Abstract
Objective:
To investigate the population-based interaction between a biological variable (APOE ε4), neuropsychiatric symptoms, and the risk of incident dementia among subjects with prevalent mild cognitive impairment (MCI).
Methods:
We prospectively followed 332 participants with prevalent MCI (aged 70 years and older) enrolled in the Mayo Clinic Study of Aging for a median of 3 years. The diagnoses of MCI and dementia were made by an expert consensus panel based on published criteria, after reviewing neurologic, cognitive, and other pertinent data. Neuropsychiatric symptoms were determined at baseline using the Neuropsychiatric Inventory Questionnaire. We used Cox proportional hazards models, with age as a time scale, to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Models were adjusted for sex, education, and medical comorbidity.
Results:
Baseline agitation, nighttime behaviors, depression, and apathy significantly increased the risk of incident dementia. We observed additive interactions between APOE ε4 and depression (joint effect HR = 2.21; 95% CI = 1.24–3.91; test for additive interaction, p < 0.001); and between APOE ε4 and apathy (joint effect HR = 1.93; 95% CI = 0.93–3.98; test for additive interaction, p = 0.031). Anxiety, irritability, and appetite/eating were not associated with increased risk of incident dementia.
Conclusions:
Among prevalent MCI cases, baseline agitation, nighttime behaviors, depression, and apathy elevated the risk of incident dementia. There was a synergistic interaction between depression or apathy and APOE ε4 in further elevating the risk of incident dementia.
Dementia is one of the leading causes of morbidity and mortality in late life. It presents several challenges, not least of which are the economic consequences.1 Therefore, it is critical to prevent or delay dementia.2 Identification of high-risk groups is a key step toward the prevention of dementia. Mild cognitive impairment (MCI) is the intermediate stage between cognitive aging and dementia and is associated with an increased risk of dementia.3
Clinic-based samples have indicated that neuropsychiatric symptoms in prevalent MCI increase the risk of incident dementia.4–6 However, only a few studies were derived from population-based settings.7,8 In addition, studies derived from clinical samples including our own team have reported the synergistic interaction between a neuropsychiatric symptom (e.g., depression) and APOE ε4 in increasing the risk of incident dementia.9–11
While APOE ε4 and neuropsychiatric symptoms are independent risk factors for incident dementia, little is known about the interaction between APOE ε4 and a broad spectrum of neuropsychiatric symptoms in increasing the risk of incident dementia in a population-based setting. Partly, this is because one needs a very large probability sample in order to investigate interactions in a population-based setting. The Mayo Clinic Study of Aging provides such a unique opportunity to examine interactions with adequate power.
Therefore, we sought to examine the risk of incident dementia among subjects with prevalent MCI with neuropsychiatric symptoms at baseline and examined whether there was an interaction between neuropsychiatric symptoms and APOE ε4 genotype (any vs none) in predicting the risk of incident dementia.
METHODS
Setting.
This study was conducted in the setting of the Mayo Clinic Study of Aging (MCSA). Details of the study procedures have been reported elsewhere.12 Briefly, the MCSA is an ongoing population-based study examining the prevalence, incidence, and risk factors for MCI and dementia in Olmsted County, Minnesota. From a target population of 9,953 elderly residents, participants were recruited on October 1, 2004, by stratified random sampling.13 In this analysis, subjects aged 70 to 91 years were enrolled from December 2004 through September 2009 and underwent baseline and 15-month interval evaluations.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards, and informed consent for participation was obtained from every subject.
Study design.
We conducted a prospective cohort study involving subjects with prevalent MCI on whom Neuropsychiatric Inventory Questionnaire (NPI-Q) data were available at baseline. Participants with a diagnosis of dementia at baseline were excluded. Subjects who had MCI with or without neuropsychiatric symptoms were followed forward in time to the outcome of incident dementia as measured by the DSM-IV criteria.14 NPI-Q data were available on 391 subjects with MCI, of whom 38 individuals were lost to follow-up and 21 died. Therefore, the final analyses included 332 subjects with MCI (figure 1).
Figure 1. Flowchart.
