Abstract
The submandibular salivary glands of non-obese diabetic (NOD) mice, a model for Sjogren’s syndrome and type-1 diabetes, show an elevated level of proliferating cell nuclear antigen (PCNA), a protein involved in cell proliferation and repair of DNA damage. We reported previously that epigallocatechin-3-gallate (EGCG), the most abundant green tea catechin, normalizes the PCNA level. PCNA’s activity can be regulated by the cyclin-dependent kinase inhibitor p21, which is also important for epithelial cell differentiation. In turn, expression of p21 and PCNA are partially regulated by Rb phosphorylation levels. EGCG was found to modulate p21 expression in epithelial cells, suggesting that EGCG-induced p21 could be associated with down-regulation of PCNA in vivo. The current study examined the protein levels of p21 and p53 (which can up-regulate p21) in NOD mice fed with either water or EGCG, and the effect of EGCG on p21 and p53 in cell line models with either normal or defective Rb. In NOD mice, the p21 level was low, and EGCG normalized it. In contrast to HSG cells with functional Rb, negligible expression of p21 in NS-SV-AC cells that lack Rb was not altered by EGCG treatment. Inhibition of p53 by siRNA demonstrated that p21 and p53 were induced independently in HSG cells by a physiological concentration range of EGCG, suggesting p53 could be an important but not conditional factor associated with p21 expression. In conclusion, PCNA and p21 levels are altered inversely in the NOD model for SS and in HSG cells, and warrant further study as candidate new markers for salivary dysfunction associated with xerostomia. Induction of p21 by EGCG could provide clinically useful normalization of salivary glands by promoting differentiation and reducing PCNA levels.
Keywords: Autoimmune disease, EGCG, non-obese diabetic mouse, p21, p53, PCNA, salivary gland
INTRODUCTION
The non-obese diabetic (NOD) mouse is a model for type I diabetes and Sjogren’s Syndrome (SS), an autoimmune disorder characterized by exocrine gland dysfunction. Normal salivary glands have at most only a moderate rate of cell turnover and replacement [1], but in the NOD mouse the submandibular gland displays marked hypertrophy and hyperplasia, particular in the granular convoluted tubules, and the proportion of cells positive for two proliferation markers, proliferating cell nuclear antigen (PCNA) and Ki67, was shown to be considerably higher than in normal BALB/c (control) mice [2]. Consistent with this, PCNA expression has also been reported to be abnormally high in patients with Sjogren’s syndrome (SS) [3]. Importantly, treatment of NOD mice with 0.2% epigallocatechin-3-gallate (EGCG) in their drinking water normalized the proportions of PCNA and Ki67-positive cells and reduced the hypertrophy and hyperplasia [2].
The mechanism leading to normalization of PCNA levels by EGCG in the submandibular salivary gland of the NOD model for SS is not known. The requirement of PCNA for DNA repair raises the possibility that in NOD mice, the higher proportion of PCNA positive cells reflects, at least in part, elevated DNA damage, perhaps due to reactive oxygen species (ROS), and subsequent PCNA-dependent repair [2]. Thus, one potential explanation for the beneficial effect of EGCG is that its antioxidant activity reduces DNA damage, with a corresponding reduction in PCNA levels.
Alteration of epithelial cell signaling could be an alternative, or complementary, mechanism for the beneficial effects of EGCG. We, and others, have shown previously that EGCG acts (at least in part) through induction of alterations in MAPK signaling pathways [4–8]. In cancer cells EGCG also induces expression of the cyclin-dependent kinase inhibitor p21waf/cip1, and p21 up-regulation by EGCG in oral carcinoma OSC2 cells is associated with growth arrest [9, 10]. p21 is a multifunction protein with various and complex roles in the regulation of both cell cycle progression and cell fate in development and differentiation, as well as in the co-ordination of the cellular response to genome damage [11]. These roles are not fully understood, but involve protein dosage effects and interactions with other proteins such as PCNA. The exact function of the p21-PCNA interaction is unclear, but may involve, in part, inhibition of potentially error-prone trans-lesion DNA synthesis [12].
Phosphorylated retinoblastoma (pRb) is a key cell cycle regulatory protein with a role in mediating certain functions of p21. In early G1, cyclin D/cdk4 and cyclin D/cdk6 complexes form that phosphorylate pRb and prime it for subsequent phosphorylation by cyclin E/cdk2 complexes. Hyperphosphorylation of pRb leads to release of E2F transcription factor and subsequent expression of genes required for S phase. Assembly of the cyclin D/cdk complexes is facilitated by basal levels of p21 (which incorporates into the complexes), while the activity of cyclin E/cdk2 complexes is inhibited by higher levels of p21 [11, 13].
Regulation of p21 expression is complex; gene transcription involves p53 dependent and independent mechanisms, while protein levels are controlled in part by proteosome-mediated degradation [11, 14]. The level of p21 protein is modulated in response to DNA damage, but the direction of change is not readily predicted. Transcription of the p21 gene is up-regulated in many cells in response to DNA damage via p53 activation [15]. Conversely, degradation of p21 protein occurs in response to various forms of DNA damage that induce different repair pathways, such as ROS-induced base excision repair. Interaction with PCNA can protect a fraction of p21 protein, but degradation is not required for PCNA-dependent recruitment of repair proteins to lesions [14].
Given the potential role of p21 in the co-ordination of DNA repair and cell cycle arrest, we hypothesized that the ROS-induced DNA damage reported in SS could account for the elevated PCNA expression in NOD mice, in conjunction with alteration in p21 protein levels, and that EGCG promotes normalization of both p21 and PCNA levels in salivary gland cells. As a first step in testing this hypothesis, the expression of p21 and p53 in the submandibular gland of the NOD mouse model was determined. Next, the levels of p21 and p53 in human salivary gland cell lines exposed to EGCG were assessed.
MATERIALS AND METHODS
Chemicals and Antibodies
EGCG (>95%) was purchased from Sigma-Aldrich (St. Louis, MO). Rabbit anti-human proliferating cell nuclear antibody (PCNA) (FL-261), rabbit anti-human/rodent p21 antibody, rabbit anti-p53 and goat anti-human actin polyclonal antibody (I-19) were purchased from Santa Cruz Biotechnology, Santa Cruz, CA.
