrolled 601 subjects with poorly controlled asthma and randomized them to usual care or one of four treatment groups: placebo with enhanced messages, placebo with neutral messages, montelukast with enhanced messages, or montelukast with neutral messages. Assignment to active or placebo treatment was double blind while assignment to enhanced or neutral messages was single blind. Subjects in the usual care group received an informational pamphlet on asthma and were then followed for 6 weeks. All other subjects received an educational session, enhanced or neutral, followed by a 2-week run-in, another information session, and then randomization to 4 weeks of treatment or placebo. Outcome measures included peak expiratory flow (PEF) and self-reported measures of asthma symptom control, including the asthma control questionnaire (ACQ). The study generated several important findings. First, PEF improved with active treatment compared to placebo, but not with enhanced messages versus neutral messages. PEF also did not improve when comparing placebo to usual care. This highlights the fact that objective measures were only improved by active treatment, not by placebos or enhanced messaging. These results are consistent with other studies that have found that the placebo response does not improve objective measures of asthma control [43, 44, 42, 45]. In contrast, patient-reported outcome measures showed improvement in the enhanced versus neutral group, but only in the placebo arm and not in the treatment arm. Subjective asthma control showed a similar magnitude of improvement when comparing active treatment to the enhanced message placebo group. However, enhanced messages in the treatment arm did not further improve subjective outcomes as compared to neutrally presented treatment. To summarize, enhanced expectations augmented subjective measures of asthma control in the placebo group but not in the treatment group (Fig. 1). This research shows that neither placebos nor patient expectancy affects objective measures of lung function in asthma. However, both have the potential to improve subjective asthma symptom control.
Fig. 1.
Effect of enhanced presentation on placebo and active treatment. Enhanced presentation shows improvement in the subjective outcome of ACQ in the placebo group but not in the treatment group. MNT montelukast, ENH enhanced, PLA placebo, NEUT neutral. A lower ACQ score indicates better asthma control. Courtesy of Robert Wise, M.D.
In light of the potential for placebos to improve subjective measures of asthma control, there has been interest in the possibility of introducing placebo treatment into clinical care. However, because placebo responses have been strongly linked to patient expectancy, there has been an almost universal perception that the placebo must be administered deceptively [46]. If patients are aware that they are to receive an inert pill, they are not likely to expect symptom improvement. To test this theory, a study of open-label placebo administration in irritable bowel syndrome (IBS) was recently undertaken [47]. Investigators truthfully informed subjects that they would be receiving an inert pill but they explained that a significant body of research has documented patient improvement with the same placebo treatment. This study demonstrated a significant improvement in symptom reports following placebo administration as compared to usual care, despite patients’knowledge that they had received pharmacologically inert pills. This proof-of-concept trial demonstrates that the placebo response can be evoked as long as its use is paired with convincing rationale. An open-label placebo response has also been elicited in a recent study of episodic migraine, in which open-label placebo treatment was found to reduce pain by 30 % during an acute attack compared to no treatment [48]. Whether open-label placebo administration has a role in asthma is yet to be determined, but the potential to prescribe placebos openly while still eliciting a response makes the introduction of placebos into clinical practice a more distinct future possibility.
Which Patient Outcomes Are affected by the Placebo Response?
While much placebo research has been conducted in diseases defined by self-report such as depression, chronic pain, and irritable bowel syndrome (IBS), asthma has been proposed as a useful model because of its easily measured objective outcomes. In patients with asthma, administration of short-acting bronchodilators can cause rapid increases in lung function as measured by spirometry, allowing objective patient outcomes to be measured over a short period of time. While most studies have repeatedly shown improvements in subjective measures of disease control, results are conflicting with regard to objective measures. There has been an abundance of research into the placebo effect on objective and subjective measures of asthma. Studies have historically reported conflicting results, with some trials showing that placebo administration leads to improvements in objective measures of lung function, such as forced expiratory volume in 1 s (FEV1), bronchial hyperreactivity, or peak expiratory flow (PEF) [37, 13, 44, 43]. Importantly, most of these trials did not include a “no treatment” arm to control for the potential contributions of natural disease progression, regression to the mean, or other outside influences, and those that did showed no placebo effect on objective measures. A landmark meta-analysis was conducted looking at the placebo response across multiple conditions in studies containing a no treatment control arm, and the results showed a significant increase in patient-reported subjective measures in the placebo arm, but not in objective measures [11, 49]. Of the studies that have been conducted in asthma with a no treatment control arm, all have also shown improvement only in patient-reported subjective measures, such as symptom severity and asthma control scores, but not in therapeutically significant objective measures [43, 44, 42, 45].