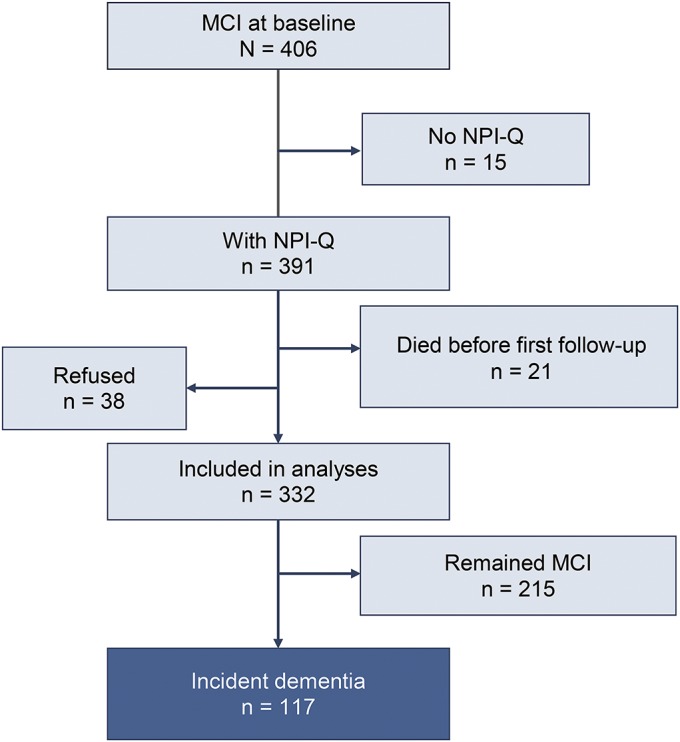
MCI = mild cognitive impairment; NPI-Q = Neuropsychiatric Inventory Questionnaire.
Cognitive evaluation.
Participants of the MCSA underwent the following 3 face-to-face evaluations: (1) risk factor ascertainment (including NPI-Q) and baseline evaluation (including Clinical Dementia Rating Scale15) performed by a nurse or study coordinator; (2) neurologic evaluation including a neurologic interview, Short Test of Mental Status,16 and neurologic examination performed by behavioral neurologists; and (3) neuropsychological evaluation of 4 cognitive domains—memory (delayed recall trials from the Auditory Verbal Learning Test17 and the Wechsler Memory Scale–Revised,18 Logical Memory and Visual Reproduction subtests); language (Boston Naming Test19 and category fluency); visuospatial (Wechsler Adult Intelligence Scale–Revised,20 Picture Completion and Block Design subtests); and executive function (Trail Making Test Part B21 and the Wechsler Adult Intelligence Scale–Revised, Digit Symbol subtest). All tests were administered by psychometrists and supervised by neuropsychologists.
An expert consensus panel of physicians, neuropsychologists, and nurses or study coordinators reviewed the data and made the diagnosis of MCI, based on the revised Mayo Clinic criteria,22 and dementia, based on the DSM-IV criteria.14
Subtypes of MCI.
MCI was classified into amnestic and nonamnestic type based on whether or not the memory domain was impaired as defined by a z score ≤−1 below the mean. In addition, MCI was further classified into single-domain or multiple-domain impairment according to the number of cognitive domains involved. For instance, an individual with language impairment only was defined as nonamnestic MCI, single-domain type, whereas an individual with memory and language domain impairment was classified as amnestic MCI, multiple-domain type.3
Measurement of neuropsychiatric symptoms.
Neuropsychiatric symptoms were measured by using the NPI-Q. The NPI-Q is a shorter version of the Neuropsychiatric Inventory, a validated clinical instrument.23 We considered the NPI-Q an appropriate screening instrument because it assesses a broad variety of neuropsychiatric symptoms and was also selected by the Uniform Data Set Initiative of the National Institute on Aging.24 It was administered as a structured interview to an informant, usually the spouse. The NPI-Q is designed to obtain information on 12 emotional behaviors (i.e., agitation, delusion, hallucination, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep, and eating/appetite). The exposure of interest in our study was the presence or absence of each symptom assessed by the NPI-Q. This is analogous to our previous work that examined the outcome of incident cognitive decline by presence/absence of depression at baseline.25 Subjects with missing NPI-Q data were excluded from our analyses.
APOE genotyping.
After obtaining informed consent, blood was drawn from the study participants. Then, APOE ε4 genotypes were determined from its DNA using a PCR amplification.26 The laboratory technicians were blinded to other study variables.
Statistical analysis.