Animal Samples
Archived mouse submandibular salivary gland samples were obtained from a study described previously [2]. Briefly, after weaning (4 weeks old) groups of NOD mice had access to either water or water containing 0.2% EGCG. Submandibular glands were harvested at the age of 22 weeks old, after autoimmune diseases developed (insulin-dependent diabetes and SS-like disorders). The tissues were fixed with buffered-formalin and paraffin embedded. Five micron-thick (5 μm) serial sections were cut from the paraffin-embedded samples.
Immunohistochemical Analysis
Immunohistochemical staining of submandibular gland sections was performed using a standard protocol with Histoplus Kits (ZYMED Laboratories, CA, USA) according to the manufacturer’s directions. Deparaffinized sections were immersed in methanol containing 3% hydrogen peroxide for 20 min to quench endogenous peroxidase activity. The sections were incubated with anti-p21 polyclonal antibody (diluted 1:100) overnight at 4°C or were incubated with anti-p53 polyclonal antibody (diluted 1:1500) for 1 hour at room temperature. The sections were then incubated with the biotinylated secondary antibody for 10 min and HRP-streptavidin for 10 min. Peroxidase staining was performed for 3~7 min using a solution of DAB chromogen. The sections were counterstained with 0.5% methyl green. To maintain consistency, slides were batch processed.
For quantification, at least 1000 cells were counted, and the percentage of cells showing positive nuclear or cytosolic staining for PCNA or p53 was calculated.
Cell Culture and Cell Treatment
The human salivary gland adenocarcinoma cell line HSG was derived from intercalated duct epithelium [16]. This cell line was maintained in DMEM/Ham’s F12 medium, with 10% fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin and 5 μg/ml hydrocortisone at 37°C with 5% CO2. The immortalized human salivary gland acinar cell line (NS-SV-AC) has been described previously [17], and was kindly provided by Dr. Masayuki Azuma (Tokushima University School of Dentistry, Tokushima, Japan). These cells were selected following stable transfection of primary human salivary gland acinar cells with origin-defective SV40 mutant DNA. Subculture of the NS-SV-AC cells was performed by detaching cells in 0.25% trypsin, and transferring into new tissue culture flasks. They were maintained in keratinocyte growth medium-2 (KGM-2, Cambrex, East Rutherford, New Jersey)
EGCG was dissolved in cell culture medium as a 50 mM stock immediately before use. To test for cytotoxicity and the effects on protein levels, cells were treated with EGCG for 12, 24 or 36 hrs, followed by MTT cell viability assays previously described [18] or Western analysis of protein extracts.
Transfection of HSG Cells with p53-siRNA-Expressing Plasmid
A plasmid encoding an siRNA specific for p53 and a control plasmid containing scrambled insert (GeneSuppressor Systen for knockdown of human p53) were purchased from Imgenex Corporation, San Diego, CA. The plasmids were amplified according to the manufacturer’s instructions. Transfection of plasmid DNA was performed using the Lipofectamine 2000 method.
Western Blot Analysis
After cell treatment, cells were washed in ice-cold PBS and lysed for 20 min in RIPA buffer containing 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 10 μg/ml leupeptin, 3 μg/ml aprotinin and 100 mM phenylmethylsulfonyl fluoride (PMSF). Samples of lysates containing the same amount of protein were loaded in each lane (we used 5–30 μg, depending on the antibody used) and electrophoretically separated on a 12% SDS polyacrylamide gel. Following electrophoresis, proteins were transferred to a PVDF membrane (Immobilon™-P, Millipore Corporation, Bedford, MA). The membrane was blocked for 1 h with 5% (w/v) non-fat dry milk powder in PBST (0.1% Tween-20 in PBS) and then incubated for 1 h with primary antibody diluted in PBST/milk (Antibodies and dilutions: goat polyclonal actin, 1:2000; p53, 1:1000; p21, 1:100). The membrane was washed three times with PBST and incubated with peroxidase-conjugated, affinity-purified anti-rabbit or anti-goat secondary antibodies (Santa Cruz Biotechnology, Inc.) for 1 h. Following extensive washing, the reaction was developed by enhanced chemiluminescent staining using ECL Western blotting detection reagents (Amersham Pharmacia Biotech Inc., Piscataway, NJ). Experiments were repeated three times with similar results and identical patterns.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Cell Viability Assay
Cells in each well were washed with 200 μl of phosphate-buffered saline (PBS) and incubated with 100 μl of 2 %(w/v) MTT in a solution of 0.05 M Tris-HCl (pH 7.6), 0.5 mM MgCl2, 2.5 mM CoCl2 and 0.25 M disodium succinate at 37 °C for 30 min. Cells were fixed by the addition of 100 μl of 4% (v/v) formalin in 0.2 M Tris-HCl (pH 7.6), and after a 5 min incubation at room temperature liquid was removed and the wells were allowed to dry. Each well was rinsed with 200 μl water and cells in each well were solubilized by the addition of 100 μl of 6.35 % (v/v) 0.1 N NaOH in DMSO. The colored formazan product was measured by a Thermo MAX micro plate reader (Molecular Devices Corp. Sunnyvale, CA) at a wavelength of 562 nm.
Statistical Analysis
All data are reported as mean ± SD. A one-way ANOVA with Tukey’s or Dunnett’s post-test, or two-tailed Student’s t tests, were used to analyze statistical significance, as appropriate. Differences were considered statistically significant at p≤0.05.