In light of the conflicting evidence generated by historical studies, Wechsler et al. sought to assess whether placebo interventions in asthma can lead to objective changes in airway caliber, self-reported subjective improvements, or both, beyond the changes in lung function and symptoms that are attributable to the natural history of the disease [45]. The effects of albuterol (an active bronchodilator), sham inhaler, sham acupuncture, and a no treatment control were compared in a randomized, double-blind, crossover study. Using a block design, one of each of the four interventions was randomly administered over four sequential visits. This process was repeated in two additional blocks of visits for a total of 12 visits per patient. Both objective (forced expiratory volume in 1 s (FEV1)) and subjective (responses to asthma control questionnaires) measures of asthma control were measured before and after intervention at each visit. As shown in Fig. 2a, the percent increase in FEV1 after active albuterol administration was significantly higher than for the two placebo interventions or the no treatment control. There was no significant difference between the three inactive interventions. In contrast, Fig. 2b shows subjective patient improvement after each of the four interventions. Subjective improvement was seen in the active treatment group as well as the two placebo intervention groups and was significantly higher than that observed in the no treatment control group. There was not a significant difference in subjective improvement seen between the active and placebo arms. These findings are consistent with previous studies that have shown a strong placebo effect for subjective measures of asthma control despite a lack of objective improvement.
Fig. 2.
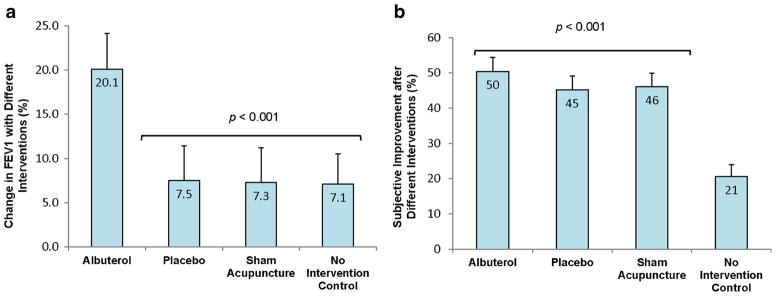
Effects of placebo on objective and subjective measures of asthma control. a Percent change in maximum FEV1 with each of the four interventions. FEV1 improvement with albuterol was significantly higher than with each of the other three interventions (p<0.001). b Percent change in subjective improvement with each of the four interventions. Subjective improvement in the albuterol and both placebo arms was greater than that of the no intervention control (p<0.001). From Wechsler et al. [45, pp. 123–124]. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission
The ability of placebos to affect subjective perceptions of disease severity without modifying objective physiologic outcomes is problematic if physicians are to pursue adjunct open-label placebo treatments clinically. In many diseases, such as depression, IBS, and chronic pain, subjective reports of symptom improvement are the only measure of disease control currently available to determine a level of treatment success. However, in asthma, where lung function measurements can be readily obtained and compared to subjective symptom score improvement, evidence indicates that subjective reports often do not correlate with objective changes in airway caliber [45]. This raises the question of whether subjective outcomes in asthma are inherently unreliable and, if so, whether treatment should be directed at increasing lung function or focused on improving the patient experience of disease. While many physicians would agree that lung function is the major determinant of asthma control, others have argued that a patient-centered care model should focus on improving perception of symptoms and quality of life [50]. According to national guidelines, the diagnosis and treatment of asthma should be based on both subjective reports of symptoms as well as the objective measurement of lung function [51]. However, in a preponderance of primary care offices, patients are diagnosed with asthma based on symptomatology alone and prescribed treatments in lieu of confirmation of disease through lung function testing. These same patients are stepped up or down on their asthma therapies based wholly on symptom reports. If research shows that subjective measures are unreliable and maximized lung function is the goal of therapy, then current treatment paradigms need to be revisited. Alternatively, if providing patient-centered care means focusing on symptom control as the goal of therapy, perhaps adjunct treatment with open-label placebos would offer an interesting clinical approach. Many reports have shown that patients are not always able to determine when their asthma is worsening and that there is incongruity between symptoms and lung function [52, 53]. Therefore, if placebos can affect subjective reports of symptom control without improving lung function, would these placebo responders potentially be at higher risk of exacerbation because of a seeming disconnect between disease severity and their ability to recognize it? In order to control for exacerbations in asthma, it seems that one would want to increase lung function to maximize patient health. However, symptom reports determine quality of life, which is also an important measure of patient treatment satisfaction. The discrepancy between these competing goals creates a dilemma in the consideration of treating patients with open-label placebo medicine, even as an adjunct to current therapy.