We conducted statistical analyses to assess the risk of incident dementia among prevalent MCI cases with or without specific neuropsychiatric symptoms at baseline. We computed hazard ratios (HRs) and 95% confidence intervals (95% CIs) to assess the association between the independent variable (neuropsychiatric symptoms as measured by the NPI-Q) and the outcome of incident dementia using Cox proportional hazards models adjusted for age, sex, education, and medical comorbidity.27 We used the following rationales to select covariates: (1) traditional confounders (age and sex) were included as covariates; (2) education is a critical covariate for our type of research. Accordingly, we have adjusted for education; (3) given that most of our participants are elderly persons who are likely to have multiple medical comorbidities, we used the standard Charlson index to adjust for comorbid medical conditions. The Charlson Comorbidity Index predicts the 10-year mortality for a patient with a total of 22 potential comorbid conditions and was calculated using the Deyo method. Herein, a composite index was calculated after numeric values were assigned to comorbid medical conditions.28
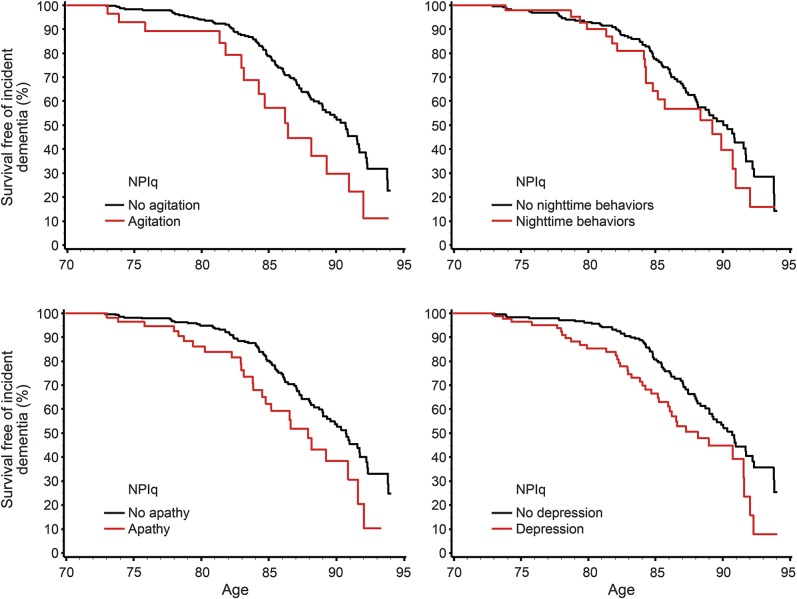
We used Kaplan-Meier survival curves for visual display of data, with age as a time scale (figure 2). We examined possible interaction effects with APOE ε4 genotype by using multivariate models to test for additive interactions. Statistical testing was done at the conventional 2-tailed α level of p < 0.05. Statistical analyses were performed using SAS System, version 9.3 software (SAS Institute, Cary, NC).
Figure 2. Survival curves for agitation, nighttime behaviors, apathy, and depression.
NPIq = NPI-Q, Neuropsychiatric Inventory Questionnaire.
RESULTS
Baseline demographic characteristics.
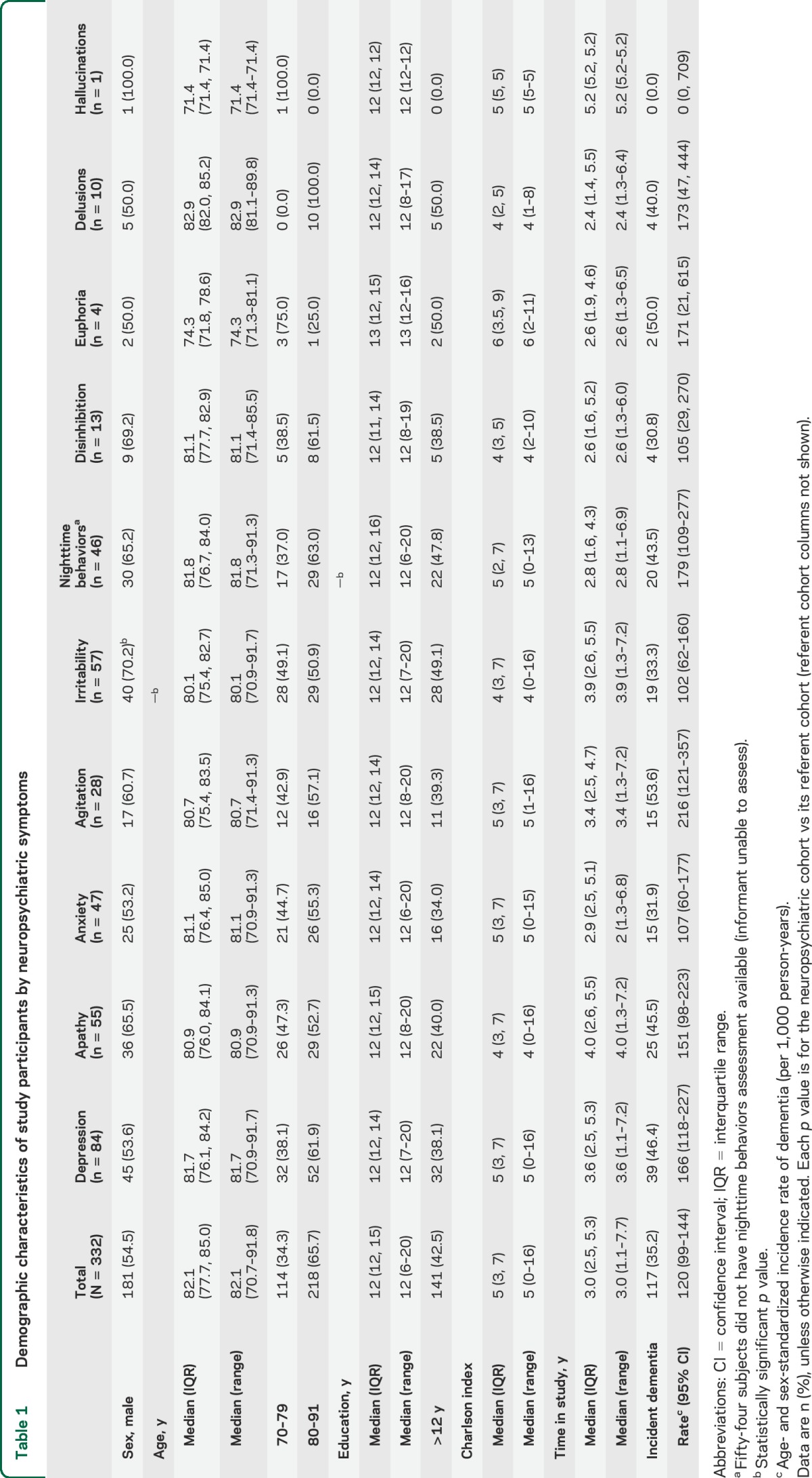
We prospectively followed subjects with MCI who had available NPI-Q data (n = 391) to the outcome of incident dementia (n = 117) or censoring variables (death, n = 21; loss to follow-up, n = 38) for a median (interquartile range [IQR]) of 3.0 (2.5, 5.3) years (figure 1). The median (IQR) age was 82.1 years (77.7, 85.0), 54.5% were males, the median (IQR) education was 12 years (12, 15), and the median (IQR) number of medical comorbidities was 5 (3, 7) as measured by the Charlson Comorbidity Index.28 Neuropsychiatric data were missing for 54 subjects with nighttime behavior, and APOE genotype data were missing for 3 subjects. All other data were complete. The complete demographic characteristics are summarized in table 1.