RESULTS
Immunostaining of p21 in the NOD Mouse Submandibular Gland
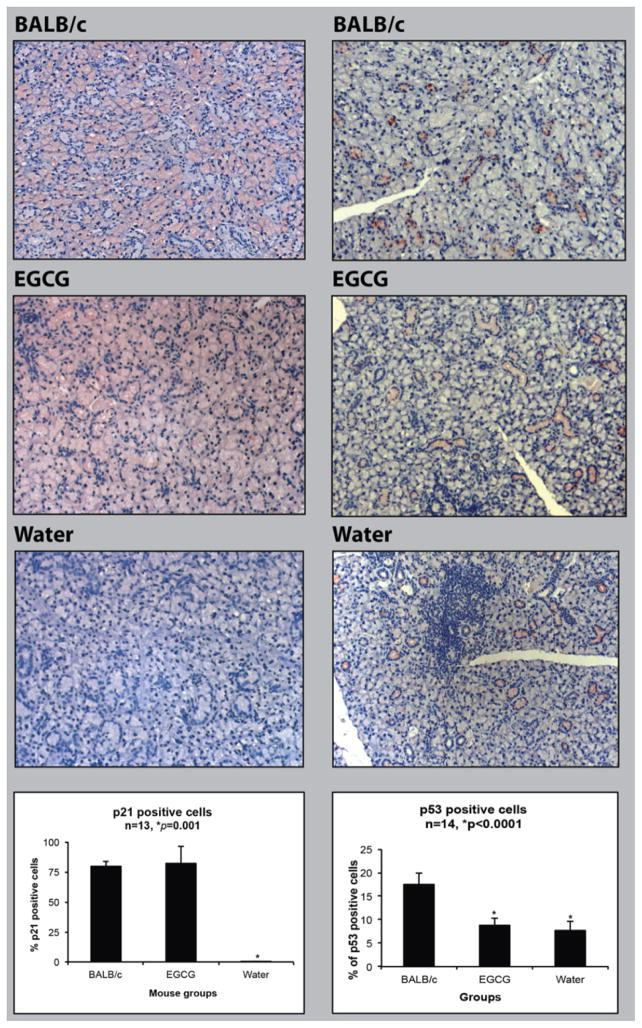
A high proportion of cells in the BALB/c submandibular gland stained positive for p21 (79.2± 4.4%; n=3). Expression of p21 was prominent in the acinar cells, with less frequent expression in granular convoluted tubule cells, and the staining was present mainly in the cytoplasm (Fig. 1, left panel). The two groups (water and EGCG fed) of NOD mouse samples harvested at 22 weeks of age showed a dramatic difference in p21 expression. In comparison to the BALB/c mouse gland tissue, tissues from water-fed animals had very few cells with p21 staining (0.21±0.16%; n=4)(p<0.0001; ANOVA, Tukey’s post-test). In contrast, the EGCG-fed group showed a far higher level of p21 in most of the epithelial cells (82.1± 13.9%; n=5), significantly higher than the water-fed animals (p<0.0001), but not significantly different from the BALB/c control (p=0.9088). EGCG-fed NOD mice showed more prominent granular convoluted tubule p21 staining than the BALB/c mice (Fig. 1, left panel). These results indicated that 22 week old NOD mice had lost almost all p21 expression, and EGCG restored p21 protein to normal control levels in acinar cells.
Fig. 1.
Quantitative analysis of immunostained submandibular gland samples of 22 week old water or EGCG-treated NOD mice, and 22 week old BALB/c mice. Left panel: representative examples of p21 protein expression in submandibular glands of BALB/c mice, EGCG-treated NOD mice, and water-treated NOD mice. Right panel: representative examples of p53 protein expression in the submandibular glands of BALB/c mice, EGCG-treated NOD mice, and water-treated NOD mice. Statistical analysis of p21 (lower left) and p53 (lower right)-positive cells were based on the number of positively stained cells (brown colored) among 1000 cells counted. * Significant difference in comparison to other groups (ANOVA, Tukey’s post-test, p<0.05).
Immunostaining of p53 in the NOD Mouse Submandibular Gland
Immunohistochemistry was used to determine if EGCG-induction of p21 in NOD mouse submandibular glands was associated with p53 up-regulation (Fig. 1, right panel). Unlike p21, only a minority of cells in BALB/c control mice (17.6±2.3%) was positive for p53 staining, and primarily in the granular convoluted tubule cells. Significantly fewer cells in glands from water-fed NOD mice were positive (7.75±1.89%, p<0.0001, ANOVA). However EGCG-water-fed NOD mice were also significantly lower (8.84 ±1.57, p<0.0001), and were not significantly different from water-fed NOD mice (p≫0.05). These results indicated that 22 week old NOD mice expressed a significantly lower level of p53 in comparison to BALB/c mice, but unlike p21, EGCG had no effect on p53 levels. Therefore, the dominant salivary gland cell types expressing p21 and p53 were different and no direct relationship was observed between p21 and p53 levels and the effect of EGCG.
Effect of EGCG on Cell Viability
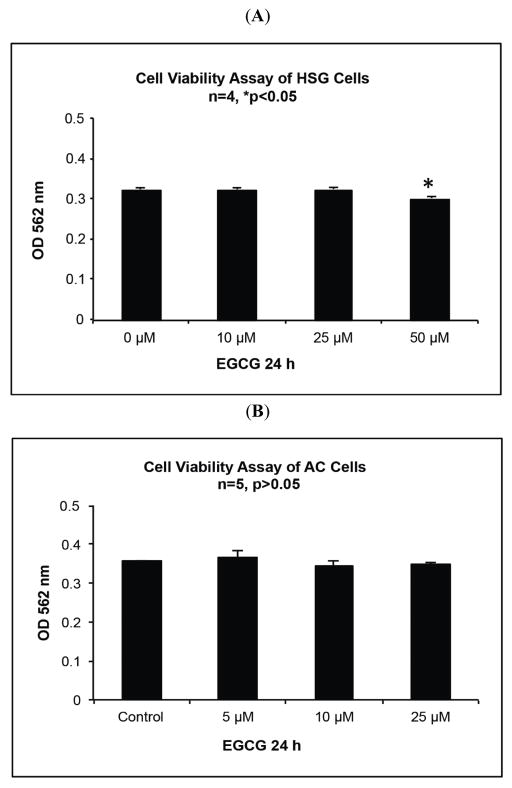
We have shown previously that EGCG up to 50 μM has no significant effect on the viability of NS-SV-AC cells [4, 17]. To assess whether treatment with EGCG in the physiological range resulted in cytotoxicity of HSG cells, they were incubated with doses of EGCG up to 50 μM for 24 h, followed by cell viability assay. The results shown in Fig. (2A, B) indicated that there was no significant decrease in cell viability in comparison to the untreated control when these cells were treated with EGCG up to 25 μM (p≫0.05; ANOVA, Dunnett’s post-test), but HSG cells showed modest cytotoxicity (93.0% control viability; p=0.0009) at a 50 μM dose.