How Does the Placebo Effect Impact Clinical Trial Design?
Since initial observations of the placebo response were realized after World War II, current standards for clinical efficacy studies indicated that active treatment should ideally be compared to placebo in order to determine a true treatment effect. The assumption of this design is that the difference between the total treatment effect in the drug arm and the nonspecific effects that occur in the placebo group is the pharmacological effect of the drug, or the specific treatment effect [54]. Because most trial designs do not include a “no treatment” control arm, the placebo response is likely to be the sum of the placebo effect and the natural progression of the disease, regression to the mean, or other outside influences. Indeed, when studies have included a “no treatment” arm, improvements are often noted in this group as well [49]. In light of this effect, current RCTs may be underestimating the true drug effect of new compounds [54]. Additionally, a recent study analyzed clinical trials published from 1966 to 2010 and found that there has been a significant decline in average effect size between drug and placebo [55]. While this is likely due in part to the fact that innovative drugs are being discovered less frequently, it emphasizes the importance of minimizing the placebo response so when an efficacious drug is trialed, it does not get overshadowed by the control. In an effort to minimize placebo responses, novel clinical trial designs are being employed for the first time since the advent of the randomized controlled clinical trial. One of these designs, known as sequential parallel comparative design (SPCD), is being used in a phase 3 trial of an opioid modulator for major depressive disorder [56]. In the first stage of the trial, more than half of the participants are assigned to the placebo arm and then placebo nonresponders are reassigned in a second portion of the trial, with half receiving drug and half receiving placebo. The benefit to this design is that studies are able to retain the same level of power while enrolling 20–50 % fewer participants [56], potentially saving the industry millions of dollars. Future trials utilizing novel designs will provide insight into the true effect of placebos and could shake the foundation of touted randomized placebo controlled clinical trials.
Conclusions
In recent years, it has been recognized that the placebo effect might be worth exploiting so that it can supplement pharmacologically active therapies to further patient improvement. While there are many benefits to this proposed method, including a lack of treatment side effects, there are many stumbling blocks that will need to be overcome. In order to appropriately introduce open-label placebos into clinical care, research needs to further elucidate the psychological and biological mechanisms of the placebo response, the subject and objective outcomes affected by placebos, and the ethics of utilizing placebo medicine in clinical practice. Research should seek to discover whether the proposed neurobiological pathways implicated in the placebo response are consistent for individuals and across various illnesses. Medical acumen will need to speak to whether promoting subjective improvement in the absence of accompanying objective changes is an ethically wise choice for patient treatment. Further research should validate the effect of open-label placebo administration so physicians can partner with their patients in implementing placebo treatment without employing deceptive means. Finally, novel clinical trial designs will need to stand the test of time to determine their superiority to the classic randomized controlled clinical trial.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the authors.
Conflict of Interest Stefanie Dutile declares that she has no conflict of interest.
Ted J Kaptchuk declares that he is supported by NCCAM/NIH grant # 2 K24 AT004095.
Michael E. Wechsler declares personal consulting fees from GlaxoSmithKline, Novartis, Cephalon/Teva, Sepracor/Sunovion, NKT Therapeutics, Asthmatx/BSCI, Genzyme, MapPharma, Genentech, Boehringer Ingelheim, Merck, Regeneron, and MedImmune outside the submitted work.