Table 1.
Demographic characteristics of study participants by neuropsychiatric symptoms
Risk of incident dementia.
We calculated the incidence of dementia as predicted by baseline neuropsychiatric status. We observed an overall age- and sex-standardized incidence rate of 120 per 1,000 person-years. Incidence rate differed by neuropsychiatric symptoms. For example, for the agitation cohort alone, the annual age- and sex-standardized incidence rate was 216 per 1,000 person-years. Details of the findings are described in table 1.
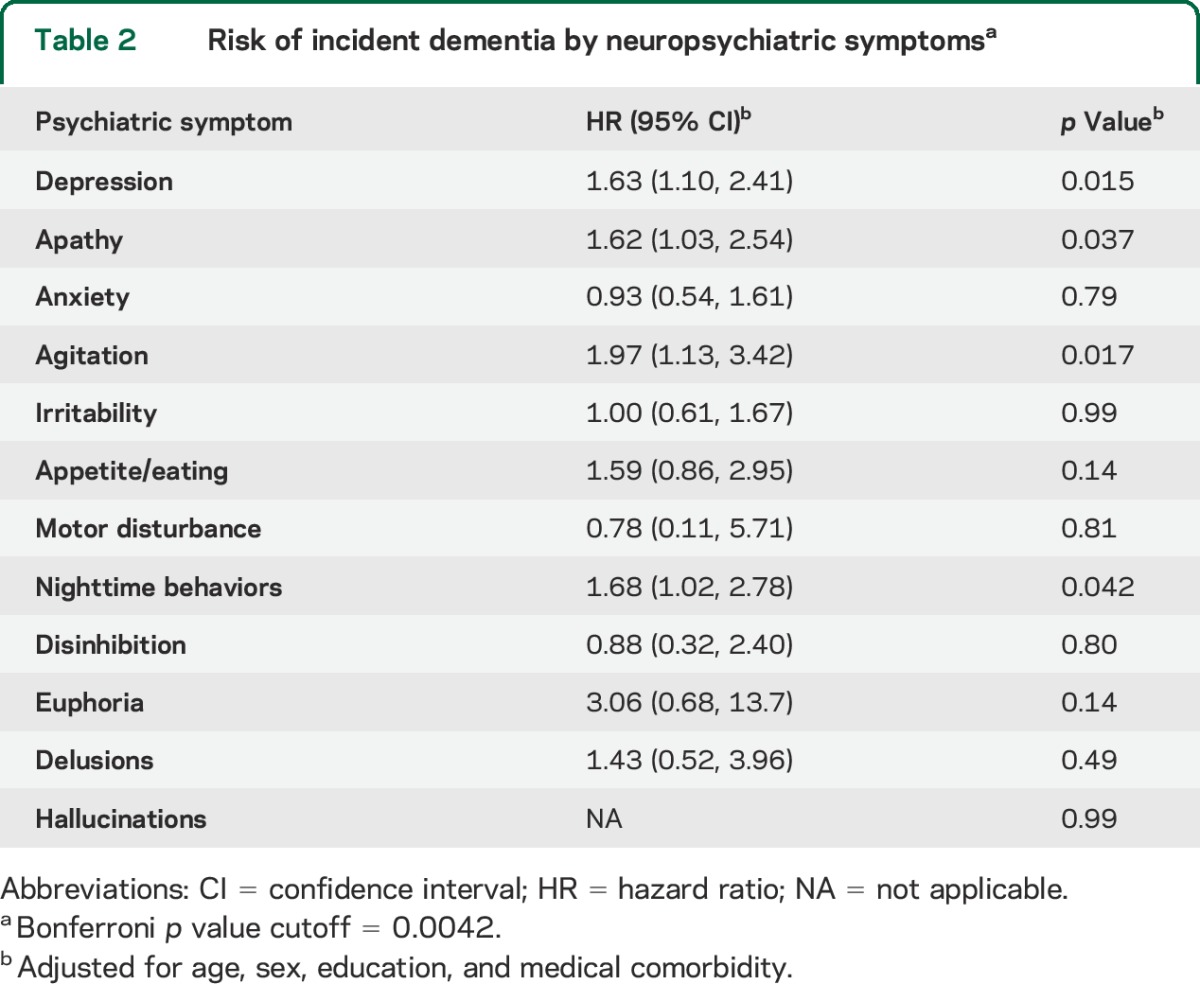
Baseline agitation, nighttime behaviors, depression, and apathy significantly increased the risk of incident dementia. Conversely, motor disturbance, anxiety, irritability, and appetite/eating were not associated with increased risk of dementia. In addition, psychotic symptoms and other emotional behaviors including disinhibition, delusions, and euphoria did not reach statistical significance. A summary of the HRs is displayed in table 2.