Fig. 2.
Cell viability assay (MTT Assay) results of NS-SV-AC cells and HSG cells treated with EGCG at indicated concentrations for 24 h. A. Optical absorption at 562 nm on HSG cells based on triplicate samples. Significant difference was found at 50 μM EGCG treated cells (ANOVA, Dunnett’s post-test, p<0.05). B. Optical absorption at 562 nm on NS-SV-AC cells based on triplicate samples. There was no significant difference among the samples (ANOVA, Dunnett’s post-test, p>0.05).
Western Analysis of Expression of p21 and p53 in HSG Cells Following EGCG Treatment
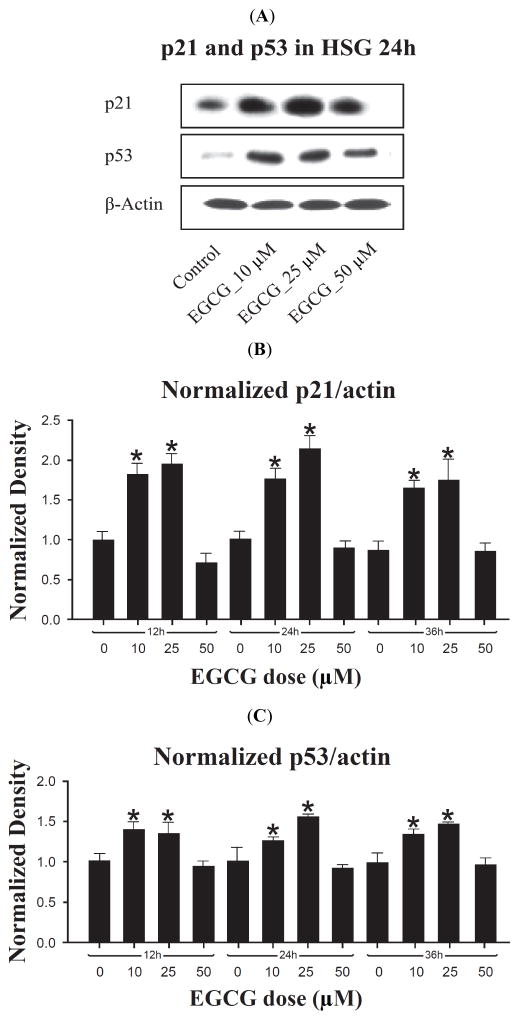
To further examine the effect of EGCG on p21 expression, HSG cells treated with different concentrations of EGCG at 12, 24 and 36 h were analyzed for p21 protein levels (Fig. 3A, B). At each time, highly significant differences in the level of p21 at different doses were seen (ANOVA, p<0.0001). With one small exception, the pattern of differences between doses was identical at each time, and for a given dose, no significant differences were seen between times in the level of p21 expression (ANOVA, p=0.088 to 0.65). That is, between 12 and 36 hrs, there was little effect of time of exposure to a given EGCG dose on the level of p21 expression. Therefore, only the results for 24 hr will be described in detail.
Fig. 3.
Western analysis of p53 and p21 protein expression in HSG cells treated with EGCG at indicated concentrations and time points. A. Representative photograph of Western blot showing protein bands of p21 and p53 in HSG cells treated with EGCG for 24 h. β-actin was used as a loading control. B. Density of p21 proteins bands. Values were normalized to the blot actin band density, and then normalized to the mean 12 hr untreated value. Thus, the normalized mean 12 hr untreated value is 1.0. Bar graph shows the mean of three independent experiments. * Significant difference observed in comparison to control cells without EGCG treatment (ANOVA, p<0.05). C. Density of p53 proteins bands relative to actin band density. Values were normalized as for p21. Bar graph is based on three independent experiments. * Significant difference in comparison to control cells without EGCG treatment (ANOVA, p<0.05).
As shown in Fig. (3A, B), at 24 hrs 10 and 25 μM EGCG induced p21 protein 1.74 fold (p=0.0006) and 2.14-fold (p<0.0001) respectively relative to untreated control. The fold difference between these doses was also significant (p=0.023) at 24 hrs, but not at 12 or 36 hrs. In contrast, between 12 and 36 hrs p21 remained at control levels at 50 μM EGCG (p≥0.05). Therefore, in HSG cells EGCG displays an optimum dose of between 10 and 25 μM for p21 induction, with a peak induction of about 2-fold, but the effect is independent of time of exposure between 12 and 36 hrs.
Western blot analysis was used to determine whether the level of p53 protein, an up-stream regulator of p21, is modulated by EGCG in HSG cells. As shown in Fig. (3A, C), p53 protein showed a response profile parallel to that seen for p21 in these cells: there was a highly significant dose effect at all three times (p=<0.0001 to 0.0004, ANOVA). For a given dose, there was no significant difference in the level of p53 at each of the three times (p>0.05); there was a maximum induction at an optimum dose of 10–25 μM EGCG, and no significant induction at 50 μM EGCG (p≫0.05). At 24 hrs, 10 and 25 μM EGCG induced p53 protein 1.28 fold (p=0.009) and 1.53-fold (p=0.0001) respectively relative to untreated control. The fold difference between these doses was also significant (p=0.017) at 24 hrs, but not at 12 or 36 hrs. Therefore, as for p21, EGCG displays an optimum dose of between 10 and 25 μM for p53 induction in HSG cells, with a peak induction of about 1.5-fold, but the effect is independent of time of exposure between 12 and 36 hrs.
These observations are consistent with a role for p53 in the EGCG-mediated induction of p21, but could also have resulted from a common EGCG modulated pathway governing both proteins with similar kinetics, and with no direct influence of p53 on p21 protein levels.