Contributor Information
Stefanie Dutile, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Ted J. Kaptchuk, Program in Placebo Studies, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Michael E. Wechsler, Email: WechslerM@NJHealth.org, Division of Pulmonary, Critical Care and Sleep Medicine, National Jewish Health, 1400 Jackson St, Denver, CO 80206, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.de Craen AJ, Kaptchuk TJ, Tijssen JG, Kleijnen J. Placebos and placebo effects in medicine: historical overview. J R Soc Med. 1999;92(10):511–5. doi: 10.1177/014107689909201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro AK, Shapiro E. The powerful placebo: from ancient priest to modern physician. Baltimore: The Johns Hopkins University Press; 1997. [Google Scholar]
- 3.Cabot RC. The use of truth and falsehood in medicine: an experimental study. Am Med. 1903;5:344–9. [Google Scholar]
- 4.Brody H, Miller FG. Lessons from recent research about the placebo effect—from art to science. JAMA. 2011;306(23):2612–3. doi: 10.1001/jama.2011.1850. [DOI] [PubMed] [Google Scholar]
- 5.Brody H. The placebo response. Recent research and implications for family medicine. J Fam Pract. 2000;49(7):649–54. [PubMed] [Google Scholar]
- 6.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA. 1994;271(20):1609–14. [PubMed] [Google Scholar]
- 7•.Benedetti F. Placebo effects: understanding the mechanisms in health and disease. Oxford: Oxford University Press; 2009. This book critically reviews the mechanisms of placebo and placebo-related effects across all medical conditions, diseases, and therapeutic interventions. [Google Scholar]
- 8.Ellenberg SS, Temple R. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: practical issues and specific cases. Ann Intern Med. 2000;133(6):464–70. doi: 10.7326/0003-4819-133-6-200009190-00015. [DOI] [PubMed] [Google Scholar]
- 9.Temple RJ. When are clinical trials of a given agent vs. placebo no longer appropriate or feasible? Control Clin Trials. 1997;18(6):613–20. doi: 10.1016/s0197-2456(97)00058-5. [DOI] [PubMed] [Google Scholar]
- 10.Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351(9117):1722–5. doi: 10.1016/s0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- 11.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344(21):1594–602. doi: 10.1056/nejm200105243442106. [DOI] [PubMed] [Google Scholar]
- 12.Ernst E, Resch K. Concept of true and perceived placebo effects. BMJ. 1995;311(7004):551–3. doi: 10.1136/bmj.311.7004.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, et al. Do “placebo responders” exist? Contemp Clin Trials. 2008;29(4):587–95. doi: 10.1016/j.cct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Program CNAC. Asthma’s impact on the nation. Atlanta, GA: 2014. http://www.cdc.gov/asthma/impacts_nation/default.htm. [Google Scholar]
- 15.Mackenzie JN. The production of the so-called “rose cold” by means of an artificial rose: with remarks and historical notes. Am J Med Sci. 1886;181:45. [Google Scholar]
- 16.Colloca L, Miller FG. Role of expectations in health. Curr Opin Psychiatry. 2011;24(2):149–55. doi: 10.1097/YCO.0b013e328343803b. [DOI] [PubMed] [Google Scholar]
- 17.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59(2):195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–90. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 19•.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–95. doi: 10.1016/s0140-6736(09)61706-2. This article discusses the ethical use of placebo mechanisms that are inherent in routine clinical care and the use of treatments that stimulate placebo effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA. The placebo effect: advances from different methodological approaches. J Neurosci. 2011;31(45):16117–24. doi: 10.1523/jneurosci.4099-11.2011. This article provides an overview of the processes involved in the formation of placebo responses by combining research findings from behavioral, psychophysiological, and neuroimaging methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaptchuk TJ. Placebo studies and ritual theory: a comparative analysis of Navajo, acupuncture and biomedical healing. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1849–58. doi: 10.1098/rstb.2010.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luparello T, Lyons H, Bleecker E, McFadden E. Influences of suggestion on airway reactivity in asthmatic subjects. Psychosom Med. 1968;30(6):819–25. doi: 10.1097/00006842-196811000-00002. [DOI] [PubMed] [Google Scholar]
- 23.McFadden E, Luparello T, Lyons H, Bleecker E. The mechanism of action of suggestion in the induction of acute asthma attacks. Psychosom Med. 1969;31(2):134–43. doi: 10.1097/00006842-196903000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Castro MRA, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, Fiss E, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–24. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedetti F. The placebo response: science versus ethics and the vulnerability of the patient. World Psychiatry. 2012;11(2):70–2. doi: 10.1016/j.wpsyc.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vits S, Cesko E, Benson S, Rueckert A, Hillen U, Schadendorf D, et al. Cognitive factors mediate placebo responses in patients with house dust mite allergy. PLoS One. 2013;8(11):e79576. doi: 10.1371/journal.pone.0079576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goebel M, Meykadeh N, Kou W, Schedlowski M, Hengge U. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother Psychosom. 2008;77(4):227–34. doi: 10.1159/000126074. [DOI] [PubMed] [Google Scholar]