Table 2.
Risk of incident dementia by neuropsychiatric symptomsa
We examined whether there was an interaction between neuropsychiatric symptoms and APOE ε4 genotype (any vs none) in predicting the risk of incident dementia. We defined the reference group as subjects who did not carry any ε4 allele and who did not have neuropsychiatric symptoms. Compared with the reference group, subjects with depression but no ε4 allele (ε3/ε4 or ε4/ε4) had an HR of 1.39 (95% CI = 0.84–2.31) for incident dementia, subjects without depression but with an ε4 allele had an HR of 1.02 (95% CI = 0.62–1.66), and subjects with both depression and an ε4 allele had an HR of 2.21 (95% CI = 1.24–3.91; table 3). A test for additive interaction was significant (p < 0.001), whereas a test for multiplicative interaction was not (p = 0.29).
Table 3.
Investigation of the interaction between neuropsychiatric symptoms and APOE ε4 genotype and the outcome of incident dementiaa
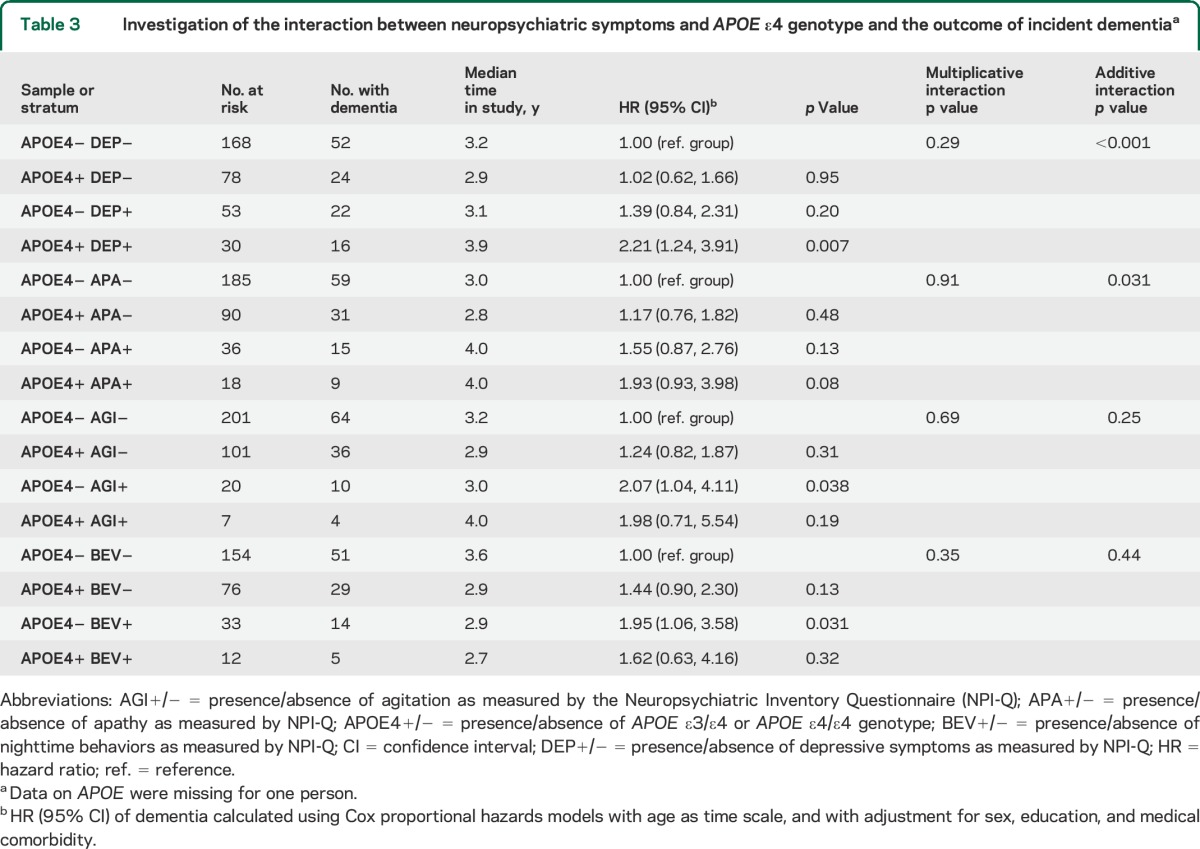
Similarly, compared with the reference group, subjects with apathy but no ε4 allele had an HR for incident dementia of 1.55 (95% CI = 0.87–2.76), subjects without apathy but with an ε4 allele had an HR of 1.17 (95% CI = 0.76–1.82), and the HR of subjects with both apathy and an ε4 allele (HR = 1.93 [95% CI = 0.93–3.98]) approached significance (table 3). A test for additive interaction was statistically significant (p = 0.031), whereas a test for multiplicative interaction was not (p = 0.91). There were no interactions between an ε4 allele and other neuropsychiatric symptoms in predicting the risk of incident dementia.