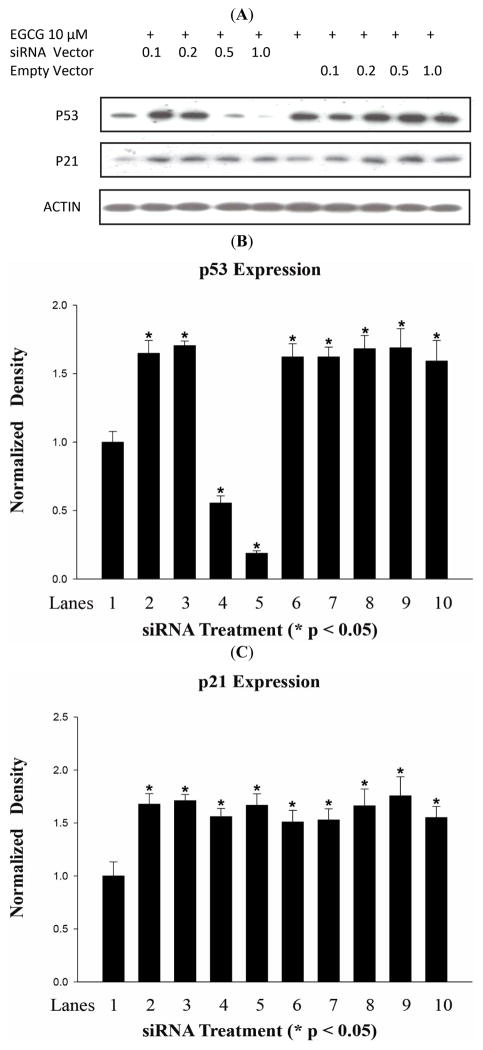
Expression of p21 and p53 Following EGCG Treatment in HSG Cells Expressing p53-siRNA
To determine whether p53 is required for EGCG regulation of p21 expression, the p53 siRNA expression vector specific for p53 mRNA and a control vector were transfected into HSG cells, followed by 10 μM EGCG treatment and Western analysis (Fig. 4A, B). At the lower siRNA doses (0.1 and 0.2μg), 10μM EGCG induced p53 1.7-fold relative to untreated control (p<0.0001), not significantly different (p≫0.05) from the 1.6-fold increase with EGCG treatment alone, consistent with no inhibition at this dose of siRNA. However, at 0.5 and 1.0μg siRNA, the p53 protein level was reduced 1.80 and 5.31-fold respectively relative to non-siRNA-treated control (p<0.0001). In contrast, the control siRNA vector had no effect on p53 protein levels following EGCG treatment (ANOVA, p≫0.05).
Fig. 4.
Western analysis of p53 and p21 protein expression in HSG cells treated with 10 μM EGCG and transfected with siRNA against p53, or empty vector, at indicated concentrations. A. Representative photograph of Western blot showing protein bands. B. Density of p53 proteins bands relative to actin band density. Bar graph shows the mean of three independent experiments. * Significant difference observed in comparison to negative control cells (Lane 1) without EGCG treatment or transfection (ANOVA, Dunnett’s post-test, p<0.05). C. Density of p21 protein bands relative to actin band density. Bar graph is based on three independent experiments. * Significant difference observed in comparison to negative control cells (Lane 1) without EGCG treatment or transfection (ANOVA, Dunnett’s post-test, p<0.05).
As shown in Fig. (4A, C), there was no significant effect of any dose of the p53 siRNA on the induction of p21 expression by EGCG. In this experiment (n=3), the mean induction at all four doses was 1.66-fold, significantly greater than the control (p<0.0002), but with no significant difference between the four p53 siRNA doses (p≫0.05), or to the EGCG control (1.509-fold; p≫0.05). The control vector had no effect on p21 expression (ANOVA, p>0.05). These results indicated that EGCG induction of p21 is not dependent on induction of p53.
Role of pRb in EGCG Modulation of p21 and PCNA Protein Levels
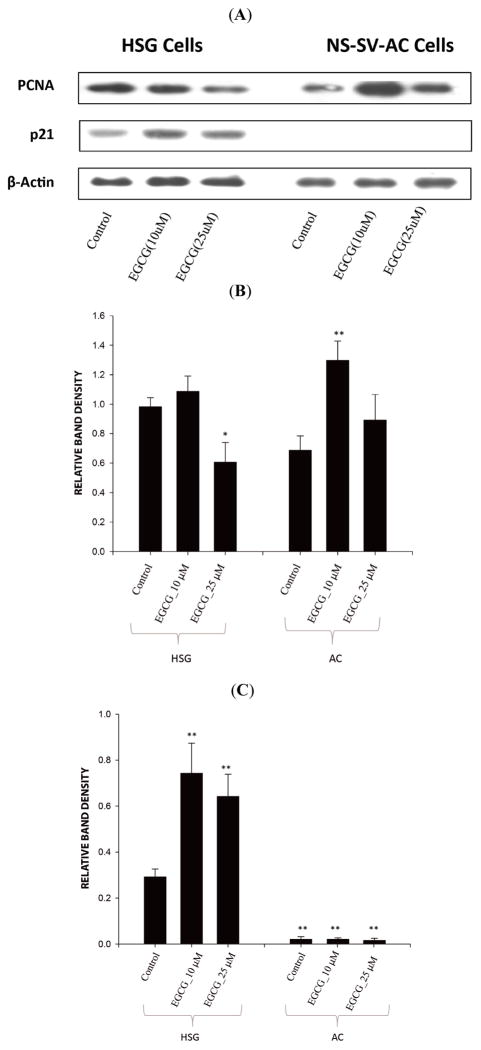
To examine the role of Rb in the effects of EGCG on p21 expression in salivary gland cells, NS-SV-AC cells (with an inactivated Rb) were treated with EGCG for 24 h. As shown in Fig. (5A, C), NS-SV-AC cells showed negligible p21 protein expression at EGCG concentrations between 0–25 μM in comparison to HSG cells, and EGCG failed to induce p21 expression. This suggested Rb is required for p21 expression in these cells, and EGCG cannot induce p21 in the absence of Rb activity.
Fig. 5.