- 28.Vits S, Cesko E, Enck P, Hillen U, Schadendorf D, Schedlowski M. Behavioural conditioning as the mediator of placebo responses in the immune system. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1799–807. doi: 10.1098/rstb.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedetti F, Amanzio M, Baldi S, Casadio C, Maggi G. Inducing placebo respiratory depressant responses in humans via opioid receptors. Eur J Neurosci. 1999;11:625–31. doi: 10.1046/j.1460-9568.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 30.de la Fuente-Fernández R, Ruth T, Sossi V, Schulzer M, Calne D, Stoessl A. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164–6. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7(6):587–8. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 32.Meissner K, Kohls N, Colloca L. Introduction to placebo effects in medicine: mechanisms and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1783–9. doi: 10.1098/rstb.2010.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albring A, Wendt L, Benson S, Witzke O, Kribben A, Engler H, et al. Placebo effects on the immune response in humans: the role of learning and expectation. PLoS One. 2012;7(11):e49477. doi: 10.1371/journal.pone.0049477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasagna L, Mosteller F, Von Felsinger JM, Beecher HK. A study of the placebo response. Am J Med. 1954;16(6):770–9. doi: 10.1016/0002-9343(54)90441-6. [DOI] [PubMed] [Google Scholar]
- 35.Joyce CR. Consistent differences in individual reactions to drugs and dummies. Br J Pharmacol Chemother. 1959;14:512–21. doi: 10.1111/j.1476-5381.1959.tb00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leigh R, MacQueen G, Tougas G, Hargreave FE, Bienenstock J. Change in forced expiratory volume in 1 second after sham bronchoconstrictor in suggestible but not suggestion-resistant asthmatic subjects: a pilot study. Psychosom Med. 2003;65(5):791–5. doi: 10.1097/01.psy.0000079454.48714.1b. [DOI] [PubMed] [Google Scholar]
- 37.Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol. 2007;119(6):1375–81. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. 2013;170(11):1335–44. doi: 10.1176/appi.ajp.2013.12030315. [DOI] [PubMed] [Google Scholar]
- 39.Hall KT, Lembo AJ, Kirsch I, Ziogas DC, Douaiher J, Jensen KB, et al. Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS One. 2012;7(10):e48135. doi: 10.1371/journal.pone.0048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16(11):1277–83. doi: 10.1038/nm.2229. This article discusses how placebo and nocebo effects are produced and what biological and psychological factors influence variances in the magnitude of the effect. [DOI] [PubMed] [Google Scholar]
- 41.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17(10):1228–30. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 42.Wise RA, Bartlett SJ, Brown ED, Castro M, Cohen R, Holbrook JT, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol. 2009;124(3):436–44. 44e1–8. doi: 10.1016/j.jaci.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.May O, Hansen NC. Comparison of terbutaline, isotonic saline, ambient air and non-treatment in patients with reversible chronic airway obstruction. Eur Respir J. 1988;1(6):527–30. [PubMed] [Google Scholar]
- 44.Isenberg SA, Lehrer PM, Hochron S. The effects of suggestion on airways of asthmatic subjects breathing room air as a suggested bronchoconstrictor and bronchodilator. J Psychosom Res. 1992;36(8):769–76. doi: 10.1016/0022-3999(92)90135-o. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365(2):119–26. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirsch I. Philosophical transactions of the Royal Society of London. Series B, biological sciences. Preface. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1781–2. doi: 10.1098/rstb.2010.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591. doi: 10.1371/journal.pone.0015591. http://www.nejm.org/doi/full/10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kam-Hansen S, Jakubowski M, Kelley J, Kirsch I, Hoaglin D, Kaptchuk T, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6(218):218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;(1):Cd003974. doi: 10.1002/14651858.CD003974.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moerman DE. Meaningful placebos—controlling the uncontrollable. N Engl J Med. 2011;365(2):171–2. doi: 10.1056/NEJMe1104010. [DOI] [PubMed] [Google Scholar]
- 51.NAEPP. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 52.Radeos MS, Leak LV, Lugo BP, Hanrahan JP, Clark S, Camargo CA., Jr Risk factors for lack of asthma self-management knowledge among ED patients not on inhaled steroids. Am J Emerg Med. 2001;19(4):253–9. doi: 10.1053/ajem.2001.21712. [DOI] [PubMed] [Google Scholar]
- 53.Kendrick AH, Higgs CM, Whitfield MJ, Laszlo G. Accuracy of perception of severity of asthma: patients treated in general practice. BMJ. 1993;307(6901):422–4. doi: 10.1136/bmj.307.6901.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lund K, Vase L, Petersen GL, Jensen TS, Finnerup NB. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLoS One. 2014;9(1):e84104. doi: 10.1371/journal.pone.0084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff (Millwood) 2013;32(6):1116–25. doi: 10.1377/hlthaff.2012.1353. [DOI] [PubMed] [Google Scholar]
- 56.Heger M. Trial designs advance to overcome bitter pill of placebo effect. Nat Med. 2013;19(11):1353. doi: 10.1038/nm1113-1353. [DOI] [PubMed] [Google Scholar]