We conducted a series of sensitivity analyses to examine whether there were interactions between MCI subtypes and any of the 12 neuropsychiatric symptoms to influence the outcome of incident dementia. We did not observe any interaction between MCI subtypes and neuropsychiatric symptoms except for nighttime behavior, which changed from significant (p = 0.042) to a trend (p = 0.11) when we specifically investigated the interaction between amnestic MCI and neuropsychiatric symptoms.
DISCUSSION
Herein, we report the population-based risk of incident dementia by baseline neuropsychiatric symptoms among subjects with MCI. In addition, we investigated the interaction between APOE ε4 and neuropsychiatric symptoms in predicting incident dementia. APOE ε4 is associated with amyloid29 and glucose hypometabolism30 as measured by PET.
Because APOE ε4 is an established risk factor for Alzheimer disease (AD), we investigated whether there was an interaction between neuropsychiatric symptoms and APOE ε4 in predicting the outcome of incident dementia among subjects with prevalent MCI. We observed a synergistic additive interaction between APOE ε4 genotype and neuropsychiatric symptoms (i.e., depression and apathy), whereas a test for multiplicative interaction was not significant. Thus, the combined presence of depression and APOE ε4, as well as apathy and APOE ε4, was greater than the expected arithmetic sum of their independent effects. Furthermore, additive interaction is more applicable to biological events than multiplicative interaction.31 This finding has relevance to recommendations that emphasize identification of APOE ε4–enriched samples for interventional studies that aim to delay dementia.32
To date, studies primarily focused on 1 or 2 neuropsychiatric symptoms5,7; only a small number of studies examined the incidence of dementia by a wide variety of neuropsychiatric symptoms in MCI subjects.4,33,34 For instance, investigators from Johns Hopkins University used a clinical sample assembled by the National Alzheimer's Coordinating Center database to examine the risk of incident dementia by baseline neuropsychiatric symptoms among 1,821 subjects with prevalent MCI whom they followed for less than 2 years. They found that any neuropsychiatric symptom was significantly associated with an increased risk of developing incident dementia.33 Our study expands on these clinic-based findings by showing more specifically that agitation, depression, nighttime behavior, and apathy increased the risk of dementia. In a relatively smaller study, investigators from the University of California, Los Angeles, followed 51 subjects with MCI to the outcome of incident dementia for a mean of 2 years and examined neuropsychiatric symptoms as predictors. They reported apathy and depression to be significant predictors of dementia.4 Our study corroborates this preliminary finding and extends it by showing that agitation and nighttime behaviors also elevate the risk of incident dementia in a population-based setting.
Consistent with our findings, other investigators have reported that apathy increases the risk of AD among MCI subjects.4,5 In addition, investigators from the Karolinska Institute in Sweden followed 47 subjects with MCI for an average of 3 years and observed that depressive symptoms and anxiety increased the risk of incident AD.7 However, we followed a larger cohort (n = 332) of subjects with MCI for more than 3 years to the outcome of incident dementia and did not observe a significant association with anxiety. The discrepancies in these findings may be attributable to methodologic differences, such as sample size and different assessment tools for neuropsychiatric symptoms.