Western analysis of PCNA and p21 protein expression in HSG cells and NS-SV-AC cells treated with EGCG at indicated concentrations for 24 h. A. Representative photograph of Western blot showing protein bands. B. Density of PCNA proteins bands relative to actin band density. Bar graph is based on three independent experiments. * Significant difference in HSG cells. ** Significant difference in NS-SV-AC cells (ANOVA, Tukey’s post-test, p<0.05). C. Density of p21 proteins bands relative to actin band density. Bar graph is based on three independent experiments. ** Significant difference in comparison to control HSG cells (ANOVA, Tukey’s post-test, p<0.05). ** Significant difference between NS-SV-AC cells and HSG cells (ANOVA, Tukey’s post-test, p<0.05).
The relationship between levels of pRb, p21 and PCNA proteins in salivary gland cells, and the effects of EGCG, was investigated by Western blot analysis. Consistent with observations in the NOD mouse model, treatment of HSG cells with 25μM EGCG caused a significant 1.62-fold decrease in the PCNA level (n=3; p=0.010; ANOVA, Tukey’s post-test). In contrast to the effect on p21 and p53 expression, treatment with 10μM EGCG had no significant effect on PCNA (p≫0.05). The ratio of p21:PCNA was significantly higher (3.70-fold) at a dose of 25μM EGCG than the control (p=0.012). Although 2.32-fold higher at a dose of 10μM EGCG, the difference was not significant (p>0.05). In contrast, in Rb-inactivated NS-SV-AC cells, 10 μM EGCG induced a 1.89-fold increase in PCNA level (p=0.004), while 25 μM EGCG had no significant effect (p>0.05, Fig. 5A, B). This indicated that in an Rb-active, p21 positive cell line, 25 μM EGCG can induce a decrease in PCNA levels in parallel with a reciprocal increase in p21 levels. However, at 10 μM EGCG, although there is a similar increase in p21, PCNA levels are unchanged, suggesting inverse levels of these two proteins are not linked directly. The induction of PCNA by 10 μM, but not 25 μM EGCG in an Rb-inactive, p21-negative cell line is also consistent with separate pathways mediating EGCG modulation of p21 and PCNA levels.
DISCUSSION
We reported previously that by 22 weeks of age (after the onset of autoimmune disease), PCNA nuclear staining in the submandibular glands of water-fed NOD mice was more than 13-fold higher than in the EGCG-fed group [2]. In comparison, BALB/c mice at 22 weeks of age showed PCNA expression similar to the EGCG-fed group (approximately 1%). That is, in water-fed NOD mice, there is a dramatic rise in PCNA-positive cells by 22 weeks of age, and EGCG considerably reduces this rise and maintains levels comparable to normal control mice [2]. The current study found, for the first time, that p21 is down-regulated in 22 week old NOD mice that had SS-like symptoms, and this down-regulation was normalized in EGCG-fed NOD mice at the same age (Fig. 1). Thus, the level of p21-positive cells in water-fed NOD mice is near zero, but in EGCG-fed NOD mice rises to match the high level (about 79%) seen in BALB/c control mice. The results presented here, combined with our previously reported PCNA data [2], are consistent with inversely related expression of PCNA and p21 in NOD mice during autoimmune disease progression, and normalization of this pattern to the control BALB/c levels by EGCG.
This reciprocal relationship in response to EGCG was recapitulated in the HSG ductal cell-derived cell line, albeit with a non-linear dose-response pattern; at 25 μM EGCG, p21 was increased >2-fold, and PCNA levels were reduced 1.62-fold. This dose is in the physiological concentration range (0–25 μM) for EGCG. Concentrations higher than this failed to modulate the protein levels (Fig. 3), suggesting that EGCG concentrations in vivo higher than the physiological range may not provide beneficial effects.
Consistent with these observations, EGCG was shown previously to significantly induce the expression of p21 in the oral squamous cell carcinoma cell line OSC2 [10]. The induction of p21 was associated with cell growth arrest, and siRNA suppression of p21 resulted in accelerated cell growth in OSC2 cells. Similarly, in epidermal growth factor (EGF) stimulated MCF10A mammary epithelial cells, EGCG significantly up-regulated the expression of p21, resulted in decreased cyclin D1-associated pRB kinase activity, decreased pRB phosphorylation, impaired progression through the late G1 restriction point, and diminished entry into the S phase of the cell cycle [19].
We observed significant amounts of p21 immunostaining in the cytoplasm of the acinar cells of normal mice. p21 is also involved in regulation of apoptosis and autophagy (a caspase-independent form of programmed cell death involving intracellular membrane-bounded autophagosomes that sequester proteins and organelles for breakdown). Interaction of p21 with the apoptosis effector precursor procaspase 3 blocks activation; conversely, caspase 3 cleaves p21 and removes a nuclear localization signal, leading to cytoplasmic localization and loss of growth arrest activity [11]. p21 inhibits pro-apoptotic signaling through the apoptosis signal regulating kinase 1 (ASK1) and the stress activated JNK pathways [20]. Low levels of p21 direct the cell to autophagy [15, 21]. The altered levels of p21 protein in NOD mice could therefore reflect alterations in apoptotic and other signaling pathways.
The level of p21 protein produced in response to DNA damage is not readily predicted. For example, in human lens epithelial cells (immortalized with SV40 large T antigen), as well as lens explants, treatment with non-cytotoxic levels of hydrogen peroxide induces an increase in p21 protein levels via ERK/JNK activation and cell cycle arrest [22], while in human fibroblasts, low levels of hydrogen peroxide can induce p21 degradation [14]. Transcription of the p21 gene is up-regulated in many cells in response to DNA damage via p53 activation [15]. Increased p21 would lead to cell cycle arrest in G1 (by both cdk inhibition and by transcriptional repression of cell cycle regulatory genes), and to protection from apoptosis [11]. Conversely, degradation of p21 protein occurs in response to various forms of DNA damage that induce different repair pathways, such as ROS-induced base excision repair. UV-induced DNA damage will also induce degradation in a wide variety of cells. The degradation appears to be related to the extent of damage [13]. Thus, low levels of p21 in NOD mice could reflect elevated cellular ROS modulation of signaling pathways, ROS-induced DNA damage, or both.