In this research, we did not investigate mechanisms linking neuropsychiatric symptoms with the outcome of incident dementia. However, in the past, our team has proposed 4 possible theoretical explanations for the link between neuropsychiatric symptoms and dementia.35 They are: (1) the etiologic pathway: a particular neuropsychiatric symptom such as depression may have a direct deleterious effect on the brain via the hypothalamus-pituitary axis and lead to incident dementia, in which case neuropsychiatric symptoms would represent “risk factors”; (2) shared risk factor or confounding pathway: a biological risk factor for dementia, for instance, β-amyloid, may be the cause of both cognitive outcome and neuropsychiatric symptoms, in which case neuropsychiatric symptoms might be better considered as “disease markers”; (3) a synergistic interaction: a neuropsychiatric symptom and a biological factor such as APOE ε4 genotype may have a synergistic interaction to elevate the risk of incident dementia; and (4) reverse causality: when a person starts noticing cognitive decline then the individual may show reactive depression. In this scenario, it is the cognitive decline that led to the genesis of neuropsychiatric symptom. It is to be noted that the above 4 theoretical constructs are not mutually exclusive. More important, these proposed constructs need to be empirically validated by mechanistic research; until then, these models remain to be hypothetical and speculative.
Our study has several strengths. First, the diagnoses of MCI and dementia were made at a center that has well-established expertise in the field of MCI. Second, we were able to screen for a broad spectrum of neuropsychiatric symptoms, and the longitudinal follow-up enabled us to observe the association between neuropsychiatric symptoms and the outcome of incident dementia. Third, the population-based nature of our study makes our findings less prone to referral bias and enhances their generalizability.36
Our study also has limitations. The NPI-Q was administered to informants, primarily spouses, knowledgeable of the study subjects. Whereas the NPI-Q has the advantage of gathering observed behaviors, informants may miss subtle signs. While our study's goal of examining the presence or absence of a baseline neuropsychiatric symptom in predicting incident dementia addresses a clinically relevant question, it is also possible that factoring in severity of symptoms might have added more depth to our findings.
Furthermore, the observed association between nighttime behaviors and incident dementia should be interpreted with caution because relevant data were missing in 54 subjects. However, the limited data we have gathered have led to a finding consistent with previous literature.37 In addition, some of the assessed symptoms were rare; hence, their analyses should be interpreted with caution. For instance, there were only 4 subjects in the euphoria cohort, of whom 2 cases developed dementia during follow-up. There were only 10 subjects with baseline delusions, of whom 4 developed dementia. Thus, the small number of study participants with motor disturbance, euphoria, delusions, psychosis, and disinhibition makes it difficult to completely assess the contribution of these symptoms to incident dementia. While a median follow-up of 3 years in the current study may be perceived as relatively short, investigators from the Pittsburgh Cardiovascular Health Study–Cognition Study reported a mean time of conversion from MCI to dementia of 2.8 years.38
Because we assessed 12 neuropsychiatric symptoms, it is reasonable to raise the question of multiplicity. Even though there is controversy in the literature as to whether it is necessary to routinely adjust for multiplicity,39,40 we opted to provide the reader with the Bonferroni cutoff value as well as the HR for each symptom including symptoms with extremely rare events. While some investigators do not recommend Bonferroni correction to avoid type 2 error,39 others have suggested routine correction for multiplicity, especially in specific types of studies, e.g., genomic research.40 We believe that our results are less prone to type I error based on the following rationales: (1) the primary outcome of our study (incident dementia) and all independent variables were defined a priori and we treated each of the 12 assessed symptoms as individual categorical variables; (2) in addition, the literature had given us some sense of the expected results of our hypothesis.11,33 Indeed, our findings are consistent with the limited available literature. This indicates that our study led to real findings.
Our team recently reported that agitation, apathy, and depression also increased the risk of incident MCI.25 Here, we report that these 3 symptoms also increased the risk of incident dementia. Therefore, agitation, apathy, and depression might be manifestations of an underlying neurobiological process driving the transitions from normal aging, to MCI, and subsequently to dementia. In a future study, we will examine possible mechanisms by investigating neuropsychiatric symptoms and their relationship with chemical and imaging biomarkers acquired by the MCSA from thousands of elderly study participants over a 10-year period.
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- HR
hazard ratio
- IQR
interquartile range
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- NPI-Q
Neuropsychiatric Inventory Questionnaire
AUTHOR CONTRIBUTIONS
Study concept and design: Dr. Pink, Dr. Stokin, Dr. Roberts, Dr. Petersen, Dr. Geda. Acquisition of data: Dr. Roberts, Dr. Knopman, Dr. Petersen, Dr. Geda. Analysis and interpretation of data: Dr. Pink, Dr. Stokin, Dr. Roberts, Ms. Christianson, Dr. Geda, Dr. Pankratz. Drafting of the manuscript: Dr. Pink, Dr. Stokin, Dr. Geda. Critical revision of the manuscript for important intellectual content: Dr. Stokin, Dr. Bartley, Dr. Roberts, Dr. Sochor, Dr. Machulda, Dr. Krell-Roesch, Dr. Knopman, Dr. Acosta, Dr. Mielke, Dr. Geda. Statistical analysis: Ms. Christianson, Dr. Pankratz. Obtained funding: Dr. Stokin, Dr. Roberts, Dr. Sochor, Dr. Petersen, Dr. Geda. Administrative, technical, and material support: Dr. Stokin, Dr. Roberts, Dr. Sochor, Dr. Petersen, Dr. Geda. Study supervision: Dr. Roberts, Dr. Petersen, Dr. Geda.