Other reports have demonstrated that in various tumor cell lines EGCG induced p53, causing cell growth arrest and apoptosis, typically in parallel with an induction of p21 [19, 24–27]. It was suggested that EGCG-induced p21 expression is p53 dependent, and p21 serves as a check-point for growth arrest [24, 27]. This p21-induced growth arrest could be protective, by allowing time for DNA repair, but p21 itself can also induce apoptosis, leading to cell-type dependent outcomes [24–26]. However, we show here that in HSG cells, although p53 is induced in parallel with p21 by EGCG, p21 induction is not dependent on p53 expression (Fig. 4), as demonstrated by retained induction of p21 despite down-regulation of p53 protein expression by siRNA. Consistent with this in vitro result, EGCG-induction of p21 and down-regulation of PCNA in NOD mouse submandibular glands is not coupled with induction of p53 expression, at least in terms of the proportion of expressing cells, which is lower than the proportion of p21-positive cells; also, p53 positive cells are primarily ductal, not acinar type cells (Fig. 1). The level of p53 expression within positive cells in NOD submandibular glands was not quantified, but could have been increased by EGCG treatment. A previous report suggested that co-localization of p53 and p21 in salivary ductal cells could provide a defensive mechanism and time for DNA repair while preventing apoptosis. In contrast, low p53/p21 expression in acinar cells, as observed in SS and the NOD model, could lead to acinar cell destruction [28].
p21 keeps Rb in a hypophosphorylated state, which could be essential for the maintenance of a differentiation state for epithelial cells [29]. The relationship between p21 and retinoblastoma (Rb) proteins is cell type-dependent. In epithelial cells, there is a positive transcriptional relationship between Rb and p21 [31]. In this study, inactivation of Rb by SV40 T antigen suppressed the expression of p21 in NS-SV-AC cells, while salivary gland-derived HSG cells, with functional Rb, express a considerable amount of p21 (Fig. 5). In contrast to HSG cells, NS-SV-AC cells showed increased levels of PCNA when the cells were exposed to 10 μM EGCG for 24 h (Fig. 5). This observation could be due to the combination of a lack of Rb function with the absence of p21, which normally serves as an inhibitor of PCNA function, but is degraded when translesional DNA synthesis is triggered by UV or oxidative agents [30, 31]. Importantly, at these concentrations (up to 25 μM), EGCG did not affect cell viability in HSG or NS-SV-AC cells in 24 h (Fig. 2).
Our previously obtained data from NOD mice indicates that PCNA up-regulation is associated with salivary dysfunction. Therefore, this PCNA elevation could serve as a biomarker for salivary dysfunction if it is confirmed in patients with this condition. Similarly, the inverse expression of p21 and PCNA observed in the current in vivo study represents an abnormal status in the salivary gland epithelial cells; and EGCG-induced p21 expression could be key to the normalization of intracellular signals, involving p53, p21 and PCNA. Combining measurement of p21 with PCNA could provide a useful tool to evaluate the clinical status of salivary glands. Expression of p21 has been reported to be sensitive to H2O2 levels; H2O2 at 5–50 μM, within a 30 min time period, causes proteasomal degradation of p21 [31]. Indeed, treatment of HSG cells with lower levels of H2O2 dose-dependently reduced the expression of p21 protein, without inducing PCNA (data not shown). Therefore, it is possible that the low levels of p21 in the submandibular glands of NOD mice are associated with an abnormally high level of H2O2, and warrants further exploration.
In conclusion, collective data from NOD mice demonstrate that there is an inverse relationship between p21 and PCNA expression in the salivary gland associated with SS-like autoimmune diseases, and EGCG at physiological doses can normalize the levels of p21 and PCNA. This suggests determination of the levels of p21 and PCNA in exocrine glands could be useful diagnostically, and that EGCG might serve as an agent to ameliorate exocrine gland autoimmune disorders. Further testing in humans studies will be required to examine these possibilities. While p53 serves as a regulator of DNA repair and the p21/PCNA complex, EGCG has little effect on p53 levels in vivo, and although EGCG is able to induce p53 expression in HSG cells, this induction of p53 is not required for p21 induction. Taken together, the effects of EGCG on the regulation of p21/PCNA are dependent on pRb status and environment (in vivo vs in vitro), indicating complex interactions that warrant further study.
Acknowledgments
Supported by NIDCR grant R15 DE019836-01 (SH). “The views expressed in this article are those of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or Department of Defense”.
Footnotes
Send Orders for Reprints to reprints@benthamscience.net
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Redman RS. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech Histochem. 2008;83:103–130. doi: 10.1080/10520290802374683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillespie K, Kodani I, Dickinson DP, Ogbureke K, DeRossi S, Yamamoto T, Hsu S. Effects of oral consumption of the green tea polyphenol EGCG in a murine model for human Sjogren’s syndrome, an autoimmune disease. Life Sciences. 2008;83:581–588. doi: 10.1016/j.lfs.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrera-Esparza R, Bollain-y-Goytia J, Ruvalcaba C, Ruvalcaba M, Pacheco-Tovar D, Avalos-Díaz E. Apoptosis and cell proliferation: the paradox of salivary glands in Sjögren’s disease. Acta Reumatol Port. 2008;33:299–303. [PubMed] [Google Scholar]
- 4.Hsu S, Dickinson DP, Qin H, Borke J, Ogbureke K, Walsh D, Bollag WB, Stoppler H, Sharawy M, Schuster G. Green tea polyphenols reduce autoimmune symptoms in a murine model for human Sjogren’s syndrome and protect human salivary acinar cells from TNF-alpha-induced cytotoxicity. Autoimmunity. 2007;40:138–147. doi: 10.1080/08916930601167343. [DOI] [PubMed] [Google Scholar]