STUDY FUNDING
Support for this research was provided by NIH grants: National Institute of Mental Health (K01 MH068351 to Y.E.G.), and National Institute on Aging (U01 AG006786 to R.C.P. and K01 AG028573 to R.O.R.). This project was also supported by the Robert Wood Johnson Foundation (to Y.E.G.), the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program (to R.C.P. and Y.E.G.), the European Regional Development Fund FNUSA-ICRC (CZ.1.05/1.1.00/02.0123; to A.P., G.B.S., O.S., J.K.-R., and Y.E.G.), the Arizona Alzheimer's Consortium (to Y.E.G.), and Department of Sport and Sport Science, Karlsruhe Institute of Technology, Germany (J.K.-R.).
DISCLOSURE
A. Pink, G. Stokin, M. Bartley, R. Roberts, O. Sochor, M. Machulda, and J. Krell-Roesch report no disclosures relevant to the manuscript. D. Knopman serves as Deputy Editor for Neurology®; serves on a data safety monitoring board for Lundbeck Pharmaceuticals and for the Dominantly Inherited Alzheimer's Disease Treatment Unit. He has served on a data safety monitoring board for Lilly Pharmaceuticals; served as a consultant to TauRx, was an investigator in clinical trials sponsored by Baxter and Elan Pharmaceuticals in the past 2 years; and receives research support from the NIH. J. Acosta did the work while she was an employee of Mayo Clinic. Currently, she is a full-time employee of Piramal Inc., Boston, MA. T. Christianson, V. Pankratz, and M. Mielke report no disclosures relevant to the manuscript. R. Petersen reports being a consultant to GE Healthcare and Elan Pharmaceuticals; serving on a data safety monitoring board in clinical trials sponsored by Pfizer Incorporated and Janssen Alzheimer Immunotherapy; and gave a CME lecture at Novartis Incorporated. Y. Geda reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement 2013;9:208–245. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC. Clinical practice: mild cognitive impairment. N Engl J Med 2011;364:2227–2234. [DOI] [PubMed] [Google Scholar]
- 4.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer's disease. Dement Geriatr Cogn Disord 2007;24:253–259. [DOI] [PubMed] [Google Scholar]
- 5.Robert PH, Berr C, Volteau M, et al. Importance of lack of interest in patients with mild cognitive impairment. Am J Geriatr Psychiatry 2008;16:770–776. [DOI] [PubMed] [Google Scholar]
- 6.Ramakers IH, Visser PJ, Aalten P, Kester A, Jolles J, Verhey FR. Affective symptoms as predictors of Alzheimer's disease in subjects with mild cognitive impairment: a 10-year follow-up study. Psychol Med 2010;40:1193–1201. [DOI] [PubMed] [Google Scholar]
- 7.Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 2007;68:1596–1602. [DOI] [PubMed] [Google Scholar]
- 8.Brodaty H, Heffernan M, Draper B, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis 2012;31:411–420. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran G, Marder K, Tang M, et al. A preliminary study of apolipoprotein E genotype and psychiatric manifestations of Alzheimer's disease. Neurology 1996;47:256–259. [DOI] [PubMed] [Google Scholar]
- 10.Scarmeas N, Brandt J, Albert M, et al. Association between the APOE genotype and psychopathologic symptoms in Alzheimer's disease. Neurology 2002;58:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol 2006;63:435–440. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, III, Rocca WA. Use of a medical records-linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 16.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The Short Test of Mental Status: correlations with standardized psychometric testing. Arch Neurol 1991;48:725–728. [DOI] [PubMed] [Google Scholar]
- 17.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 18.Wechsler D. Wechsler Memory Scale–Revised. New York: The Psychological Corporation; 1987. [Google Scholar]
- 19.Kaplan E, Goodglass H, Brand S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 21.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276. [Google Scholar]
- 22.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 23.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 25.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014;171:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990;31:545–548. [PubMed] [Google Scholar]
- 27.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 29.Fleisher AS, Chen K, Liu X, et al. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 2013;34:1–12. [DOI] [PubMed] [Google Scholar]
- 30.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996;334:752–758. [DOI] [PubMed] [Google Scholar]
- 31.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Gaithersburg: Aspen; 2000. [Google Scholar]
- 32.Selkoe DJ. Preventing Alzheimer's disease. Science 2012;337:1488–1492. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013;21:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan WC, Lam LC, Tam CW, et al. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing 2011;40:30–35. [DOI] [PubMed] [Google Scholar]
- 35.Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimers Dement 2013;9:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kokmen E, Ozsarfati Y, Beard CM, O'Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer's disease. J Clin Epidemiol 1996;49:79–83. [DOI] [PubMed] [Google Scholar]
- 37.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez OL, Becker JT, Chang YF, et al. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study–Cognition Study. Neurology 2012;79:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- 40.Patel CJ, Ioannidis JPA. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J Epidemiol Community Health 2014;68:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]