- 5.Hsu S, Dickinson D, Borke J, Walsh DS, Wood J, Qin H, Winger J, Pearl H, Schuster G, Bollag WB. Green tea polyphenol induces caspase 14 in epidermal keratinocytes via MAPK pathways reduces psoriasiform lesions in the flaky skin mouse model. Exp Dermatol. 2007;16:678–684. doi: 10.1111/j.1600-0625.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Digumarthi H, Aranbayeva Z, Wataha J, Lewis J, Messer R, Qin H, Dickinson D, Osaki T, Schuster GS, Hsu S. EGCG-targeted p57/KIP2 reduces tumorigenicity of oral carcinoma cells: role of c-Jun N-terminal kinase. Toxicol Appl Pharmacol. 2007;224:318–325. doi: 10.1016/j.taap.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Pullikotil P, Chen H, Muniyappa R, Greenberg CC, Yang S, Reiter CE, Lee JW, Chung JH, Quon MJ. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J Nutr Biochem. 2012;23:1134–1145. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adachi S, Shimizu M, Shirakami Y, Yamauchi J, Natsume H, Matsushima-Nishiwaki R, To S, Weinstein IB, Moriwaki H, Kozawa O. (−)-Epigallocatechin gallate downregulates EGF receptor via phosphorylation at Ser1046/1047 by p38 MAPK in colon cancer cells. Carcinogenesis. 2009;30:1544–1552. doi: 10.1093/carcin/bgp166. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Min KW, Wimalasena J, Baek SJ. Cyclin D1 degradation and p21 induction contribute to growth inhibition of colorectal cancer cells induced by epigallocatechin-3-gallate. J Cancer Res Clin Oncol. 2012;138:2051–2060. doi: 10.1007/s00432-012-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu S, Farrey K, Wataha J, Lewis J, Borke J, Singh B, Qin H, Lapp C, Lapp D, Nguyen T, Schuster G. Role of p21WAF1 in green tea polyphenol-induced growth arrest and apoptosis of oral carcinoma cells. Anticancer Res. 2005;25:63–67. [PubMed] [Google Scholar]
- 11.Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704:12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara K, Daido S, Yamamoto A, Kobayashi R, Yokoyama T, Aoki H, Iwado E, Shinojima N, Kondo Y, Kondo S. Pivotal role of the cyclin-dependent kinase inhibitor p21WAF1/CIP1 in apoptosis and autophagy. J Biol Chem. 2008;283:388–397. doi: 10.1074/jbc.M611043200. [DOI] [PubMed] [Google Scholar]
- 13.Prives C, Gottifredi V. The p21 and PCNA partnership: a new twist for an old plot. Cell Cycle. 2008;7:3840–3846. doi: 10.4161/cc.7.24.7243. [DOI] [PubMed] [Google Scholar]
- 14.Cazzalini O, Donà F, Savio M, Tillhon M, Maccario C, Perucca P, Stivala LA, Scovassi AI, Prosperi E. p21CDKN1A participates in base excision repair by regulating the activity of poly(ADP-ribose) polymerase-1. DNA Repair (Amst) 2010;9:627–635. doi: 10.1016/j.dnarep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 16.Ota M, Kyakumoto S, Sato N. Proliferation and differentiation of human salivary gland adenocarcinoma cell line HSG. Hum Cell. 1996;9:79–88. [PubMed] [Google Scholar]
- 17.Yamamoto T, Staples J, Wataha J, Lewis J, Lockwood P, Schoenlein P, Rao S, Osaki T, Dickinson D, Kamatani T, Schuster G, Hsu S. Protective effects of EGCG on salivary gland cells treated with gamma-radiation or cis-platinum(II)diammine dichloride. Anticancer Res. 2004;24:3065–3073. [PubMed] [Google Scholar]
- 18.Wu M, Kodani I, Dickinson D, Huff F, Ogbureke KU, Qin H, Arun S, Dulebohn R, Al-Shabrawey M, Tawfik A, Prater S, Lewis J, Wataha J, Messer R, Hsu S. Exogenous expression of caspase-14 induces tumor suppression in human salivary cancer cells by inhibiting tumor vascularization. Anticancer Res. 2009;29:3811–3818. [PMC free article] [PubMed] [Google Scholar]
- 19.Liberto M, Cobrinik D. Growth factor-dependent induction of p21(CIP1) by the green tea polyphenol, epigallocatechin gallate. Cancer Lett. 2000;154:151–161. doi: 10.1016/s0304-3835(00)00378-5. [DOI] [PubMed] [Google Scholar]
- 20.Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704:12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg WC, Denning MF. P21Waf1 control of epithelial cell cycle and cell fate. Crit Rev Oral Biol Med. 2002;13:453–464. doi: 10.1177/154411130201300603. [DOI] [PubMed] [Google Scholar]
- 22.Savio M, Coppa T, Cazzalini O, Perucca P, Necchi D, Nardo T, Stivala LA, Prosperi E. Degradation of p21CDKN1A after DNA damage is independent of type of lesion, and is not required for DNA repair. DNA Repair (Amst) 2009;8:778–785. doi: 10.1016/j.dnarep.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Stearns ME, Wang M. Synergistic effects of the green tea extract epigallocatechin-3-gallate and taxane in eradication of malignant human prostate tumors. Transl Oncol. 2011;4:147–156. doi: 10.1593/tlo.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes Cip1/p21 and p16INK4a by reducing DNA methylation increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32:537–544. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakur VS, Ruhul AR, Paul RK, Gupta K, Hastak K, Agarwal MK, Jackson MW, Wald DN, Mukhtar H, Agarwal ML. p53-Dependent p21-mediated growth arrest preempts and protects HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer Lett. 2010;296:225–232. doi: 10.1016/j.canlet.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna S, Banerjee S, Mukherjee S, Das S, Panda CK. Epigallocatechin gallate induced apoptosis in Sarcoma180 cells in vivo: mediated by p53 pathway and inhibition in U1B, U4–U6 UsnRNAs expression. Apoptosis. 2006;11:2267–2276. doi: 10.1007/s10495-006-0198-2. [DOI] [PubMed] [Google Scholar]
- 27.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 28.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 29.Decesse JT, Medjkane S, Datto MB, Crémisi CE. RB regulates transcription of the p21/WAF1/CIP1 gene. Oncogene. 2001;20:962–971. doi: 10.1038/sj.onc.1204169. [DOI] [PubMed] [Google Scholar]
- 30.Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, Fotedar A, Fotedar R. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote. DNA repair Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Savio M, Coppa T, Cazzalini O, Perucca P, Necchi D, Nardo T, Stivala LA, Prosperi E. Degradation of p21CDKN1A after DNA damage is independent of type of lesion, and is not required for DNA repair. DNA Repair (Amst) 2009;8:778–785. doi: 10.1016/j.dnarep.2009.02.005. [DOI] [PubMed] [Google Scholar]